PD1, CTLA4 and TIGIT Expression on T and NK Cells in Granulomatous Diseases: Sarcoidosis and ANCA-Associated Vasculitis
Abstract
1. Introduction
2. Results
2.1. Patients
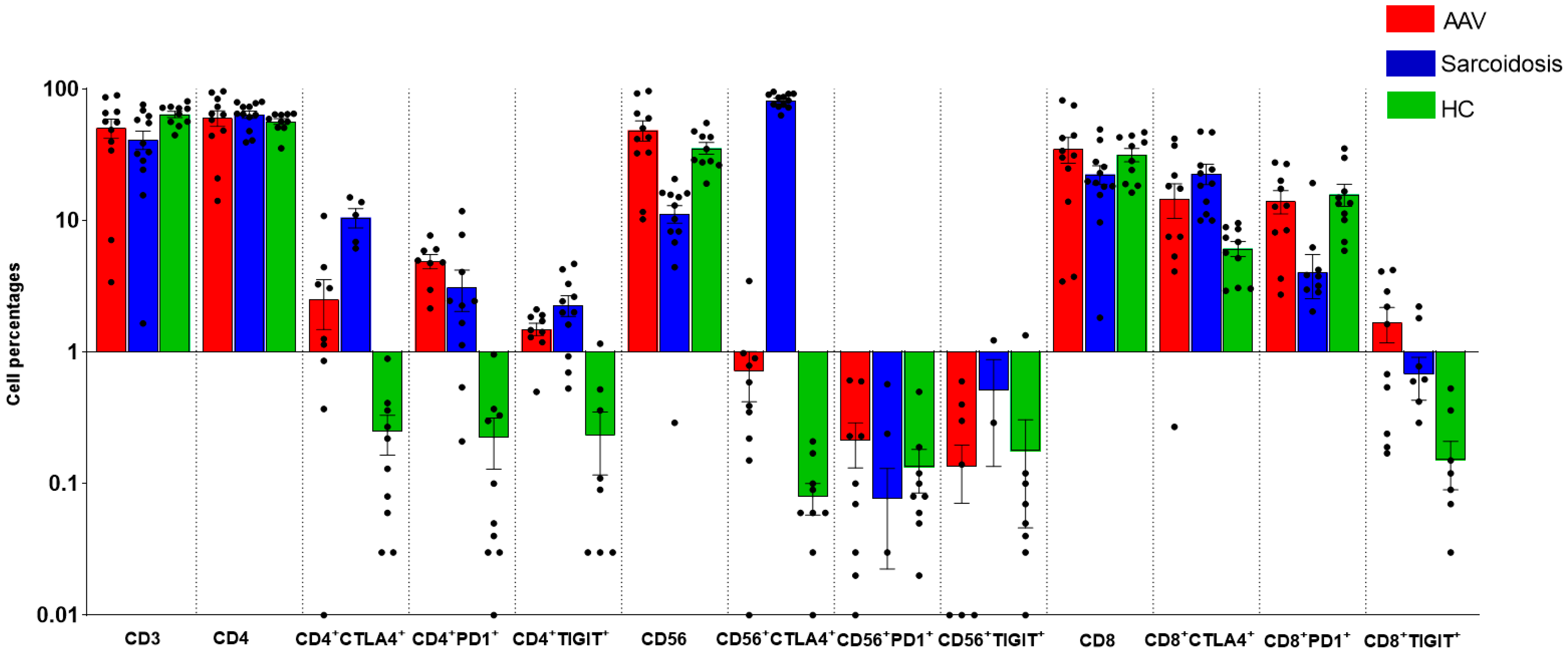
2.2. Comparison of Immunological Features between GPA, MPA, Sarcoidosis and HC
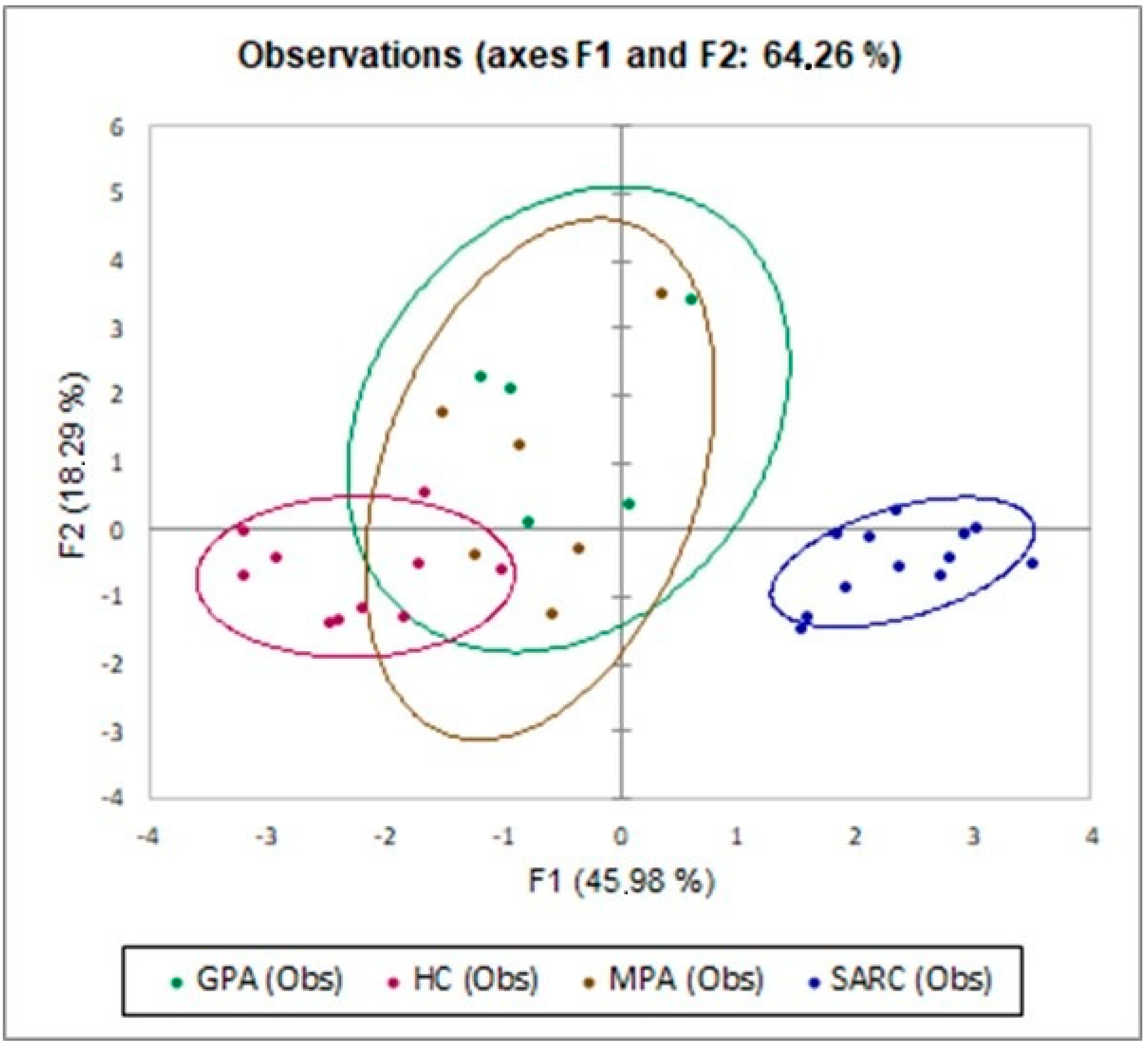
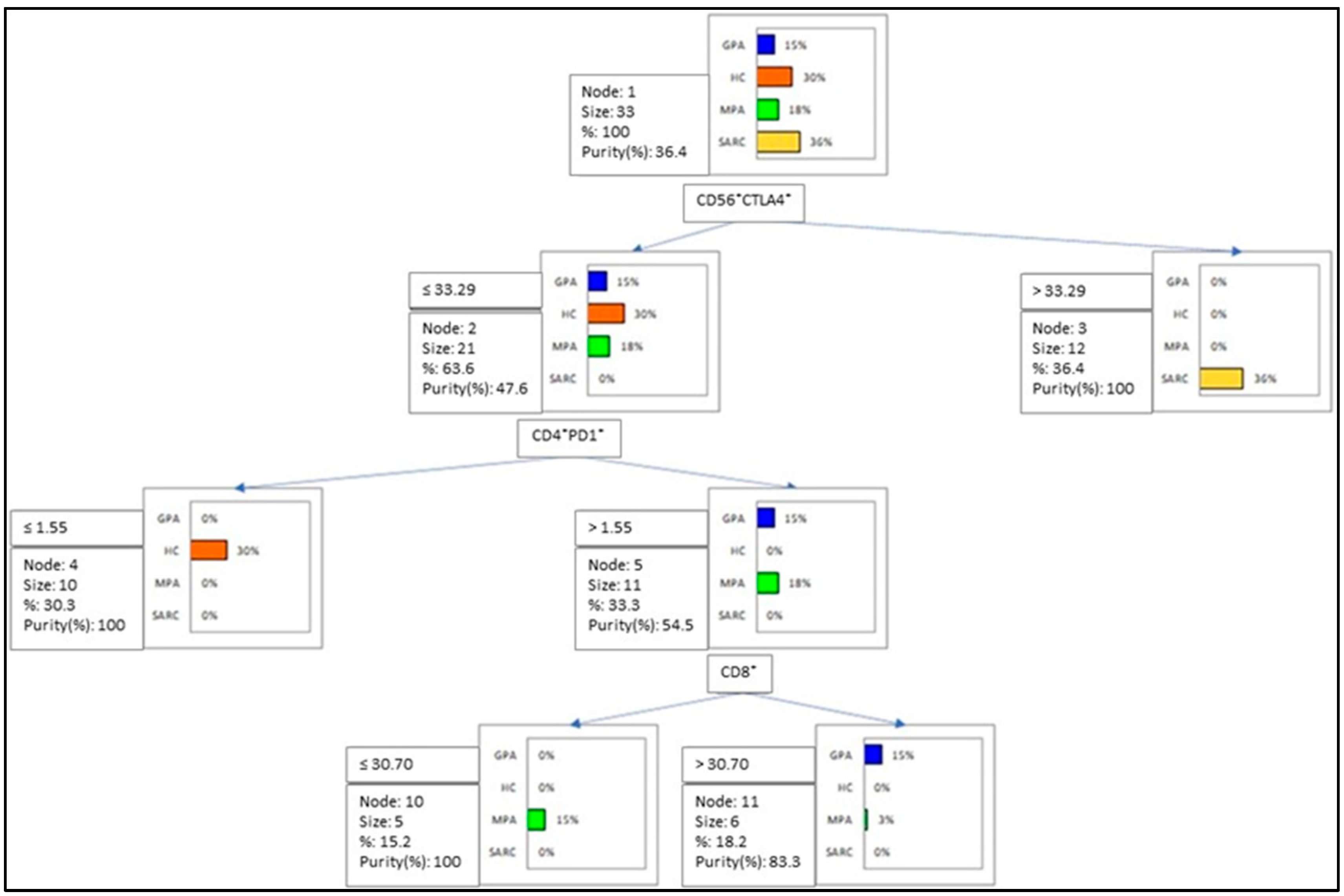
2.3. Multivariate Analysis of IC Molecules in Patients and Controls
3. Materials and Methods
3.1. Study Population
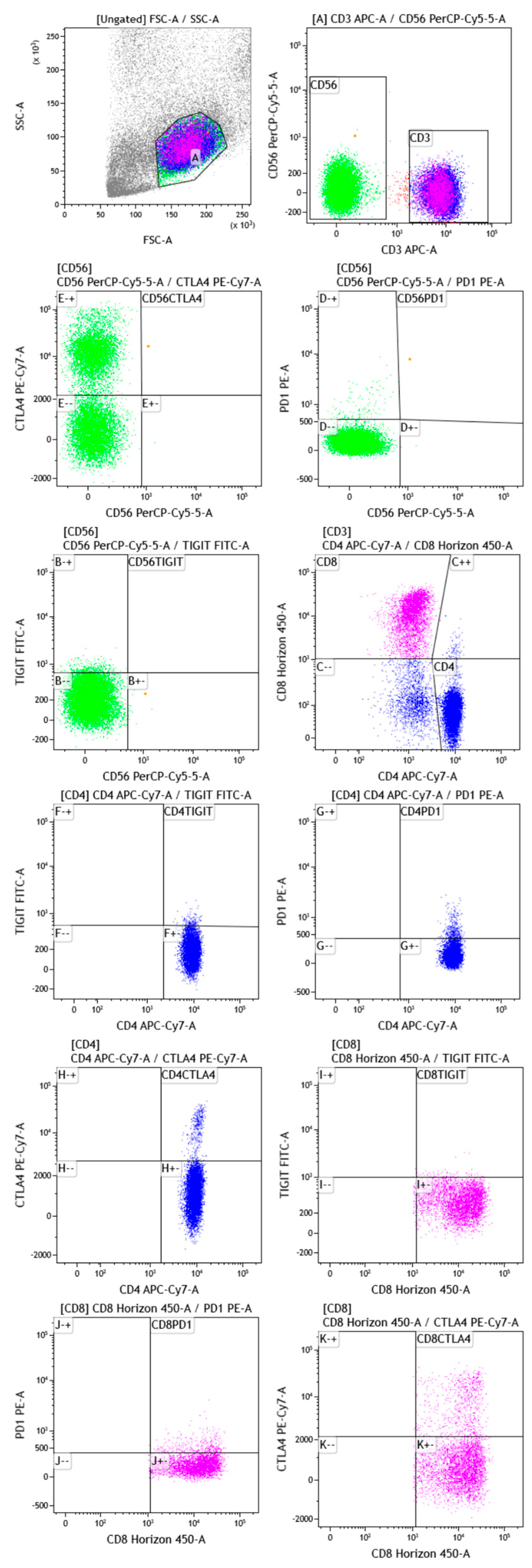
3.2. Gating Strategy
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jain, R.; Yadav, D.; Puranik, N.; Guleria, R.; Jin, J.-O. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J. Clin. Med. 2020, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Müller, L.J.; Volkers, S.M.; Heinrich, F.; Mashreghi, M.-F.; Ruppert, C.; Sander, L.E.; Hutloff, A. Follicular Helper-like T Cells in the Lung Highlight a Novel Role of B Cells in Sarcoidosis. Am. J. Respir. Crit. Care Med. 2021, 204, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Starshinova, A.A.; Malkova, A.M.; Basantsova, N.Y.; Zinchenko, Y.S.; Kudryavtsev, I.V.; Ershov, G.A.; Soprun, L.A.; Mayevskaya, V.A.; Churilov, L.P.; Yablonskiy, P.K. Sarcoidosis as an Autoimmune Disease. Front. Immunol. 2020, 10, 2933. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.-J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Watts, R.A. Classification of ANCA-associated vasculitis. Curr. Rheumatol. Rep. 2013, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Scheffschick, A.; Gunnarsson, I.; Brauner, H. Natural Killer Cells in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis—A Review of the Literature. Front. Immunol. 2021, 12, 796640. [Google Scholar] [CrossRef] [PubMed]
- Prasse, A.; Georges, C.G.; Biller, H.; Hamm, H.; Matthys, H.; Luttmann, W.; Virchow, J.C., Jr. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin. Exp. Immunol. 2000, 122, 241–248. [Google Scholar] [CrossRef]
- Hilhorst, M.; Shirai, T.; Berry, G.; Goronzy, J.J.; Weyand, C.M. T cell-macrophage interactions and granuloma formation in vasculitis. Front. Immunol. 2014, 5, 432. [Google Scholar] [CrossRef]
- Zhang, H.; Costabel, U.; Dai, H. The Role of Diverse Immune Cells in Sarcoidosis. Front. Immunol. 2021, 12, 788502. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-cell exhaustion: Characteristics, causes and conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef]
- Al-Mterin, M.A.; Alsalman, A.; Elkord, E. Inhibitory Immune Checkpoint Receptors and Ligands as Prognostic Biomarkers in COVID-19 Patients. Front. Immunol. 2022, 13, 870283. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Bergantini, L.; Mezzasalma, F.; Cavallaro, D.; Gangi, S.; Baglioni, S.; Armati, M.; Abbritti, M.; Cattelan, S.; Cameli, P.; et al. Immune-Checkpoint Expression on CD4, CD8 and NK Cells in Blood, Bronchoalveolar Lavage and Lymph Nodes of Sarcoidosis. Mol. Diagn. Ther. 2022, 26, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Oyewole-Said, D.; Konduri, V.; Vazquez-Perez, J.; Weldon, S.A.; Levitt, J.M.; Decker, W.K. Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 608024. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef]
- Nozaki, Y. New Insights Into Novel Therapeutic Targets in ANCA-Associated Vasculitis. Front. Immunol. 2021, 12, 631055. [Google Scholar] [CrossRef]
- Esen, F.; Deniz, G.; Aktas, E.C. PD-1, CTLA-4, LAG-3, and TIGIT: The roles of immune checkpoint receptors on the regulation of human NK cell phenotype and functions. Immunol. Lett. 2021, 240, 15–23. [Google Scholar] [CrossRef]
- Luo, Q.; Li, X.; Fu, B.; Zhang, L.; Deng, Z.; Qing, C.; Su, R.; Xu, J.; Gio, Y.; Huang, Z.; et al. Decreased expression of TIGIT in NK cells correlates negatively with disease activity in systemic lupus erythematosus. Int. J. Clin. Exp. Pathol. 2018, 11, 2408–2418. [Google Scholar]
- Sarhan, D.; Cichocki, F.; Zhang, B.; Yingst, A.; Spellman, S.R.; Cooley, S.; Verneris, M.R.; Blazar, B.R.; Miller, J.S. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res 2016, 76, 5696–5706. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.; Wu, S.; Tang, Q.; Liu, W.; Huang, M.; Yin, B.; Huang, J.; Mao, L.; Lu, Y.; et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur. J. Immunol. 2015, 45, 2886–2897. [Google Scholar] [CrossRef]
- Yates, M.; Watts, R.A.; Bajema, I.M.; Cid, M.C.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.U.; Laudien, M.; Little, M.; et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016, 75, 1583–1594. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; Du Bois, R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc. Diffuse Lung Dis. 1999, 16, 149–173. [Google Scholar] [PubMed]
- Vietri, L.; Fui, A.; Bergantini, L.; d’Alessandro, M.; Cameli, P.; Sestini, P.; Rottoli, P.; Bargagli, E. Serum amyloid A: A potential biomarker of lung disorders. Respir. Investig. 2020, 58, 21–27. [Google Scholar] [CrossRef]
- Bennett, D.; Cameli, P.; Lanzarone, N.; Carobene, L.; Bianchi, N.; Fui, A.; Rizzi, L.; Bergantini, L.; Cillis, G.; d’Alessandro, M.; et al. Chitotriosidase: A biomarker of activity and severity in patients with sarcoidosis. Respir. Res. 2020, 21, 6, Erratum in Respir Res. 2020, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zheng, S.G. Regulatory T cells and B cells: Implication on autoimmune diseases. Int. J. Clin. Exp. Pathol. 2013, 6, 2668–2674. [Google Scholar] [PubMed]
- Lechler, R.; Chai, J.G.; Marelli-Berg, F.; Lombardi, G. The contributions of T-cell anergy to peripheral T-cell tolerance. Immunology 2001, 103, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Schietinger, A.; Greenberg, P.D. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2014, 35, 51–60. [Google Scholar] [CrossRef]
- Timmermans, W.M.C.; van Laar, J.A.M.; van Hagen, P.M.; van Zelm, M.C. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin. Transl. Immunol. 2016, 5, e118. [Google Scholar] [CrossRef]
- Xia, A.; Zhang, Y.; Xu, J.; Yin, T.; Lu, X.-J. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Front. Immunol. 2019, 10, 1719. [Google Scholar] [CrossRef]
- Ando, M.; Ito, M.; Srirat, T.; Kondo, T.; Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 2020, 43, 1–9. [Google Scholar] [CrossRef]
- Wei, Y.-Y.; Fan, J.; Shan, M.-X.; Yin, D.D.; Wang, L.L.; Ye, W.; Zhao, W. TIGIT marks exhausted T cells and serves as a target for immune restoration in patients with chronic HBV infection. Am. J. Transl. Res. 2022, 14, 942–954. [Google Scholar]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining “T cell exhaustion”. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Hua, F.; Dolff, S.; Jun, C.; Cai, X.; Specker, C.; Feldkamp, T.; Kribben, A.; Tervaert, J.W.C.; Witzke, O. Aberrant expression of the negative costimulator PD-1 on T cells in granulomatosis with polyangiitis. Rheumatology 2012, 51, 1188–1197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Manika, K.; Domvri, K.; Kyriazis, G.; Kontakiotis, T.; Papakosta, D. BALF and BLOOD NK-cells in different stages of pulmonary sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2022, 38, e2021039. [Google Scholar] [CrossRef] [PubMed]
- Hannani, D.; Vétizou, M.; Enot, D.; Rusakiewicz, S.; Chaput, N.; Klatzmann, D.; Desbois, M.; Jacquelot, N.; Vimond, N.; Chouaib, S.; et al. Anticancer immunotherapy by CTLA-4 blockade: Obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015, 25, 208–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Alessandro, M.; Conticini, E.; Bergantini, L.; Mezzasalma, F.; Cameli, P.; Baglioni, S.; Armati, M.; Abbritti, M.; Bargagli, E. PD1, CTLA4 and TIGIT Expression on T and NK Cells in Granulomatous Diseases: Sarcoidosis and ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2023, 24, 256. https://doi.org/10.3390/ijms24010256
d’Alessandro M, Conticini E, Bergantini L, Mezzasalma F, Cameli P, Baglioni S, Armati M, Abbritti M, Bargagli E. PD1, CTLA4 and TIGIT Expression on T and NK Cells in Granulomatous Diseases: Sarcoidosis and ANCA-Associated Vasculitis. International Journal of Molecular Sciences. 2023; 24(1):256. https://doi.org/10.3390/ijms24010256
Chicago/Turabian Styled’Alessandro, Miriana, Edoardo Conticini, Laura Bergantini, Fabrizio Mezzasalma, Paolo Cameli, Stefano Baglioni, Martina Armati, Marta Abbritti, and Elena Bargagli. 2023. "PD1, CTLA4 and TIGIT Expression on T and NK Cells in Granulomatous Diseases: Sarcoidosis and ANCA-Associated Vasculitis" International Journal of Molecular Sciences 24, no. 1: 256. https://doi.org/10.3390/ijms24010256
APA Styled’Alessandro, M., Conticini, E., Bergantini, L., Mezzasalma, F., Cameli, P., Baglioni, S., Armati, M., Abbritti, M., & Bargagli, E. (2023). PD1, CTLA4 and TIGIT Expression on T and NK Cells in Granulomatous Diseases: Sarcoidosis and ANCA-Associated Vasculitis. International Journal of Molecular Sciences, 24(1), 256. https://doi.org/10.3390/ijms24010256







