E as in Enigma: The Mysterious Role of the Voltage-Dependent Anion Channel Glutamate E73
Abstract
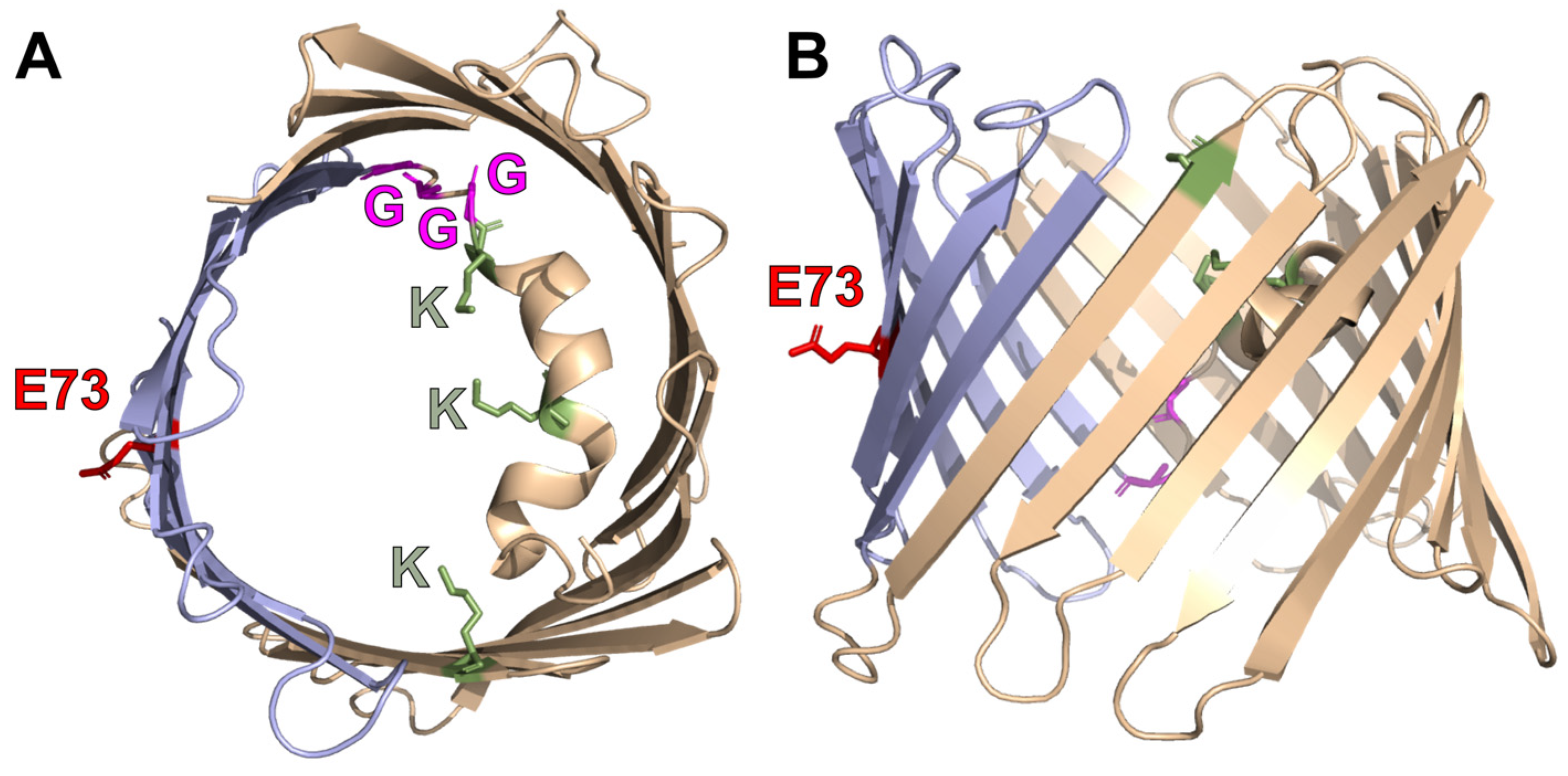
1. Introduction
2. Intrinsic Channel Function
3. Calcium
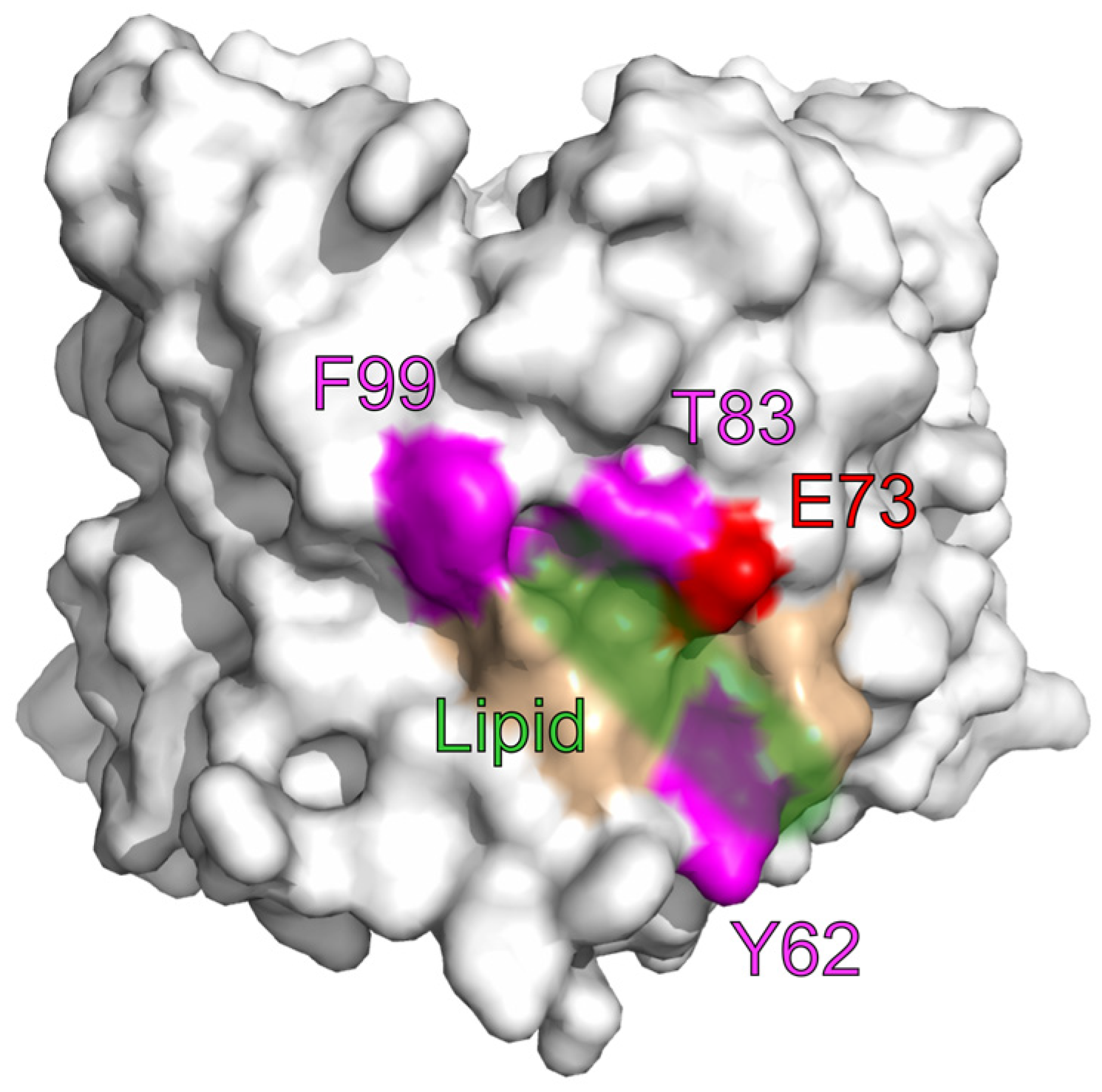
4. Lipids
5. Hexokinase
| Molecular Process | Results in Favor of an Involvement of E73 | Results Contradicting a Direct Involvement of E73 |
|---|---|---|
| Voltage-gating | - In molecular dynamics simulations E73 enhances barrel flexibility to allow transition to a more elliptical state [29,30]. | - Replacing E73 by Q did not alter voltage gating in lipid bilayers [13]. |
| Selectivity | - The flexible area around E73 was suggested to interact with the helix to determine ion selectivity [20,31,32]. | - No differences in ion selectivity were observed in lipid bilayers upon exchange of E73 for Q [49]. |
| Modulation of channel function through interaction (scaffolding) | - In simulation experiments the charge E73 induces a thinning of the membrane to facilitate molecular interactions [28,33]. - Experimental data shows reduced formation of dimers [26], as well as binding of lipids [14] and hexokinase [69] for E73Q mutants. | - The molecular structure reveals a location of E73 within the membrane making it poorly accessible [5,6]. |
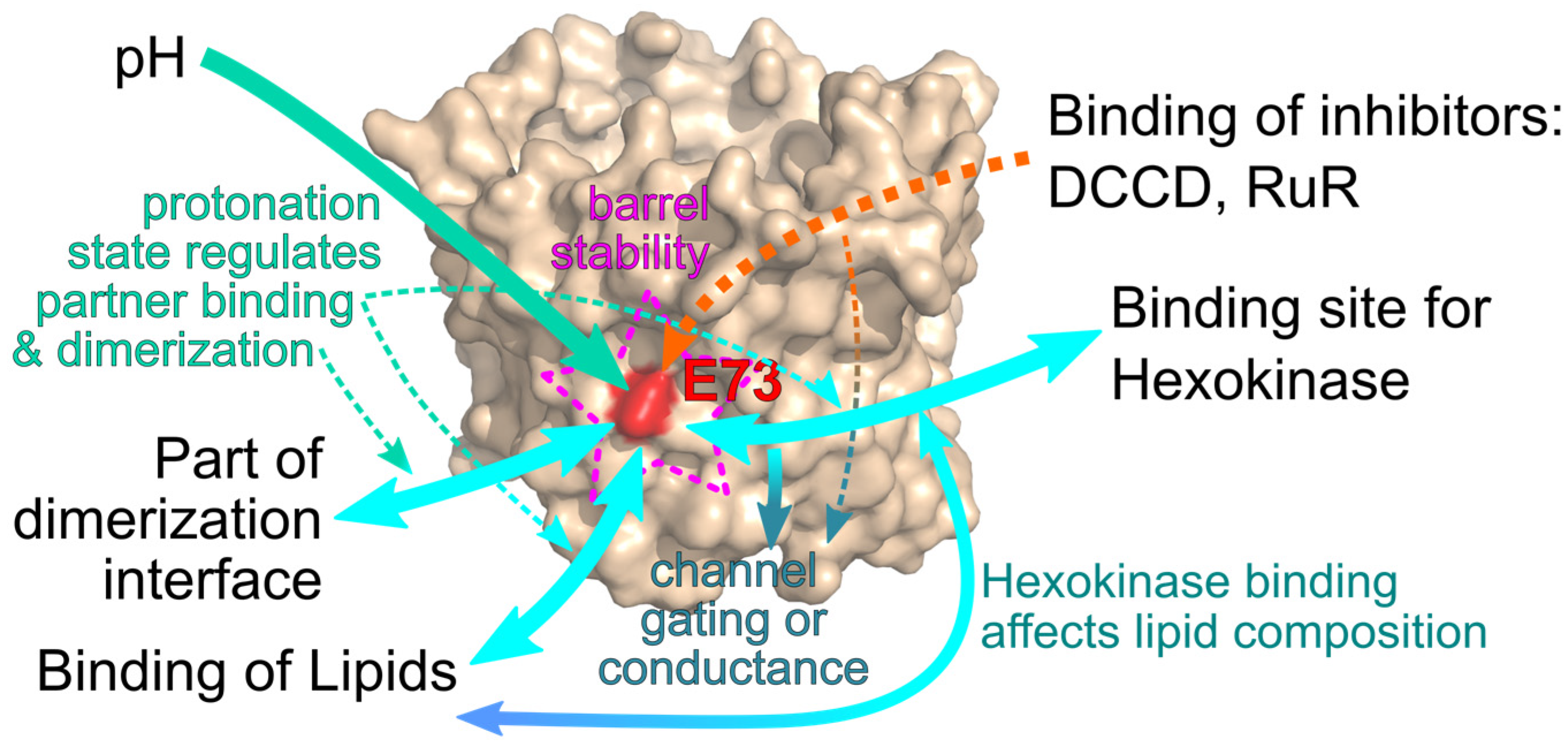
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-Lysosome Contacts Regulate Mitochondrial Ca2+ Dynamics via Lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef] [PubMed]
- Min, C.K.; Yeom, D.R.; Lee, K.-E.; Kwon, H.-K.; Kang, M.; Kim, Y.-S.; Park, Z.Y.; Jeon, H.; Kim, D.H. Coupling of Ryanodine Receptor 2 and Voltage-Dependent Anion Channel 2 Is Essential for Ca2+ Transfer from the Sarcoplasmic Reticulum to the Mitochondria in the Heart. Biochem. J. 2012, 447, 371–379. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Reina, S.; Nibali, S.C.; Tomasello, M.F.; Magrì, A.; Messina, A.; De Pinto, V. Voltage Dependent Anion Channel 3 (VDAC3) Protects Mitochondria from Oxidative Stress. Redox Biol. 2022, 51, 102264. [Google Scholar] [CrossRef] [PubMed]
- Ujwal, R.; Cascio, D.; Colletier, J.-P.; Faham, S.; Zhang, J.; Toro, L.; Ping, P.; Abramson, J. The Crystal Structure of Mouse VDAC1 at 2.3 Å Resolution Reveals Mechanistic Insights into Metabolite Gating. Proc. Natl. Acad. Sci. USA 2008, 105, 17742–17747. [Google Scholar] [CrossRef]
- Schredelseker, J.; Paz, A.; López, C.J.; Altenbach, C.; Leung, C.S.; Drexler, M.K.; Chen, J.-N.; Hubbell, W.L.; Abramson, J. High-Resolution Structure and Double Electron-Electron Resonance of the Zebrafish Voltage Dependent Anion Channel 2 Reveal an Oligomeric Population. J. Biol. Chem. 2014, 289, 12566–12577. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Bezrukov, S.M. VDAC Regulation: Role of Cytosolic Proteins and Mitochondrial Lipids. J. Bioenerg. Biomembr. 2008, 40, 163–170. [Google Scholar] [CrossRef]
- Sander, P.; Gudermann, T.; Schredelseker, J. A Calcium Guard in the Outer Membrane: Is VDAC a Regulated Gatekeeper of Mitochondrial Calcium Uptake? Int. J. Mol. Sci. 2021, 22, 946. [Google Scholar] [CrossRef]
- Colombini, M. Voltage Gating in the Mitochondrial Channel, VDAC. J. Membr. Biol. 1989, 111, 103–111. [Google Scholar] [CrossRef]
- Menzel, V.A.; Cassará, M.C.; Benz, R.; De Pinto, V.; Messina, A.; Cunsolo, V.; Saletti, R.; Hinsch, K.; Hinsch, E. Molecular and Functional Characterization of VDAC2 Purified from Mammal Spermatozoa. Biosci. Rep. 2009, 29, 351–362. [Google Scholar] [CrossRef]
- Wilting, F.; Kopp, R.; Gurnev, P.A.; Schedel, A.; Dupper, N.J.; Kwon, O.; Nicke, A.; Gudermann, T.; Schredelseker, J. The Antiarrhythmic Compound Efsevin Directly Modulates Voltage-dependent Anion Channel 2 by Binding to Its Inner Wall and Enhancing Mitochondrial Ca2+ Uptake. Br. J. Pharmacol. 2020, 177, 2947–2958. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Kazemi, N.; Weinrich, M.; Bezrukov, S.M. Voltage Gating of VDAC Is Regulated by Nonlamellar Lipids of Mitochondrial Membranes. J. Biol. Chem. 2006, 281, 37496–37506. [Google Scholar] [CrossRef]
- Queralt-Martín, M.; Bergdoll, L.; Jacobs, D.; Bezrukov, S.M.; Abramson, J.; Rostovtseva, T.K. Assessing the Role of Residue E73 and Lipid Headgroup Charge in VDAC1 Voltage Gating. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 22–29. [Google Scholar] [CrossRef]
- Cheng, W.W.L.; Budelier, M.M.; Sugasawa, Y.; Bergdoll, L.; Queralt-Martín, M.; Rosencrans, W.; Rostovtseva, T.K.; Chen, Z.W.; Abramson, J.; Krishnan, K.; et al. Multiple Neurosteroid and Cholesterol Binding Sites in Voltage-Dependent Anion Channel-1 Determined by Photo-Affinity Labeling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1269–1279. [Google Scholar] [CrossRef]
- Bera, A.K.; Ghosh, S. Dual Mode of Gating of Voltage-Dependent Anion Channel as Revealed by Phosphorylation. J. Struct. Biol. 2001, 135, 67–72. [Google Scholar] [CrossRef]
- Báthori, G.; Csordás, G.; Garcia-Perez, C.; Davies, E.; Hajnóczky, G. Ca2+-Dependent Control of the Permeability Properties of the Mitochondrial Outer Membrane and Voltage-Dependent Anion-Selective Channel (VDAC). J. Biol. Chem. 2006, 281, 17347–17358. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Zaid, H.; Israelson, A.; Nahon, E.; Shoshan-Barmatz, V. Hexokinase-I Protection against Apoptotic Cell Death Is Mediated via Interaction with the Voltage-Dependent Anion Channel-1: Mapping the Site of Binding. J. Biol. Chem. 2008, 283, 13482–13490. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Israelson, A.; Brdiczka, D.; Sheu, S. The Voltage-Dependent Anion Channel (VDAC): Function in Intracellular Signalling, Cell Life and Cell Death. Curr. Pharm. Des. 2006, 12, 2249–2270. [Google Scholar] [CrossRef]
- Shimizu, S.; Narita, M.; Tsujimoto, Y. Bcl-2 Family Proteins Regulate the Release of Apoptogenic Cytochrome c by the Mitochondrial Channel VDAC. Nature 1999, 399, 483–487. [Google Scholar] [CrossRef]
- Krammer, E.M.; Vu, G.T.; Homblé, F.; Prévost, M. Dual Mechanism of Ion Permeation through VDAC Revealed with Inorganic Phosphate Ions and Phosphate Metabolites. PLoS ONE 2015, 10, e0121746. [Google Scholar] [CrossRef]
- Geula, S.; Ben-Hail, D.; Shoshan-Barmatz, V. Structure-Based Analysis of VDAC1: N-Terminus Location, Translocation, Channel Gating and Association with Anti-Apoptotic Proteins. Biochem. J. 2012, 444, 475–485. [Google Scholar] [CrossRef] [PubMed]
- De Pinto, V.; Al Jamal, J.A.; Palmieri, F. Location of the Dicyclohexylcarbodiimide-Reactive Glutamate Residue in the Bovine Heart Mitochondrial Porin. J. Biol. Chem. 1993, 268, 12977–12982. [Google Scholar] [CrossRef] [PubMed]
- Israelson, A.; Abu-Hamad, S.; Zaid, H.; Nahon, E.; Shoshan-Barmatz, V. Localization of the Voltage-Dependent Anion Channel-1 Ca2+-Binding Sites. Cell Calcium 2007, 41, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Budelier, M.M.; Cheng, W.W.L.; Bergdoll, L.; Chen, Z.-W.; Janetka, J.W.; Abramson, J.; Krishnan, K.; Mydock-McGrane, L.; Covey, D.F.; Whitelegge, J.P.; et al. Photoaffinity Labeling with Cholesterol Analogues Precisely Maps a Cholesterol-Binding Site in Voltage-Dependent Anion Channel-1. J. Biol. Chem. 2017, 292, 9294–9304. [Google Scholar] [CrossRef] [PubMed]
- Budelier, M.M.; Cheng, W.W.L.; Bergdoll, L.; Chen, Z.W.; Abramson, J.; Krishnan, K.; Qian, M.; Covey, D.F.; Janetka, J.W.; Evers, A.S. Click Chemistry Reagent for Identification of Sites of Covalent Ligand Incorporation in Integral Membrane Proteins. Anal. Chem. 2017, 89, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Bergdoll, L.A.; Lerch, M.T.; Patrick, J.W.; Belardo, K.; Altenbach, C.; Bisignano, P.; Laganowsky, A.; Grabe, M.; Hubbell, W.L.; Abramson, J. Protonation State of Glutamate 73 Regulates the Formation of a Specific Dimeric Association of mVDAC1. Proc. Natl. Acad. Sci. USA 2018, 115, E172–E179. [Google Scholar] [CrossRef]
- Bayrhuber, M.; Meins, T.; Habeck, M.; Becker, S.; Giller, K.; Villinger, S.; Vonrhein, C.; Griesinger, C.; Zweckstetter, M.; Zeth, K. Structure of the Human Voltage-Dependent Anion Channel. Proc. Natl. Acad. Sci. USA 2008, 105, 15370–15375. [Google Scholar] [CrossRef]
- Villinger, S.; Briones, R.; Giller, K.; Zachariae, U.; Lange, A.; de Groot, B.L.; Griesinger, C.; Becker, S.; Zweckstetter, M. Functional Dynamics in the Voltage-Dependent Anion Channel. Proc. Natl. Acad. Sci. USA 2010, 107, 22546–22551. [Google Scholar] [CrossRef]
- Jaremko, M.; Jaremko, Ł.; Villinger, S.; Schmidt, C.D.; Griesinger, C.; Becker, S.; Zweckstetter, M. High-Resolution NMR Determination of the Dynamic Structure of Membrane Proteins. Angew. Chem. Int. Ed. 2016, 55, 10518–10521. [Google Scholar] [CrossRef]
- Najbauer, E.E.; Becker, S.; Giller, K.; Zweckstetter, M.; Lange, A.; Steinem, C.; de Groot, B.L.; Griesinger, C.; Andreas, L.B. Structure, Gating and Interactions of the Voltage-Dependent Anion Channel. Eur. Biophys. J. 2021, 50, 159–172. [Google Scholar] [CrossRef]
- Amodeo, G.F.; Scorciapino, M.A.; Messina, A.; De Pinto, V.; Ceccarelli, M. Charged Residues Distribution Modulates Selectivity of the Open State of Human Isoforms of the Voltage Dependent Anion-Selective Channel. PLoS ONE 2014, 9, e103879. [Google Scholar] [CrossRef]
- Rui, H.; Lee, K.; Pastor, R.W.; Im, W. Molecular Dynamics Studies of Ion Permeation in VDAC. Biophys. J. 2011, 100, 602–610. [Google Scholar] [CrossRef]
- Briones, R.; Weichbrodt, C.; Paltrinieri, L.; Mey, I.; Villinger, S.; Giller, K.; Lange, A.; Zweckstetter, M.; Griesinger, C.; Becker, S.; et al. Voltage Dependence of Conformational Dynamics and Subconducting States of VDAC-1. Biophys. J. 2016, 111, 1223–1234. [Google Scholar] [CrossRef]
- Zalk, R.; Israelson, A.; Garty, E.S.; Azoulay-Zohar, H.; Shoshan-Barmatz, V. Oligomeric States of the Voltage-Dependent Anion Channel and Cytochrome c Release from Mitochondria. Biochem. J. 2005, 386, 73. [Google Scholar] [CrossRef]
- Keinan, N.; Tyomkin, D.; Shoshan-Barmatz, V. Oligomerization of the Mitochondrial Protein Voltage-Dependent Anion Channel Is Coupled to the Induction of Apoptosis. Mol. Cell. Biol. 2010, 30, 5698. [Google Scholar] [CrossRef]
- Diaz-Juarez, J.; Suarez, J.A.; Dillmann, W.H.; Suarez, J. Mitochondrial Calcium Handling and Heart Disease in Diabetes Mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165984. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial Calcium Overload Is a Key Determinant in Heart Failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Graier, W.F.; Malli, R. Mitochondrial Calcium: A Crucial Hub for Cancer Cell Metabolism? Transl. Cancer Res. 2017, 6, S1124–S1127. [Google Scholar] [CrossRef]
- Sterea, A.M.; El Hiani, Y. The Role of Mitochondrial Calcium Signaling in the Pathophysiology of Cancer Cells. Adv. Exp. Med. Biol. 2020, 1131, 747–770. [Google Scholar] [CrossRef]
- Rodríguez, L.R.; Lapeña-Luzón, T.; Benetó, N.; Beltran-Beltran, V.; Pallardó, F.V.; Gonzalez-Cabo, P.; Navarro, J.A. Therapeutic Strategies Targeting Mitochondrial Calcium Signaling: A New Hope for Neurological Diseases? Antioxidants 2022, 11, 165. [Google Scholar] [CrossRef]
- Arduino, D.M.; Perocchi, F. Pharmacological Modulation of Mitochondrial Calcium Homeostasis. J. Physiol. 2018, 596, 2717–2733. [Google Scholar] [CrossRef] [PubMed]
- Rosencrans, W.M.; Rajendran, M.; Bezrukov, S.M.; Rostovtseva, T.K. VDAC Regulation of Mitochondrial Calcium Flux: From Channel Biophysics to Disease. Cell Calcium 2021, 94, 102356. [Google Scholar] [CrossRef] [PubMed]
- Subedi, K.P.; Kim, J.-C.; Kang, M.; Son, M.-J.; Kim, Y.-S.; Woo, S.-H. Voltage-Dependent Anion Channel 2 Modulates Resting Ca2+ Sparks, but Not Action Potential-Induced Ca2+ Signaling in Cardiac Myocytes. Cell Calcium 2011, 49, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.K.; Wilting, F.; Sedej, S.; Dreizehnter, L.; Dupper, N.J.; Tian, Q.; Moretti, A.; My, I.; Kwon, O.; Priori, S.G.; et al. Suppression of Arrhythmia by Enhancing Mitochondrial Ca2+ Uptake in Catecholaminergic Ventricular Tachycardia Models. JACC Basic to Transl. Sci. 2017, 2, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Schredelseker, J.; Huang, J.; Lu, K.; Naghdi, S.; Lu, F.; Franklin, S.; Fiji, H.D.; Wang, K.; Zhu, H.; et al. Mitochondrial Ca2+ Uptake by the Voltage-Dependent Anion Channel 2 Regulates Cardiac Rhythmicity. eLife 2015, 4, e04801. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Huber, S.; Langenbacher, A.D.; Crisman, L.; Huang, J.; Wang, K.; Wilting, F.; Gudermann, T.; Schredelseker, J.; Chen, J.-N. Glutamate 73 Promotes Anti-Arrhythmic Effects of Voltage-Dependent Anion Channel Through Regulation of Mitochondrial Ca2+ Uptake. Front. Physiol. 2021, 12, 1284. [Google Scholar] [CrossRef]
- Tan, W.; Colombini, M. VDAC Closure Increases Calcium Ion Flux. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2510–2515. [Google Scholar] [CrossRef]
- Pavlov, E.; Grigoriev, S.M.; Dejean, L.M.; Zweihorn, C.L.; Mannella, C.A.; Kinnally, K.W. The Mitochondrial Channel VDAC Has a Cation-Selective Open State. Biochim. Biophys. Acta Bioenerg. 2005, 1710, 96–102. [Google Scholar] [CrossRef]
- Rosencrans, W.M.; Aguilella, V.M.; Rostovtseva, T.K.; Bezrukov, S.M. α-Synuclein Emerges as a Potent Regulator of VDAC-Facilitated Calcium Transport. Cell Calcium 2021, 95, 102355. [Google Scholar] [CrossRef]
- Gincel, D.; Zaid, H.; Shoshan-Barmatz, V. Calcium Binding and Translocation by the Voltage-Dependent Anion Channel: A Possible Regulatory Mechanism in Mitochondrial Function. Biochem. J. 2001, 358, 147–155. [Google Scholar] [CrossRef]
- Hiller, S.; Garces, R.G.; Malia, T.J.; Orekhov, V.Y.; Colombini, M.; Wagner, G. Solution Structure of the Integral Human Membrane Protein VDAC-1 in Detergent Micelles. Science 2008, 321, 1206–1210. [Google Scholar] [CrossRef]
- Van Liefferinge, F.; Krammer, E.-M.; Sengupta, D.; Prévost, M. Lipid Composition and Salt Concentration as Regulatory Factors of the Anion Selectivity of VDAC Studied by Coarse-Grained Molecular Dynamics Simulations. Chem. Phys. Lipids 2019, 220, 66–76. [Google Scholar] [CrossRef]
- Rovini, A.; Gurnev, P.A.; Beilina, A.; Queralt-Martín, M.; Rosencrans, W.; Cookson, M.R.; Bezrukov, S.M.; Rostovtseva, T.K. Molecular Mechanism of Olesoxime-Mediated Neuroprotection through Targeting α-Synuclein Interaction with Mitochondrial VDAC. Cell. Mol. Life Sci. 2020, 77, 3611–3626. [Google Scholar] [CrossRef]
- Morad, S.A.F.; Cabot, M.C. Ceramide-Orchestrated Signalling in Cancer Cells. Nat. Rev. Cancer 2012, 13, 51–65. [Google Scholar] [CrossRef]
- Dadsena, S.; Bockelmann, S.; Mina, J.G.M.; Hassan, D.G.; Korneev, S.; Razzera, G.; Jahn, H.; Niekamp, P.; Müller, D.; Schneider, M.; et al. Ceramides Bind VDAC2 to Trigger Mitochondrial Apoptosis. Nat. Commun. 2019, 10, 1832. [Google Scholar] [CrossRef]
- De Pinto, V.; Benz, R.; Palmieri, F. Interaction of Non-Classical Detergents with the Mitochondrial Porin. Eur. J. Biochem. 1989, 183, 179–187. [Google Scholar] [CrossRef]
- Hulce, J.J.; Cognetta, A.B.; Niphakis, M.J.; Tully, S.E.; Cravatt, B.F. Proteome-Wide Mapping of Cholesterol-Interacting Proteins in Mammalian Cells. Nat. Methods 2013, 10, 259–264. [Google Scholar] [CrossRef]
- Ferens, F.G.; Patel, T.R.; Oriss, G.; Court, D.A.; Stetefeld, J. A Cholesterol Analog Induces an Oligomeric Reorganization of VDAC. Biophys. J. 2019, 116, 847–859. [Google Scholar] [CrossRef]
- Campbell, A.M.; Chan, S.H.P. The Voltage Dependent Anion Channel Affects Mitochondrial Cholesterol Distribution and Function. Arch. Biochem. Biophys. 2007, 466, 203–210. [Google Scholar] [CrossRef]
- Campbell, A.M.; Chan, S.H.P. Mitochondrial Membrane Cholesterol, the Voltage Dependent Anion Channel (VDAC), and the Warburg Effect. J. Bioenerg. Biomembr. 2008, 40, 193–197. [Google Scholar] [CrossRef]
- Weiser, B.P.; Salari, R.; Eckenhoff, R.G.; Brannigan, G. Computational Investigation of Cholesterol Binding Sites on Mitochondrial VDAC. J. Phys. Chem. B 2014, 118, 9852–9860. [Google Scholar] [CrossRef] [PubMed]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s Double-Edged Sword Acting as Both Facilitator and Gatekeeper of Malignancy When Bound to Mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Messer, J.L.; Wilson, J.E. Purification of a Hexokinase-Binding Protein from the Outer Mitochondrial Membrane. J. Biol. Chem. 1979, 254, 4946–4949. [Google Scholar] [CrossRef] [PubMed]
- Fiek, C.; Benz, R.; Roos, N.; Brdiczka, D. Evidence for Identity between the Hexokinase-Binding Protein and the Mitochondrial Porin in the Outer Membrane of Rat Liver Mitochondria. Biochim. Biophys. Acta Biomembr. 1982, 688, 429–440. [Google Scholar] [CrossRef]
- Pedersen, P.L.; Mathupala, S.; Rempel, A.; Geschwind, J.F.; Ko, Y.H. Mitochondrial Bound Type II Hexokinase: A Key Player in the Growth and Survival of Many Cancers and an Ideal Prospect for Therapeutic Intervention. Biochim. Biophys. Acta Bioenerg. 2002, 1555, 14–20. [Google Scholar] [CrossRef]
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl Jasmonate Binds to and Detaches Mitochondria-Bound Hexokinase. Oncogene 2008, 27, 4636–4643. [Google Scholar] [CrossRef]
- Azoulay-Zohar, H.; Israelson, A.; Abu-Hamad, S.; Shoshan-Barmatz, V. In Self-Defence: Hexokinase Promotes Voltage-Dependent Anion Channel Closure and Prevents Mitochondria-Mediated Apoptotic Cell Death. Biochem. J. 2004, 377, 347–355. [Google Scholar] [CrossRef]
- Nakashima, R.A.; Pedersen, P.L.; Mangan, P.S.; Colombini, M. Hexokinase Receptor Complex in Hepatoma Mitochondria: Evidence from N,N′-Dicyclohexylcarbodiimide-Labeling Studies for the Involvement of the Pore-Forming Protein VDAC. Biochemistry 1986, 25, 1015–1021. [Google Scholar] [CrossRef]
- Zaid, H.; Abu-Hamad, S.; Israelson, A.; Nathan, I.; Shoshan-Barmatz, V. The Voltage-Dependent Anion Channel-1 Modulates Apoptotic Cell Death. Cell Death Differ. 2005, 12, 751–760. [Google Scholar] [CrossRef]
- Quach, C.H.T.; Jung, K.H.; Lee, J.H.; Park, J.W.; Moon, S.H.; Cho, Y.S.; Choe, Y.S.; Lee, K.H. Mild Alkalization Acutely Triggers the Warburg Effect by Enhancing Hexokinase Activity via Voltage-Dependent Anion Channel Binding. PLoS ONE 2016, 11, e0159529. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rister, A.B.; Gudermann, T.; Schredelseker, J. E as in Enigma: The Mysterious Role of the Voltage-Dependent Anion Channel Glutamate E73. Int. J. Mol. Sci. 2023, 24, 269. https://doi.org/10.3390/ijms24010269
Rister AB, Gudermann T, Schredelseker J. E as in Enigma: The Mysterious Role of the Voltage-Dependent Anion Channel Glutamate E73. International Journal of Molecular Sciences. 2023; 24(1):269. https://doi.org/10.3390/ijms24010269
Chicago/Turabian StyleRister, Alexander Bernhard, Thomas Gudermann, and Johann Schredelseker. 2023. "E as in Enigma: The Mysterious Role of the Voltage-Dependent Anion Channel Glutamate E73" International Journal of Molecular Sciences 24, no. 1: 269. https://doi.org/10.3390/ijms24010269
APA StyleRister, A. B., Gudermann, T., & Schredelseker, J. (2023). E as in Enigma: The Mysterious Role of the Voltage-Dependent Anion Channel Glutamate E73. International Journal of Molecular Sciences, 24(1), 269. https://doi.org/10.3390/ijms24010269






