Evolution of Ceftriaxone Resistance of Penicillin-Binding Proteins 2 Revealed by Molecular Modeling
Abstract
1. Introduction
2. Results
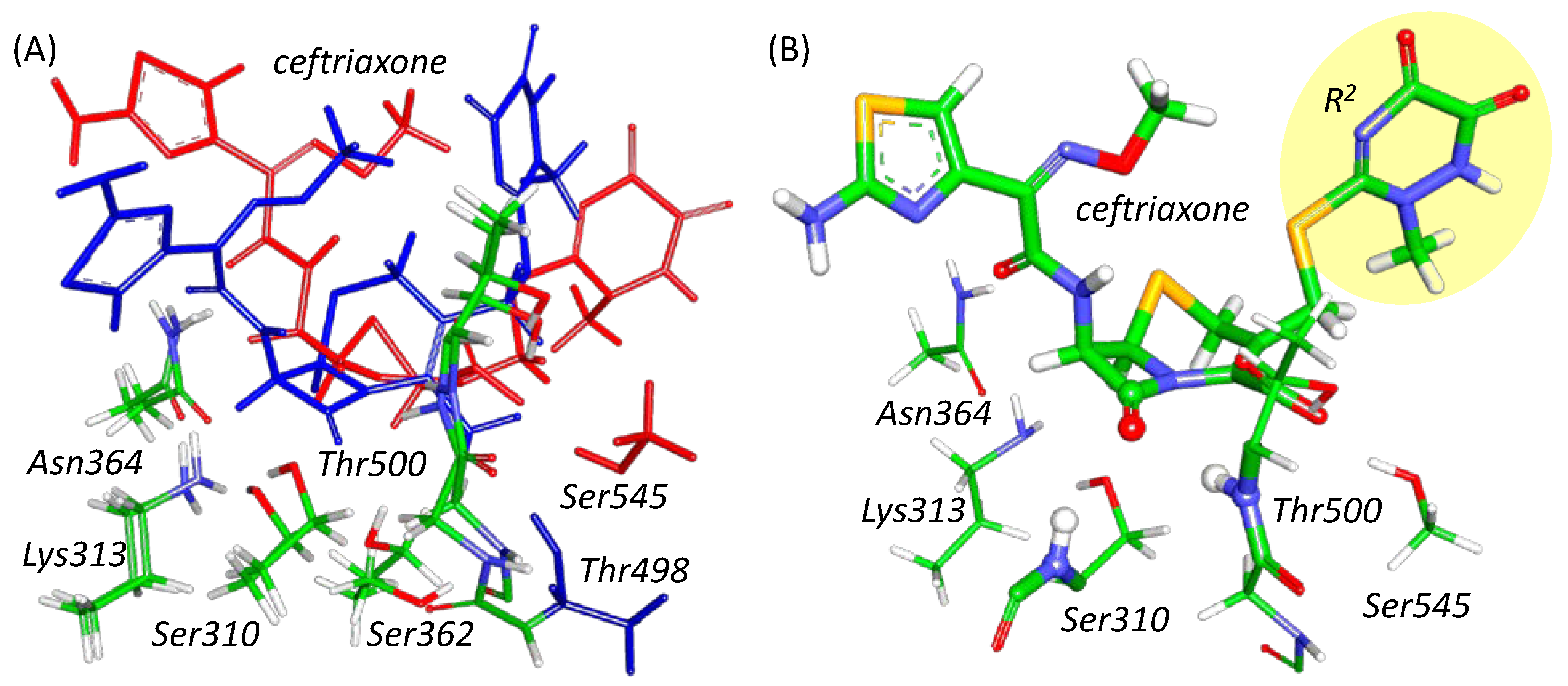
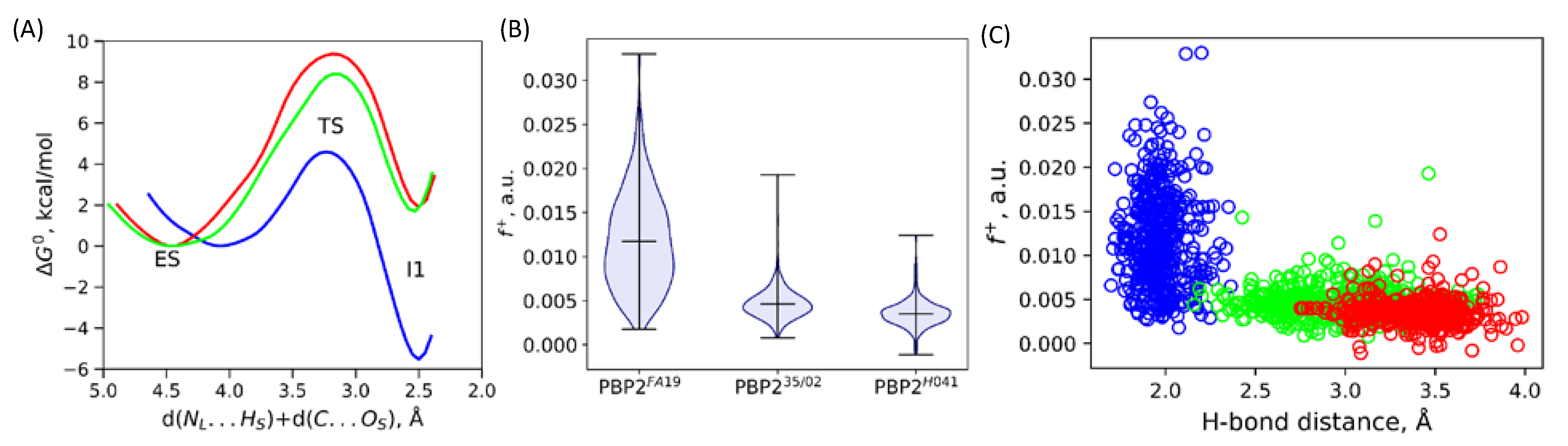
2.1. Ceftriaxone Activation in the Active Site of Enzyme and Nucleophilic Attack
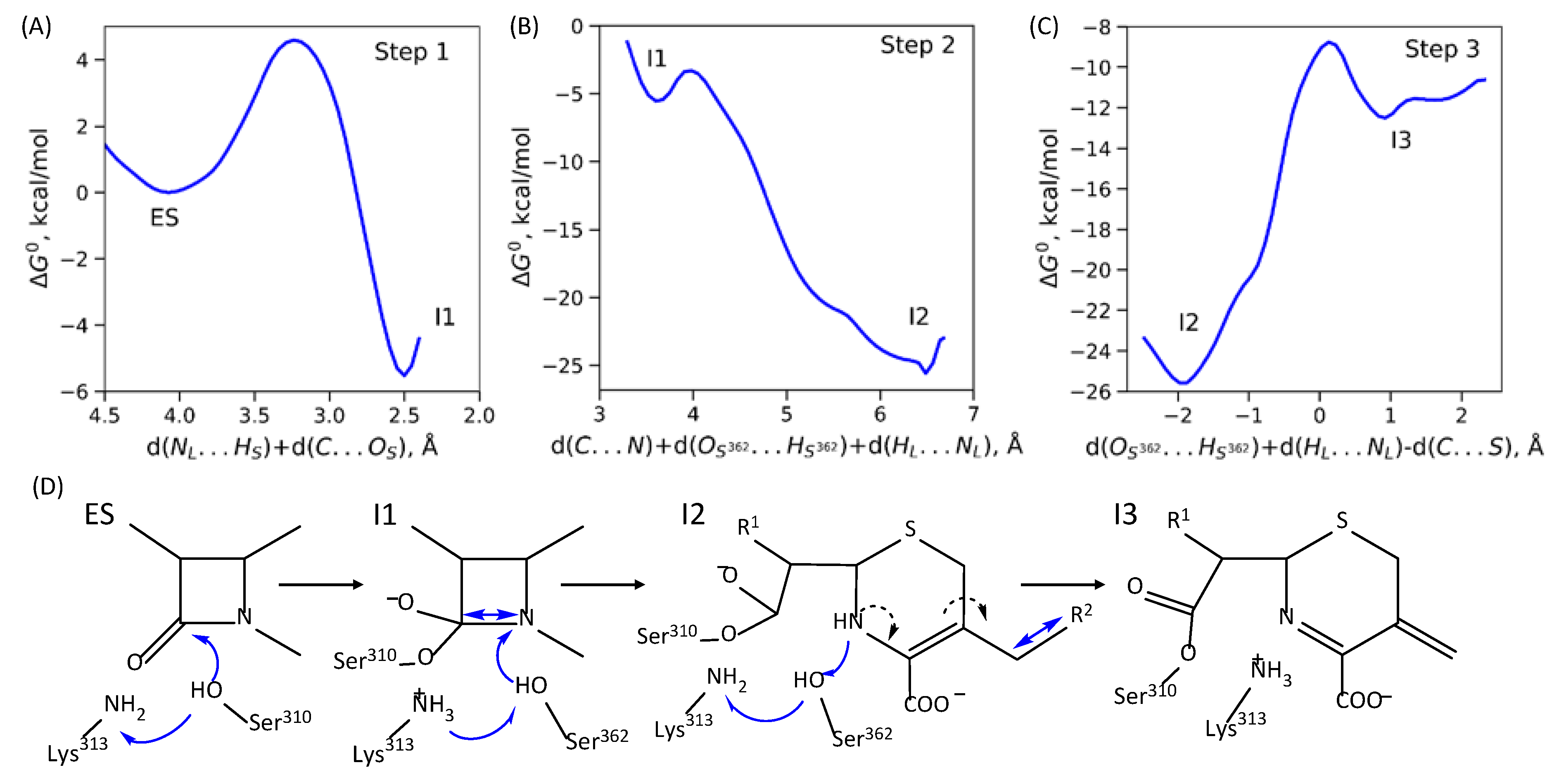
2.2. Mechanism of Acyl–Enzyme Complex Formation in PBP2 from FA19 Strain
2.3. Mechanisms of Acyl–Enzyme Complex Formation in PBP2 from 35/02 and H041 Strains
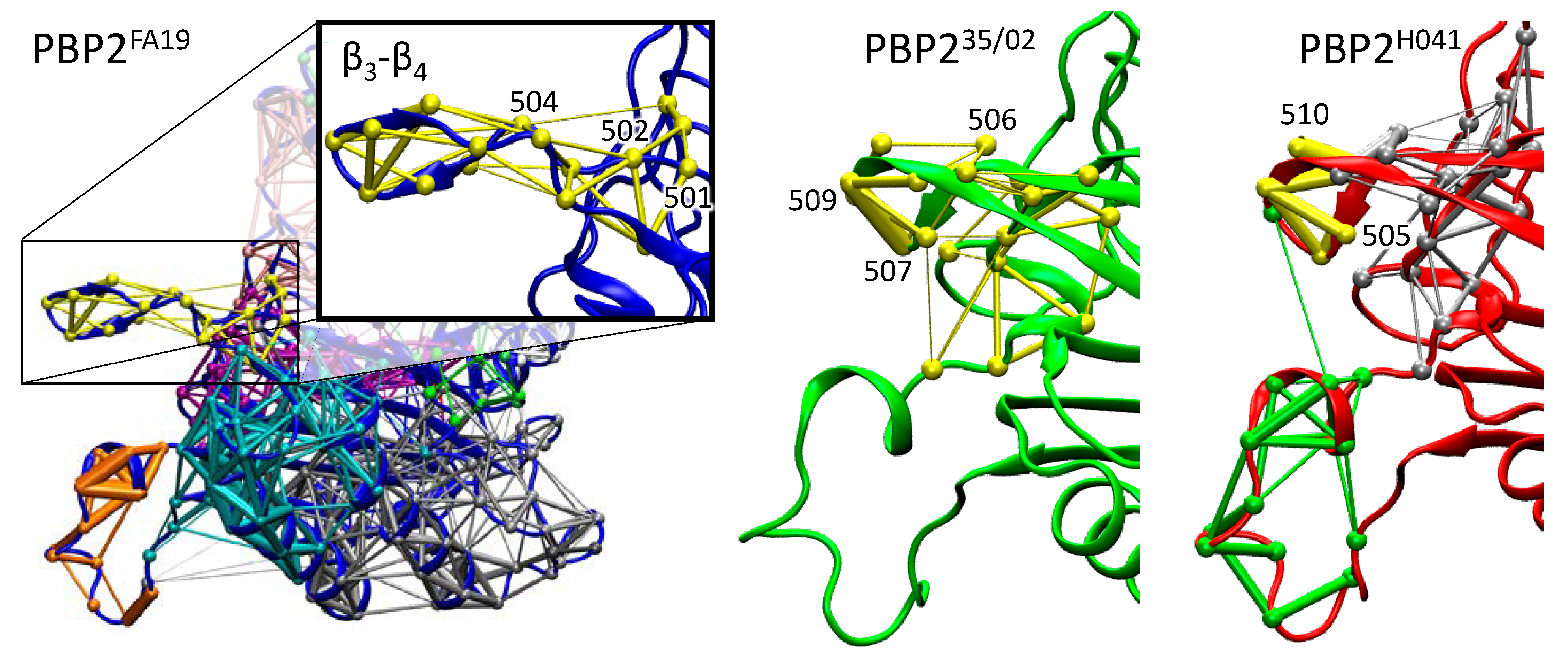
2.4. Dynamical Network Analysis of β3-β4 Loop
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: Oral Cephalosporins No Longer a Recommended Treatment for Gonococcal Infections. JAMA 2012, 308, 1850. [CrossRef][Green Version]
- Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2006: Fluoroquinolones No Longer Recommended for Treatment of Gonococcal Infections. Ann. Emerg. Med. 2007, 50, 232–234. [CrossRef]
- St. Cyr, S.; Barbee, L.; Workowski, K.A.; Bachmann, L.H.; Pham, C.; Schlanger, K.; Torrone, E.; Weinstock, H.; Kersh, E.N.; Thorpe, P. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; McNulty, A.; Avery, A.; Whiley, D.; Tabrizi, S.N.; Hardy, D.; Das, A.F.; Nenninger, A.; Fairley, C.K.; Hocking, J.S.; et al. Solithromycin versus ceftriaxone plus azithromycin for the treatment of uncomplicated genital gonorrhoea (SOLITAIRE-U): A randomised phase 3 non-inferiority trial. Lancet Infect. Dis. 2019, 19, 833–842. [Google Scholar] [CrossRef]
- Fernandes, P.; Craft, J.C. Phase 3 trial of treating gonorrhoea with solithromycin. Lancet Infect. Dis. 2019, 19, 928. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; Schim-van der Loeff, M.F. Solithromycin for the treatment of drug-resistant gonorrhoea. Lancet Infect. Dis. 2019, 19, 791–792. [Google Scholar] [CrossRef]
- Goytia, M.; Thompson, S.T.; Jordan, S.V.L.; King, K.A. Antimicrobial Resistance Profiles of Human Commensal Neisseria Species. Antibiotics 2021, 10, 538. [Google Scholar] [CrossRef]
- Lindberg, R.; Fredlund, H.; Nicholas, R.; Unemo, M. Neisseria gonorrhoeae Isolates with Reduced Susceptibility to Cefixime and Ceftriaxone: Association with Genetic Polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 2007, 51, 2117–2122. [Google Scholar] [CrossRef]
- Spratt, B.G.; Balthazar, J.T.; Hagman, K.E.; Pan, W.; Judd, R.C.; Shafer, W.M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995, 141, 611–622. [Google Scholar] [CrossRef]
- Veal, W.L.; Nicholas, R.A.; Shafer, W.M. Overexpression of the MtrC-MtrD-MtrE Efflux Pump Due to an mtrR Mutation Is Required for Chromosomally Mediated Penicillin Resistance in Neisseria gonorrhoeae. J. Bacteriol. 2002, 184, 5619–5624. [Google Scholar] [CrossRef]
- Gill, M.J.; Simjee, S.; Al-Hattawi, K.; Robertson, B.D.; Easmon, C.S.F.; Ison, C.A. Gonococcal Resistance to β-Lactams and Tetracycline Involves Mutation in Loop 3 of the Porin Encoded at the penB Locus. Antimicrob. Agents Chemother. 1998, 42, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Olesky, M.; Zhao, S.; Rosenberg, R.L.; Nicholas, R.A. Porin-Mediated Antibiotic Resistance in Neisseria gonorrhoeae: Ion, Solute, and Antibiotic Permeation through PIB Proteins with penB Mutations. J. Bacteriol. 2006, 188, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Ropp, P.A.; Hu, M.; Olesky, M.; Nicholas, R.A. Mutations in ponA, the Gene Encoding Penicillin-Binding Protein 1, and a Novel Locus, penC, Are Required for High-Level Chromosomally Mediated Penicillin Resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2002, 46, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Tomberg, J.; Unemo, M.; Ohnishi, M.; Davies, C.; Nicholas, R.A. Identification of Amino Acids Conferring High-Level Resistance to Expanded-Spectrum Cephalosporins in the penA Gene from Neisseria gonorrhoeae Strain H041. Antimicrob. Agents Chemother. 2013, 57, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 1988, 332, 173–176. [Google Scholar] [CrossRef]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Powell, A.J.; Tomberg, J.; Deacon, A.M.; Nicholas, R.A.; Davies, C. Crystal Structures of Penicillin-binding Protein 2 from Penicillin-susceptible and -resistant Strains of Neisseria gonorrhoeae Reveal an Unexpectedly Subtle Mechanism for Antibiotic Resistance. J. Biol. Chem. 2009, 284, 1202–1212. [Google Scholar] [CrossRef]
- Stefanova, M.E.; Tomberg, J.; Olesky, M.; Höltje, J.-V.; Gutheil, W.G.; Nicholas, R.A. Neisseria gonorrhoeae Penicillin-Binding Protein 3 Exhibits Exceptionally High Carboxypeptidase and β-Lactam Binding Activities. Biochemistry 2003, 42, 14614–14625. [Google Scholar] [CrossRef]
- Stefanova, M.E.; Tomberg, J.; Davies, C.; Nicholas, R.A.; Gutheil, W.G. Overexpression and enzymatic characterization of Neisseria gonorrhoeae penicillin-binding protein 4. Eur. J. Biochem. 2003, 271, 23–32. [Google Scholar] [CrossRef]
- Barbour, A.G. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 1981, 19, 316–322. [Google Scholar] [CrossRef]
- Tomberg, J.; Unemo, M.; Davies, C.; Nicholas, R.A. Molecular and Structural Analysis of Mosaic Variants of Penicillin-Binding Protein 2 Conferring Decreased Susceptibility to Expanded-Spectrum Cephalosporins in Neisseria gonorrhoeae: Role of Epistatic Mutations. Biochemistry 2010, 49, 8062–8070. [Google Scholar] [CrossRef]
- Singh, A.; Turner, J.M.; Tomberg, J.; Fedarovich, A.; Unemo, M.; Nicholas, R.A.; Davies, C. Mutations in penicillin-binding protein 2 from cephalosporin-resistant Neisseria gonorrhoeae hinder ceftriaxone acylation by restricting protein dynamics. J. Biol. Chem. 2020, 295, 7529–7543. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Golparian, D.; Shimuta, K.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.; Kitawaki, J.; Unemo, M. Is Neisseria gonorrhoeae Initiating a Future Era of Untreatable Gonorrhea?: Detailed Characterization of the First Strain with High-Level Resistance to Ceftriaxone. Antimicrob. Agents Chemother. 2011, 55, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Fenton, B.A.; Tomberg, J.; Sciandra, C.A.; Nicholas, R.A.; Davies, C.; Zhou, P. Mutations in PBP2 from ceftriaxone-resistant Neisseria gonorrhoeae alter the dynamics of the β3–β4 loop to favor a low-affinity drug-binding state. J. Biol. Chem. 2021, 297, 101188. [Google Scholar] [CrossRef]
- Kamerlin, S.C.L.; Chu, Z.T.; Warshel, A. On Catalytic Preorganization in Oxyanion Holes: Highlighting the Problems with the Gas-Phase Modeling of Oxyanion Holes and Illustrating the Need for Complete Enzyme Models. J. Org. Chem. 2010, 75, 6391–6401. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Goodman, J.M. Hydrogen-bond stabilization in oxyanion holes: Grand jeté to three dimensions. Org. Biomol. Chem. 2012, 10, 1905. [Google Scholar] [CrossRef]
- Amyes, T.L.; Richard, J.P. Specificity in Transition State Binding: The Pauling Model Revisited. Biochemistry 2013, 52, 2021–2035. [Google Scholar] [CrossRef]
- Krivitskaya, A.V.; Khrenova, M.G. Molecular modeling of ceftriaxone activation in the active sites of penicillin-binding proteins 2. Russ. Chem. Bull. 2022, 71, 915–920. [Google Scholar] [CrossRef]
- Khrenova, M.G.; Tsirelson, V.G.; Nemukhin, A.V. Dynamical properties of enzyme–substrate complexes disclose substrate specificity of the SARS-CoV-2 main protease as characterized by the electron density descriptors. Phys. Chem. Chem. Phys. 2020, 22, 19069–19079. [Google Scholar] [CrossRef]
- Krivitskaya, A.V.; Khrenova, M.G.; Nemukhin, A.V. Two Sides of Quantum-Based Modeling of Enzyme-Catalyzed Reactions: Mechanistic and Electronic Structure Aspects of the Hydrolysis by Glutamate Carboxypeptidase. Molecules 2021, 26, 6280. [Google Scholar] [CrossRef]
- Krivitskaya, A.V.; Khrenova, M.G. Influence of the Active Site Flexibility on the Efficiency of Substrate Activation in the Active Sites of Bi-Zinc Metallo-β-Lactamases. Molecules 2022, 27, 7031. [Google Scholar] [CrossRef]
- Frère, J.M.; Nguyen-Distèche, M.; Coyette, J.; Joris, B. Mode of action: Interaction with the penicillin binding proteins. In The Chemistry of β-Lactams; Springer: Dordrecht, The Netherlands, 1992; pp. 148–197. [Google Scholar]
- Singh, A.; Tomberg, J.; Nicholas, R.A.; Davies, C. Recognition of the β-lactam carboxylate triggers acylation of Neisseria gonorrhoeae penicillin-binding protein 2. J. Biol. Chem. 2019, 294, 14020–14032. [Google Scholar] [CrossRef] [PubMed]
- Word, J.M.; Lovell, S.C.; Richardson, J.S.; Richardson, D.C. Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 1999, 285, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Denning, E.J.; Priyakumar, U.D.; Nilsson, L.; Mackerell, A.D. Impact of 2′-hydroxyl sampling on the conformational properties of RNA: Update of the CHARMM all-atom additive force field for RNA. J. Comput. Chem. 2011, 32, 1929–1943. [Google Scholar] [CrossRef]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field (CGenFF): A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- TeraChem v 1.9, PetaChem, LLC. Available online: www.petachem.com (accessed on 21 November 2022).
- Melo, M.C.R.; Bernardi, R.C.; Rudack, T.; Scheurer, M.; Riplinger, C.; Phillips, J.C.; Maia, J.D.C.; Rocha, G.B.; Ribeiro, J.V.; Stone, J.E.; et al. NAMD goes quantum: An integrative suite for hybrid simulations. Nat. Methods 2018, 15, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Krivitskaya, A.V.; Khrenova, M.G. Boronic Acids as Prospective Inhibitors of Metallo-β-Lactamases: Efficient Chemical Reaction in the Enzymatic Active Site Revealed by Molecular Modeling. Molecules 2021, 26, 2026. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Liu, S.; Rong, C.; Lu, T. Information Conservation Principle Determines Electrophilicity, Nucleophilicity, and Regioselectivity. J. Phys. Chem. A 2014, 118, 3698–3704. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Kästner, J.; Thiel, W. Bridging the gap between thermodynamic integration and umbrella sampling provides a novel analysis method: “Umbrella integration”. J. Chem. Phys. 2005, 123, 144104. [Google Scholar] [CrossRef]
- Kästner, J. Umbrella sampling. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 932–942. [Google Scholar] [CrossRef]
- Sethi, A.; Eargle, J.; Black, A.A.; Luthey-Schulten, Z. Dynamical networks in tRNA:protein complexes. Proc. Natl. Acad. Sci. USA 2009, 106, 6620–6625. [Google Scholar] [CrossRef]
- Glykos, N.M. Software news and updates carma: A molecular dynamics analysis program. J. Comput. Chem. 2006, 27, 1765–1768. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivitskaya, A.V.; Khrenova, M.G. Evolution of Ceftriaxone Resistance of Penicillin-Binding Proteins 2 Revealed by Molecular Modeling. Int. J. Mol. Sci. 2023, 24, 176. https://doi.org/10.3390/ijms24010176
Krivitskaya AV, Khrenova MG. Evolution of Ceftriaxone Resistance of Penicillin-Binding Proteins 2 Revealed by Molecular Modeling. International Journal of Molecular Sciences. 2023; 24(1):176. https://doi.org/10.3390/ijms24010176
Chicago/Turabian StyleKrivitskaya, Alexandra V., and Maria G. Khrenova. 2023. "Evolution of Ceftriaxone Resistance of Penicillin-Binding Proteins 2 Revealed by Molecular Modeling" International Journal of Molecular Sciences 24, no. 1: 176. https://doi.org/10.3390/ijms24010176
APA StyleKrivitskaya, A. V., & Khrenova, M. G. (2023). Evolution of Ceftriaxone Resistance of Penicillin-Binding Proteins 2 Revealed by Molecular Modeling. International Journal of Molecular Sciences, 24(1), 176. https://doi.org/10.3390/ijms24010176




