Colourimetric Plate Assays Based on Functionalized Gelatine Hydrogel Useful for Various Screening Purposes in Enzymology
Abstract
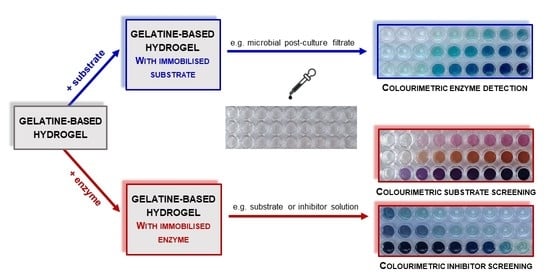
1. Introduction
2. Results and Discussion
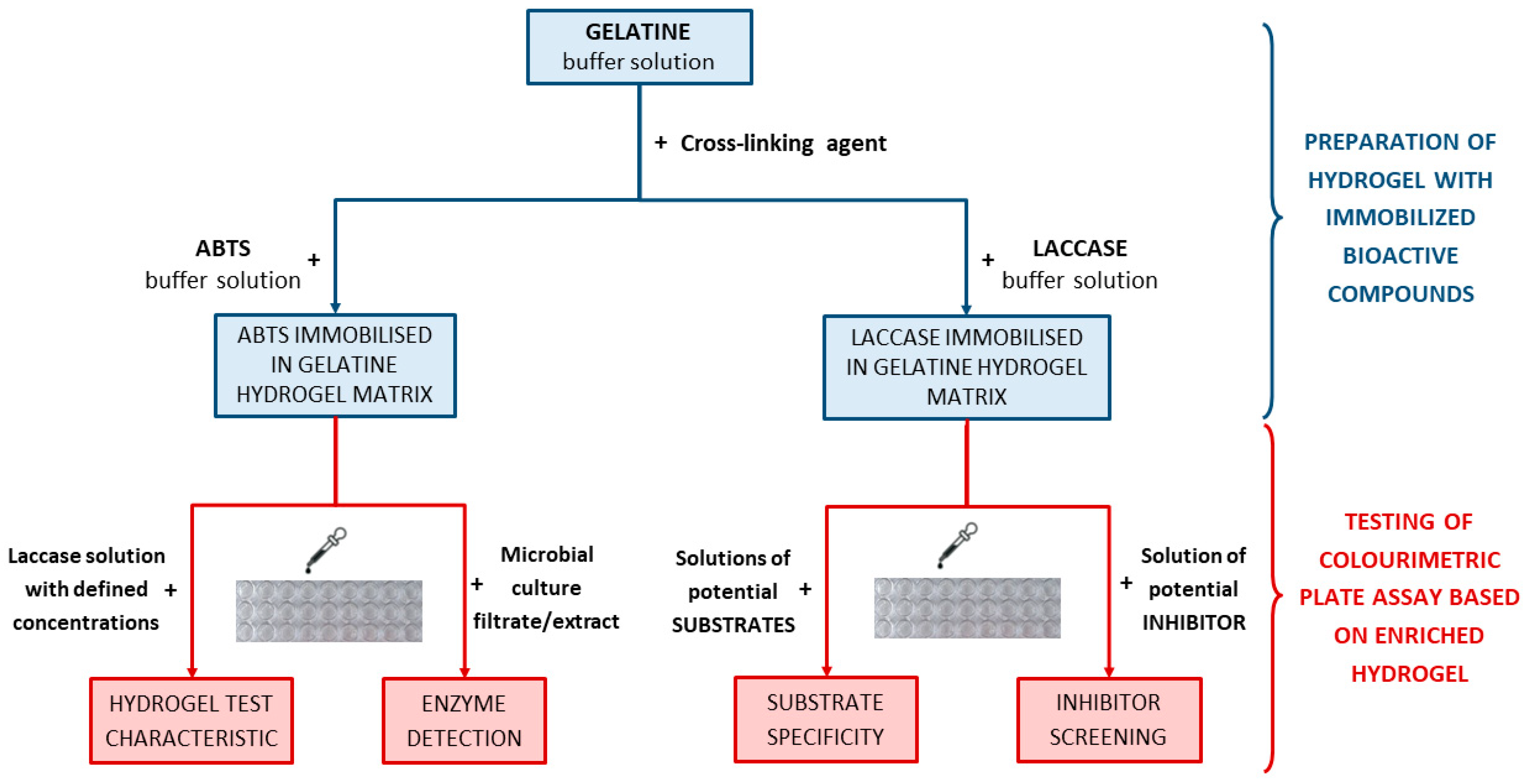
2.1. Hydrogel-Based Test for Colourimetric Detection of Laccase
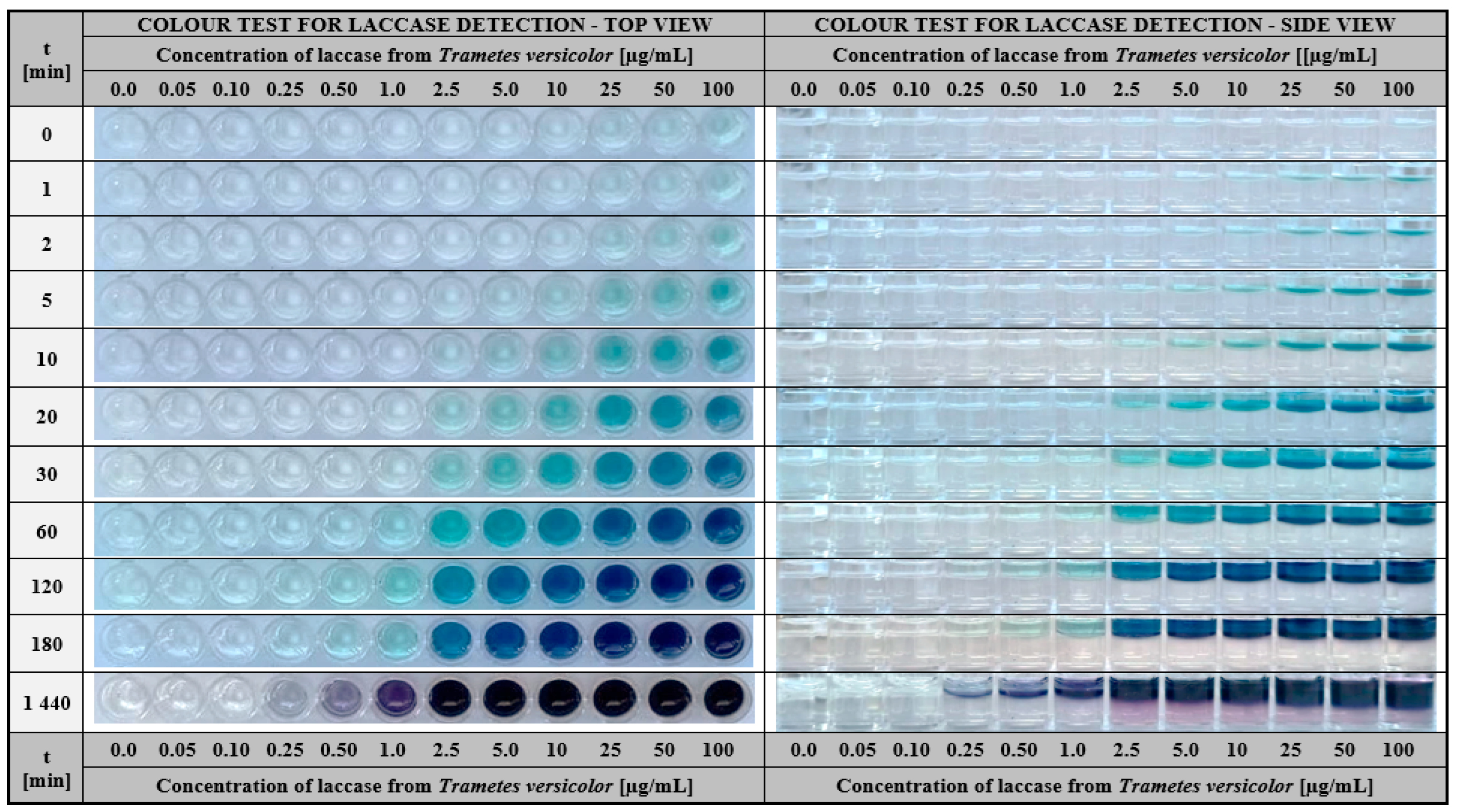
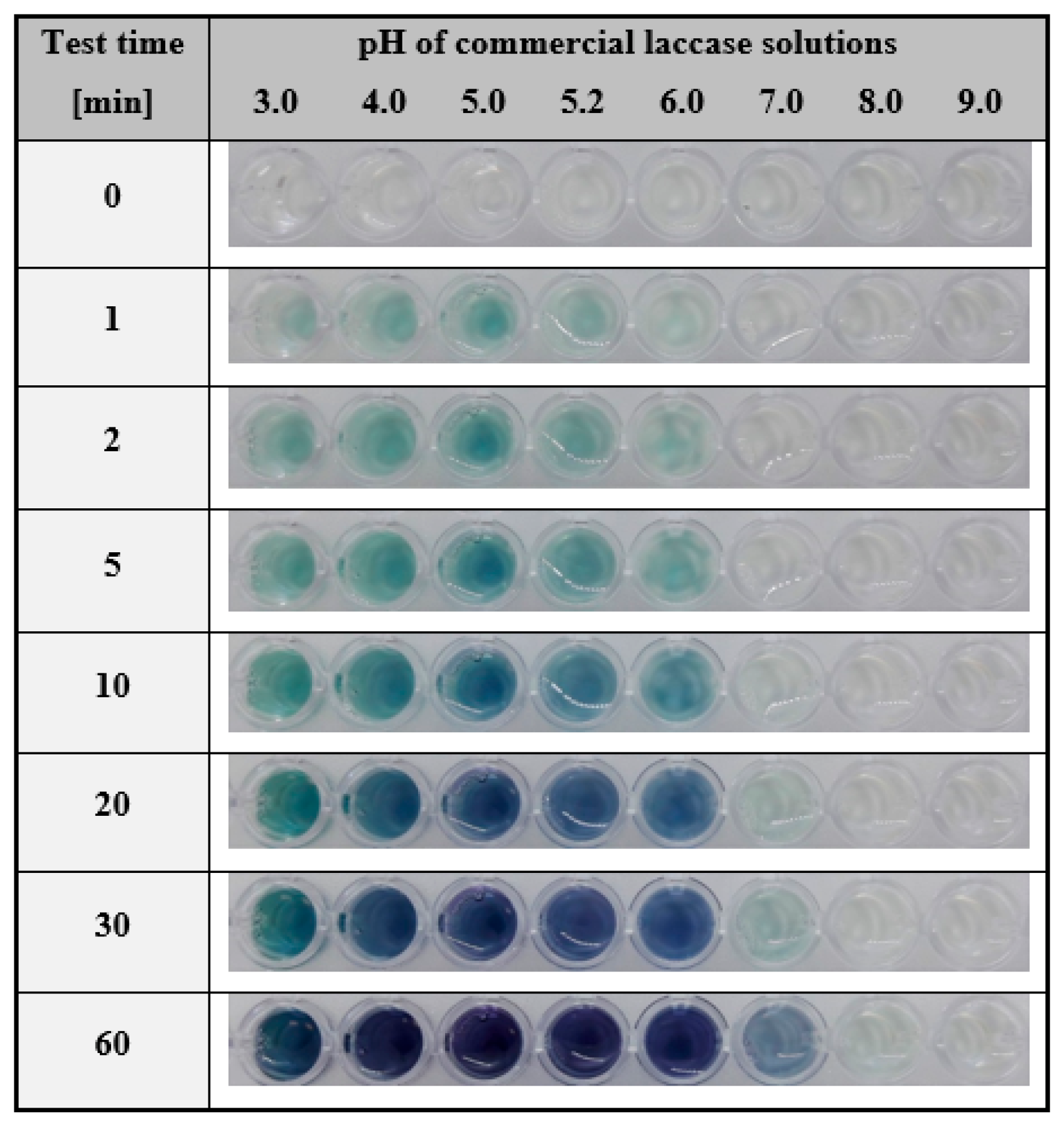
2.1.1. Development of the Test for the Detection of Laccase in Aqueous Solutions
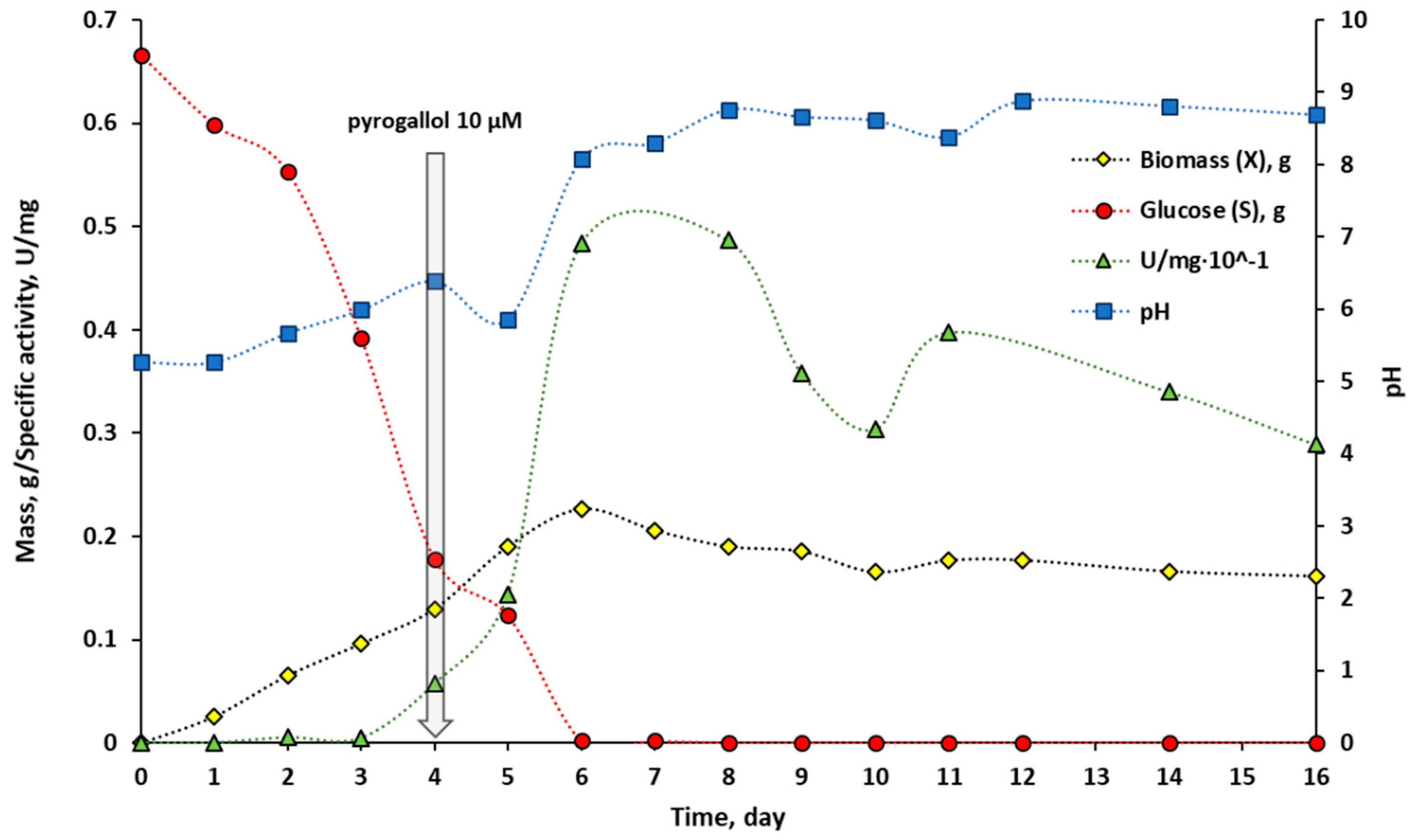
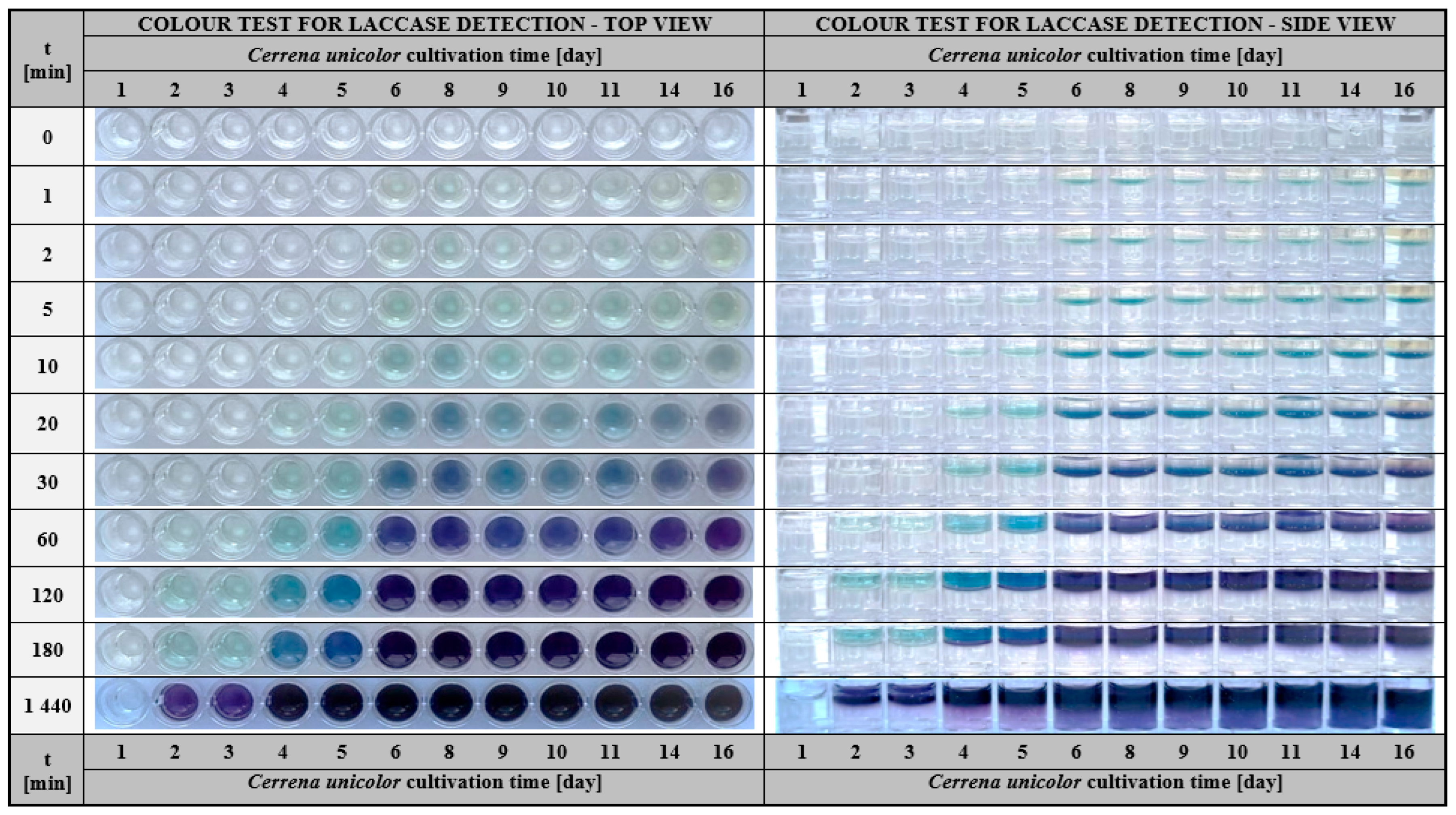
2.1.2. Application of the Hydrogel-Based Colourimetric Assay in Microbiological Cultures
2.2. Gelatine Hydrogels Containing Immobilised Laccase for Various Purposes in Enzymology
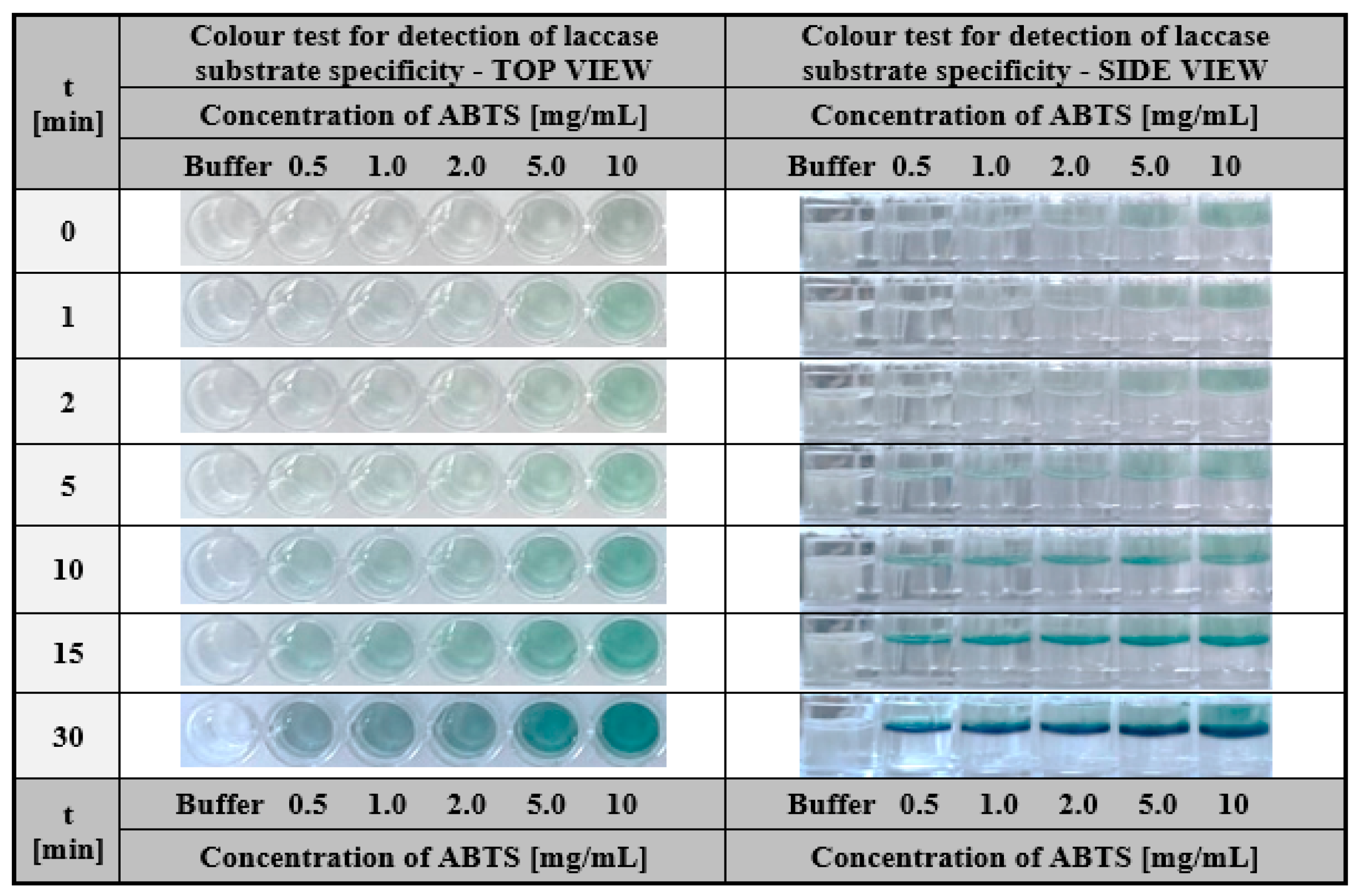
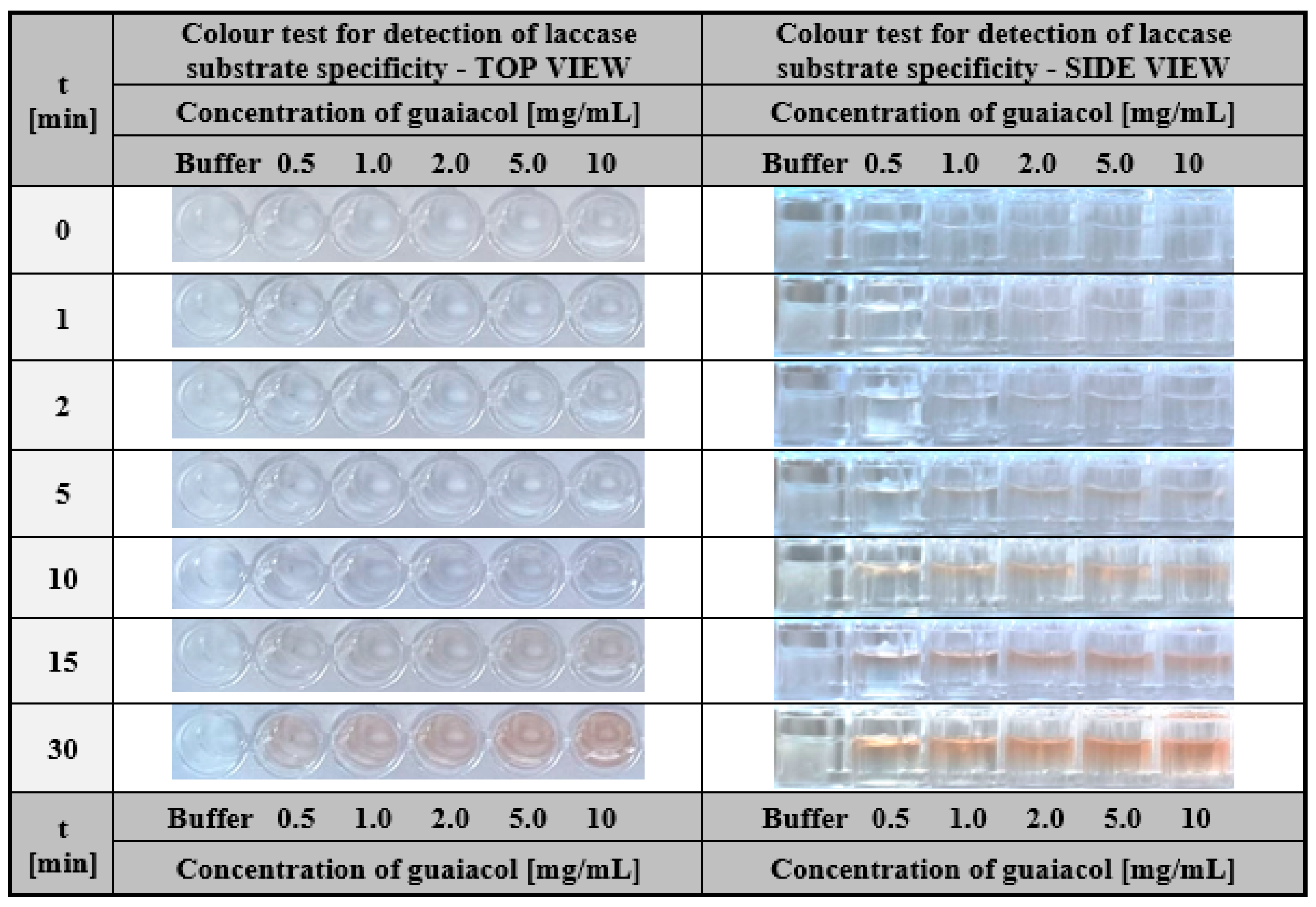
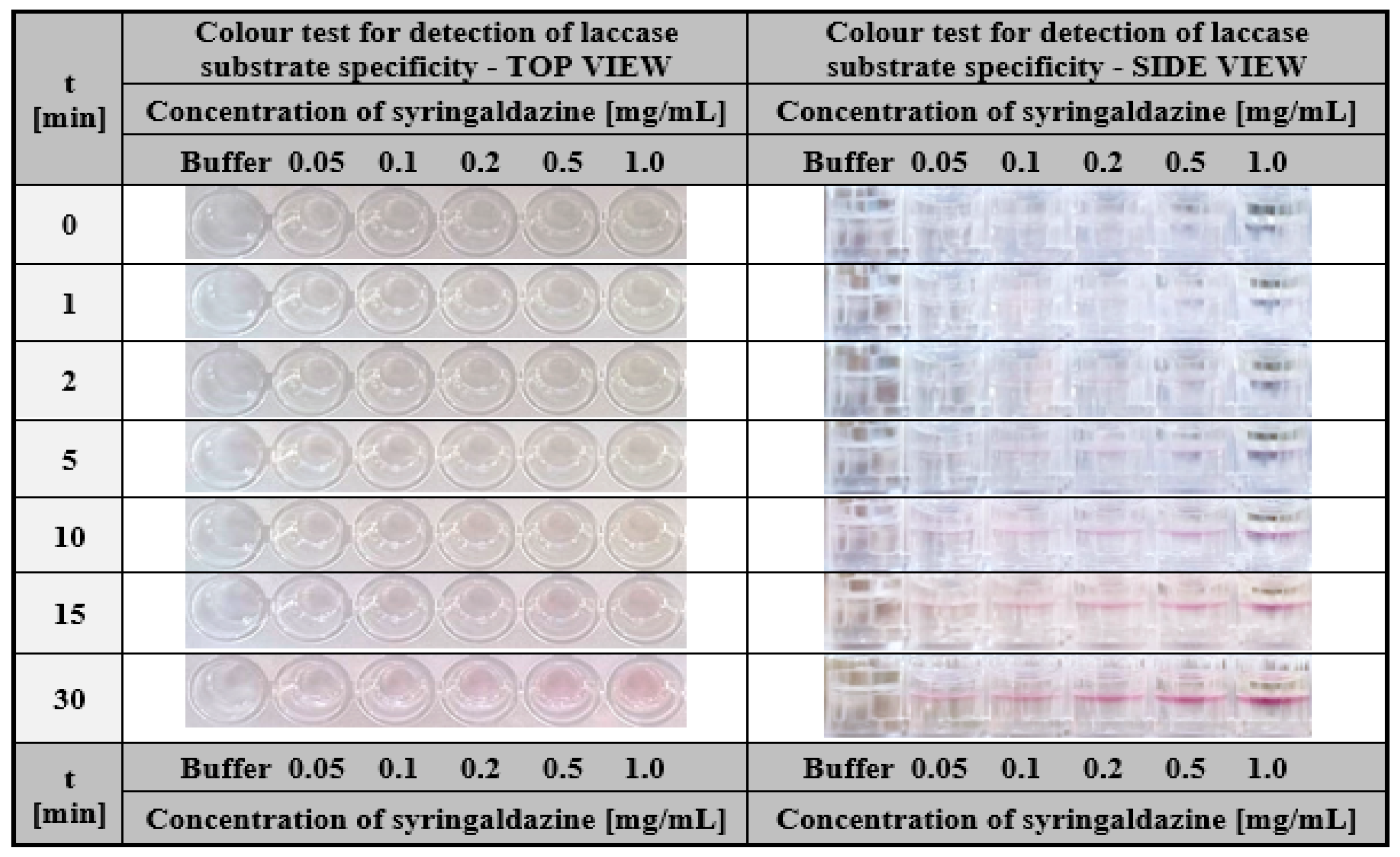
2.2.1. Hydrogel-Based Assay for Colourimetric Screening a Substrate Specificity of Laccase
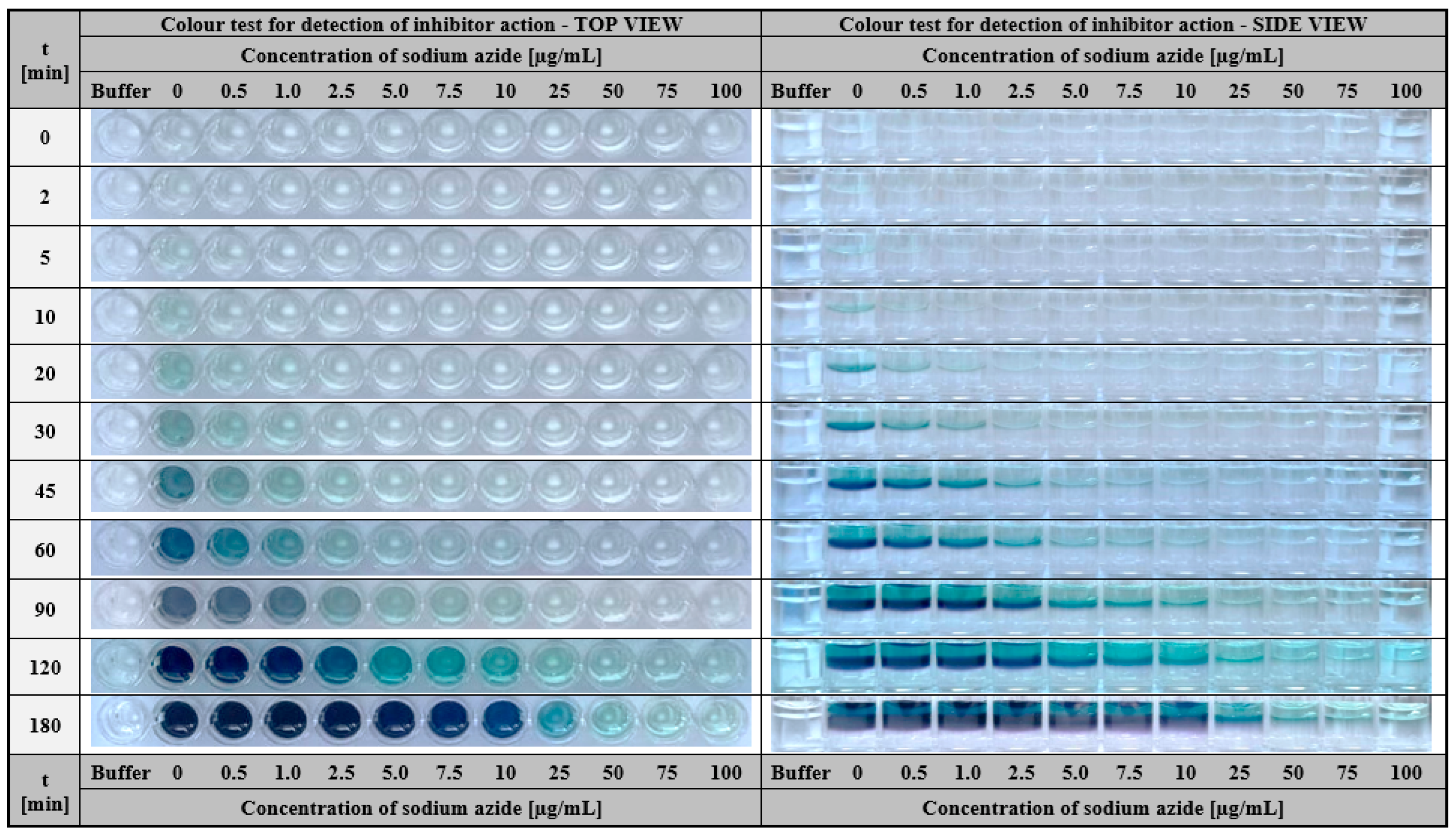
2.2.2. Hydrogel-Based Assay for Colourimetric Screening Potential Inhibitors of Laccase
2.3. Results Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Gelatine Hydrogel Matrices Containing Reactive Compounds
3.2.2. Hydrogel-Based Assay for Colourimetric Detection of Laccase
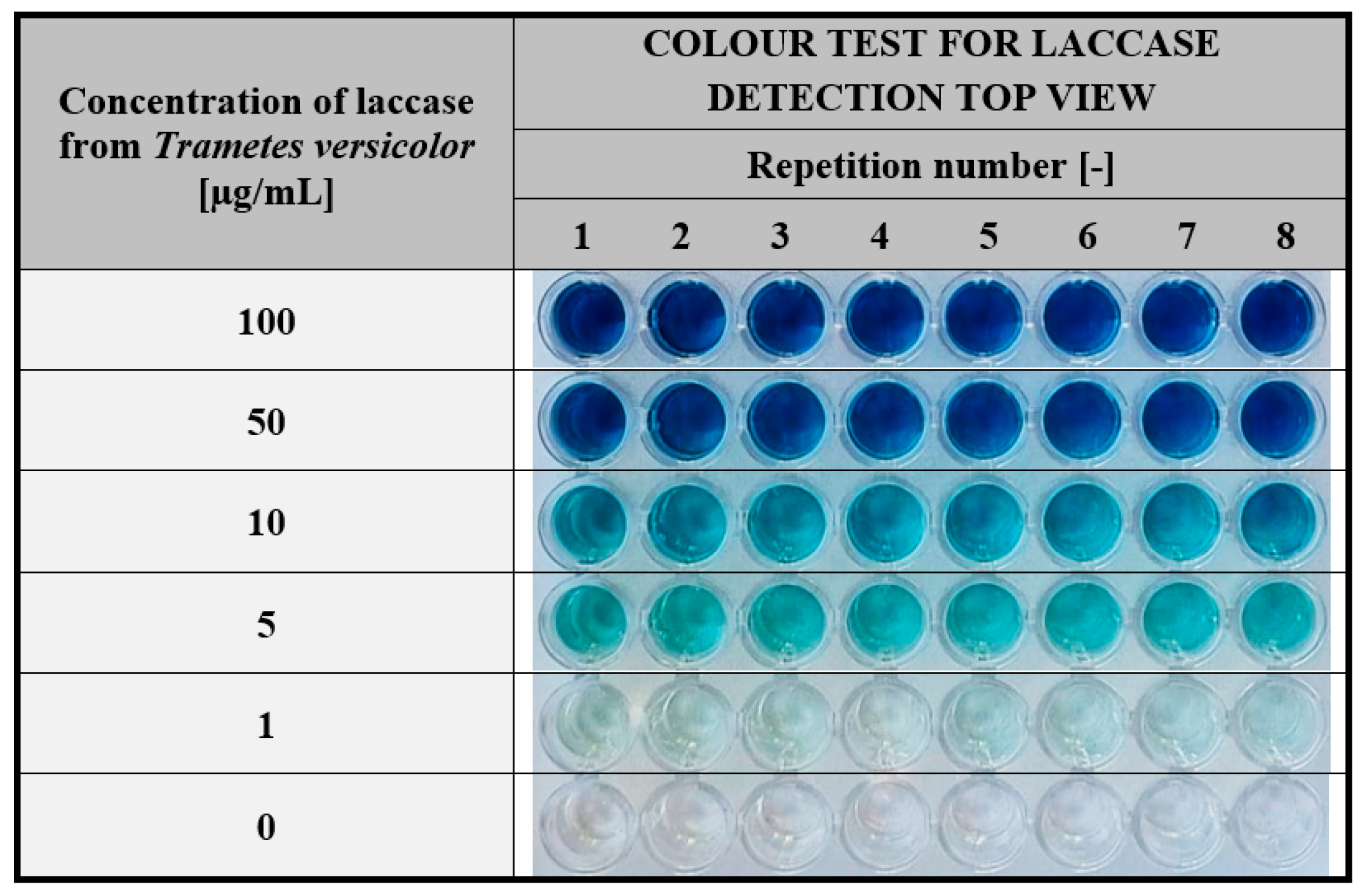
3.2.3. Determination of Laccase Concentration Range Effectively Detected by the Hydrogel-Based Assay
3.2.4. Determination of the pH Range in which Laccase Is Effectively Detected by the Hydrogel-Based Assay
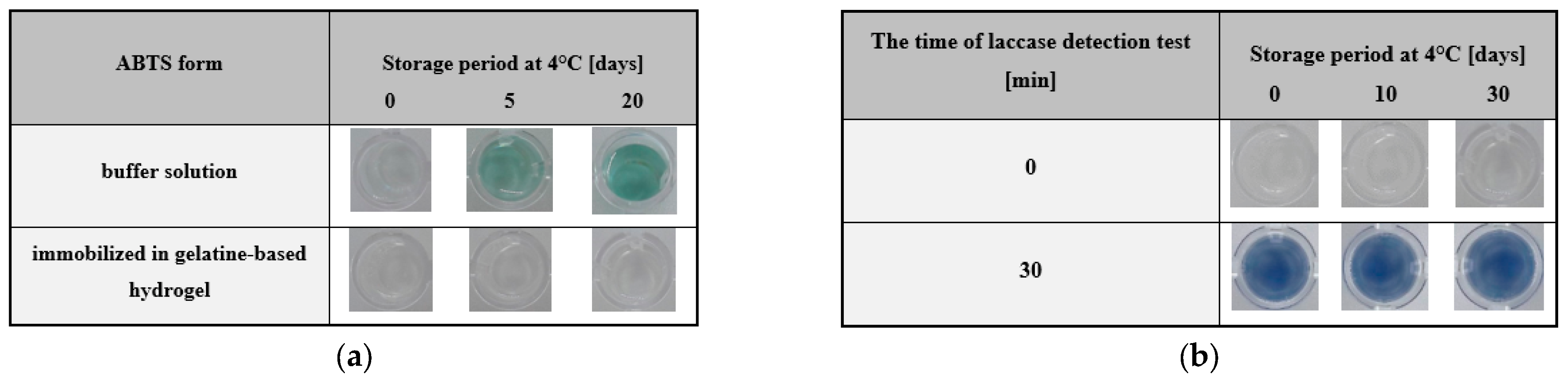
3.2.5. Determination of Storage Stability of 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonate) Sodium Salt
3.2.6. Determination of Storage Stability of the Hydrogel-Based Test
3.2.7. Hydrogel-Based Assay for Colourimetric Screening a Substrate Specificity of Laccase
3.2.8. Hydrogel-Based Assay for Colourimetric Screening Potential Inhibitors of Laccase
3.2.9. Microorganism and Cultivation Conditions
3.2.10. Analytical Procedures
3.2.11. Determination of Laccase Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Scanes, C.G. Human activity and habitat loss: Destruction, fragmentation, and degradation. In Animals and Human Society; Elsevier: Amsterdam, The Netherlands, 2018; pp. 451–482. ISBN 9780128052471. [Google Scholar]
- Karak, T.; Bhagat, R.M.; Bhattacharyya, P. Municipal solid waste generation, composition, and management: The world scenario. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1509–1630. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Joshi, M.; Bhatnagar, A.; Chaurasia, A.K.; Nigam, S. Pharmaceutical residues: One of the significant problems in achieving ‘clean water for all’ and its solution. Environ. Res. 2022, 215, 114219. [Google Scholar] [CrossRef] [PubMed]
- Papagiannaki, D.; Belay, M.H.; Gonçalves, N.P.F.; Robotti, E.; Bianco-Prevot, A.; Binetti, R.; Calza, P. From monitoring to treatment, how to improve water quality: The pharmaceuticals case. Chem. Eng. J. Adv. 2022, 10, 100245. [Google Scholar] [CrossRef]
- Kayode-Afolayan, S.D.; Ahuekwe, E.F.; Nwinyi, O.C. Impacts of pharmaceutical effluents on aquatic ecosystems. Sci. Afr. 2022, 17, e01288. [Google Scholar] [CrossRef]
- Pandey, A. Food wastage: Causes, impacts and solutions. Sci. Herit. J. 2021, 5, 17–20. [Google Scholar] [CrossRef]
- Scholz, K.; Eriksson, M.; Strid, I. Carbon footprint of supermarket food waste. Resour. Conserv. Recycl. 2015, 94, 56–65. [Google Scholar] [CrossRef]
- Conti, I.; Simioni, C.; Varano, G.; Brenna, C.; Costanzi, E.; Neri, L.M. Legislation to limit the environmental plastic and microplastic pollution and their influence on human exposure. Environ. Pollut. 2021, 288, 117708. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Kate, A.; Sahu, L.K.; Pandey, J.; Mishra, M.; Sharma, P.K. Green catalysis for chemical transformation: The need for the sustainable development. Curr. Res. Green Sustain. Chem. 2022, 5, 100248. [Google Scholar] [CrossRef]
- Woodley, J.M. New frontiers in biocatalysis for sustainable synthesis. Curr. Opin. Green Sustain. Chem. 2020, 21, 22–26. [Google Scholar] [CrossRef]
- Alcántara, A.R.; Domínguez de María, P.; Littlechild, J.A.; Schürmann, M.; Sheldon, R.A.; Wohlgemuth, R. Biocatalysis as Key to Sustainable Industrial Chemistry. ChemSusChem 2022, 15, e202102709. [Google Scholar] [CrossRef]
- Alcalde, M.; Ferrer, M.; Plou, F.J.; Ballesteros, A. Environmental biocatalysis: From remediation with enzymes to novel green processes. Trends Biotechnol. 2006, 24, 281–287. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Kumaran, A.; Vashishth, R.; Singh, S.; Surendran, U.; James, A.; Velayudhaperumal Chellam, P. Biosensors for detection of organophosphate pesticides: Current technologies and future directives. Microchem. J. 2022, 178, 107420. [Google Scholar] [CrossRef]
- Sarkar, A.; Sarkar, K.D.; Amrutha, V.; Dutta, K. An overview of enzyme-based biosensors for environmental monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–329. ISBN 9780128146798. [Google Scholar]
- Farias, T.C.; Kawaguti, H.Y.; Bello Koblitz, M.G. Microbial amylolytic enzymes in foods: Technological importance of the Bacillus genus. Biocatal. Agric. Biotechnol. 2021, 35, 102054. [Google Scholar] [CrossRef]
- Deckers, M.; Deforce, D.; Fraiture, M.A.; Roosens, N.H.C. Genetically modified micro-organisms for industrial food enzyme production: An overview. Foods 2020, 9, 326. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Dziuba, R.; Kucharska, M.; Madej-Kiełbik, L.; Sulak, K.; Wiśniewska-Wrona, M. Biopolymers and biomaterials for special applications within the context of the circular economy. Materials 2021, 14, 7704. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural polymeric materials: A solution to plastic pollution from the agro-food sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A Comprehensive Review on Recent Advances in Preparation, Physicochemical Characterization, and Bioengineering Applications of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 0123456789. [Google Scholar]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradación de bioplásticos desechados en entornos naturales e industriales. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Silva, M.P.; Fabi, J.P. Food biopolymers-derived nanogels for encapsulation and delivery of biologically active compounds: A perspective review. Food Hydrocoll. Health 2022, 2, 100079. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Machado, T.O.; Grabow, J.; Sayer, C.; de Araújo, P.H.H.; Ehrenhard, M.L.; Wurm, F.R. Biopolymer-based nanocarriers for sustained release of agrochemicals: A review on materials and social science perspectives for a sustainable future of agri- and horticulture. Adv. Colloid Interface Sci. 2022, 303, 102645. [Google Scholar] [CrossRef]
- Rivero Berti, I.; Islan, G.A.; Castro, G.R. Enzymes and biopolymers. The opportunity for the smart design of molecular delivery systems. Bioresour. Technol. 2021, 322, 124546. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bashir Yahya, E.; Jummaat, F.; Adnan, A.S.; Olaiya, N.G.; Rizal, S.; Abdullah, C.K.; Pasquini, D.; Thomas, S. Biopolymers based aerogels: A review on revolutionary solutions for smart therapeutics delivery. Prog. Mater. Sci. 2023, 131, 101014. [Google Scholar] [CrossRef]
- Czyzewska, K.; Trusek, A. Encapsulated nolaTM fit 5500 lactase—An economically beneficial way to obtain lactose-free milk at low temperature. Catalysts 2021, 11, 527. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Jain, U. Hydrogel based materials: A progressive approach towards advancement in biomedical applications. Mater. Today Commun. 2022, 33, 104369. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel beads for designing future foods: Structures, mechanisms, applications, and challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- ALSamman, M.T.; Sánchez, J. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water. Arab. J. Chem. 2021, 14, 103455. [Google Scholar] [CrossRef]
- Meyer, J.; Meyer, L.E.; Kara, S. Enzyme immobilization in hydrogels: A perfect liaison for efficient and sustainable biocatalysis. Eng. Life Sci. 2022, 22, 165–177. [Google Scholar] [CrossRef]
- Tan, Z.; Bilal, M.; Raza, A.; Cui, J.; Ashraf, S.S.; Iqbal, H.M.N. Expanding the biocatalytic scope of enzyme-loaded polymeric hydrogels. Gels 2021, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, Y.; Song, W.; Yu, B.; Liu, H. Rational design in functional hydrogels towards biotherapeutics. Mater. Des. 2022, 223, 111086. [Google Scholar] [CrossRef]
- Zhang, F.; Lian, M.; Alhadhrami, A.; Huang, M.; Li, B.; Mersal, G.A.M.; Ibrahim, M.M.; Xu, M. Laccase immobilized on functionalized cellulose nanofiber/alginate composite hydrogel for efficient bisphenol A degradation from polluted water. Adv. Compos. Hybrid Mater. 2022, 5, 1852–1864. [Google Scholar] [CrossRef]
- Piao, M.; Zou, D.; Yang, Y.; Ren, X.; Qin, C.; Piao, Y. Multi-functional laccase immobilized hydrogel microparticles for efficient removal of Bisphenol A. Materials 2019, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Mogharabi, M.; Nassiri-Koopaei, N.; Bozorgi-Koushalshahi, M.; Nafissi-Varcheh, N.; Bagherzadeh, G.; Faramarzi, M.A. Immobilization of laccase in alginate-gelatin mixed gel and decolorization of synthetic dyes. Bioinorg. Chem. Appl. 2012, 2012, 823830. [Google Scholar] [CrossRef]
- Kiiskinen, L.L.; Rättö, M.; Kruus, K. Screening for novel laccase-producing microbes. J. Appl. Microbiol. 2004, 97, 640–646. [Google Scholar] [CrossRef]
- Fu, K.; Fu, S.; Zhan, H.; Zhou, P.; Liu, M.; Liu, H. A newly isolated wood-rot fungus for laccase production in submerged cultures. BioResources 2013, 8, 1385–1397. [Google Scholar] [CrossRef]
- Dias, A.A.; Matos, A.J.S.; Fraga, I.; Sampaio, A.; Bezerra, R.M.F. An easy method for screening and detection of laccase activity. Open Biotechnol. J. 2017, 11, 89–93. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Lv, L.C.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Sun, G.; Huang, Z.; Zhang, Z.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. A two-step cross-linked hydrogel immobilization strategy for diacetylchitobiose deacetylase. Catalysts 2022, 12, 932. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Irfan, N.I.; Mohd Zubir, A.Z.; Suwandi, A.; Haris, M.S.; Jaswir, I.; Lestari, W. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent. J. 2022, 34, 699–707. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Z.; Zheng, M.; Yue, O.; Hou, M.; Cui, B.; Su, R.; Wei, C.; Liu, X. Engineered gelatin-based conductive hydrogels for flexible wearable electronic devices: Fundamentals and recent advances. J. Sci. Adv. Mater. Devices 2022, 7, 100451. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, H.; Liu, S.; Pu, L.; Hu, X.; Ding, J.; Xu, G.; Xu, W.; Xiang, S.; Yuan, Z. A review of protein hydrogels: Protein assembly mechanisms, properties, and biological applications. Colloids Surf. B Biointerfaces 2022, 220, 112973. [Google Scholar] [CrossRef]
- Labus, K.; Wolanin, K.; Radosiński, Ł. Comparative study on enzyme immobilization using natural hydrogel matrices—Experimental studies supported by molecular models analysis. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Labus, K. Effective detection of biocatalysts with specified activity by using a hydrogel-based colourimetric assay - β-galactosidase case study. PLoS ONE 2018, 13, e0205532. [Google Scholar] [CrossRef]
- Labus, K. Żelatynowe Matryce Hydrożelowe, Sposób ich Wytwarzania Oraz ich Zastosowanie. 2019; pp. 1–10. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.421412?lng=en (accessed on 18 December 2022).
- Labus, K.; Krystek, K. Zastosowanie L-3,4-dihydroksyfenyloalaniny Immobilizowanej w Żelatynowych Matrycach Hydrożelowych. 2021; pp. 1–11. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.422983 (accessed on 18 December 2022).
- Lonergan, G.; Mew, E.; Schliephake, K.; Baker, W.L. Phenolic substrates for fluorometric detection of laccase activity. FEMS Microbiol. Lett. 1997, 153, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C.; Narayani, T. Screening and production of a potential extracellular fungal laccase from Penicillium chrysogenum: Media optimization by response surface methodology (RSM) and central composite rotatable design (CCRD). Biotechnol. Rep. 2019, 23, e00344. [Google Scholar] [CrossRef] [PubMed]
- Lisov, A.V.; Trubitsina, L.I.; Lisova, Z.A.; Trubitsin, I.V.; Zavarzina, A.G.; Leontievsky, A.A. Transformation of humic acids by two-domain laccase from Streptomyces anulatus. Process Biochem. 2019, 76, 128–135. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S. Kinetic studies of laccase enzyme of Coriolus versicolor MTCC 138 in an inexpensive culture medium. Biochem. Eng. J. 2009, 46, 252–256. [Google Scholar] [CrossRef]
- Grassin, C.; Dubourdieu, D. Quantitative determination of Botrytis laccase in musts and wines by the syringaldazine test. J. Sci. Food Agric. 1989, 48, 369–376. [Google Scholar] [CrossRef]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzyme Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Harkin, J.M.; Obst, J.R. Syringaldazine, an effective reagent for detecting laccase and peroxidase in fungi. Experientia 1973, 29, 381–387. [Google Scholar] [CrossRef]
- Tetianec, L.; Chaleckaja, A.; Vidziunaite, R.; Kulys, J.; Bachmatova, I.; Marcinkeviciene, L.; Meskys, R. Development of a laccase/syringaldazine system for NAD(P)H oxidation. J. Mol. Catal. B Enzym. 2014, 101, 28–34. [Google Scholar] [CrossRef]
- Maniak, H.; Talma, M.; Matyja, K.; Trusek, A.; Giurg, M. Synthesis and structure-activity relationship studies of hydrazide-hydrazones as inhibitors of laccase from Trametes versicolor. Molecules 2020, 25, 1255. [Google Scholar] [CrossRef]
- Maniak, H.; Talma, M.; Giurg, M. Inhibitory potential of new phenolic hydrazide-hydrazones with a decoy substrate fragment towards laccase from a phytopathogenic fungus: SAR and molecular docking studies. Int. J. Mol. Sci. 2021, 22, 12307. [Google Scholar] [CrossRef]
- Abd El Monssef, R.A.; Hassan, E.A.; Ramadan, E.M. Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann. Agric. Sci. 2016, 61, 145–154. [Google Scholar] [CrossRef]
- Singh, G.; Bhalla, A.; Kaur, P.; Capalash, N.; Sharma, P. Laccase from prokaryotes: A new source for an old enzyme. Rev. Environ. Sci. Biotechnol. 2011, 10, 309–326. [Google Scholar] [CrossRef]
- Mandic, M.; Djokic, L.; Nikolaivits, E.; Prodanovic, R.; O’connor, K.; Jeremic, S.; Topakas, E.; Nikodinovic-Runic, J. Identification and characterization of new laccase biocatalysts from Pseudomonas species suitable for degradation of synthetic textile dyes. Catalysts 2019, 9, 629. [Google Scholar] [CrossRef]
- Han, M.-J.; Choi, H.-T.; Song, H.-G. Purification and characterization of laccase from white rot fungi Trametes versicolor. J. Microbiol. 2005, 43, 555–560. [Google Scholar]
- Stoilova, I.; Krastanov, A.; Stanchev, V. Properties of crude laccase from Trametes versicolor produced by solid-substrate fermentation. Adv. Biosci. Biotechnol. 2010, 1, 208–215. [Google Scholar] [CrossRef]
- Asgher, M.; Nasir Iqbal, H.M.; Asad, M.J. Kinetic characterization of purified laccase produced from Trametes versicolor IBL-04 in solid state bio-processing of corncobs. BioResources 2012, 7, 1171–1188. [Google Scholar]
- Lisova, Z.A.; Lisov, A.V.; Leontievsky, A.A. Two laccase isoforms of the basidiomycete Cerrena unicolor VKMF-3196. Induction, isolation and properties. J. Basic Microbiol. 2010, 50, 72–82. [Google Scholar] [CrossRef]
- Rogalski, J.; Janusz, G. Purification of extracellular laccase from Cerrena unicolor. Prep. Biochem. Biotechnol. 2010, 40, 242–255. [Google Scholar] [CrossRef]
- Rola, B.; Karaśkiewicz, M.; Majdecka, D.; Mazur, I.; Bilewicz, R.; Rogalski, J.; Ohga, S. Scale up of Cerrena unicolor lacease production. J. Fac. Agric. Kyushu Univ. 2013, 58, 231–238. [Google Scholar] [CrossRef]
- Antecka, A.; Blatkiewicz, M.; Głuszcz, P.; Ledakowicz, S. Improvement of laccase biosynthesis by various feeding strategies and in situ integration of biomass separation. Chem. Eng. Process. Process Intensif. 2021, 159. [Google Scholar] [CrossRef]
- Labus, K.; Drozd, A.; Trusek-Holownia, A. Preparation and characterisation of gelatine hydrogels predisposed to use as matrices for effective immobilisation of biocatalystst. Chem. Pap. 2016, 70, 523–530. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fahraeus, G.; Reinhammar, B. Large scale production and purification of laccase from cultures of the fungus Polyporus versicolor and some properties of laccase A. Acta Chem. Scand. 1967, 21, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Al-adhami, A.J.H.; Bryjak, J.; Greb-Markiewicz, B.; Peczynska-Czoch, W. Immobilization of wood-rotting fungi laccases on modified cellulose and acrylic carriers. Process Biochem. 2002, 37, 1387–1394. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labus, K.; Maniak, H. Colourimetric Plate Assays Based on Functionalized Gelatine Hydrogel Useful for Various Screening Purposes in Enzymology. Int. J. Mol. Sci. 2023, 24, 33. https://doi.org/10.3390/ijms24010033
Labus K, Maniak H. Colourimetric Plate Assays Based on Functionalized Gelatine Hydrogel Useful for Various Screening Purposes in Enzymology. International Journal of Molecular Sciences. 2023; 24(1):33. https://doi.org/10.3390/ijms24010033
Chicago/Turabian StyleLabus, Karolina, and Halina Maniak. 2023. "Colourimetric Plate Assays Based on Functionalized Gelatine Hydrogel Useful for Various Screening Purposes in Enzymology" International Journal of Molecular Sciences 24, no. 1: 33. https://doi.org/10.3390/ijms24010033
APA StyleLabus, K., & Maniak, H. (2023). Colourimetric Plate Assays Based on Functionalized Gelatine Hydrogel Useful for Various Screening Purposes in Enzymology. International Journal of Molecular Sciences, 24(1), 33. https://doi.org/10.3390/ijms24010033