Potential Anti-Inflammatory and Chondroprotective Effect of Luzula sylvatica
Abstract
1. Introduction
2. Results
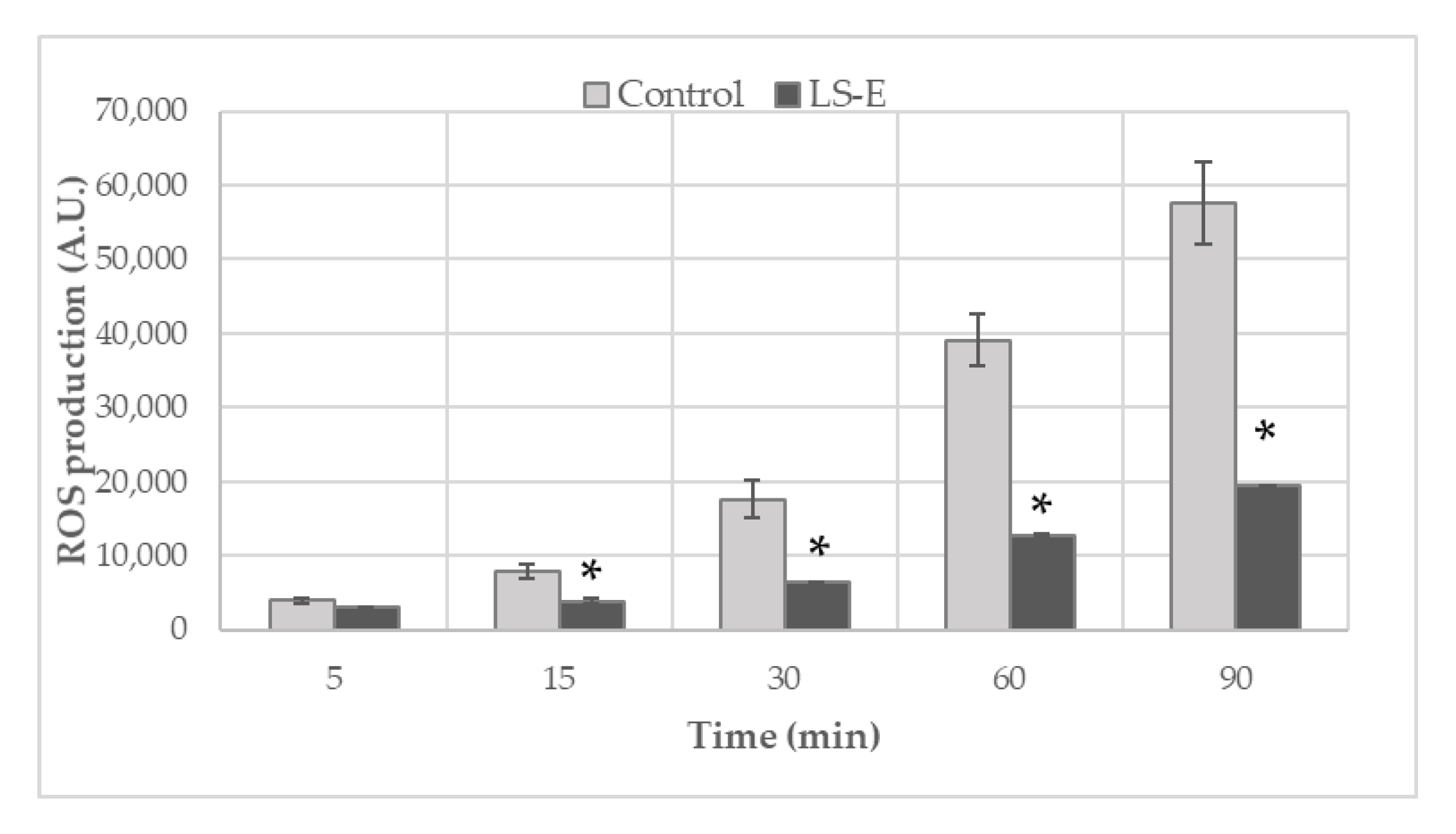
2.1. Inhibition of Reactive Species Generation
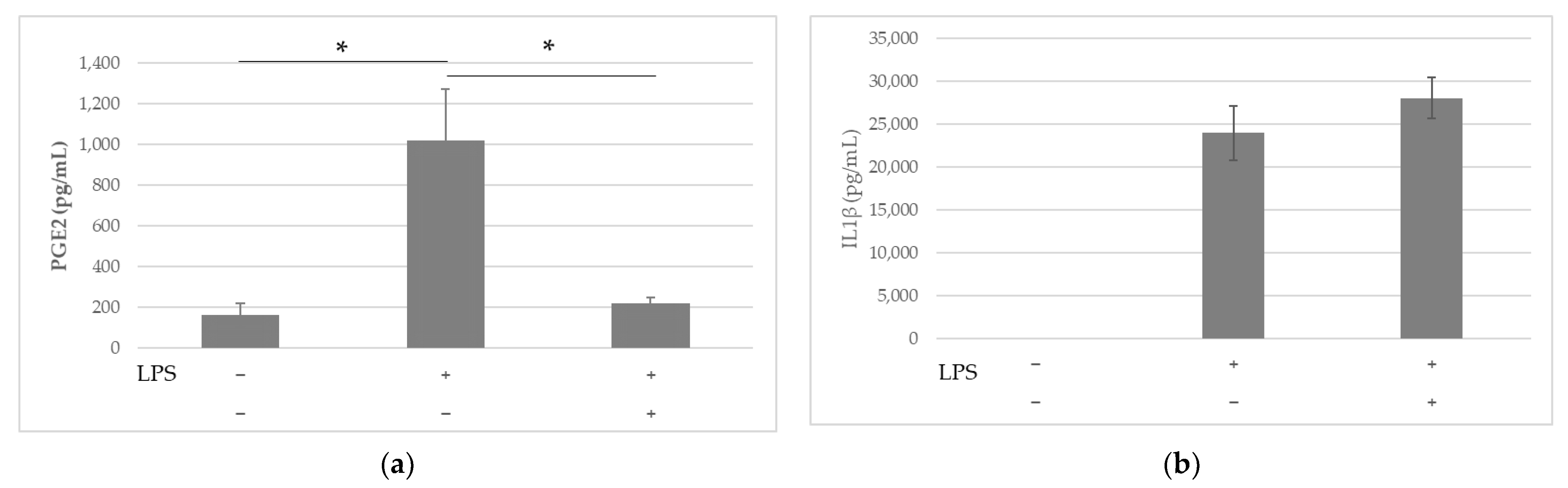
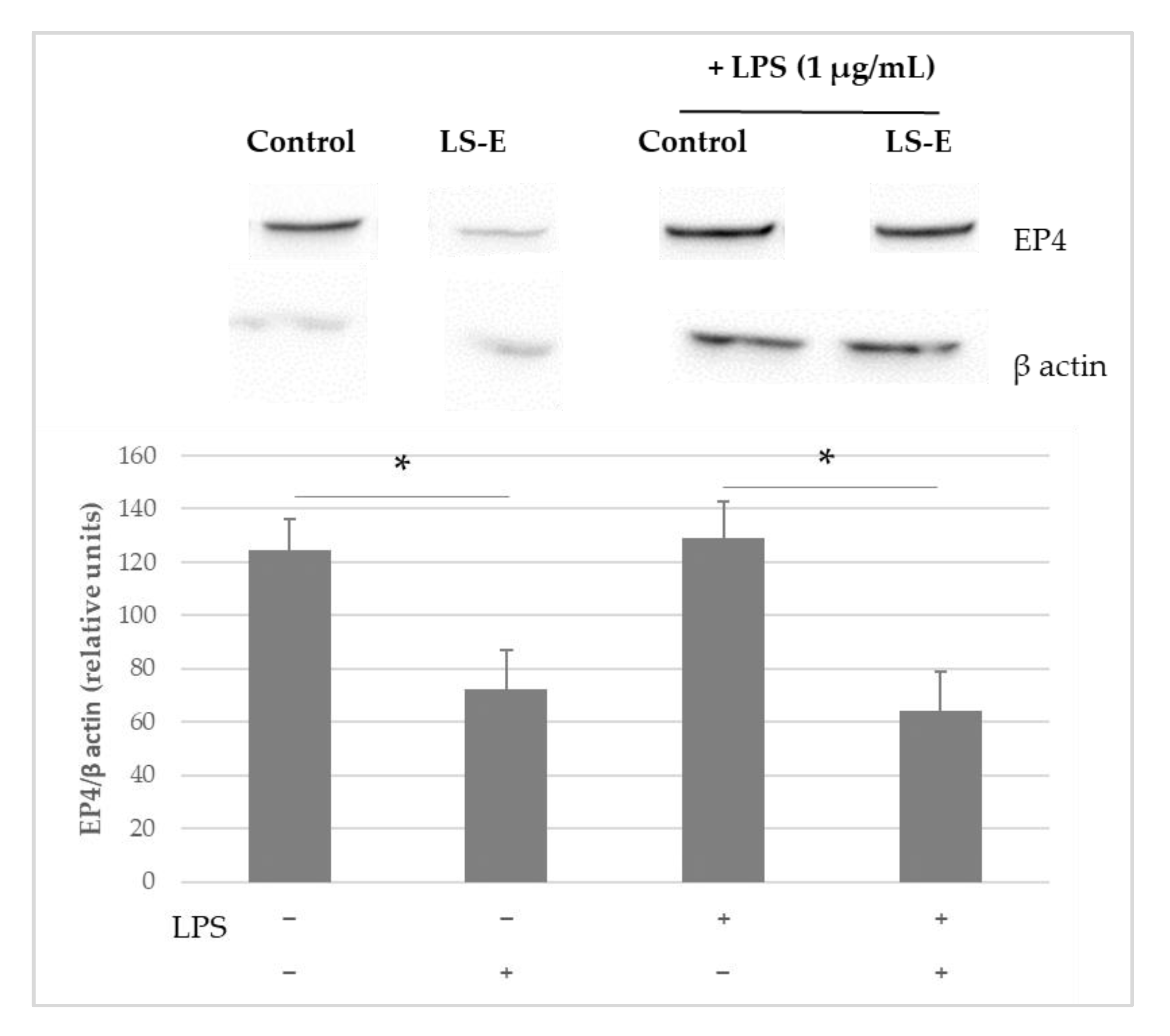
2.2. Anti-Inflammatory Effects of LS-E in Leucocytes via the Inhibition of PGE2
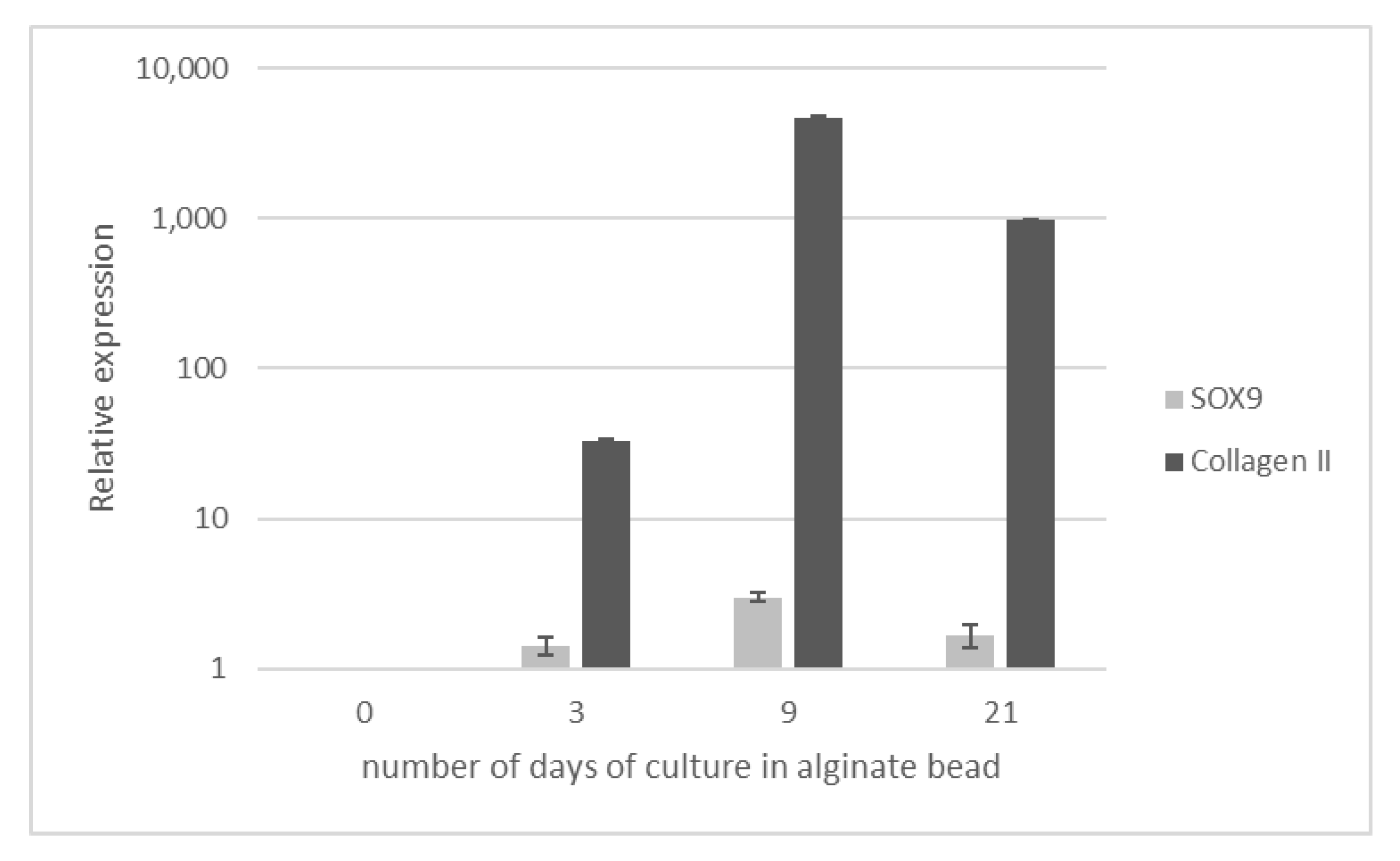
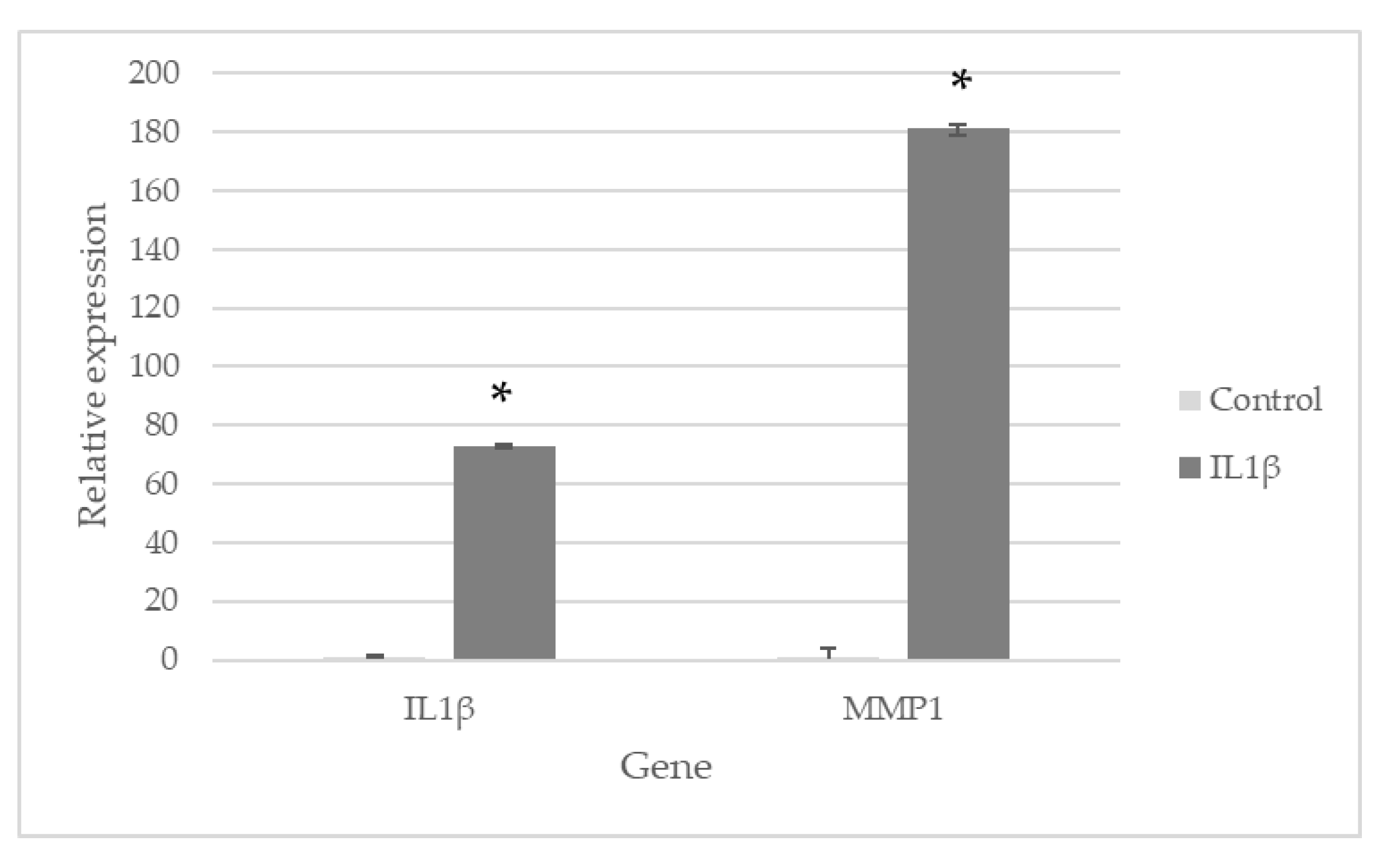
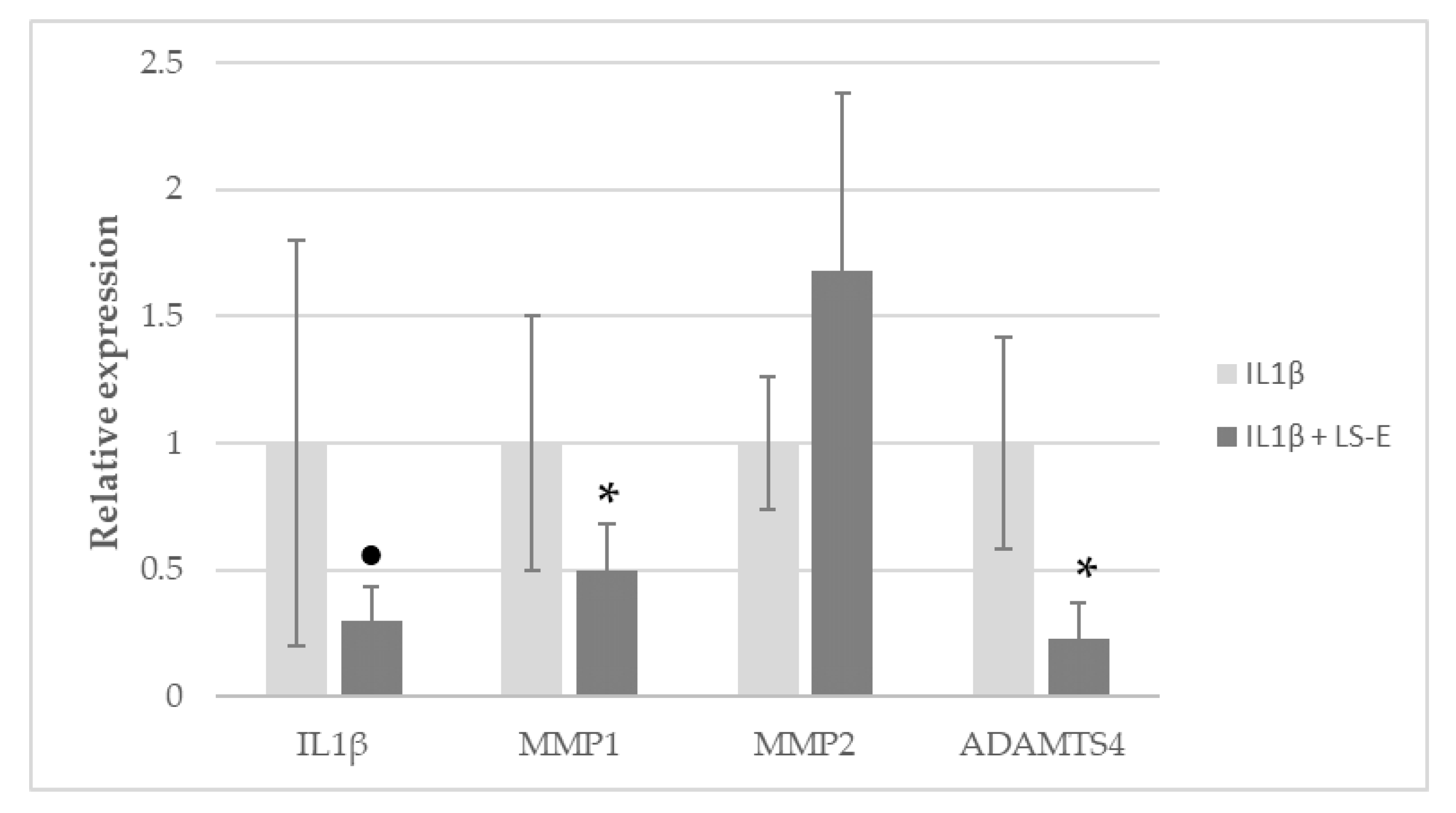
2.3. LS-E Modulates Gene Expression in IL1β-Activated Normal Human Articular Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extract and Composition
4.2. Production of Reactive Oxygen Species (ROS) by Blood Leucocytes
4.3. IL1β and PGE2 Production of PBMCs
4.4. Western Blot Analysis of EP4 and NFκB
4.5. Chondrocyte Culture and 3D Modeling
4.6. Analyses of Gene Expression
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Preliminary Work: Effect of a Methanolic Extract of Luzula sylvatica on ROS Production of Blood Leukocytes Stimulated by PMA
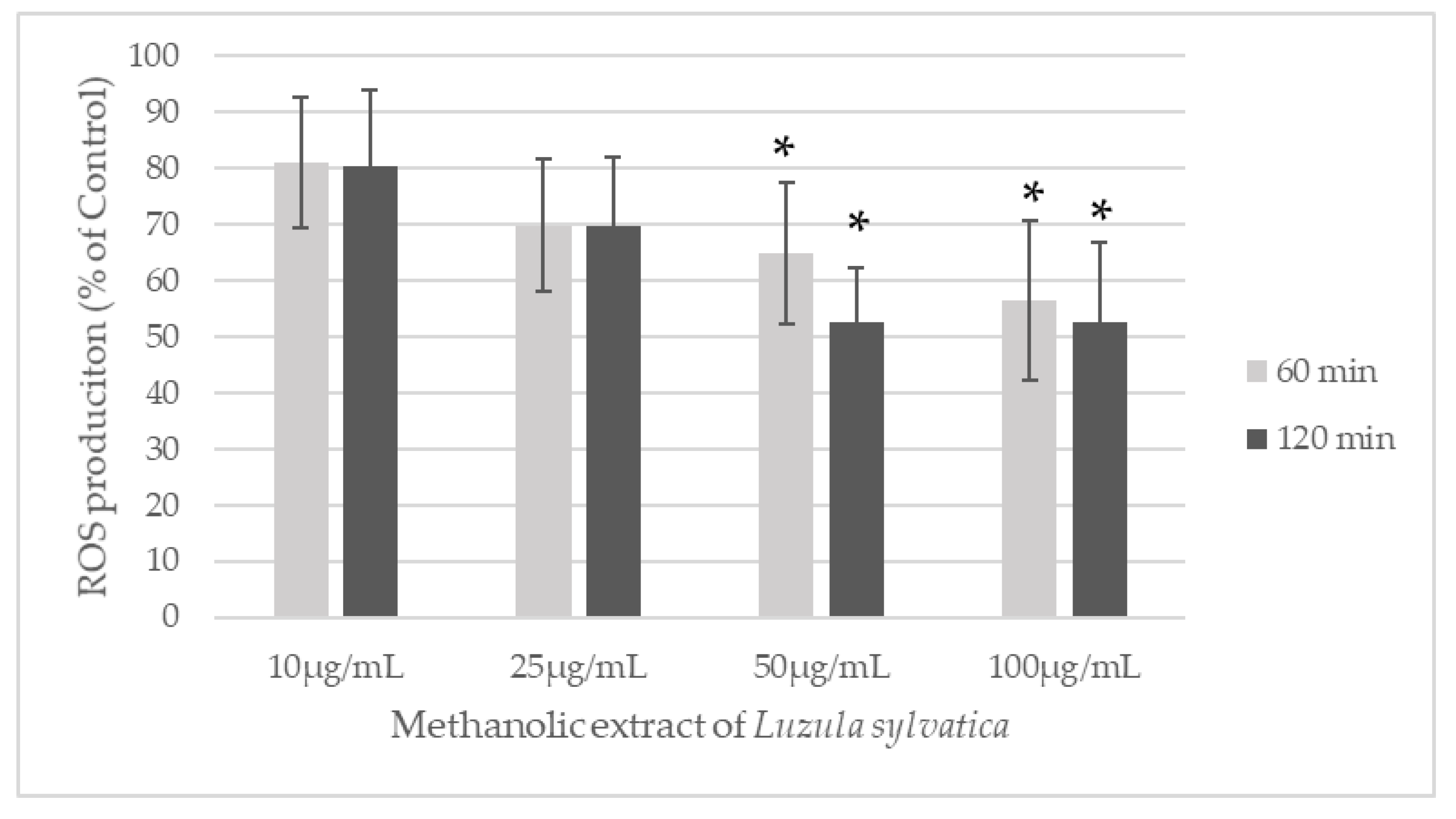
Appendix A.2. Impact of LS-E on PBMCs Viability
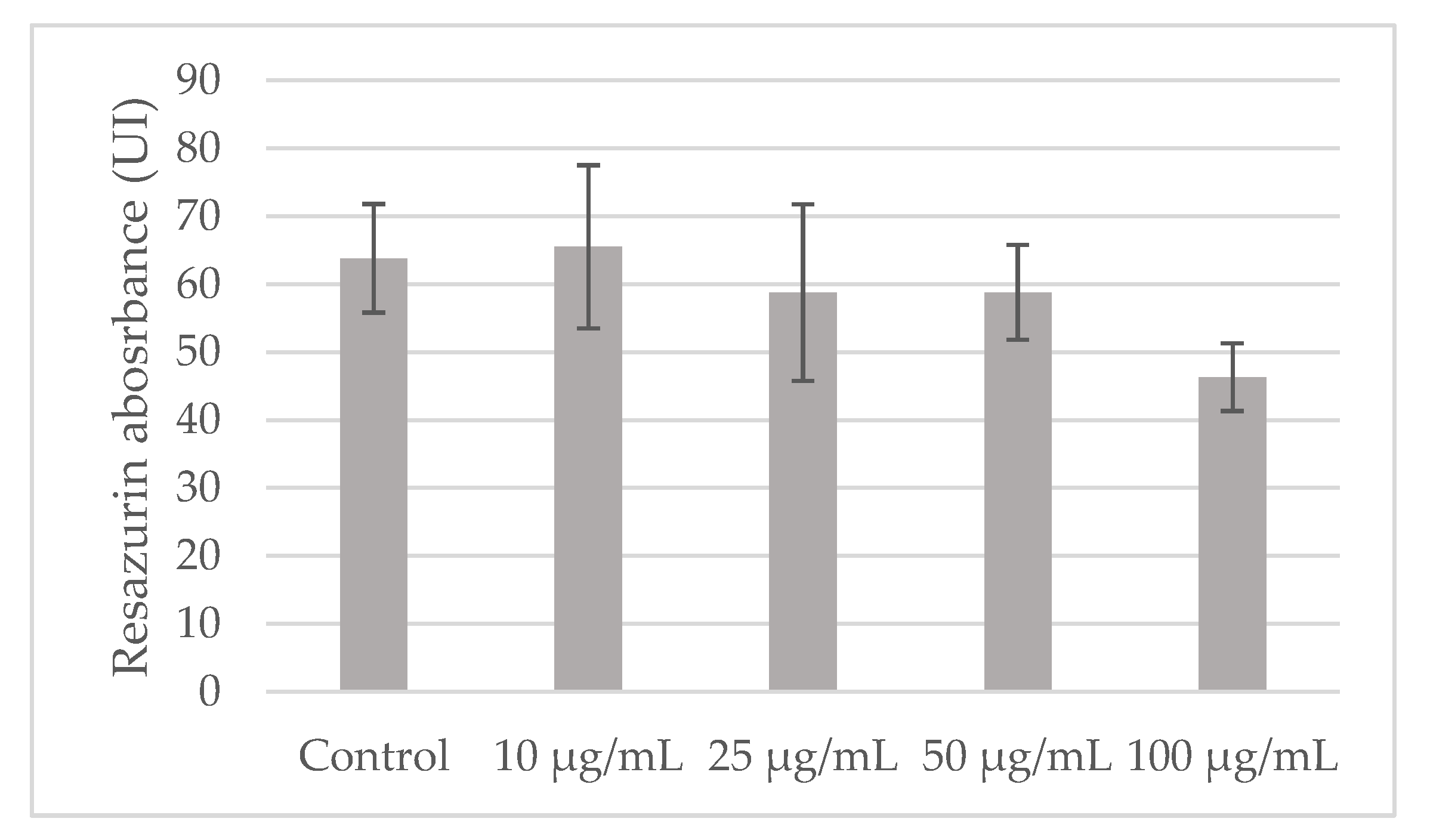
Appendix B
Appendix B.1. Gels Blots for β Actin
Appendix B.2. Gels Blots for EP4
Appendix C
Appendix C.1. Aspect of the Alginate Beads
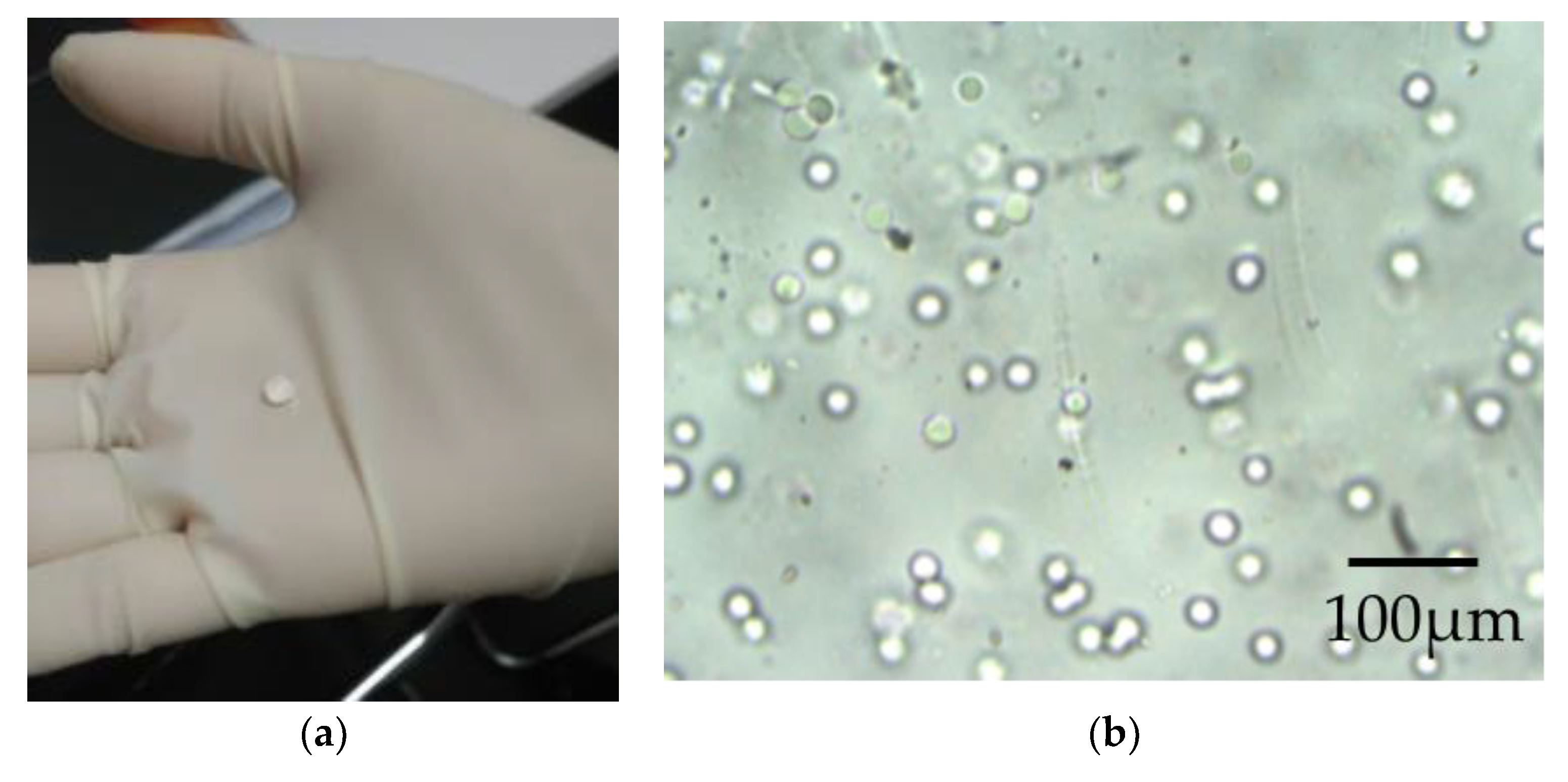
Appendix C.2. Alcian Blue Staining
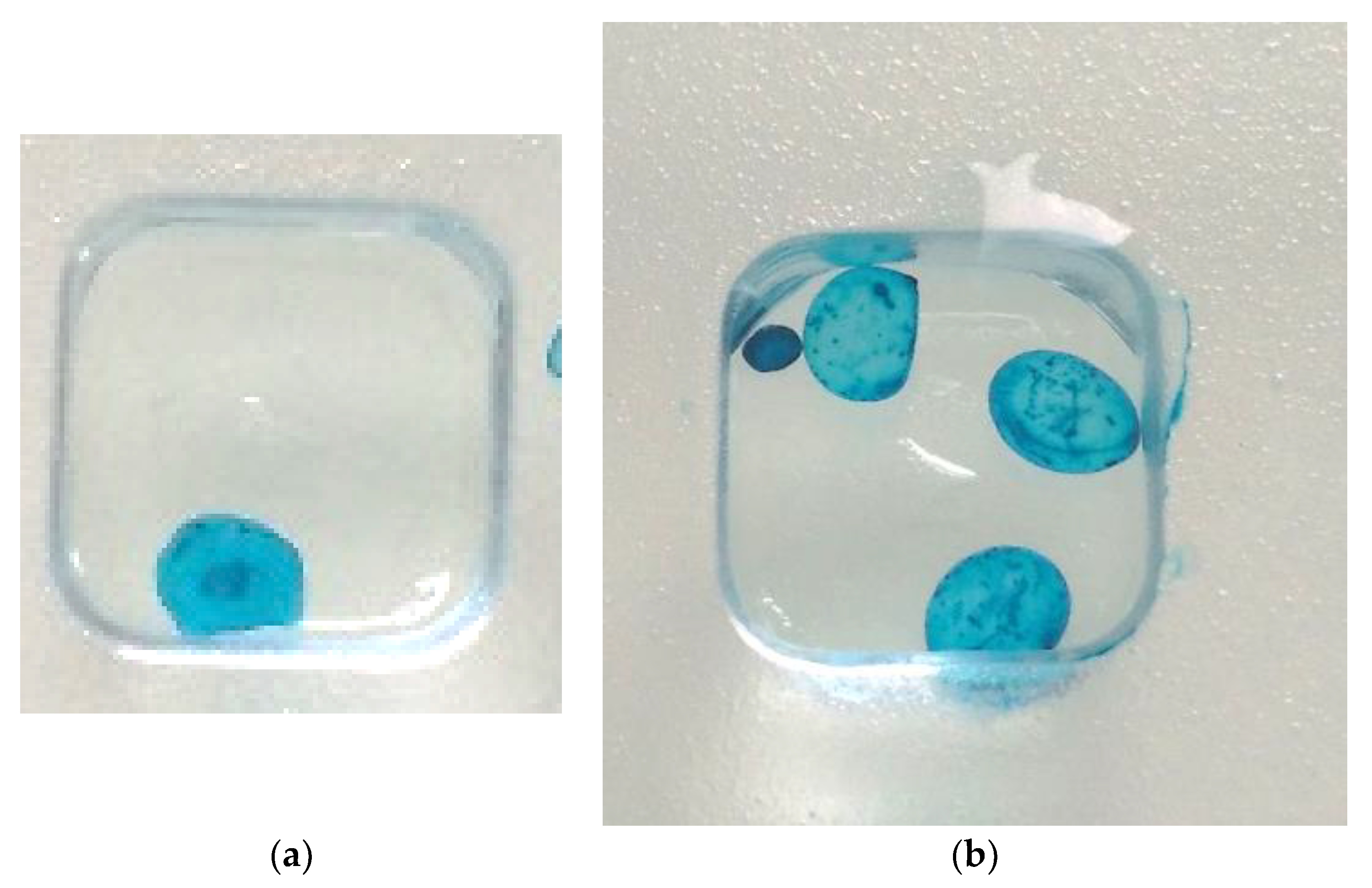
Appendix C.3. Differentiation of Chondrocytes
References
- World Health Organization. Chronic Diseases and Health Promotion. Available online: https://www.who.int/chp/topics/rheumatic/en/ (accessed on 20 August 2019).
- Abramson, S.B. Osteoarthritis and Nitric Oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Min, G.-Y.; Park, J.-M.; Joo, I.-H.; Kim, D.-H. Inhibition Effect of Caragana Sinica Root Extracts on Osteoarthritis through MAPKs, NF-ΚB Signaling Pathway. Int. J. Med. Sci. 2021, 18, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wylde, V.; Moore, A.J.; Anderson, E.; Donovan, R.; Blom, A.W.; Judge, A.; Whitehouse, M.R. Research Priorities for Intra-Articular Corticosteroid Injections for Osteoarthritis: A Delphi Study. Osteoarthr. Cartil. Open 2022, 4, 100291. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, E.; Elia, R.; Mattiuzzo, E.; Faggian, A.; Pozzuoli, A.; Ruggieri, P.; Brun, P. The Viability and Anti-Inflammatory Effects of Hyaluronic Acid-Chitlac-Tracimolone Acetonide- β-Cyclodextrin Complex on Human Chondrocytes. Cartilage 2021, 13, 920S–924S. [Google Scholar] [CrossRef]
- Ragle, R.L.; Sawitzke, A.D. Nutraceuticals in the Management of Osteoarthritis: A Critical Review. Drugs Aging 2012, 29, 717–731. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules 2016, 21, 465. [Google Scholar] [CrossRef]
- Bús, C.; Tóth, B.; Stefkó, D.; Hohmann, J.; Vasas, A. Family Juncaceae: Promising Source of Biologically Active Natural Phenanthrenes. Phytochem. Rev. 2018, 17, 833–851. [Google Scholar] [CrossRef]
- El-Shamy, A.I.; Abdel-Razek, A.F.; Nassar, M.I. Phytochemical Review of Juncus L. Genus (Fam. Juncaceae). Arab. J. Chem. 2015, 8, 614–623. [Google Scholar] [CrossRef]
- Park, N.-Y.; Kim, S.-G.; Park, H.-H.; Jeong, K.-T.; Lee, Y.J.; Lee, E. Anti-Inflammatory Effects of Juncus Effusus Extract (JEE) on LPS-Stimulated RAW 264.7 Cells and Edema Models. Pharm. Biol. 2016, 54, 243–250. [Google Scholar] [CrossRef]
- Tóth, B.; Chang, F.-R.; Hwang, T.-L.; Szappanos, Á.; Mándi, A.; Hunyadi, A.; Kurtán, T.; Jakab, G.; Hohmann, J.; Vasas, A. Screening of Luzula Species Native to the Carpathian Basin for Anti-Inflammatory Activity and Bioactivity-Guided Isolation of Compounds from Luzula Luzuloides (Lam.) Dandy & Wilmott. Fitoterapia 2017, 116, 131–138. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Luteolin and Luteolin-7-O-Glucoside from Dandelion Flower Suppress INOS and COX-2 in RAW264.7 Cells. Mol. Cell. Biochem. 2004, 265, 107–113. [Google Scholar] [CrossRef]
- Mayer, A.C.; Huovinen, C. Silvopastoralism in the Alps: Native Plant Species Selection under Different Grazing Pressure. Ecol. Eng. 2007, 29, 372–381. [Google Scholar] [CrossRef]
- McEvoy, P.M.; Flexen, M.; McAdam, J.H. The Effects of Livestock Grazing on Ground Flora in Broadleaf Woodlands in Northern Ireland. For. Ecol. Manag. 2006, 225, 39–50. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A. Anti-Inflammatory and Antioxidant Activity of an Extract of Luzula Sylvatica in a Co-Culture Model of Fibroblasts and Macrophages. Curr. Res. Complement. Alt. Med. 2022, 6, 152. [Google Scholar]
- Delfino, F.; Walker, W.H. Hormonal Regulation of the NF-ΚB Signaling Pathway. Mol. Cell. Endocrinol. 1999, 157, 1–9. [Google Scholar] [CrossRef]
- Harris, P.J.; Hartley, R.D. Phenolic Constituents of the Cell Walls of Monocotyledons. Biochem. Syst. Ecol. 1980, 8, 153–160. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B. Luteolin and Daphnetin Derivatives in the Juncaceae and Their Systematic Significance. Biochem. Syst. Ecol. 1975, 3, 181–190. [Google Scholar] [CrossRef]
- Gainche, M.; Ripoche, I.; Senejoux, F.; Cholet, J.; Ogeron, C.; Decombat, C.; Danton, O.; Delort, L.; Vareille-Delarbre, M.; Berry, A.; et al. Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula Sylvatica. Molecules 2020, 25, 2372. [Google Scholar] [CrossRef]
- Li, D.; Wang, W.; Xie, G. Reactive Oxygen Species: The 2-Edged Sword of Osteoarthritis. Am. J. Med. Sci. 2012, 344, 486–490. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Ying, L.; De Bruyne, T.; Apers, S.; Cos, P.; Hermans, N.; Bakana, P.; Tona, L.; Kambu, K.; Kalenda, D.T.; et al. Radical Scavenging and Xanthine Oxidase Inhibitory Activity of Phenolic Compounds from Bridelia Ferruginea Stem Bark. J. Pharm. Pharmacol. 2010, 53, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Watanabe, H.; Kadota, S. Xanthine Oxidase Inhibitory Activity of Vietnamese Medicinal Plants. Biol. Pharm. Bull. 2004, 27, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Gainche, M.; Ogeron, C.; Ripoche, I.; Senejoux, F.; Cholet, J.; Decombat, C.; Delort, L.; Berthon, J.-Y.; Saunier, E.; Caldefie Chezet, F.; et al. Xanthine Oxidase Inhibitors from Filipendula Ulmaria (L.) Maxim. and Their Efficient Detections by HPTLC and HPLC Analyses. Molecules 2021, 26, 1939. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Hohmann, J.; Vasas, A. Phenanthrenes: A Promising Group of Plant Secondary Metabolites. J. Nat. Prod. 2018, 81, 661–678. [Google Scholar] [CrossRef]
- Mattiuzzo, E.; Faggian, A.; Venerando, R.; Benetti, A.; Belluzzi, E.; Abatangelo, G.; Ruggieri, P.; Brun, P. In Vitro Effects of Low Doses of β-Caryophyllene, Ascorbic Acid and d-Glucosamine on Human Chondrocyte Viability and Inflammation. Pharmaceuticals 2021, 14, 286. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Minatani, A.; Uchida, K.; Inoue, G.; Takano, S.; Aikawa, J.; Miyagi, M.; Fujimaki, H.; Iwase, D.; Onuma, K.; Matsumoto, T.; et al. Activation of Calcitonin Gene-Related Peptide Signaling through the Prostaglandin E2-EP1/EP2/EP4 Receptor Pathway in Synovium of Knee Osteoarthritis Patients. J. Orthop. Surg. 2016, 11, 117. [Google Scholar] [CrossRef]
- Sung, M.-S.; Lee, E.-G.; Jeon, H.-S.; Chae, H.-J.; Park, S.J.; Lee, Y.C.; Yoo, W.-H. Quercetin Inhibits IL-1β-Induced Proliferation and Production of MMPs, COX-2, and PGE2 by Rheumatoid Synovial Fibroblast. Inflammation 2012, 35, 1585–1594. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Chen, M. Effect of luteolin on COX-2 and mPGES-1 expression in LPS-induced RAW264.7 cells. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2007, 30, 1263–1266. [Google Scholar]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Laufer, S.; Lima, J.L.F.C.; Fernandes, E. Flavonoids Inhibit COX-1 and COX-2 Enzymes and Cytokine/Chemokine Production in Human Whole Blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, Y.; Zhang, S.; Ding, Y.; Huo, K.; Yang, J.; Zhao, L.; Nian, B.; Zhong, T.P.; Lu, W.; et al. PGE2 Activates EP4 in Subchondral Bone Osteoclasts to Regulate Osteoarthritis. Bone Res. 2022, 10, 27. [Google Scholar] [CrossRef]
- Majumder, M.; Xin, X.; Liu, L.; Girish, G.V.; Lala, P.K. Prostaglandin E2 Receptor EP 4 as the Common Target on Cancer Cells and Macrophages to Abolish Angiogenesis, Lymphangiogenesis, Metastasis, and Stem-like Cell Functions. Cancer Sci. 2014, 105, 1142–1151. [Google Scholar] [CrossRef]
- Melero-Martin, J.; Al-Rubeai, M. In Vitro Expansion of Chondrocytes. Top. Tissue Eng. 2007, 3, 37. [Google Scholar]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte Dedifferentiation and Osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Kang, B.-J.; Ryu, J.; Lee, C.J.; Hwang, S.-C. Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee. Biomol. Ther. 2014, 22, 239–245. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Q.; Ahn, J.H.; Kim, S.B.; Kim, Y.C.; Sung, S.H.; Hwang, B.Y.; Lee, M.K. Luteolin Downregulates IL-1β-Induced MMP-9 and -13 Expressions in Osteoblasts via Inhibition of ERK Signalling Pathway. J. Enzym. Inhib. Med. Chem. 2012, 27, 261–266. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Senejoux, F.; Ripoche, I.; Delort, L.; Vermerie, M.; Fraisse, D.; et al. In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum Rotundifolium. Medicines 2019, 6, 101. [Google Scholar] [CrossRef]
| Gene | +LS-E 50 µg/mL Fold Change | p Value | |
|---|---|---|---|
| ADAMTS1 | 0.599 ± 0.15 | 0.0806 ● | ↓ |
| ADAMTS13 | 0.8122 ± 0.21 | 0.2295 | |
| ADAMTS8 | 1.7912 ± 1.9 | 0.4708 | |
| CCL2 | 0.5588 ± 0.13 | 0.0219 * | ↓ |
| CCL5 | 0.5747 ± 0.09 | 0.0313 * | |
| COL11A1 | 1.079 ± 0.23 | 0.7312 | |
| COL1A2 | 1.0031 ± 0.38 | 0.9898 | |
| COL2A1 | 0.9586 ± 0.21 | 0.8669 | |
| COL4A2 | 0.9211 ± 0.21 | 0.6976 | |
| COL4A4 | 0.8229 ± 0.32 | 0.4409 | |
| COL5A1 | 0.6321 ± 0.34 | 0.2195 | |
| COL6A1 | 0.6815 ± 0.39 | 0.2555 | |
| COL6A2 | 0.7738 ± 0.12 | 0.1648 | |
| COL9A2 | 0.8771 ± 0.38 | 0.575 | |
| CXCL8 | 0.7075 ± 0.23 | 0.1224 | |
| IL1B | 0.4355 ± 0.42 | 0.0948 ● | ↓ |
| MMP1 | 0.5865 ± 0.17 | 0.0771 ● | ↓ |
| MMP10 | 0.6017 ± 0.23 | 0.2729 | |
| MMP13 | 1.1403 ± 0.8 | 0.7569 | |
| MMP2 | 0.8845 ± 0.35 | 0.5721 | |
| MMP3 | 0.5915 ± 0.13 | 0.0725 ● | ↓ |
| MMP7 | 0.7141 ± 0.1 | 0.2272 | |
| MMP8 | 0.8229 ± 0.32 | 0.4409 | |
| MMP9 | 0.5816 ± 0.2 | 0.0326 * | ↓ |
| NFKB1 | 0.7712 ± 0.27 | 0.2674 | |
| NFKBIA | 0.9849 ± 0.18 | 0.9256 | |
| COX-2 | 0.5002 ± 0.13 | 0.0339 * | ↓ |
| RUNX2 | 0.9286 ± 0.15 | 0.83 | |
| SMAD1 | 0.8633 ± 0.21 | 0.3566 | |
| SMAD2 | 0.9163 ± 0.4 | 0.7469 | |
| SMAD3 | 0.7854 ± 0.34 | 0.3234 | |
| SMAD4 | 0.763 ± 0.21 | 0.1142 | |
| SMAD5 | 0.7914 ± 0.25 | 0.2319 | |
| SMAD7 | 0.8855 ± 0.17 | 0.6368 | |
| SOX9 | 0.648 ± 0.24 | 0.0676 ● | ↓ |
| TGFBR1 | 0.7061 ± 0.31 | 0.1747 | |
| TGFBR2 | 0.8929 ± 0.36 | 0.6717 | |
| TIMP1 | 1.5087 ± 1.54 | 0.5586 | |
| TIMP2 | 0.6291 ± 0.1 | 0.0127 * | ↓ |
| TIMP3 | 0.765 ± 0.38 | 0.3715 | |
| TNF | 0.7866 ± 0.16 | 0.2445 | |
| VEGFA | 0.6396 ± 0.12 | 0.0274 * | ↓ |
| VEGFB | 0.9229 ± 0.46 | 0.7932 | |
| VEGFC | 0.5561 ± 0.21 | 0.0514 ● | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholet, J.; Decombat, C.; Delort, L.; Gainche, M.; Berry, A.; Ogeron, C.; Ripoche, I.; Vareille-Delarbre, M.; Vermerie, M.; Fraisse, D.; et al. Potential Anti-Inflammatory and Chondroprotective Effect of Luzula sylvatica. Int. J. Mol. Sci. 2023, 24, 127. https://doi.org/10.3390/ijms24010127
Cholet J, Decombat C, Delort L, Gainche M, Berry A, Ogeron C, Ripoche I, Vareille-Delarbre M, Vermerie M, Fraisse D, et al. Potential Anti-Inflammatory and Chondroprotective Effect of Luzula sylvatica. International Journal of Molecular Sciences. 2023; 24(1):127. https://doi.org/10.3390/ijms24010127
Chicago/Turabian StyleCholet, Juliette, Caroline Decombat, Laetitia Delort, Maël Gainche, Alexandre Berry, Clémence Ogeron, Isabelle Ripoche, Marjolaine Vareille-Delarbre, Marion Vermerie, Didier Fraisse, and et al. 2023. "Potential Anti-Inflammatory and Chondroprotective Effect of Luzula sylvatica" International Journal of Molecular Sciences 24, no. 1: 127. https://doi.org/10.3390/ijms24010127
APA StyleCholet, J., Decombat, C., Delort, L., Gainche, M., Berry, A., Ogeron, C., Ripoche, I., Vareille-Delarbre, M., Vermerie, M., Fraisse, D., Felgines, C., Rossary, A., Ranouille, E., Berthon, J.-Y., Tourrette, A., Priam, J., Saunier, E., Troin, Y., Senejoux, F., ... Caldefie-Chézet, F. (2023). Potential Anti-Inflammatory and Chondroprotective Effect of Luzula sylvatica. International Journal of Molecular Sciences, 24(1), 127. https://doi.org/10.3390/ijms24010127





