Abstract
The mechanisms by which immune systems identify and destroy tumors, known as immunosurveillance, have been discussed for decades. However, several factors that lead to tumor persistence and escape from the attack of immune cells in a normal immune system have been found. In the process known as immunoediting, tumors decrease their immunogenicity and evade immunosurveillance. Furthermore, tumors exploit factors such as regulatory T cells, myeloid-derived suppressive cells, and inhibitory cytokines that avoid cytotoxic T cell (CTL) recognition. Current immunotherapies targeting tumors and their surroundings have been proposed. One such immunotherapy is autologous cancer vaccines (ACVs), which are characterized by enriched tumor antigens that can escalate specific CTL responses. Unfortunately, ACVs usually fail to activate desirable therapeutic effects, and the low immunogenicity of ACVs still needs to be elucidated. This difficulty highlights the significance of immunogenic antigens in antitumor therapies. Previous studies have shown that defective host immunity triggers tumor development by reprogramming tumor antigenic expressions. This phenomenon sheds new light on ACVs and provides a potential cue to improve the effectiveness of ACVs. Furthermore, synergistically with the ACV treatment, combinational therapy, which can reverse the suppressive tumor microenvironments, has also been widely proposed. Thus, in this review, we focus on tumor immunogenicity sculpted by the immune systems and discuss the significance and application of restructuring tumor antigens in precision medicine.
1. Introduction
The tumor immunoediting process includes elimination, equilibrium, and escape. The elimination phase is the same as the theory of immune surveillance, in which the immune system prevents tumor growth [1]. Tumors will be spontaneously eliminated by the immune cells, including innate [2,3] and adaptive cells [1,4,5,6,7]. In general, tumor regression can be completed in this phase. However, when only a portion of tumor cells are eliminated, the resulting tumors survive and enter the subsequent two phases, dampening the immunogenicity and facilitating tumor progression. Whether tumors remain dormant or continue to evolve during this period, the accumulation of DNA mutations or changes in gene expression still occur. These result in the modulation of tumor antigens, and the immune systems possess selective pressures by attacking susceptible tumor clones. Under selective pressures, the tumors continue to change their phenotypes and downregulate their immunogenic targets. The immune system ultimately fails to recognize and destroy tumors due to the decreased immunogenicity, so the tumors escape from the immune system and thus progressively develop [8].
The immune theory, which addresses the interaction between cancer development and host immunity, has been investigated. Thus, tumor immunotherapy is regarded as a prospective antitumor strategy and aims to strengthen the host immunity, especially cytotoxic T lymphocytes (CTLs), against tumors. The activation of antigen-specific CTL responses is triggered by antigens with high tumor specificity [5]. This highlights the significance of tumor antigens in immunotherapy. Among the immunotherapies currently used, autologous cancer vaccines (ACVs) are characterized by the expression of “individual” tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs), which can escalate stronger personalized and antigen-specific immunity. Therefore, studies using antitumor vaccines with the design of individual tumor antigens are highly recommended. Furthermore, in a previous study, more cancer patients benefited from whole tumor cell-based vaccines than from specific tumor antigens [9]. This provided strong evidence for the use of whole tumor cell-based vaccines rather than immunization with the specific antigens. However, many clinical trials using ACVs have failed due to their low immunogenicity [10,11,12]. Therefore, recent studies have been focusing on increasing the effectiveness of ACVs by the induction of tumor antigens.
The sculpting of the immunogenicity of tumors by the immune system is well understood. Tumors developed in immunocompetent hosts undergo immunoediting, which reduces the expression of TSAs and TAAs [13]. In contrast, tumors grow more aggressively in immunocompromised hosts, which increases their immunogenicity [14,15,16,17]. Furthermore, our previous studies have also indicated that tumors developed in immunocompromised hosts lead to the reprogramming of the gene and/or protein profiles with the overexpression of tumor-associated antigens [18,19]. Herein, we update the progress on the interaction between host immunity and tumor development, the impacts of host immunity on the manipulation of tumor immunogenicity, and the effective strategies of precision medicine using boosted autologous cancer vaccines.
2. The Sentry of the Immune System: Immunosurveillance
2.1. Tumor Recognition and Rejection by the Immune System
T lymphocytes are essential in antitumor immunity, such as in the surveillance, detection, and destruction of neoplastic cells [1]. CTLs are the most potent effectors and are considered significant drivers in antitumor effects [4], and they are activated by dendritic cells (DCs). DCs initiate and maintain the antitumor T cell immunity [5]. During tumor initiation, the dying tumor cells release danger signals, such as molecules called damage-associated molecular patterns (DAMPs). Upon sensing these signals and capturing tumor cells, DCs undergo maturation, migrate to the draining lymph nodes (dLNs), and present the tumor antigens onto major histocompatibility complex I (MHC I) for presentation to CD8+ T cells [20]. These activate the antigen-specific CTLs, which naïve CD8+ T cells differentiate into CTLs and memory CD8+ T cells. The educated CTLs can recognize the antigenic targets expressed on the tumors and secrete cytokines, IFN-γ, perforin, and granzyme, thus performing tumor lytic functions [6,7]. In addition to priming naïve CD8+ T cells, DCs interact with memory T cells and induce their differentiation into the tumor sites [21]. Furthermore, DCs produce IL-12, which triggers IFN-γ release from the CTLs, which in turn enhances CTL activation and functions [22]. The close interaction between DCs and CTLs suggests tight T cell–DC cross-talk in the tumor microenvironment (TME).
CTLs exerting antitumor effects rely on the help of other immune cells, such as DCs and helper CD4+ T cells. Helper CD4+ T cells play a prominent role in maintaining CD8+ cytolytic responses and preventing CTL exhaustion [4,23]. Interestingly, the help signals originating from the CD40 ligand (CD40L) on the helper CD4+ T cells stimulate CD40 on the DCs, which deliver CD4+ T cell-derived help signals to CD8+ T cells and elicit CTL responses [24]. During the first antigen priming step, naïve CD4+ T cells and CD8+ T cells are separately activated by different populations of DCs in the peripheral lymph nodes [25,26,27], where MHC I-expressing cDC1 usually interacts with CD8+ T cells. In contrast, MHC II-expressing cDC2 interacts with CD4+ T cells. Next, in the second priming step, CD4+ T cells and CD8+ T cells interact with the same cDC1, and the help signal occurs [28,29]. cDC1 engages in cognate interaction with pre-activated CD4+ T cells, which optimizes the cDC1 to relay signals for the differentiation of effector T cells and memory CTLs to pre-activated CD8+ T cells [30]. In summary, antigen-specific (pre-activated) CD4+ T cells assist the pre-activated CD8+ T cells in the differentiation into effector T or memory T cells. Helper CD4+ T cells dictate the quality of the CTL differentiation and promote the expansion of antigen-specific CTLs by cytokine signals. CD4+ T cells promote the development of antigen-specific CTLs through the amplification of IL-12 and IL-15 production induced by IFN-γ in DCs [31,32]. Furthermore, IL-2 signaling promotes CD8 proliferation [33,34], and the expression of IL-2 receptor α-chain (IL-2Rα) by recently primed CD8+ T cells depends on CD4+ T cells [35]. In addition to the assistant roles of CD4+ T cells, the cytolytic activities in antitumor effects of CD4+ T cells have been proposed. CD4+ T cells produce IFN-γ and express granzymes and perforin to kill tumors [36,37]. Recent studies using RNA sequencing in human cancers have reported cytotoxic characteristics of CD4+ T cells, which express cytolytic molecules such as granzymes, perforin, and granulysin in the tumors and circulation of cancer patients [38,39,40,41].
Natural killer (NK) cells are also essential effectors in tumor immunosurveillance. For example, NK cells can be activated by MHC class I polypeptide-related sequence A (MICA) and MICB, which are expressed on tumors [2]. NK cells directly kill tumor cells by releasing perforin and granzymes [3] or trigger cell apoptosis through the ligation of cell death receptor-mediated pathways (FasL/Fas) [42]. Healthy cells express high levels of MHC I, which ligates to the killer immunoglobulin-like family inhibitory receptors (KIRs) on NK cells. However, tumors will downregulate MHC I expression to evade CTL-mediated cytotoxicity and simultaneously activate NK cells due to the decreased inhibitory signaling [42,43] (Figure 1).
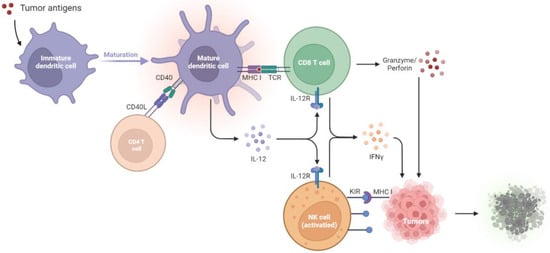
Figure 1.
Tumor suppression by the immune systems during immunosurveillance. Once tumor antigens are captured, immature DCs undergo maturation. The mature DCs present the antigens onto MHC I molecules for presentation to CD8+ T cells. The CD40L on the CD4+ T cell stimulates CD40 on the DCs, which delivers help signals to CD8+ T cells. With the CD40–CD40L interaction, the amplification of IL-12 in DCs promotes the development of antigen-specific CD8+ T cells and thus increases the IFN-γ, granzyme, and perforin production to kill tumors. NK cells will be activated by the diminished expression of MHC I on tumors, which relieves the KIR inhibitory signaling and activates the NK cell toxicity towards tumors. CD40L, CD40 ligand; DCs, dendritic cells; IL-12R, IL-12 receptor; IFN-γ, interferon-γ; KIR, killer immunoglobulin-like receptor; MHC I, major histocompatibility complex class I; NK cells, natural killer cells; TCR, T cell receptor.
2.2. Failure of Immunosurveillance Enables Cancer Progression
Accumulating evidence indicates the increased risk of tumor development in immunosuppressed patients, suggesting the crucial antitumor role of intact immunity. Mice with a deficiency in adaptive immunity provide a practical animal model for testing cancer immunosurveillance. In one study, mice with severe combined immunodeficiency (SCID) exhibited impaired differentiation of both T and B lymphocytes, and thus 15% of these mice developed T cell lymphomas [44]. In another study, RAG2−/− mice, another kind of mice without B and T cells, developed spontaneous intestinal adenomas (50%), intestinal adenocarcinomas (35%), and lung cancers (15%) at the age of 15–16 months [45]. In addition to adaptive immunity, NK cells also manifested cancer immunoediting by producing IFN-γ and induced M1 macrophages.
Mice with a deficiency in effector cells, such as T cells and NK cells, usually develop spontaneous cancers with aging. In STAT1-deficient mice, the JAK/STAT1 signaling pathway is down-regulated and thus inhibits type I and II IFN production. This leads to the increased formation of mammary carcinomas [46]. Mice lacking death-inducing molecule tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) [47], or with the inactivation of the Fas-mediated cell death pathway by Fas or FasL mutation, have accelerated hematological malignancies [48]. Decreased cytokine production and/or antigen presentation in gene-deficient mice, including Perforin−/− (lack perforin) [49], Ifng−/− (lack IFN-γ) [50], Perforin−/−Ifng−/− (lack perforin and IFN-γ) [50], Perforin−/−B2m−/− (lack perforin and MHC class I expression) [51], and Lmp2−/− (defective MHC class I antigen presentation) [52], facilitates tumor growth. Due to the decreased IFN-γ secretion, IL-12 and IL-18, two IFN-γ-inducing cytokines, are down-regulated. A previous study found that, compared with wild-type mice, neither IL-12 nor IL-18 deficient mice exhibited increased incidence of tumor development [50]. These results suggest that immune effector cells, cytokines, and their related pathways are essential for the regression of tumor development in immunosurveillance. The following findings support the same conclusion. Due to the presence of the constitutively expressing oncogene, Kras, and inhibiting tumor suppressor, p53, tumors growing in immunocompetent mice were retarded compared to those in immunocompromised mice.
3. Immunoediting: How Tumors Hijack Host Immunity and Establish a Favorable Microenvironment
Tumors escape from the immune system via several mechanisms, including the formation of regulatory cells, reduced immune recognition, and production of suppressive cytokines. The immune system fails to restrict tumor development; as a result, tumors evade immune recognition (due to diminished tumor antigens and immunogenicity) and release suppressive cytokines. The regulatory immune cells, Tregs, MDSCs, dysfunctional DCs, and M2 macrophages also express immune regulatory factors, such as IDO and arginase, to construct a tumor microenvironment (TME) that enhances tumor progression and dampens T cell functions. In this escape phase, the balance is toward tumor progression, with the infiltrations of inhibitory cells, cytokines, and factors. The results are that the immune system is incapable of inhibiting tumor progression. By generating the appropriate TME via the mechanisms listed below, tumors dampen the immune responses, supporting tumor growth and even metastasis [8] (Figure 2).
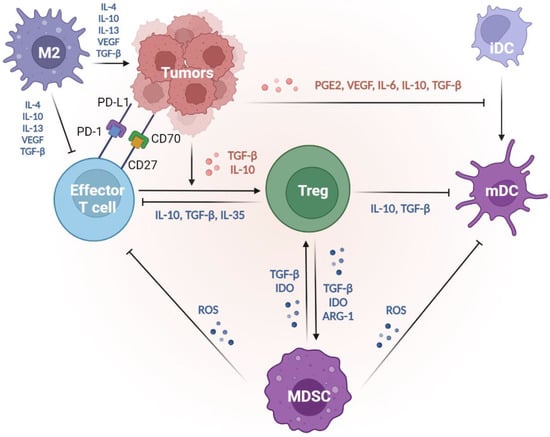
Figure 2.
Suppressive tumor microenvironment created by tumors, thus decreasing tumor immunogenicity and evading immunosurveillance. Tumors suppress the DC functions to reduce their antigenicity. With the impacts of PGE2, VEGF, IL-6, IL-10, and TGF-β produced by tumors, immature DCs fail to undergo maturation, so the antigen presentation abilities are suppressed. Tumors evade the attack from T effectors from several mechanisms. First, tumors mediate T-cell apoptosis via dysregulation of the CD70–CD25 axis and the ligation of PD-L1 and PD-1. Second, tumors produce IL-10 and TGF-β to trigger effector T cells into regulatory phenotypes (Treg), which play central roles in immune suppression. Through the release of IL-10, TGF-β, and/or IL-35, Tregs dampen the effector T cell activities and suppress the antigen presentation of mature DCs. Furthermore, factors such as TGF-β, IDO, and ARG-1 produced by both Tregs and/or MDSCs shape a positive feedback correlation to facilitate the expansion of each group and strengthen the suppressive TME. MDSCs secrete a high level of ROS, suppressing the antigen presentation from mature DCs and the anti-tumor immunity from effector T cells. In the TME, M2 macrophages are the most abundant tolerogenic cells. M2 macrophages produce IL-4, IL-10, IL-13, VEGF, and TGF-β to inhibit effector T cells and promote tumor progression. Thus, tumors can decrease their antigenicity by manipulating DC functions and inhibiting effector T cells, thereby evading immunosurveillance. ARG-1, arginase 1; DCs, dendritic cells; IDO, indoleamine 2, 3-dioxygenase; MDSCs, myeloid-derived suppressive cells; PGE2, prostaglandin E2; TGF-β, transforming growth factor-β; TME, tumor microenvironment; Tregs, regulatory T cells; VEGF, vascular endothelial growth factor.
3.1. Recruitment of Regulatory Cells
3.1.1. Regulatory T Cells
Immune suppression mediated by the regulatory cells and other suppressive factors in the TME is a major mechanism by which tumors avoid attacks from the immune system. CD4+CD25+FoxP3+ tumor-derived regulatory T cells (Tregs) play central roles in immune suppression. Studies also show that different cytokines, such as IL-10 and TGF-β produced by the tumors, trigger the conversion of CD4+ T cells into suppressive Tregs [53]. Tregs then secret IL-10, TGF-β, and IL-35 to downregulate antitumor immunity, suppress the antigen presentation by DCs, and decrease the tumor-specific CTLs [54]. Meanwhile, since IL-2 is essential to T cell activation and maintenance, these regulatory T cells compete with effector T cells by largely consuming IL-2 [33,34]. Tregs can also kill the CTLs by the secretion of perforin and granzyme, leading to osmotic lysis and apoptosis, just as CTLs and NK cells do to tumor cells. Furthermore, Tregs interfere with the memory T cells by repressing their effector and proliferation activities through the upregulation of CTLA-4 ligands [55]. The aforementioned evidence supports the mediation of the comprehensive suppression of antitumor immunity by Tregs.
3.1.2. Myeloid-Derived Suppressive Cells
Both Tregs and myeloid-derived suppressive cells (MDSCs) facilitate their own expansion by the overexpression of CD73 [56,57], TGF-β [58], and indoleamine 2, 3-dioxygenase (IDO) [59,60,61]. In addition, MDSCs may promote the recruitment of Tregs by producing CCR5 ligands, CCL3, CCL4, and CCL5 [62]. MDSCs express a high level of inducible nitric oxide (iNO), which produces nitric oxide (NO) [63,64]. NO inhibits the JAK/STAT5 pathway and/or suppresses the antigen presentation from DCs, leading to the suppressive proliferation of effector T cells [64,65]. Furthermore, MDSC-derived NO also triggers the effector T cell apoptosis [66] and reduces E-selectin expression on endothelial cells, which hampers the migration of T cells to the tumor sites [67,68]. MDSCs also secrete a high level of arginase 1 (ARG1), which promotes Treg expansion [69] and depletes the L-arginine, a conditionally essential amino acid in effector T cell functions. These actions lead to the dysregulation of effector T cells and the propagation of Tregs [70].
3.1.3. Tumor-Associated Macrophages
Among the regulatory cell types, tumor-associated macrophages (TAMs) are the most abundant population in TME [71]. There are two polarization states of TAMs: classically activated M1 and alternatively activated M2 subtypes [72]. M2 macrophages produce several anti-inflammatory cytokines, such as IL-4, IL-10, IL-13, vascular endothelial growth factor (VEGF), and TGF-β, to inhibit immune systems and promote tumor progression [73,74,75]. Therefore, CD11b+F4/80+ macrophages having an M2 phenotype are considered regulatory (or “bad”) macrophages [76], which are essential for tumor growth and metastasis. [77]. Furthermore, M2 macrophages can support tumor-related vasculature by accumulating vascular endothelial cells [78]. M2 polarization is induced by several factors, one of which is a colony-stimulating factor 1 (CSF1). CSF1 plays a fundamental role in pro-angiogenesis and tumor burden increase [79], revealing M2 macrophages as the efficient cancer development enabler within the TME [77]. In fact, M2 participates in the angiogenesis cascade, which consists of a series of pro-tumor functions, including degradation of extracellular matrix (ECM), endothelial cell proliferation, and migration [80]. M2-related enzymes promote ECM deposition and the proteolysis of collagens. The degraded collagen fragments may stimulate M2, which could further re-arrange stroma and enhance the angiogenesis activities [81]. Therefore, M2 macrophages are immune cells that promote tumors by releasing suppressive cytokines, triggering tumor angiogenesis, and reorganizing the ECM in the TME.
3.2. Defective Antigen Presentation
3.2.1. Manipulation of the DC Lineage
Defective antigen presentation is another fundamental mechanism by which tumors evade immune surveillance. Immunogenic tumors will be recognized and destroyed by immunosurveillance. However, tumors evolve multiple skills for escaping from immune recognition, including the suppression of DC functions and downregulation of MHC-I expression [82]. DCs play essential roles in the initiation of antitumor T cell immunity [5]. Among the DC subsets, cDC1s (c: conventional) are the most important [83], for the abundance of cDC1s in the TME is associated with T cell infiltration and overall survival in cancer patients [20,84]. cDC1s are recruited to tumor regions by chemokines released by the tumors, such as CCL4 and CCL5 [85,86]. After taking up tumor cells, cDC1s will mature in the tumor sites and migrate to the dLNs to process tumor antigens onto MHC-I for presentation to CD8+ T cells [20]. These processes result in the activation of antigen-specific CTLs. Thus, cDC1s participate in antitumor activities and serve as targets for tumors to escape from the immune system. Tumors prevent the accumulation of cDC1s by activating their β-catenin signaling pathways, thereby decreasing the infiltration of cDC1s and T cells [85,86]. Furthermore, tumors release high levels of prostaglandin E2 (PGE2) [87], VEGF [88,89], IL-6 [90], IL-10 [91], and TGF-β [92] to suppress DC maturation and differentiation. Thus, tumors can decrease their antigenicity by manipulating DC functions, which become tolerogenic and immunosuppressive phenotypes.
3.2.2. Sabotage of the Machinery of Antigen Presentation
The downregulation of tumor antigenicity, such as MHC class I loss, is a well-studied characteristic of immune evasion from tumor-specific cytolytic effects. Tumor antigenicity is sculpted during immunoediting in an immunocompetent host. Some of these changes include antigen depletion [93], gene alterations in MHC-I and B2M [94], and modulation of other antigen processing and presentation machinery (APM). Several TAAs are the by-products during tumorigenesis, which indicates that these TAAs are not necessarily functional for tumor growth. The loss of such antigens in tumors can prevent immune predation [82]. MHC-I and B2M mutations are usually found in tumors [94], and they lead to reduced surface expression of MHC-I [95]. Diminished B2M expression is correlated to a cold immune environment, with low T cell infiltration [93]. Tumors evading immune surveillance by downregulation of APM components are also widely described. Deficiency in proteasome subunits [96], transporters associated with antigen processing (TAP) proteins [97], and Tapasin [98] result in inadequate antigen presentation, thereby escaping CTL recognition. In conclusion, the downregulation of tumor antigens and antigen-presenting processes leads to enhanced tumor growth and metastasis as the CTLs fail to identify the targets on the tumor cells.
3.3. Immune Suppressive Mediators
Tumors can also inhibit host immunity by releasing regulatory cytokines. Within the TME, tumor-induced cytokines and inflammation facilitate cancer development [99,100]. Several tumor-promoting cytokines are regulated by nuclear factor-κB (NF-κB), a central orchestrator of inflammation. Previous research has revealed that the inactivation of NF-κB in immune cells decreases the expression of proinflammatory cytokines and thus relieves the tumor burden [101]. These results indicate that NF-κB-mediated cytokines, including IL-1 [102], IL-6 [103], IL-8 [104], and TNF-α [102], are highly correlated to tumorigenesis. For example, IL-6 interacts with the receptor JAK and induces STAT-3 activation [103], which triggers oncogenes such as myeloid cell leukemia-1 (MCL-1) and upregulates proliferative genes, Cyclin-D1, in tumor cells [105]. In addition, TGF-β is one of the most important contributors to tumor growth. TGF-β is released by the cancer cells, Tregs, fibroblasts, and other types of cells within the TME. The elevated TGF-β levels inhibit effector T cell differentiation, promote Treg proliferation, and dampen DC functions [106]. Furthermore, TGF-β enhances the epithelial-to-mesenchymal transition (EMT), synergistically triggering tumor metastasis with IL-6 via the overactivation of JAK/STAT signaling pathways [107]. Therefore, TGF-β is usually regarded as a chief mediator within the immunosuppressive factors [108].
The overproduction of PGE2 [87], VEGF [88,89], IL-6 [90], IL-10 [91], and TGF-β [92] from tumors will inhibit DC differentiation. The undifferentiated DCs fail to appropriately present antigens and are unable to educate T cells. Immunosuppressive enzymes, such as IDO and arginase, also cause tumor progression through the induction of T-cell tolerance and tumor cell proliferation. IDO impairs CTL functions through the downregulation of the T cell receptor ζ chain and enhances Treg generation [109,110]. Elevated intertumoral IDO expression is correlated to T cell dysfunction, such as decreased levels of granzyme B in tumor-infiltrating CD8+ T cells [111], impaired degranulation of γδ T cells [112], and upregulation of PD-1 and PD-L1 ligands [113,114]. Tumor-infiltrating myeloid cells produce high levels of arginase, which inhibit T cell receptor expression, dampen antigen-specific T cell antitumor immunity, and induce Treg proliferation [115,116].
3.4. Deletion of Tumor-Specific CTLs
Tumors and several immunosuppressive cells trigger T cell apoptosis by Fas and Fas ligand (FasL) signaling pathways. FasL is expressed by tumors and can induce apoptosis of Fas-expressing antitumor CTLs [117]. FasL is reported to be expressed on the tumoral endothelium but not normal vasculature [118], suggesting that FasL-expressing endothelial cells in tumors induce Fas-mediated apoptosis in CTLs. Furthermore, these phenomena have only been observed in CTLs, not Tregs [119,120]. FasL-expressing MDSCs also result in the deletion of CTLs [121,122].
The high expression of CD70 and PD-L1 on the surfaces of tumors also mediates T cell death. The TNF receptor family member CD27 is usually expressed on T cells [123], but its ligand, CD70, is overexpressed in some tumors [124]. The dysregulation of the CD70–CD27 axis within the TME is associated with immunosuppression [125]. One explanation is that the over-activation of CD27 can lead to T-cell deletion, for Siva, a pro-apoptotic protein, can interact with CD27 through a caspase-dependent pathway [126]. Therefore, tumors can induce immunosuppression by promoting T-cell apoptosis via the expression of CD70.
PD-L1 and PD-1 induce immune suppression and facilitate tumor growth by induction of T cell apoptosis. PD-L1 is highly expressed in numerous types of cancers, including numerous solid tumors and hematological cancers [127]. When tumor-expressing PD-L1 combines with PD-1 on the T cells, SHP-1/2 are recruited to the C-terminal PD-1. This causes the de-phosphorylation of several vital signal transducers, including ZAP70, CD3δ, and PI3K pathways, and thus inhibits T cell proliferation, reduces cytokine production, and triggers T cell apoptosis [127,128].
4. Greater Immunogenicity of Tumors Developed in Immunodeficient Hosts
4.1. Increased Immunogenicity of Tumors Developed in Immunocompromised Hosts (Figure 3)
Genomic instability, such as mutation, is one of the essential features of tumors [129]. The uncontrolled growth in tumors ultimately results in alterations of tumor antigens, some of which may harbor high immunogenicity [17]. A previous study showed that immunodeficient (RAG2−/−x γc−/−) mice were more susceptible to MCA (3-methylcholanthrene)-induced sarcoma than were syngeneic RAG2−/− and wide-type (WT) mice. Furthermore, the authors harvested the tumors from these three strains of mice and re-inoculated the primary tumors into immunocompetent mice. MCA-induced sarcoma cell lines derived from RAG2−/− (30% regression) and RAG2−/−x γc−/− (70% regression) mice failed to form tumors when transplanted into syngeneic WT mice. In contrast, all the MCA-induced sarcoma cell lines from WT mice successfully developed when re-inoculated into immunocompetent mice. These results suggest that tumors derived from an immunodeficient host are more malignant with the increasing tumor antigens. Therefore, with their high immunogenicity, tumors derived from RAG2−/−x γc−/− mice attract the incremental infiltration of immune cells, followed by tumor remission in WT mice [14].
Tumor immunoediting is the consequence of immunoselection; this is especially true for the T-cell-dominant responses, which lead to the generation or tumors that exhibit reduced immunogenicity [13,15]. In contrast, tumors developed in immunodeficient hosts will not undergo the selective process from immune cells, so they can develop more aggressively with a higher tumor mutational burden (TMB). Schreiber et al. [130] reported that tumors in RAG2-deficient mice were more immunogenic than those in immunocompetent hosts. Furthermore, exome sequence analysis was conducted to determine the mutational landscape of highly immunogenic (unedited) tumors derived from RAG2−/− mice. Unlike the WT mice, the RAG2−/− mice had a point mutation in Spectrin- β2, which was validated as the source of a neo-epitope in tumors grown in RAG2−/− mice [15]. These results reveal that desirable antigens and/or increased immunogenicity will be generated when tumors develop in immunodeficient hosts [16].
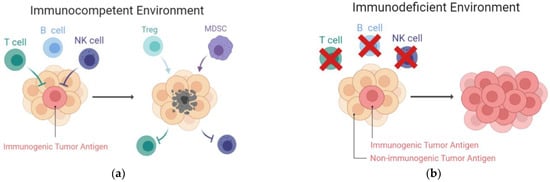
Figure 3.
Increased immunogenicity of tumors developed in immunodeficient hosts. (a) In immunocompetent hosts, tumors with high immunogenicity will be spontaneously destroyed by the T cells and NK cells. The resulting tumors, which express low immunogenicity, survive and create a suppressive TME. Within the TME, inhibitory cells, such as Tregs and MDSCs, facilitate tumor progression and deplete the effector cells; (b) In contrast, tumors established in an immunocompromised host are more malignant and immunogenic. Without the selective pressures from T and NK cells, antigenic tumors are retained. MDSCs, myeloid-derived suppressive cells; NK cells, natural killer cells; TME, tumor microenvironment; Tregs, regulatory T cells.
4.2. Differentially Expressed Profiles in Tumors Developed in Immunodeficient Hosts
According to the immunoediting theory, tumors can alter the antigen expression profiles to escape the hunt for host immunity. Our previous findings have provided significant evidence supporting this theory. The canine transmissible venereal tumor (CTVT) is one of the few spontaneously occurring cancer models for investigating the interaction between tumor development and the host immune system [131]. Though well-proven not to be induced by a virus infection, CTVT is a contagious tumor that can break the MHC barriers and is transmissible among dogs. This phenomenon reveals the strong living instinct of this tumor, but it remains a problem to explain this characteristic. So far, CTVT cannot be prepared as a cell line, and the inoculation of this tumor into dogs or immunodeficient mice is the only solution for CTVT maintenance. By chance, we observed the rapid growth pattern of CTVT that had previously been inoculated into NOD.CB17-Prkdcscid/NcrCrl (NOD.SCID) mice for live tumor conservation (named as XCTVT, X: xenogeneic). XCTVT developed in dogs showed an excellent proliferation ability and even metastasized in some cases, which seldom occurs in the original CTVT. We then analyzed the whole transcriptomes between the parent CTVT and CTVT once sojourned in NOD.SCID mice (named as MCTVT, M: mice). The result show that the gene expression profiles significantly differed between the two tumors. In MCTVT, the mRNA involved in the dysregulated interaction of chronic inflammation, chemotaxis, and extracellular space modification are highly transcribed, which implies their roles in promoting cancer progression [19]. This raised the question of whether the altered tumor antigenic topology in CTVT once seeded in NOD.SCID mice were just an exception, or whether the competent versus defective host immunity actually did have impacts on the profiles of TAAs. We then verified this issue using the colorectal carcinoma model, known as CT26 tumors, and similar results were found. Comprehensive analysis of protein profiles from CT26 tumors (wild type) and CT26 tumors developed in NOD.SCID mice (CT26/SCID) by liquid chromatography/mass spectrometry (LC-MS/MS) revealed that CT26 cells inoculated on NOD.SCID mice display more than 400 significantly differential expression proteins (DEPs). Several novel therapeutic targets were identified as overexpressed on CT26/SCID tumors, including KNG1, apoA-I, and β2-GPI, which could effectively activate cytotoxic T cells and directly suppress tumor proliferation [18].
4.3. Increased Antigenicity of Tumors Developed in Immunocompromised Hosts
The DEP landscapes shown in the CT26/SCID model have proven the quantitative modulation or qualitative alteration of the antigen repertoire presented on tumors. A variety of TAAs have bloomed on CT26/SCID cells because the tumor development within immunodeficient hosts may relieve the microenvironmental stress to tumors and reshape the TAA expression profiles. The customized TAAs from tumors that have ever been through the immunocompromised host immunity may evolve to produce heterogenic tumor antigens and operatively trigger antitumor immunity. To clarify the immune-stimulatory effects of these TAAs expressed in CT26/SCID, we prepared the proteins from CT26/SCID tumors and vaccinated CT26 tumor-bearing BALB/c mice. Compared with CT26/WT, CT26/SCID tumor lysates could significantly suppress tumor growth, increase CD3+ tumor-infiltrating lymphocytes (TILs), enhance CTL functions, and trigger Th-1 predominant immune responses. These T cells could produce IFN-𝛾 and possessed specific tumor cytotoxicity [18]. Therefore, tumors inoculated into hosts with defective immunity could reprogram the tumor antigenic expressions. Harvesting these cells as the major component of cancer vaccines could be a promising and effective strategy.
5. The Era of Tumor Antigens in Immunotherapies
5.1. Tumor-Associated Antigens
Tumor antigens recognized by the immune system can be classified by their tumor specificity. TSAs are only found in cancer cells, and TAAs are variably expressed in normal cells [132]. TSAs, such as mutation-derived antigens, are distinguishable from normal cells. For example, basal and Her2-positive tumors contain more mutated proteins and TP53 mutations than luminal A/B breast tumors do [133]. Mutations of β-catenin [134], caspase-8 [135], KRAS [136], and BCR-ABL [137] have been found in different tumors, and they could be effective therapeutic targets for cancer management. The aberrant expression of TAAs on the tumors also makes them targets for antitumor immunity. RAGE-1 [138], MUC-1 [139], and Her2/neu [140] are well-known TAAs and have been applied in cancer vaccines. Immunogenic antigens have high potential for immunotherapy, as these antigens can be captured by DCs, thereby triggering T-cell recruitment to the tumor sites [141]. The well-educated CTLs can release IFN-𝛾, perforin, and TNF-α, thus precisely eradicating the tumors [142]. Though TAAs are presumed to induce a minor immune response, which is insufficient for tumor rejection, these antigens are essential factors in the development of immunotherapy agents.
5.2. Differentially Expressed Profiles in Tumors Developed in Immunodeficient Hosts
Tumors are heterogeneous populations containing tumor cells and the surrounding microenvironment, such as fibroblasts, endothelial cells, Tregs, TAMs, MDSCs, and the ECM. These cells and factors help create an immunosuppressive TME leading to tumorigenesis, tumor progression, and metastasis. Stroma cells are more genetically stable than tumor cells [143]. Furthermore, tumors will decrease immunogenicity by several mechanisms to escape CTL recognition. These characteristics make stroma cells potential candidates for therapeutic targets.
Tumor stroma cells display physiological functions distinct from their normal counterparts under the impacts of TME, and they exhibit elevated tumor stroma-associated antigens (TSAAs). Well-known tumor stromal cells are cancer-associated fibroblasts (CAFs), tumor endothelial cells (TECs), and TAMs, all of which have been proposed as possible cellular targets in cancer therapies [144]. As the most abundant cell type in TME, CAFs secrete growth and angiogenic factors that remodel the tumor ECM, inhibit the antitumor immunity, and support tumor cell proliferation [143,144]. Furthermore, CAFs overproduce fibroblast activation protein α (FAP-α), a surface glycoprotein selectively expressed on the stroma of numerous solid tumors [145,146], to enable tumor growth, angiogenesis, and metastasis [147,148]. These known findings make TSAA-expressing stromal cells ideal therapeutic targets for cancer treatments.
5.3. Tumor Cell-Based Vaccine in Current Antitumor Strategies
Targeting both tumor cells and stroma is considered a promising strategy to remove tumors [149]. The aforementioned findings have spurred the development of autologous tumor cell-based vaccines. Autologous tumor cell-based vaccines can be prepared by different methods, and their immunogenicity can be improved through various approaches. Whole tumor cell lysates are usually prepared by homogenization [18], UV-ray irradiation [150], repeated cycles of freezing and thawing [151], and hyperthermia [152,153,154]. Cancer vaccines processed by homogenization express the total tumor proteins, preserving personalized TAAs, TSAs, and antigenic elements from the surrounding stroma. These vaccines can induce robust antigen-specific immunity against tumors and stromal cells [155]. The UV-treated tumor lysates induce apoptosis in tumor cells, and the exposure of phosphatidylserine (PS) on the surface of tumors facilitates the uptake and cross-presentation by DCs [150]. The apoptotic cells release high mobility group box1 (HMGB1) [156] and pentraxin-3 (PTX3) [157] and thus induce DC maturation. Tumor cell lysates prepared by a repeated freeze–thaw process undergo necrosis, and the necrotic cells can induce DC maturation [151]. Hyperthermia is an effective method of manufacturing tumor vaccines and improving the immunogenicity of tumors [154]. After being heated, tumors enhance their surface expression of MHC I-related molecules [153] and the antigens, leading to higher cytolytic effects and antigen-specific CD8+ T cell accumulation [152].
Therapeutic tumor cell-based vaccines rely on the concept of expressing “unique” and “personalized” TAAs, which can trigger more cancer-specific CTLs. As previously described, tumors are heterogeneous, and multiple treatments simultaneously inhibiting tumor proliferation and reversing the suppressive TME are promising antitumor strategies. Therefore, numerous clinical trials using cancer vaccines in combination with other therapies have been widely reported (Table 1). For example, the Her2/neu cancer vaccines combined with granulocyte macrophage-colony stimulating factor (GM-CSF) significantly increased the antigen-specific T responses and prolonged the disease-free survival (DFS) in patients with triple-negative breast cancers [158]. Her2-targeted cancer vaccines with trastuzumab treatments improved the DFS in high-risk breast cancer patients [159]. In advanced melanoma cases, the gp100 cancer vaccine combined with IL-2 therapy significantly improved the median survival in patients as compared with the use of IL-2 alone [160,161]. Furthermore, combining cancer vaccines with chemotherapy reduced myeloid suppressive cells and enhanced the immune responses in advanced cervical cancer patients [162]. Radiotherapy with cancer vaccination induced elevated infiltrations of vaccine-induced CD8+ T cell responses in neck cancer patients [163]. Anti-angiogenic molecules, which reverse the suppressive TME, synergistically enhance the therapeutic effects of cancer vaccines [164]. Antagonists of inhibitory receptors or agonists of co-stimulatory molecules are widely proposed as a combinational therapy with cancer vaccines. Anti-CTLA-4, anti-PD-1, anti-PD-L1, and other blockades of inhibitory receptors (Lag-3 and Tim-3) have shown synergy with cancer vaccines [165,166,167]. Co-stimulatory molecules, including CD40 [168], OX40 [169], and 4-1BB [170] antibodies, have been used in combination with cancer vaccines to augment cancer vaccine efficacy by increasing immunogenicity. These successful clinical trials have shown that both the cancer-specific immunity induced by tumor antigens and the factors that are able to effectively reverse the immunosuppressive TME are both important in tumor control.

Table 1.
Selected cancer cell-based vaccines achieved to clinical trials. DCs, dendritic cells; PFS, progression-free survival; GM-CSF, granulocyte macrophage-colony stimulating factor; TNBC, triple negative breast cancer; DFS, disease-free survival; RT, radiotherapy.
6. Conclusions
It is well proven that tumors developed in defective immunity can stimulate tumor antigenic expressions. Therefore, harvesting these cells as the major component of ACVs could effectively boost immunogenicity and antitumor efficacy (Figure 4). Furthermore, combinational immunotherapy has benefited cancer patients greatly and been viewed as a prospective choice for cancer treatments. To date, many immune-boosting strategies can be used in combination with ACVs. The blockade pathways (CTLA-4, CD80, CD86, PD-1, PD-L1), co-stimulatory signaling (CD40, OX40, 4-1BB), cytokines (IL-2, GM-CSF), chemotherapy, radiation, or any strategies that can reverse the suppressive TME are all potential options. The optimization of ACVs and collaborative treatments will lead to future clinical success.
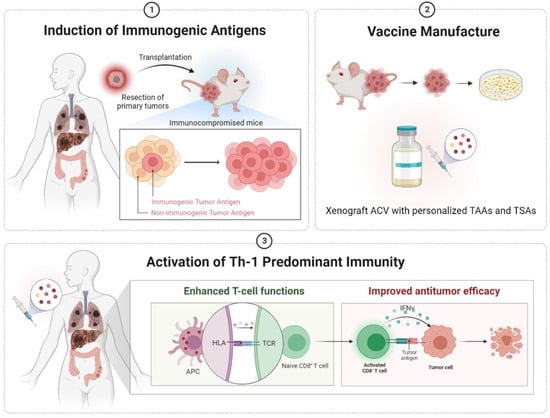
Figure 4.
Diagrams illustrating the hypothesis in the current study. Tumors developed in a defective immune environment trigger the overexpression of immunogenic antigens. Based on this theory, we hoped to inoculate a few primary tumors from clinical cancer patients into immunocompromised mice. Then, the tumors progressively developed with the generation of personalized tumor antigens. The tumors were harvested and manufactured into xenograft ACVs. With the xenograft cancer vaccination, the APCs could recognize the unique tumor antigens and educate the naïve CD8+ T cells. The activated (educated) CD8+ T cells exerted cytolytic effects by releasing cytokines, such as IFN-γ. ACV, autologous cancer vaccine; APC, antigen-presenting cells; HLA, human leukocyte antigen; IFN-γ, interferon-gamma; TAAs, tumor-associated antigens; TCR, T-cell receptor; TSAs, tumor-specific antigens.
Author Contributions
Conceptualization, C.-H.K. and C.-S.L.; investigation, C.-H.K.; resources, Y.-H.C. and K.-C.H.; writing—original draft preparation, C.-H.K.; writing—review and editing, Y.-H.C. and C.-S.L.; supervision, C.-S.L.; project administration, C.-H.K. and Y.-H.C.; funding acquisition, C.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Taiwan University (NTU-CC-111L892703), and VGHUST Joint Research Program (111-G6-2-2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Amin, P.J.; Shankar, B.S. Sulforaphane induces ROS mediated induction of NKG2D ligands in human cancer cell lines and enhances susceptibility to NK cell mediated lysis. Life Sci. 2015, 126, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Nolz, J.C. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell Mol. Life Sci. 2015, 72, 2461–2473. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Neller, M.A.; López, J.A.; Schmidt, C.W. Antigens for cancer immunotherapy. Semin. Immunol. 2008, 20, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef]
- Melief, C.J.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Blanc, C.; Granier, C.; Saldmann, A.; Tanchot, C.; Tartour, E. Therapeutic cancer vaccine: Building the future from lessons of the past. Semin. Immunopathol. 2019, 41, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; Saddawi-Konefka, R.; Vermi, W.; Koebel, C.M.; Arthur, C.; White, J.M.; Uppaluri, R.; Andrews, D.M.; Ngiow, S.F.; Teng, M.W.; et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med. 2012, 209, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Vesely, M.D.; Koboldt, D.C.; Rickert, C.G.; Uppaluri, R.; Magrini, V.J.; Arthur, C.D.; White, J.M.; Chen, Y.S.; Shea, L.K.; et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012, 482, 400–404. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Escors, D. Tumour Immunogenicity, Antigen Presentation, and Immunological Barriers in Cancer Immunotherapy. New J. Sci. 2014, 2014, 734515. [Google Scholar] [CrossRef]
- Ke, C.-H.; Wang, Y.-S.; Chiang, H.-C.; Wu, H.-Y.; Liu, W.-J.; Huang, C.-C.; Huang, Y.-C.; Lin, C.-S. Xenograft cancer vaccines prepared from immunodeficient mice increase tumor antigen diversity and host T cell efficiency against colorectal cancers. Cancer Lett. 2022, 526, 66–75. [Google Scholar] [CrossRef]
- Ke, C.-H.; Tomiyasu, H.; Lin, Y.-L.; Huang, W.-H.; Huang, H.-H.; Chiang, H.-C.; Lin, C.-S. Canine transmissible venereal tumour established in immunodeficient mice reprograms the gene expression profiles associated with a favourable tumour microenvironment to enable cancer malignancy. BMC Vet. Res. 2022, 18, 4. [Google Scholar] [CrossRef]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef]
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martínez-Cano, S.; Mejías-Pérez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 2017, 8, 16073. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ridge, J.P.; Di Rosa, F.; Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998, 393, 474–478. [Google Scholar] [CrossRef]
- Bedoui, S.; Heath, W.R.; Mueller, S.N. CD4(+) T-cell help amplifies innate signals for primary CD8(+) T-cell immunity. Immunol. Rev. 2016, 272, 52–64. [Google Scholar] [CrossRef]
- Calabro, S.; Liu, D.; Gallman, A.; Nascimento, M.S.; Yu, Z.; Zhang, T.T.; Chen, P.; Zhang, B.; Xu, L.; Gowthaman, U.; et al. Differential Intrasplenic Migration of Dendritic Cell Subsets Tailors Adaptive Immunity. Cell Rep. 2016, 16, 2472–2485. [Google Scholar] [CrossRef]
- Gerner, M.Y.; Casey, K.A.; Kastenmuller, W.; Germain, R.N. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J. Exp. Med. 2017, 214, 3105–3122. [Google Scholar] [CrossRef]
- Eickhoff, S.; Brewitz, A.; Gerner, M.Y.; Klauschen, F.; Komander, K.; Hemmi, H.; Garbi, N.; Kaisho, T.; Germain, R.N.; Kastenmüller, W. Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell 2015, 162, 1322–1337. [Google Scholar] [CrossRef]
- Hor, J.L.; Whitney, P.G.; Zaid, A.; Brooks, A.G.; Heath, W.R.; Mueller, S.N. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4+ and CD8+ T Cell Activation to Localized Viral Infection. Immunity 2015, 43, 554–565. [Google Scholar] [CrossRef]
- Groom, J.R.; Richmond, J.; Murooka, T.T.; Sorensen, E.W.; Sung, J.H.; Bankert, K.; von Andrian, U.H.; Moon, J.J.; Mempel, T.R.; Luster, A.D. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 2012, 37, 1091–1103. [Google Scholar] [CrossRef]
- Wang, J.C.; Livingstone, A.M. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J. Immunol. 2003, 171, 6339–6343. [Google Scholar] [CrossRef]
- Greyer, M.; Whitney, P.G.; Stock, A.T.; Davey, G.M.; Tebartz, C.; Bachem, A.; Mintern, J.D.; Strugnell, R.A.; Turner, S.J.; Gebhardt, T.; et al. T Cell Help Amplifies Innate Signals in CD8(+) DCs for Optimal CD8(+) T Cell Priming. Cell. Rep. 2016, 14, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Carmenate, T.; Ortíz, Y.; Enamorado, M.; García-Martínez, K.; Avellanet, J.; Moreno, E.; Graça, L.; León, K. Blocking IL-2 Signal In Vivo with an IL-2 Antagonist Reduces Tumor Growth through the Control of Regulatory T Cells. J. Immunol. 2018, 200, 3475. [Google Scholar] [CrossRef] [PubMed]
- Obar, J.J.; Molloy, M.J.; Jellison, E.R.; Stoklasek, T.A.; Zhang, W.; Usherwood, E.J.; Lefrançois, L. CD4+T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA 2010, 107, 193–198. [Google Scholar] [CrossRef]
- Xie, Y.; Akpinarli, A.; Maris, C.; Hipkiss, E.L.; Lane, M.; Kwon, E.K.; Muranski, P.; Restifo, N.P.; Antony, P.A. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 2010, 207, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.S.; Rancan, C.; et al. Intratumoral CD4(+) T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer. Cell 2020, 181, 1612–1625.e13. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Yizhak, K.; Bjorgaard, S.L.; Ray, J.P.; de Boer, C.G.; Jenkins, R.W.; Lieb, D.J.; Chen, J.H.; Frederick, D.T.; Barzily-Rokni, M.; et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 2018, 175, 998–1013.e20. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Lang, P.; André, M.C. Exploitation of natural killer cells for the treatment of acute leukemia. Blood 2016, 127, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- Chan, S.R.; Vermi, W.; Luo, J.; Lucini, L.; Rickert, C.; Fowler, A.M.; Lonardi, S.; Arthur, C.; Young, L.J.T.; Levy, D.E.; et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Res. 2012, 14, R16. [Google Scholar] [CrossRef]
- Zerafa, N.; Westwood, J.A.; Cretney, E.; Mitchell, S.; Waring, P.; Iezzi, M.; Smyth, M.J. Cutting Edge: TRAIL Deficiency Accelerates Hematological Malignancies. J. Immunol. 2005, 175, 5586–5590. [Google Scholar] [CrossRef]
- Davidson, W.F.; Giese, T.; Fredrickson, T.N. Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. J. Exp. Med. 1998, 187, 1825–1838. [Google Scholar] [CrossRef]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; MacGregor, D.; Godfrey, D.I.; Trapani, J.A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000, 192, 755–760. [Google Scholar] [CrossRef]
- Street, S.E.; Trapani, J.A.; MacGregor, D.; Smyth, M.J. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J. Exp. Med. 2002, 196, 129–134. [Google Scholar] [CrossRef]
- Street, S.E.; Hayakawa, Y.; Zhan, Y.; Lew, A.M.; MacGregor, D.; Jamieson, A.M.; Diefenbach, A.; Yagita, H.; Godfrey, D.I.; Smyth, M.J. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004, 199, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Faustman, D.L. Development of spontaneous uterine tumors in low molecular mass polypeptide-2 knockout mice. Cancer. Res. 2002, 62, 24–27. [Google Scholar] [PubMed]
- Paluskievicz, C.M.; Cao, X.; Abdi, R.; Zheng, P.; Liu, Y.; Bromberg, J.S. T Regulatory Cells and Priming the Suppressive Tumor Microenvironment. Front. Immunol. 2019, 10, 2453. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e35. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Morello, S.; Pinto, A.; Blandizzi, C.; Antonioli, L. Myeloid cells in the tumor microenvironment: Role of adenosine. Oncoimmunology 2016, 5, e1108515. [Google Scholar] [CrossRef]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Puig, P.E.; Roux, S.; Parcellier, A.; Schmitt, E.; Solary, E.; Kroemer, G.; Martin, F.; Chauffert, B.; Zitvogel, L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005, 202, 919–929. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Moon, Y.W.; Hajjar, J.; Hwu, P.; Naing, A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer 2015, 3, 51. [Google Scholar] [CrossRef]
- Jitschin, R.; Braun, M.; Büttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; Le Blanc, K.; et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Schlecker, E.; Stojanovic, A.; Eisen, C.; Quack, C.; Falk, C.S.; Umansky, V.; Cerwenka, A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 2012, 189, 5602–5611. [Google Scholar] [CrossRef] [PubMed]
- Raber, P.L.; Thevenot, P.; Sierra, R.; Wyczechowska, D.; Halle, D.; Ramirez, M.E.; Ochoa, A.C.; Fletcher, M.; Velasco, C.; Wilk, A.; et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int. J. Cancer. 2014, 134, 2853–2864. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.; Wang, J.; Vangundy, Z.; You, J.; Yildiz, V.; Yu, L.; Foote, I.P.; Branson, O.E.; Stiff, A.R.; Brooks, T.R.; et al. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration. Sci. Rep. 2017, 7, 15424. [Google Scholar] [CrossRef]
- Bingisser, R.M.; Tilbrook, P.A.; Holt, P.G.; Kees, U.R. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol. 1998, 160, 5729–5734. [Google Scholar] [PubMed]
- Wang, Z.; Jiang, J.; Li, Z.; Zhang, J.; Wang, H.; Qin, Z. A myeloid cell population induced by Freund adjuvant suppresses T-cell-mediated antitumor immunity. J. Immunother. 2010, 33, 167–177. [Google Scholar] [CrossRef]
- Gehad, A.E.; Lichtman, M.K.; Schmults, C.D.; Teague, J.E.; Calarese, A.W.; Jiang, Y.; Watanabe, R.; Clark, R.A. Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J. Investig. Dermatol. 2012, 132, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef]
- Serafini, P.; Mgebroff, S.; Noonan, K.; Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer. Res. 2008, 68, 5439–5449. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef]
- Dehne, N.; Mora, J.; Namgaladze, D.; Weigert, A.; Brüne, B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr. Opin. Pharmacol. 2017, 35, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and cancer: From mechanisms to therapeutic implications. Trends. Immunol. 2015, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, L.C.; Ruiter, D.J.; van Muijen, G.N.; Coussens, L.M. The tumor microenvironment: A critical determinant of neoplastic evolution. Eur. J. Cell Biol. 2003, 82, 539–548. [Google Scholar] [CrossRef]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Jeannin, P.; Paolini, L.; Adam, C.; Delneste, Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 2018, 285, 680–699. [Google Scholar] [CrossRef]
- Spring, H.; Schüler, T.; Arnold, B.; Hämmerling, G.J.; Ganss, R. Chemokines direct endothelial progenitors into tumor neovessels. Proc. Natl. Acad. Sci. USA 2005, 102, 18111–18116. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Strachan, D.C.; Ruffell, B.; Oei, Y.; Bissell, M.J.; Coussens, L.M.; Pryer, N.; Daniel, D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology 2013, 2, e26968. [Google Scholar] [CrossRef]
- Butoi, E.; Gan, A.M.; Tucureanu, M.M.; Stan, D.; Macarie, R.D.; Constantinescu, C.; Calin, M.; Simionescu, M.; Manduteanu, I. Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim. Biophys. Acta 2016, 1863, 1568–1578. [Google Scholar] [CrossRef]
- Hu, W.Q.; Fang, M.; Zhao, H.L.; Yan, S.G.; Yuan, J.P.; Peng, C.W.; Yang, G.F.; Li, Y.; Li, J.D. Tumor invasion unit in gastric cancer revealed by QDs-based in situ molecular imaging and multispectral analysis. Biomaterials 2014, 35, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Reis e Sousa, C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends. Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Hangai, S.; Ao, T.; Kimura, Y.; Matsuki, K.; Kawamura, T.; Negishi, H.; Nishio, J.; Kodama, T.; Taniguchi, T.; Yanai, H. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc. Natl. Acad. Sci. USA 2016, 113, 3844–3849. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef]
- Ohm, J.E.; Carbone, D.P. VEGF as a mediator of tumor-associated immunodeficiency. Immunol. Res. 2001, 23, 263–272. [Google Scholar] [CrossRef]
- Park, S.J.; Nakagawa, T.; Kitamura, H.; Atsumi, T.; Kamon, H.; Sawa, S.; Kamimura, D.; Ueda, N.; Iwakura, Y.; Ishihara, K.; et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004, 173, 3844–3854. [Google Scholar] [CrossRef]
- Yang, A.S.; Lattime, E.C. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003, 63, 2150–2157. [Google Scholar] [PubMed]
- Papaspyridonos, M.; Matei, I.; Huang, Y.; do Rosario Andre, M.; Brazier-Mitouart, H.; Waite, J.C.; Chan, A.S.; Kalter, J.; Ramos, I.; Wu, Q.; et al. Id1 suppresses anti-tumour immune responses and promotes tumour progression by impairing myeloid cell maturation. Nat. Commun. 2015, 6, 6840. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef]
- Castro, A.; Ozturk, K.; Pyke, R.M.; Xian, S.; Zanetti, M.; Carter, H. Elevated neoantigen levels in tumors with somatic mutations in the HLA-A, HLA-B, HLA-C and B2M genes. BMC. Med. Genom. 2019, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.A.; Rooney, M.S.; Rajasagi, M.; Tiao, G.; Dixon, P.M.; Lawrence, M.S.; Stevens, J.; Lane, W.J.; Dellagatta, J.L.; Steelman, S.; et al. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat. Biotechnol. 2015, 33, 1152–1158. [Google Scholar] [CrossRef]
- Boulpicante, M.; Darrigrand, R.; Pierson, A.; Salgues, V.; Rouillon, M.; Gaudineau, B.; Khaled, M.; Cattaneo, A.; Bachi, A.; Cascio, P.; et al. Tumors escape immunosurveillance by overexpressing the proteasome activator PSME3. Oncoimmunology 2020, 9, 1761205. [Google Scholar] [CrossRef]
- Tabassum, A.; Samdani, M.N.; Dhali, T.C.; Alam, R.; Ahammad, F.; Samad, A.; Karpiński, T.M. Transporter associated with antigen processing 1 (TAP1) expression and prognostic analysis in breast, lung, liver, and ovarian cancer. J. Mol. Med. 2021, 99, 1293–1309. [Google Scholar] [CrossRef]
- Shionoya, Y.; Kanaseki, T.; Miyamoto, S.; Tokita, S.; Hongo, A.; Kikuchi, Y.; Kochin, V.; Watanabe, K.; Horibe, R.; Saijo, H.; et al. Loss of tapasin in human lung and colon cancer cells and escape from tumor-associated antigen-specific CTL recognition. Oncoimmunology 2017, 6, e1274476. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Quinn, K.M.; Kartikasari, A.E.R.; Cooke, R.E.; Koldej, R.M.; Ritchie, D.S.; Plebanski, M. Impact of age-, cancer-, and treatment-driven inflammation on T cell function and immunotherapy. J. Leukoc. Biol. 2020, 108, 953–965. [Google Scholar] [CrossRef]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis. Rev. 2010, 29, 273–283. [Google Scholar] [CrossRef]
- Manna, S.K.; Ramesh, G.T. Interleukin-8 induces nuclear transcription factor-kappaB through a TRAF6-dependent pathway. J. Biol. Chem. 2005, 280, 7010–7021. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.; Lang, C.; Devgan, G.; Azare, J.; Berishaj, M.; Gerald, W.; Kim, Y.B.; Paz, K.; Darnell, J.E.; Albanese, C.; et al. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006, 66, 2544–2552. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, B.; Datta, J.; Teknos, T.N.; Kumar, P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol. Cancer Res. 2011, 9, 1658–1667. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Chen, W.; Liang, X.; Peterson, A.J.; Munn, D.H.; Blazar, B.R. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 2008, 181, 5396–5404. [Google Scholar] [CrossRef]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Cao, Y.; Liu, X.; Chen, Y.; Qi, Y.; Wang, J.; Yu, K.; Lin, C.; Liu, H.; et al. Lauren classification identifies distinct prognostic value and functional status of intratumoral CD8(+) T cells in gastric cancer. Cancer Immunol. Immunother. 2020, 69, 1327–1336. [Google Scholar] [CrossRef]
- Jonescheit, H.; Oberg, H.H.; Gonnermann, D.; Hermes, M.; Sulaj, V.; Peters, C.; Kabelitz, D.; Wesch, D. Influence of Indoleamine-2,3-Dioxygenase and Its Metabolite Kynurenine on γδ T Cell Cytotoxicity against Ductal Pancreatic Adenocarcinoma Cells. Cells 2020, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [PubMed]
- Gide, T.N.; Allanson, B.M.; Menzies, A.M.; Ferguson, P.M.; Madore, J.; Saw, R.P.M.; Thompson, J.F.; Long, G.V.; Wilmott, J.S.; Scolyer, R.A. Inter- and intrapatient heterogeneity of indoleamine 2,3-dioxygenase expression in primary and metastatic melanoma cells and the tumour microenvironment. Histopathology 2019, 74, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Wakkach, A.; Fournier, N.; Brun, V.; Breittmayer, J.P.; Cottrez, F.; Groux, H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 2003, 18, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, B.C.; Legembre, P.; Pietras, E.; Bubici, C.; Franzoso, G.; Peter, M.E. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004, 23, 3175–3185. [Google Scholar] [CrossRef]
- Yu, J.S.; Lee, P.K.; Ehtesham, M.; Samoto, K.; Black, K.L.; Wheeler, C.J. Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J. Neurooncol. 2003, 64, 55–61. [Google Scholar] [CrossRef]
- Bajou, K.; Peng, H.; Laug, W.E.; Maillard, C.; Noel, A.; Foidart, J.M.; Martial, J.A.; DeClerck, Y.A. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell 2008, 14, 324–334. [Google Scholar] [CrossRef]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef]
- Horton, B.L.; Williams, J.B.; Cabanov, A.; Spranger, S.; Gajewski, T.F. Intratumoral CD8(+) T-cell Apoptosis Is a Major Component of T-cell Dysfunction and Impedes Antitumor Immunity. Cancer Immunol. Res. 2018, 6, 14–24. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Dai, Z.; Jaffarzad, N.; Ye, Y.; Medina, M.A.; Huang, X.-F.; Dorta-Estremera, S.M.; Greeley, N.R.; Nitti, G.; Peng, W.; et al. Persistent antigen at vaccination sites induces tumor-specific CD8⁺ T cell sequestration, dysfunction and deletion. Nat. Med. 2013, 19, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Wasiuk, A.; Testa, J.; Weidlick, J.; Sisson, C.; Vitale, L.; Widger, J.; Crocker, A.; Thomas, L.J.; Goldstein, J.; Marsh, H.C.; et al. CD27-Mediated Regulatory T Cell Depletion and Effector T Cell Costimulation Both Contribute to Antitumor Efficacy. J. Immunol. 2017, 199, 4110–4123. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Deschoolmeester, V.; Zwaenepoel, K.; Rolfo, C.; Silence, K.; Rottey, S.; Lardon, F.; Smits, E.; Pauwels, P. CD70: An emerging target in cancer immunotherapy. Pharmacol. Ther. 2015, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Flieswasser, T.; Van den Eynde, A.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Slomianny, C.; Auberger, P.; Petit, P.X.; Benichou, S. Siva-1 and an alternative splice form lacking the death domain, Siva-2, similarly induce apoptosis in T lymphocytes via a caspase-dependent mitochondrial pathway. J. Immunol. 2004, 172, 4008–4017. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Horn, L.A.; Haile, S.T. The programmed death-1 immune-suppressive pathway: Barrier to antitumor immunity. J. Immunol. 2014, 193, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Sonugür, F.G.; Akbulut, H. The Role of Tumor Microenvironment in Genomic Instability of Malignant Tumors. Front. Genet. 2019, 10, 1063. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Chiang, H.C.; Liao, A.T.; Jan, T.R.; Wang, Y.S.; Lei, H.J.; Tsai, M.H.; Chen, M.F.; Lee, C.Y.; Lin, Y.C.; Chu, R.M.; et al. Gene-expression profiling to identify genes related to spontaneous tumor regression in a canine cancer model. Vet. Immunol. Immunopathol. 2013, 151, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.K.; Bright, J.D.; Byrne, J.A. Overexpressed oncogenic tumor-self antigens. Hum. Vaccin. Immunother. 2014, 10, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.C.; Uduman, M.; Pabla, S.; Stein, R.B.; Buell, J.S. Mutation-Derived Neoantigens for Cancer Immunotherapy. Front. Immunol. 2019, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, S. Mutation Hotspots in the β-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol. Cells 2019, 42, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Egloff, A.M.; Sen, M.; Grandis, J.R.; Johnson, D.E. Caspase-8 mutations in head and neck cancer confer resistance to death receptor-mediated apoptosis and enhance migration, invasion, and tumor growth. Mol. Oncol. 2014, 8, 1220–1230. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Curr. Signal Transduct. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL family kinases in cancer: From leukaemia to solid tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef]
- Guinn, B.-a.; Gilkes, A.F.; Mufti, G.J.; Burnett, A.K.; Mills, K.I. The tumour antigens RAGE-1 and MGEA6 are expressed more frequently in the less lineage restricted subgroups of presentation acute myeloid leukaemia. Br. J. Haematol. 2006, 134, 238–239. [Google Scholar] [CrossRef]
- Finn, O.J.; Gantt, K.R.; Lepisto, A.J.; Pejawar-Gaddy, S.; Xue, J.; Beatty, P.L. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol. Res. 2011, 50, 261–268. [Google Scholar] [CrossRef]
- Disis, M.L.; Wallace, D.R.; Gooley, T.A.; Dang, Y.; Slota, M.; Lu, H.; Coveler, A.L.; Childs, J.S.; Higgins, D.M.; Fintak, P.A.; et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 2009, 27, 4685–4692. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakui, M.; Sato, M.; Iwakabe, K.; Kitamura, H.; Sekimoto, M.; Ohta, A.; Koda, T.; Nishimura, S. The critical role of Th1-dominant immunity in tumor immunology. Cancer. Chemother. Pharmacol. 2000, 46, S52–S61. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.-L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, V.; Vetter, C.; Schrama, D.; Bröcker, E.B.; Becker, J.C. Tumor stroma-associated antigens for anti-cancer immunotherapy. Cancer Immunol. Immunother. 2006, 55, 481–494. [Google Scholar] [CrossRef]
- Mori, Y.; Kono, K.; Matsumoto, Y.; Fujii, H.; Yamane, T.; Mitsumata, M.; Chen, W.T. The expression of a type II transmembrane serine protease (Seprase) in human gastric carcinoma. Oncology 2004, 67, 411–419. [Google Scholar] [CrossRef]
- Huber, M.A.; Kraut, N.; Park, J.E.; Schubert, R.D.; Rettig, W.J.; Peter, R.U.; Garin-Chesa, P. Fibroblast activation protein: Differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J. Investig. Dermatol. 2003, 120, 182–188. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Kelly, T. Seprase promotes rapid tumor growth and increased microvessel density in a mouse model of human breast cancer. Cancer. Res. 2004, 64, 2712–2716. [Google Scholar] [CrossRef]
- Iwasa, S.; Jin, X.; Okada, K.; Mitsumata, M.; Ooi, A. Increased expression of seprase, a membrane-type serine protease, is associated with lymph node metastasis in human colorectal cancer. Cancer. Lett. 2003, 199, 91–98. [Google Scholar] [CrossRef]
- Sadeghi Najafabadi, S.A.; Bolhassani, A.; Aghasadeghi, M.R. Tumor cell-based vaccine: An effective strategy for eradication of cancer cells. Immunotherapy 2022, 14, 639–654. [Google Scholar] [CrossRef]
- Jenne, L.; Arrighi, J.F.; Jonuleit, H.; Saurat, J.H.; Hauser, C. Dendritic cells containing apoptotic melanoma cells prime human CD8+ T cells for efficient tumor cell lysis. Cancer Res. 2000, 60, 4446–4452. [Google Scholar]
- Herzog, G.I.; Solgi, G.; Wiegmann, D.S.; Nienhaus, C.; Schrezenmeier, H.; Yildiz, T.; Lotfi, R. Quality of tumor lysates used for pulsing dendritic cells is influenced by the method used to harvest adherent tumor cells. BMC Res. Notes 2011, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, T.; Connolly, J.E.; Monnet, L.; Bennett, L.; Chapel, S.; Bagnis, C.; Mannoni, P.; Davoust, J.; Palucka, A.K.; et al. Hyperthermia Enhances CTL Cross-Priming. J. Immunol. 2006, 176, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, J.R.; Dayanc, B.E.; Yuan, M.; Oflazoglu, E.; Repasky, E.A. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J. Leukoc. Biol. 2007, 82, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Skitzki, J.J.; Repasky, E.A.; Evans, S.S. Hyperthermia as an immunotherapy strategy for cancer. Curr. Opin. Investig. Drugs. 2009, 10, 550–558. [Google Scholar]
- Convit, J.; Montesinos, H.; Oviedo, H.; Romero, G.; Maccarone, B.; Essenfeld, E.; Convit, A.; Palacios, L.E. Autologous tumor lysate/Bacillus Calmette-Guérin immunotherapy as an adjuvant to conventional breast cancer therapy. Clin. Transl. Oncol. 2015, 17, 884–887. [Google Scholar] [CrossRef][Green Version]
- Bell, C.W.; Jiang, W.; Reich, C.F., 3rd; Pisetsky, D.S. The extracellular release of HMGB1 during apoptotic cell death. Am. J. Physiol. Cell Physiol. 2006, 291, C1318-1325. [Google Scholar] [CrossRef]
- Rovere, P.; Peri, G.; Fazzini, F.; Bottazzi, B.; Doni, A.; Bondanza, A.; Zimmermann, V.S.; Garlanda, C.; Fascio, U.; Sabbadini, M.G.; et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 2000, 96, 4300–4306. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Ardavanis, A.; Symanowski, J.; Murray, J.L.; Shumway, N.M.; Litton, J.K.; Hale, D.F.; Perez, S.A.; Anastasopoulou, E.A.; Pistamaltzian, N.F.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 2016, 27, 1241–1248. [Google Scholar] [CrossRef]
- Clifton, G.T.; Hale, D.; Vreeland, T.J.; Hickerson, A.T.; Litton, J.K.; Alatrash, G.; Murthy, R.K.; Qiao, N.; Philips, A.V.; Lukas, J.J.; et al. Results of a Randomized Phase IIb Trial of Nelipepimut-S + Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast Cancer. Clin. Cancer Res. 2020, 26, 2515–2523. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Schwartzentruber, D.J.; Hwu, P.; Marincola, F.M.; Topalian, S.L.; Restifo, N.P.; Dudley, M.E.; Schwarz, S.L.; Spiess, P.J.; et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998, 4, 321–327. [Google Scholar] [CrossRef]
- Schwartzentruber, D.J.; Lawson, D.H.; Richards, J.M.; Conry, R.M.; Miller, D.M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 2011, 364, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.; van der Sluis, T.C.; van Meir, H.; Loof, N.M.; van Ham, V.J.; van Duikeren, S.; Santegoets, S.J.; Arens, R.; de Kam, M.L.; Cohen, A.F.; et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 2016, 8, 334ra352. [Google Scholar] [CrossRef] [PubMed]
- Mondini, M.; Nizard, M.; Tran, T.; Mauge, L.; Loi, M.; Clémenson, C.; Dugue, D.; Maroun, P.; Louvet, E.; Adam, J.; et al. Synergy of Radiotherapy and a Cancer Vaccine for the Treatment of HPV-Associated Head and Neck Cancer. Mol. Cancer Ther. 2015, 14, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhao, Y.; Satterlee, A.B.; Wang, Y.; Xu, Y.; Huang, L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J. Control. Release 2017, 245, 81–94. [Google Scholar] [CrossRef]
- Grosso, J.F.; Kelleher, C.C.; Harris, T.J.; Maris, C.H.; Hipkiss, E.L.; De Marzo, A.; Anders, R.; Netto, G.; Getnet, D.; Bruno, T.C.; et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Investig. 2007, 117, 3383–3392. [Google Scholar] [CrossRef]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef]
- Baghdadi, M.; Nagao, H.; Yoshiyama, H.; Akiba, H.; Yagita, H.; Dosaka-Akita, H.; Jinushi, M. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol. Immunother. 2013, 62, 629–637. [Google Scholar] [CrossRef]
- Carlring, J.; Szabo, M.J.; Dickinson, R.; De Leenheer, E.; Heath, A.W. Conjugation of lymphoma idiotype to CD40 antibody enhances lymphoma vaccine immunogenicity and antitumor effects in mice. Blood 2012, 119, 2056–2065. [Google Scholar] [CrossRef]
- Murata, S.; Ladle, B.H.; Kim, P.S.; Lutz, E.R.; Wolpoe, M.E.; Ivie, S.E.; Smith, H.M.; Armstrong, T.D.; Emens, L.A.; Jaffee, E.M.; et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J. Immunol. 2006, 176, 974–983. [Google Scholar] [CrossRef]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290-5299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).