Transcriptome Analysis in Response to Infection of Xanthomonas oryzae pv. oryzicola Strains with Different Pathogenicity
Abstract
1. Introduction
2. Results
2.1. Resistance Identification Results
2.2. RNA Sequencing Results
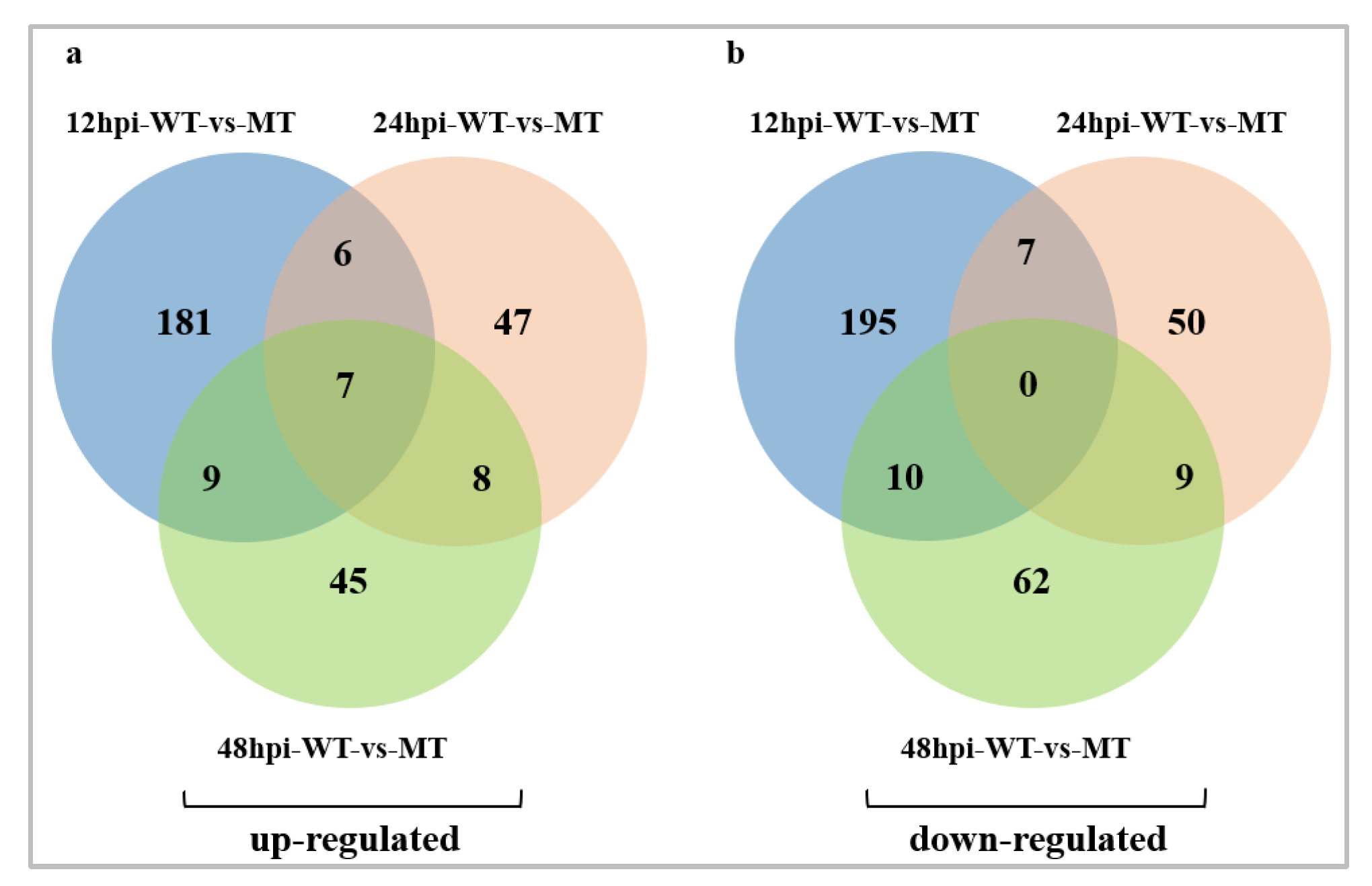
2.3. DEGs Identification Number Analysis
2.4. DEGs Resistance Gene Distribution
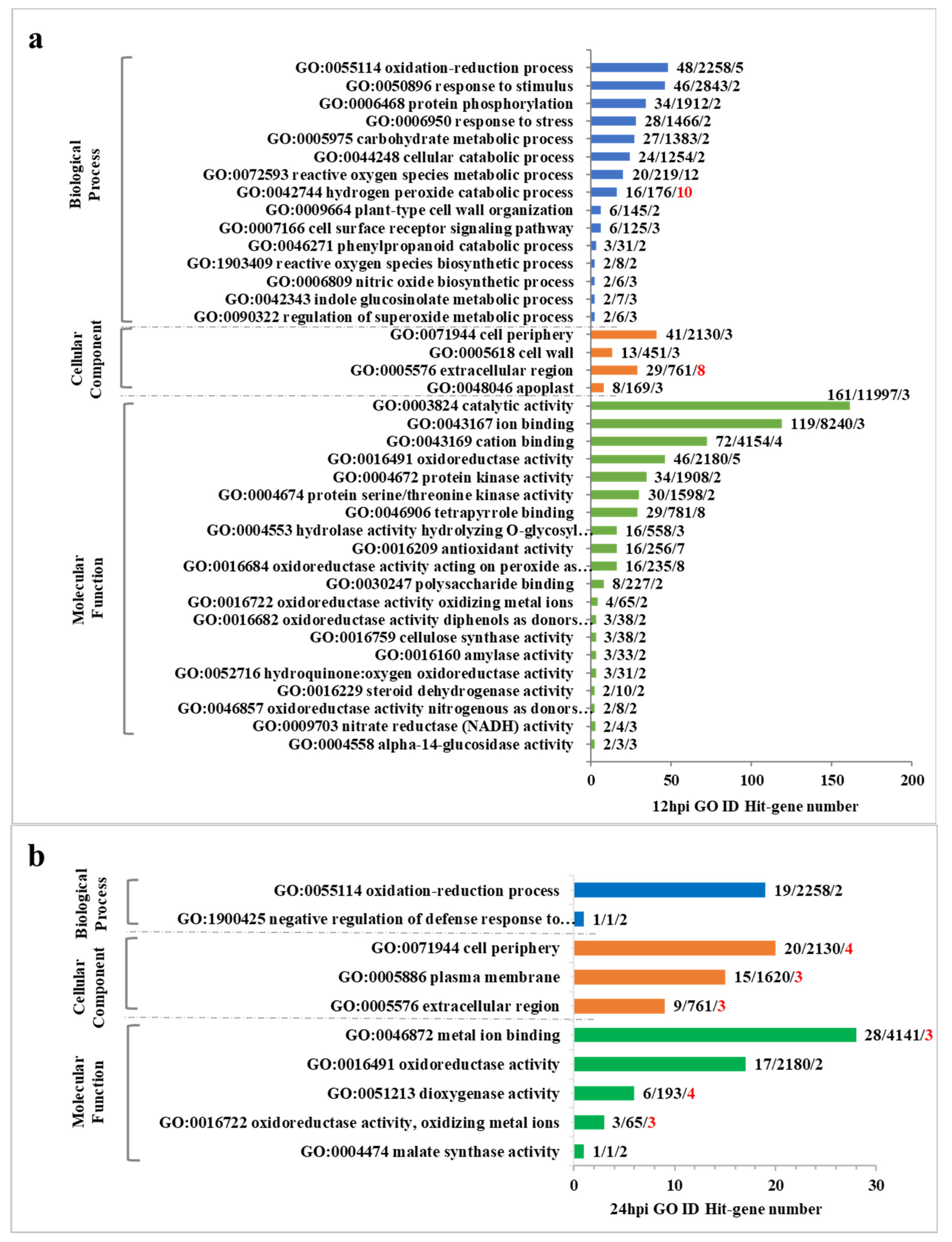
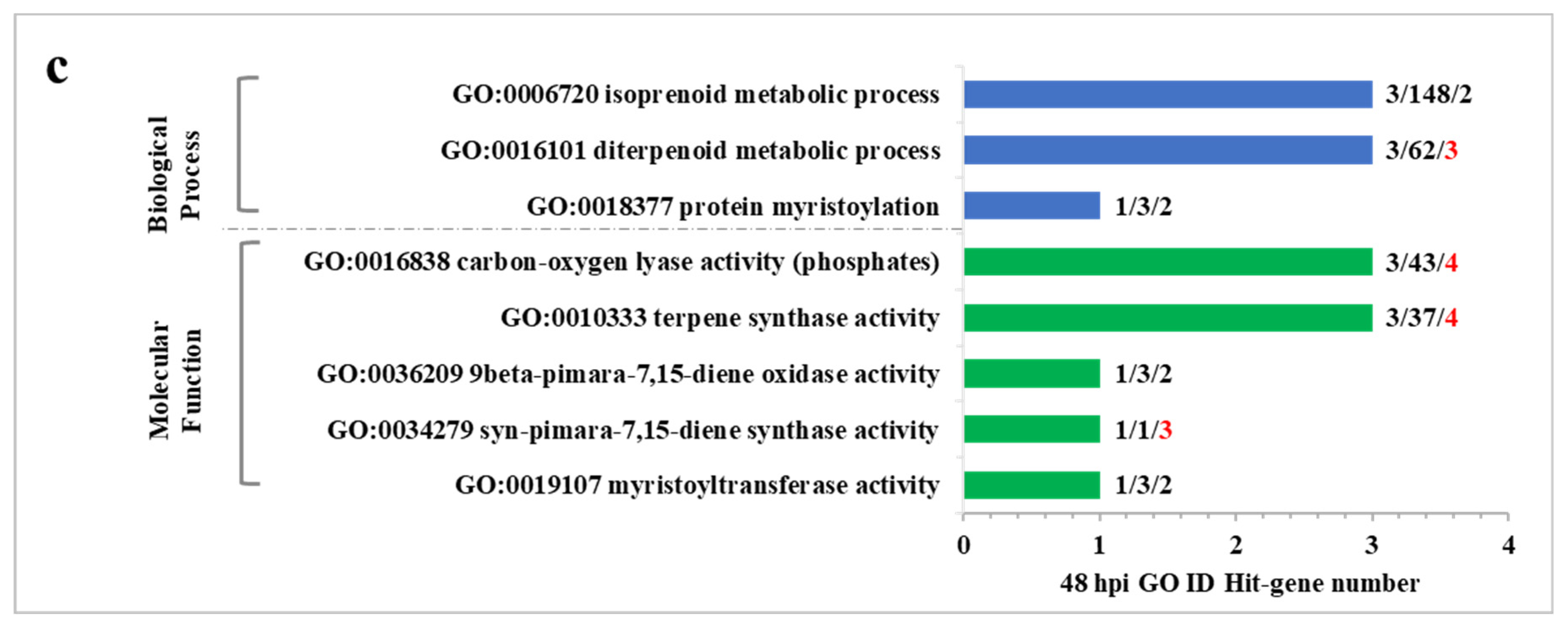
2.5. GO Enrichment Analysis of DEGs
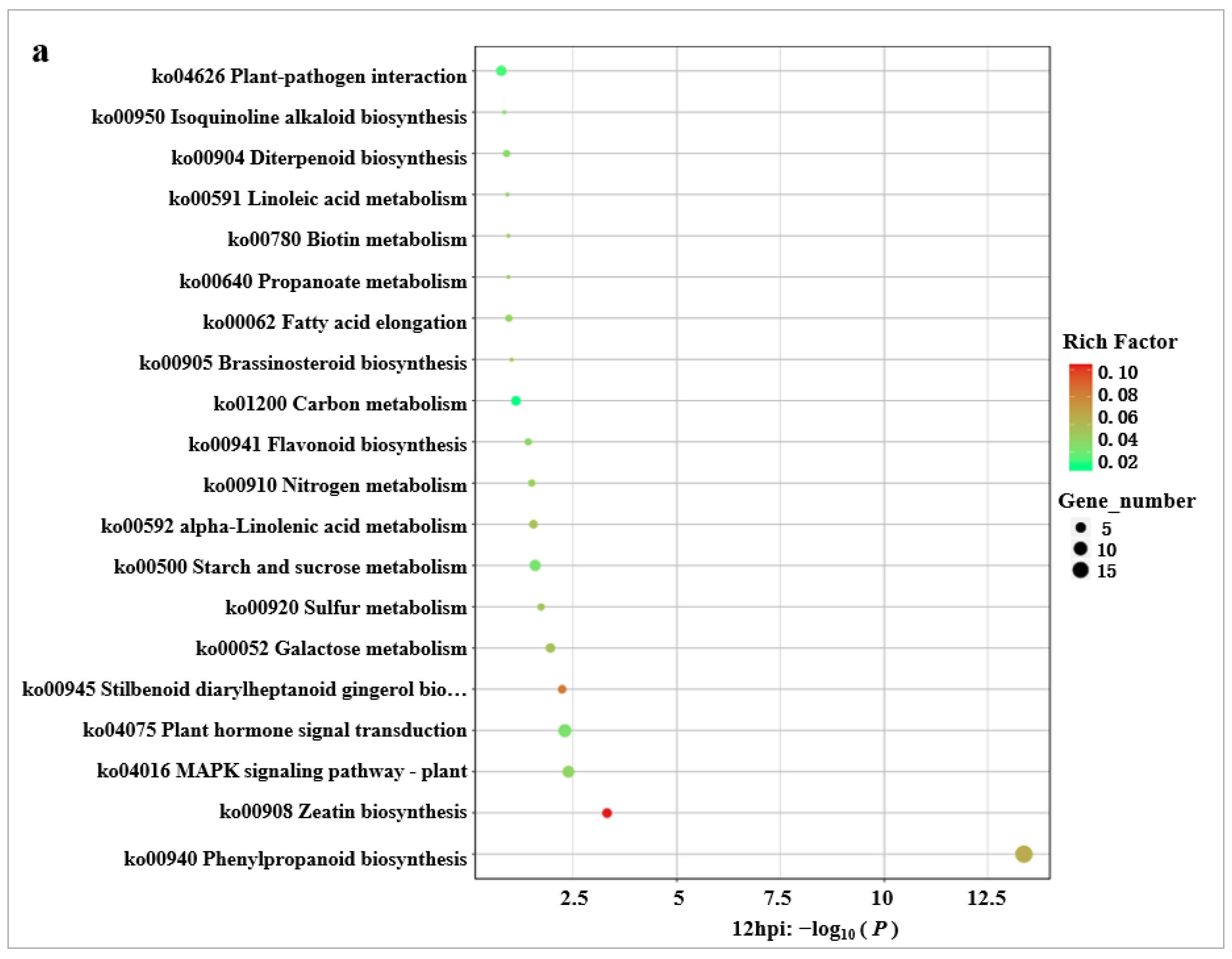
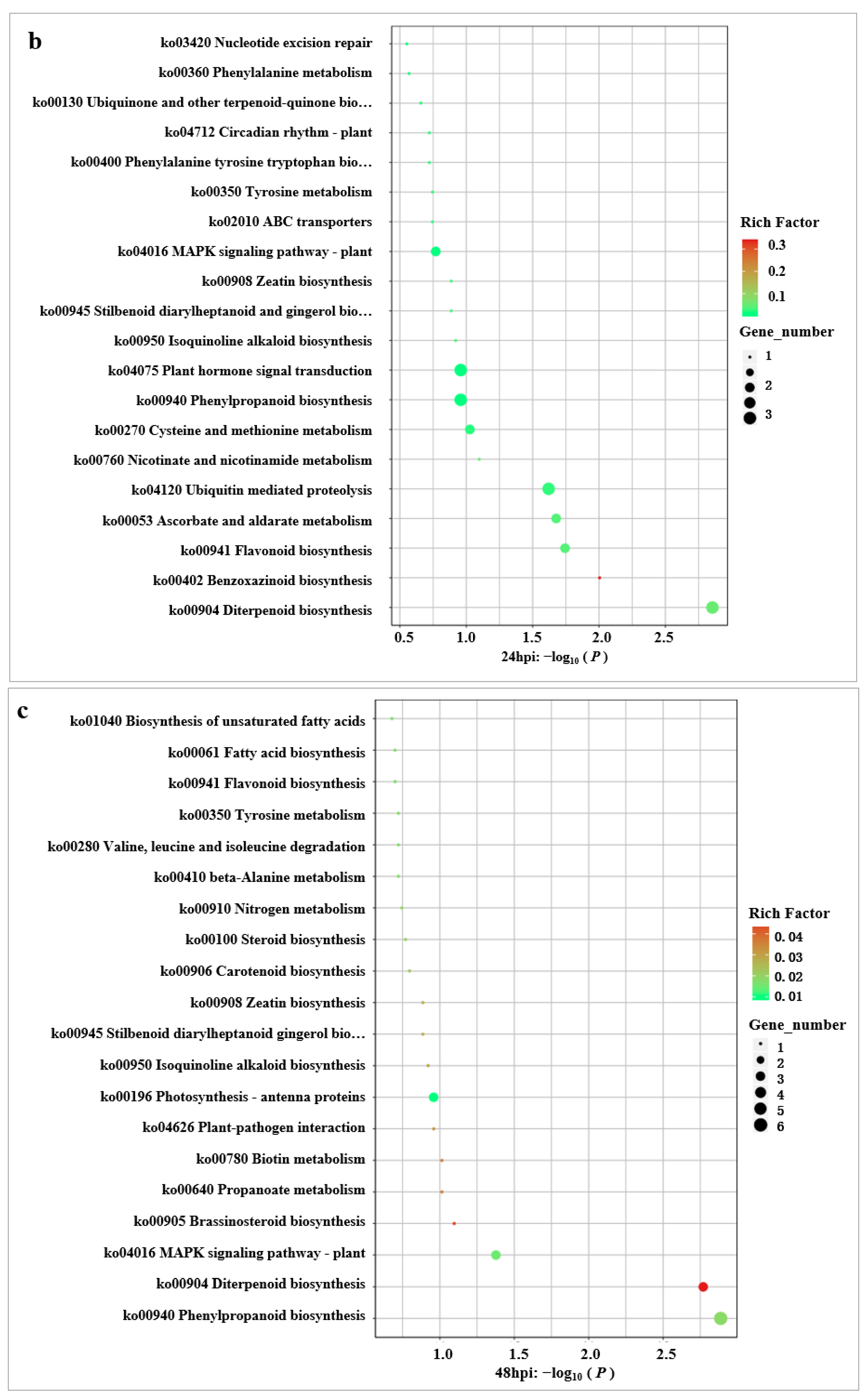
2.6. KEGG Enrichment Analysis of DEGs
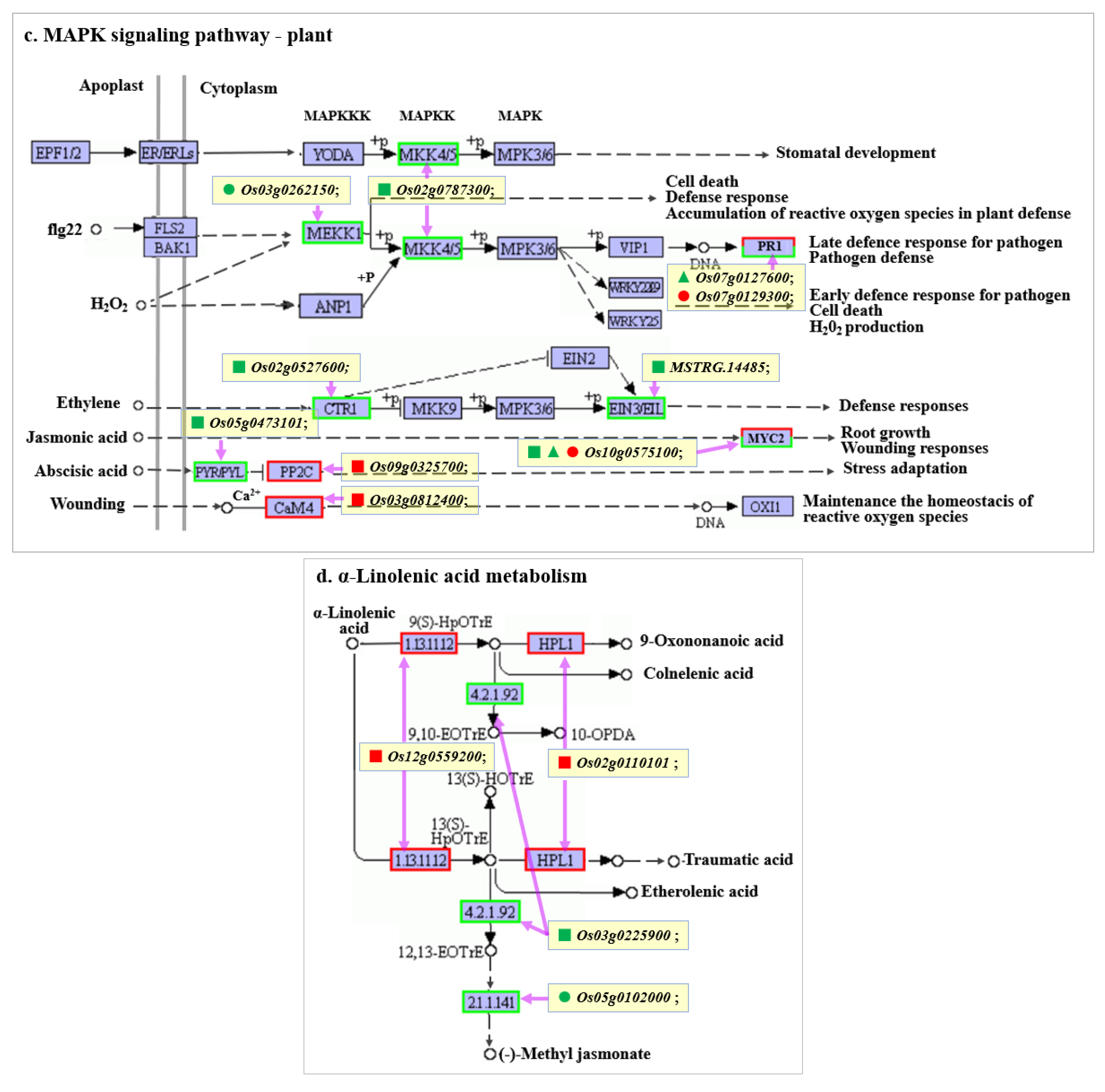
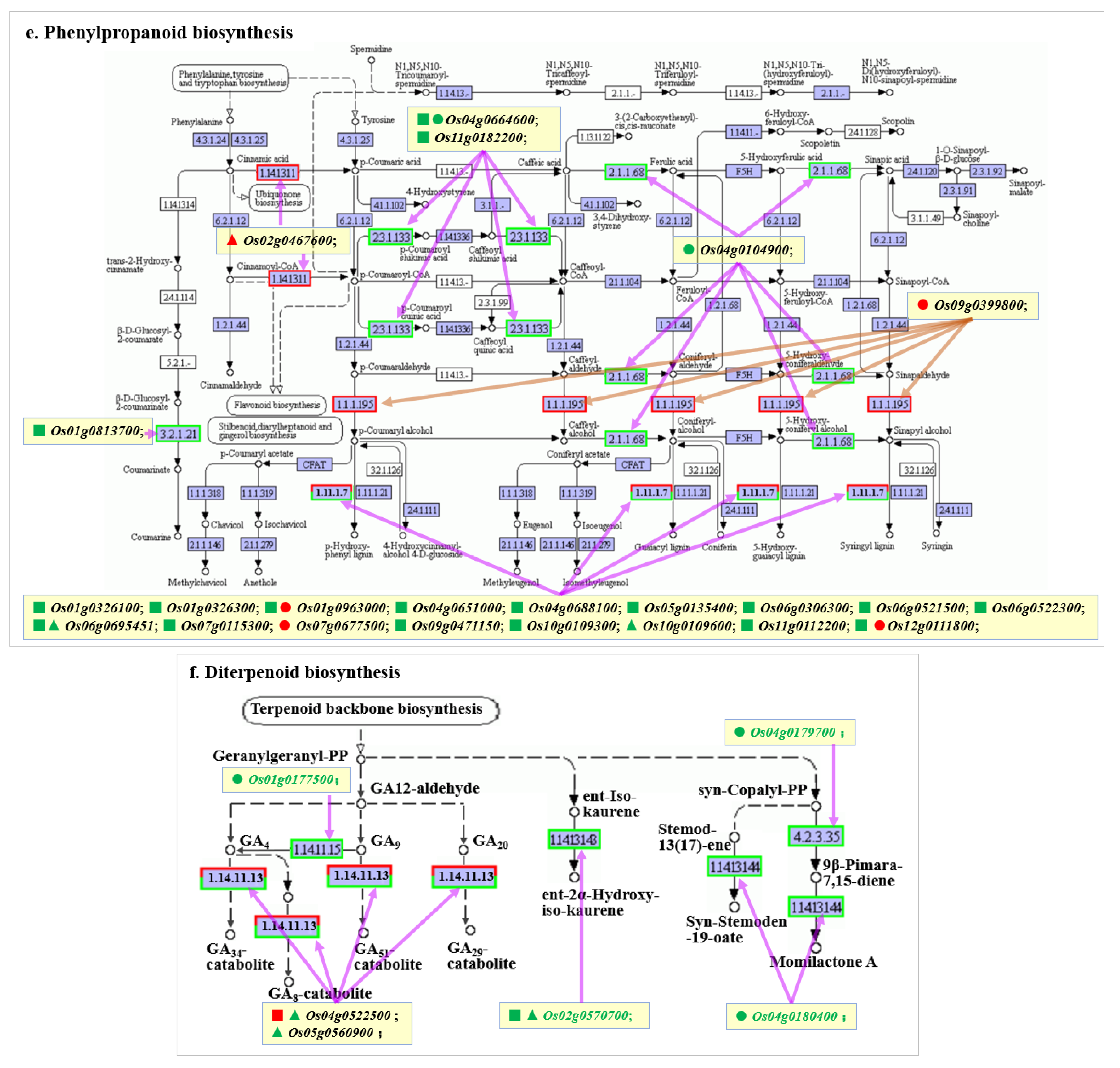
2.7. Key KEGG Pathway Analysis
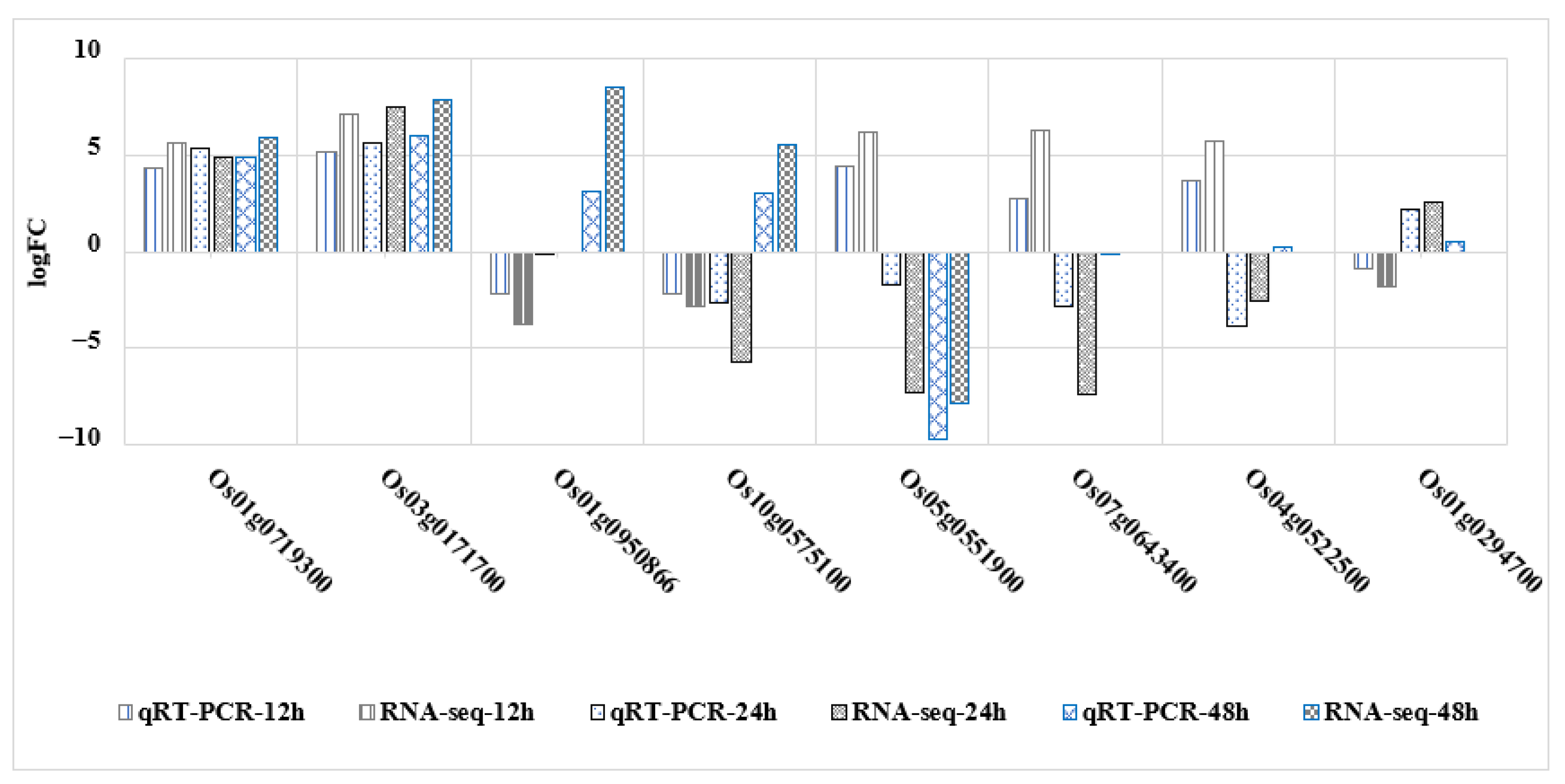
2.8. qRT-PCR Confirms Gene Expression Profiles
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
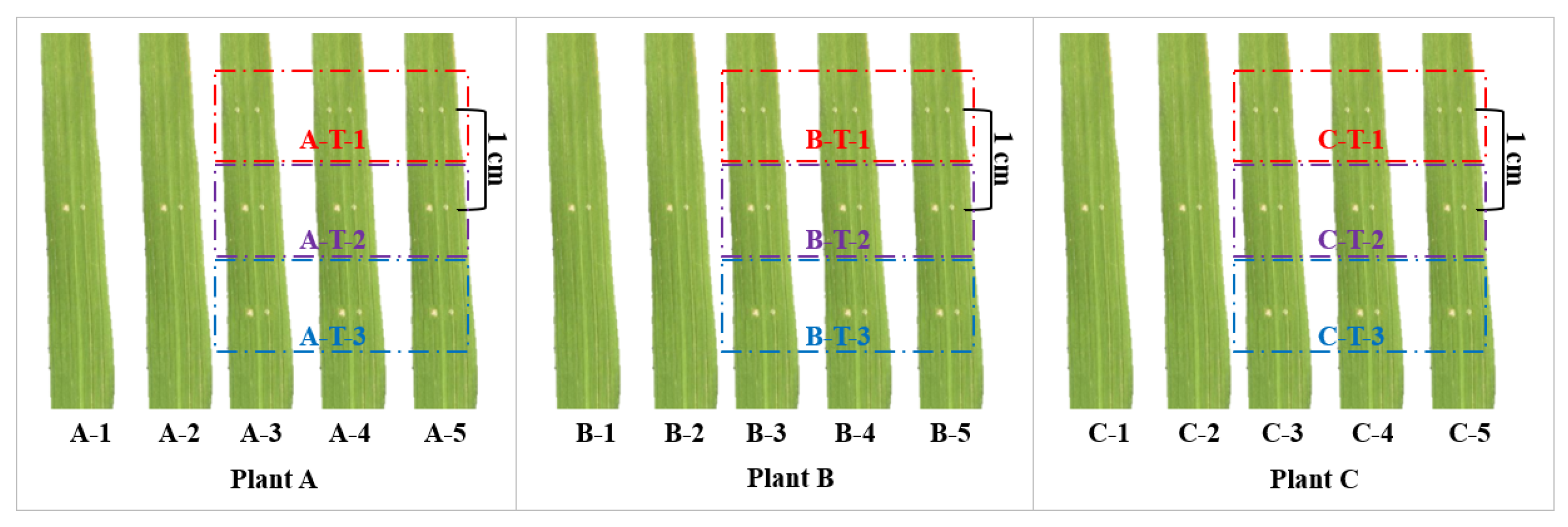
4.2. Bacterial Inoculation and Identification of Diseased Spots
4.3. RNA Sequencing
4.4. RNA Data Analysis
4.5. qRT-PCR Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Makino, S.; Subedee, A.; Bogdanove, A.J. Novel Candidate Virulence Factors in Rice Pathogen Xanthomonas oryzae pv. oryzicola as Revealed by Mutational Analysis. Appl. Environ. Microb. 2007, 73, 8023–8027. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Wang, L.; Lui, A.C.W.; Liu, H.; Takeda-Kimura, Y.; Chen, M.; Zhu, F.; Zhang, J.; Umezawa, T.; Tobimatsu, Y.; et al. Deficiency in flavonoid biosynthesis genesCHS, CHI, and CHIL alters rice flavonoid and lignin profiles. Plant Physiol. 2022, 188, 1993–2011. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.J.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Del Río, J.C. Structural Characteristics of the Guaiacyl-Rich Lignins From Rice (Oryza sativa L.) Husks and Straw. Front. Plant Sci. 2021, 12, 640475. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Jiang, H.; Ding, T. Genome-wide dissection of the chalcone synthase gene family in Oryza sativa. Mol. Breeding 2017, 37, 119. [Google Scholar] [CrossRef]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! Surveying the evolution of (di)terpenoid metabolism. Ann. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar] [CrossRef]
- Cibils Stewart, X.; Mace, W.J.; Popay, A.J.; Lattanzi, F.A.; Hartley, S.S.E.; Hall, C.R.; Powell, J.R.; Johnson, S.N. Interactions between silicon and alkaloid defences in endophyte-infected grasses and the consequences for a folivore. Funct. Ecol. 2022, 36, 249–261. [Google Scholar] [CrossRef]
- Smith, J.M.; Salamango, D.J.; Leslie, M.E.; Collins, C.A.; Heese, A. Sensitivity to Flg22 Is Modulated by Ligand-Induced Degradation and de Novo Synthesis of the Endogenous Flagellin-Receptor FLAGELLIN-SENSING2. Plant Physiol. 2014, 164, 440–454. [Google Scholar] [CrossRef]
- Hutin, M.; Césari, S.; Chalvon, V.; Michel, C.; Tran, T.T.; Boch, J.; Koebnik, R.; Szurek, B.; Kroj, T. Ectopic activation of the rice NLR heteropair RGA4/RGA5 confers resistance to bacterial blight and bacterial leaf streak diseases. Plant J. 2016, 88, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, X.; Wu, T.; Yuan, B.; Ding, X.; Yao, F.; Chu, Z. The polygalacturonase-inhibiting protein 4 (OsPGIP4), a potential component of the qBlsr5a locus, confers resistance to bacterial leaf streak in rice. Planta 2016, 243, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, J.; Zhang, Z.; Ma, L.; Yang, Z.; Zhang, Q.; Li, X.; Xiao, J.; Wang, S. MAPK kinase 10.2 promotes disease resistance and drought tolerance by activating different MAPKs in rice. Plant J. 2017, 92, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, B.; Qi, G.; Shang, L.; Liu, H.; Ding, X.; Chu, Z. Identification of the phytosulfokine receptor 1 (OsPSKR1) confers resistance to bacterial leaf streak in rice. Planta 2019, 250, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Tian, H.; Zhang, R.; Zuo, L.; Jin, G.; Xu, Q.; Ding, X.; Li, X.; Chu, Z. Overexpression of OsHSP18.0-CI Enhances Resistance to Bacterial Leaf Streak in Rice. Rice 2017, 10, 12. [Google Scholar] [CrossRef]
- Fu, J.; Liu, H.; Li, Y.; Yu, H.; Li, X.; Xiao, J.; Wang, S. Manipulating Broad-Spectrum Disease Resistance by Suppressing Pathogen-Induced Auxin Accumulation in Rice. Plant Physiol. 2011, 155, 589–602. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, H.; Qiu, D.; Zhou, Y.; Li, X.; Xu, C.; Wang, S. A Pair of Allelic WRKY Genes Play Opposite Roles in Rice-Bacteria Interactions. Plant Physiol. 2009, 151, 936–948. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Wang, W.; Wang, L.; Hao, G.; Guo, C.; Chen, L. Over-expression in the nucleotide-binding site-leucine rich repeat gene DEPG1 increases susceptibility to bacterial leaf streak disease in transgenic rice plants. Mol. Biol. Rep. 2012, 39, 3491–3504. [Google Scholar] [CrossRef][Green Version]
- Guo, L.; Guo, C.; Li, M.; Wang, W.; Luo, C.; Zhang, Y.; Chen, L. Suppression of expression of the putative receptor-like kinase gene NRRB enhances resistance to bacterial leaf streak in rice. Mol. Biol. Rep. 2014, 41, 2177–2187. [Google Scholar] [CrossRef]
- Shen, X.; Yuan, B.; Liu, H.; Li, X.; Xu, C.; Wang, S. Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J. 2010, 64, 86–99. [Google Scholar] [CrossRef]
- Ma, Z.; Qin, G.; Zhang, Y.; Liu, C.; Wei, M.; Cen, Z.; Yan, Y.; Luo, T.; Li, Z.; Liang, H.; et al. Bacterial leaf streak 1 encoding a mitogen-activated protein kinase confers resistance to bacterial leaf streak in rice. Plant J. 2021, 107, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Munir, S.; Dai, Z.; Liu, Q.; Zhang, J.; Wang, Y.; Xu, W.; Miao, X.; Wei, L.; Ji, G. Transcriptome profiles of a native rice variety Hongyou-4 responding to infections of hypervirulent and hypovirulent Xanthomonas oryzae pv. oryzicola strains. Physiol. Mol. Plant Pathol. 2020, 110, 101462. [Google Scholar] [CrossRef]
- Lu, L.; Yang, D.; Tang, D.; Li, S.; Chen, Z. Transcriptome analysis of different rice cultivars provides novel insights into the rice response to bacterial leaf streak infection. Funct. Integr. Genom. 2020, 20, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Z.; Zhang, B.; Guan, H.; Zheng, Y.; Lan, T.; Zhang, J.; Qin, M.; Wu, W. Transcriptome analysis of xa5-mediated resistance to bacterial leaf streak in rice (Oryza sativa L.). Sci. Rep. 2020, 10, 19439. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tariq, R.; Ji, Z.; Wei, Z.; Zheng, K.; Mishra, R.; Zhao, K. Transcriptome analysis of a rice cultivar reveals the differentially expressed genes in response to wild and mutant strains of Xanthomonas oryzae pv. oryzae. Sci. Rep. 2019, 9, 3757. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Bagnaresi, P.; Biselli, C.; Orru, L.; Amaral Carneiro, G.; Siciliano, I.; Valé, G.; Gullino, M.L.; Spadaro, D. Comparative transcriptome profiling of resistant and susceptible rice genotypes in response to the seedborne pathogen Fusarium fujikuroi. BMC Genom. 2016, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Bagnaresi, P.; Biselli, C.; Orru, L.; Urso, S.; Crispino, L.; Abbruscato, P.; Piffanelli, P.; Lupotto, E.; Cattivelli, L.; Vale, G. Comparative transcriptome profiling of the early response to Magnaporthe oryzae in durable resistant vs susceptible rice (Oryza sativa L.) genotypes. PLoS ONE 2012, 7, e51609. [Google Scholar] [CrossRef]
- Shi, L.; Luo, D.; Zhao, Y.; Chen, Z.; Liu, F.; Li, R. Genetic analysis and mapping of bacterial leaf streak resistance genes in Oryzae rufipogon Griff. J. South China Agric. Univ. 2019, 40, 1–5. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Li, Z.; Zakria, M.; Zou, L.; Chen, G. Increasing resistance to bacterial leaf streak in rice by editing the promoter of susceptibility gene OsSULRT3;6. Plant Biotechnol. J. 2021, 19, 1101–1103. [Google Scholar] [CrossRef]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.S. SWEET11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef]
- Streubel, J.; Pesce, C.; Hutin, M.; Koebnik, R.; Boch, J.; Szurek, B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013, 200, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, H.; Yuan, B.; Liu, H.; Kong, L.; Chu, Z.; Ding, X. Tal2b targets and activates the expression of OsF3H03g to hijack OsUGT74H4 and synergistically interfere with rice immunity. New Phytol. 2022, 233, 1864–1880. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH Transcription Factors MYC3 and MYC4 Are Targets of JAZ Repressors and Act Additively with MYC2 in the Activation of Jasmonate Responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, C.; Cao, J.; Chen, H.; Pang, J.; Zhao, Q.; Wang, Z.; Qing, F.Z.; Liu, J. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J. Integr. Plant Biol. 2020, 62, 1552–1573. [Google Scholar] [CrossRef]
- Nong, X.; Liao, H.; Liu, Z. Studies on the methodology for resistance identification of rice cultivar to bacterial leaf streak. Guangxi Plant Prot. 1992, 3, 5–9. (In Chinese) [Google Scholar]



| Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Os01g0719300 | CTAGCACTGGCAGAAGGAATAG | CATGATACCAAACGCGATCATC |
| Os01g0294700 | ACAACACCTACTACCACAACAA | TACAAGCATAGTCGTCGTTGAT |
| Os01g0950866 | GTATTGATCCCTCCAGATGCGT | GCTTGCTCAAAGTCACCAGACA |
| Os03g0171700 | TCTCCTGAAAAGGGAAAAGTGT | TCGATCTCTATTAGCCCTGGTA |
| Os04g0522500 | CCTCCCAAACTTTTTCAAGCAT | TCGAGTTCACATCGACCATAAA |
| Os05g0551900 | CATCAAGCCCCACAGAGCA | CCCACCAATCCACAAAACG |
| Os07g0643400 | GATCCACTCCTTCTACGTCTTC | AATTAAGCATTCGATTTGGGCG |
| Os10g0575100 | CCATCCTAACACAGATCTTT | ATACTCTCTACTCTCCCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Zhang, H.; Wan, Y.; Deng, Z.; Qin, X.; Li, R.; Liu, F. Transcriptome Analysis in Response to Infection of Xanthomonas oryzae pv. oryzicola Strains with Different Pathogenicity. Int. J. Mol. Sci. 2023, 24, 14. https://doi.org/10.3390/ijms24010014
Tang M, Zhang H, Wan Y, Deng Z, Qin X, Li R, Liu F. Transcriptome Analysis in Response to Infection of Xanthomonas oryzae pv. oryzicola Strains with Different Pathogenicity. International Journal of Molecular Sciences. 2023; 24(1):14. https://doi.org/10.3390/ijms24010014
Chicago/Turabian StyleTang, Min, Hui Zhang, Yao Wan, Ziqiu Deng, Xuemei Qin, Rongbai Li, and Fang Liu. 2023. "Transcriptome Analysis in Response to Infection of Xanthomonas oryzae pv. oryzicola Strains with Different Pathogenicity" International Journal of Molecular Sciences 24, no. 1: 14. https://doi.org/10.3390/ijms24010014
APA StyleTang, M., Zhang, H., Wan, Y., Deng, Z., Qin, X., Li, R., & Liu, F. (2023). Transcriptome Analysis in Response to Infection of Xanthomonas oryzae pv. oryzicola Strains with Different Pathogenicity. International Journal of Molecular Sciences, 24(1), 14. https://doi.org/10.3390/ijms24010014







