Lactoferrin as a Human Genome “Guardian”—An Overall Point of View
Abstract
1. Introduction
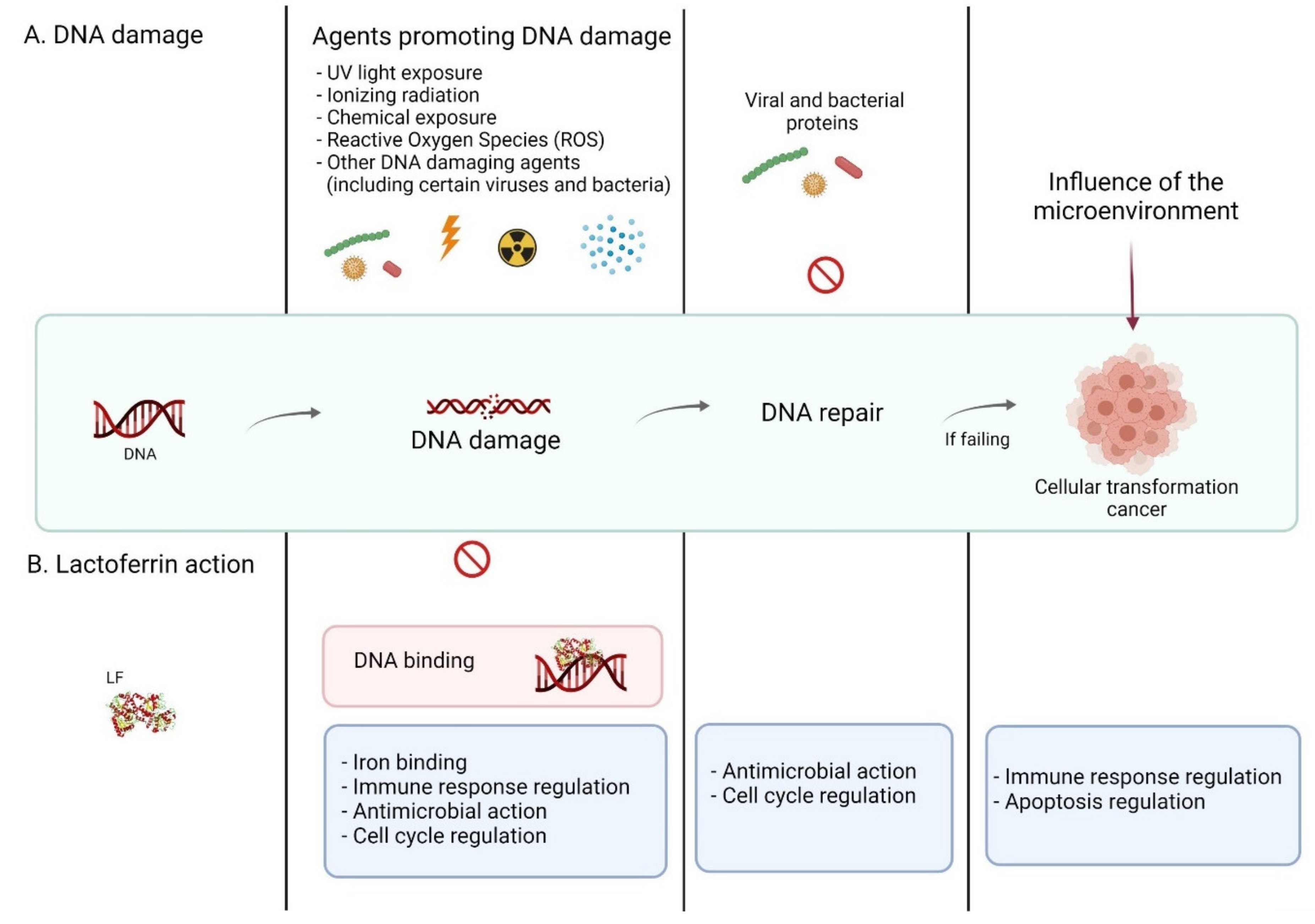
2. DNA Damage
2.1. DNA–Damaging Factors
2.1.1. Chemical Factors
2.1.2. Biological Factors
2.1.3. Physiological Conditions
2.2. Oxidative Stress and Repair Mechanisms
3. Lactoferrin
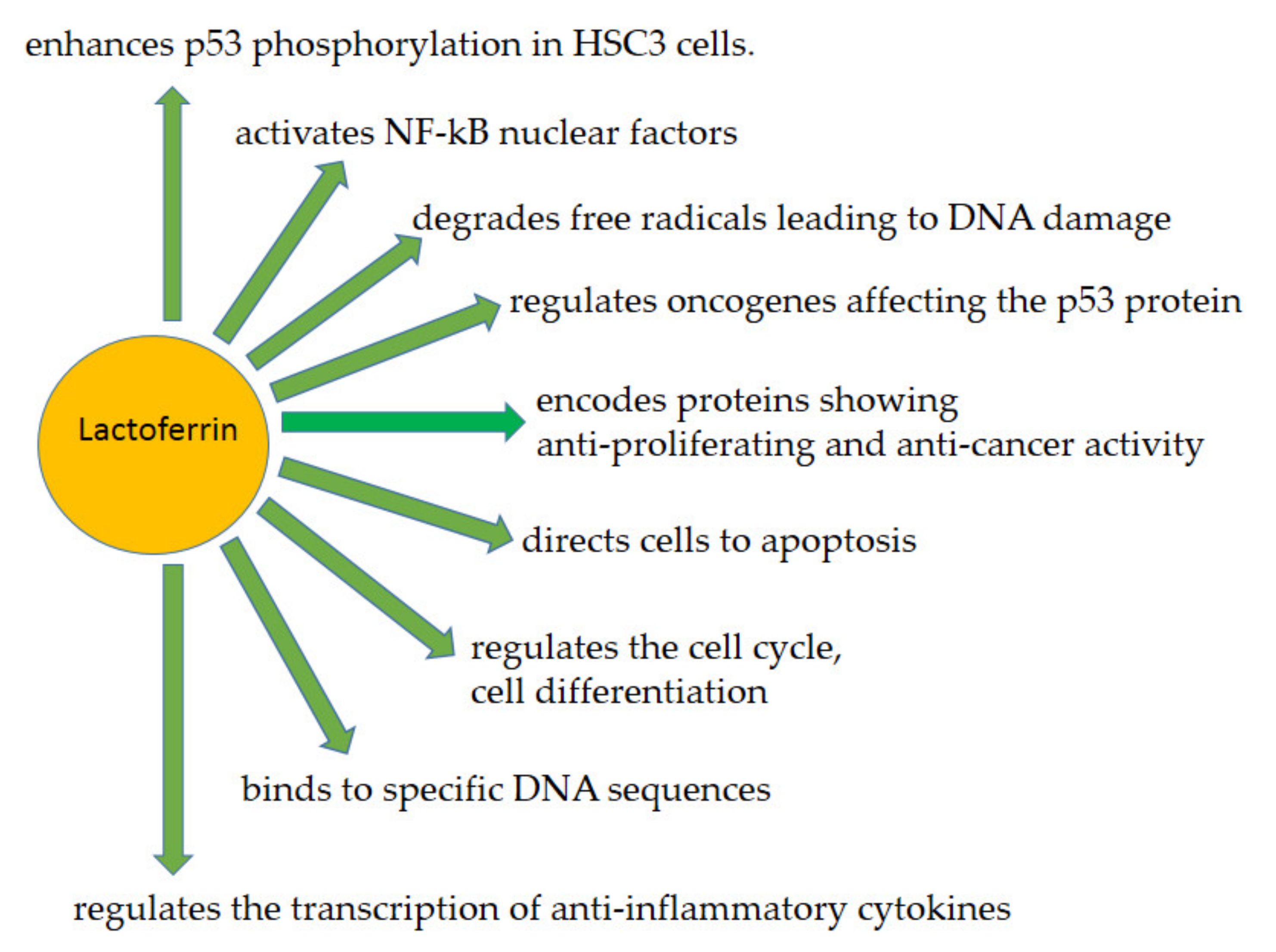
3.1. Lactoferrin as a Human Genome “Guardian” (Indirect and Direct Action)
3.2. Examples of LF (Indirect and Direct) Functions Providing Protection of the Human Genome—An Overview
3.2.1. LF as a Transcription Factor—A Similar to p53 Mode of Action
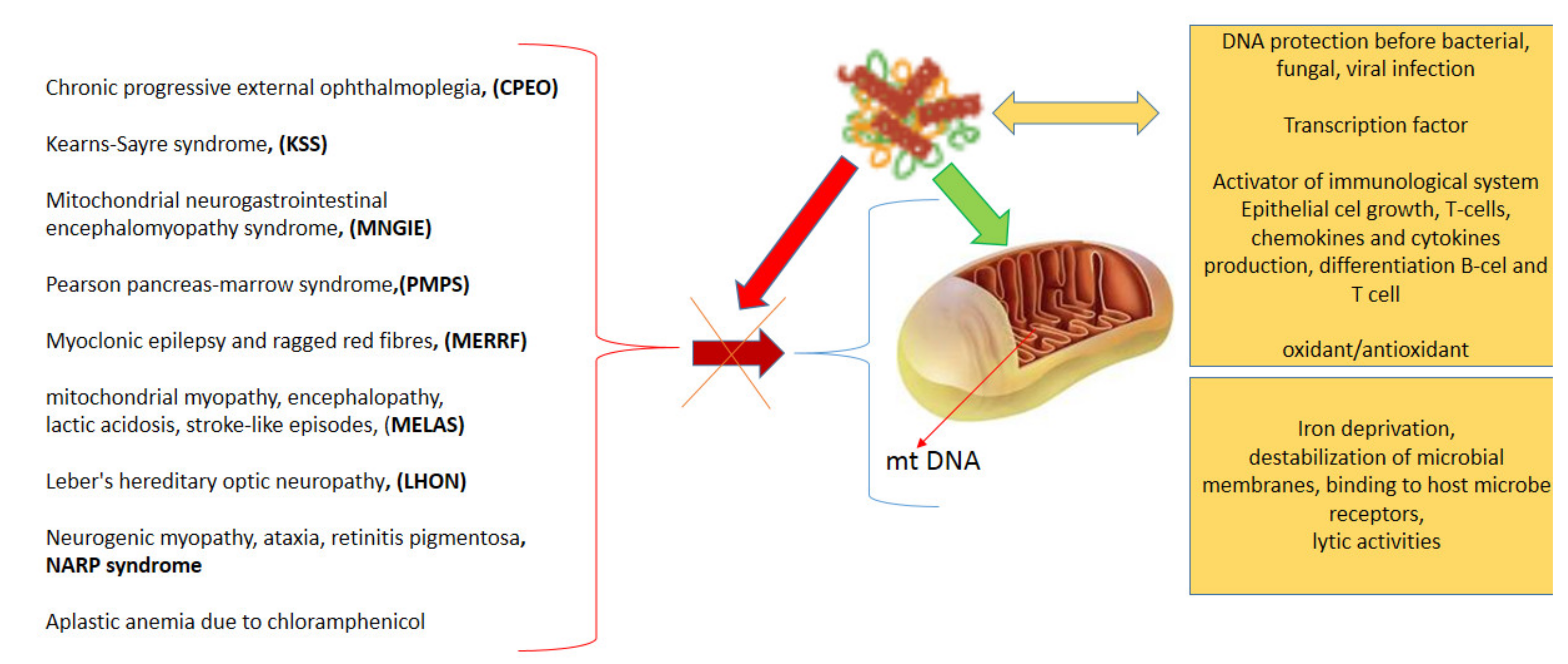
3.2.2. LF in ROS Ratio Control
3.2.3. Human Immune System Regulation
Decrease of Inflammation and Cell Damages—Immunosuppression
Immunostimulation
3.2.4. Antitumor Action of LF
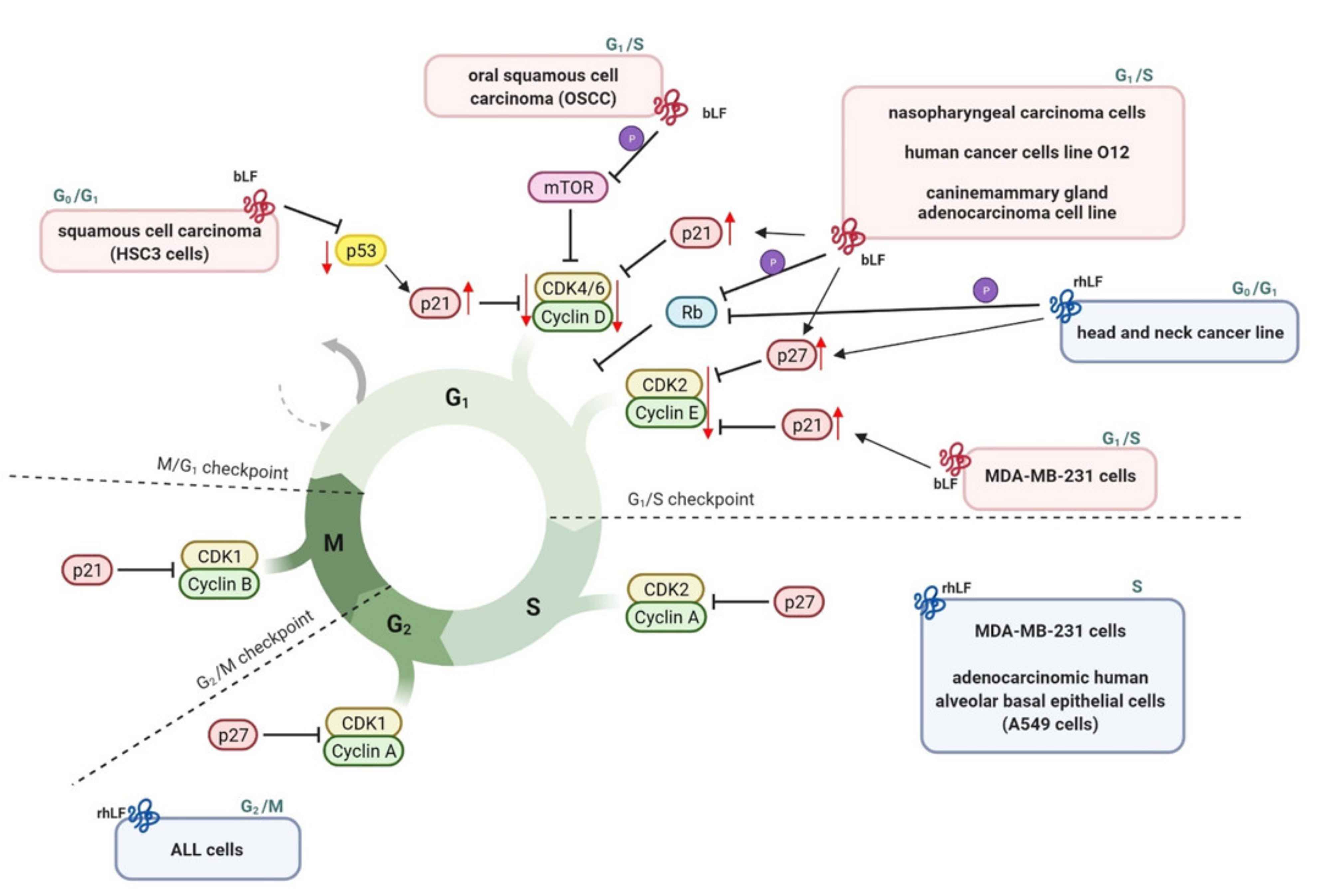
3.2.5. Cell Cycle Arrest
3.2.6. Apoptosis
3.2.7. Antimicrobial Action
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A review. Vet. Med.-Czech 2008, 53, 457–468. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin and its biological functions. Biochemistry 2001, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Mazurier, J. A critical review of the roles of host lactoferrin in immunity. Biometals 2010, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Mazurier, J.; Spik, G.; Kapp, J.A. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology 1995, 86, 122–127. [Google Scholar]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Babina, S.E.; Semenov, D.V.; Isaeva, N.; Vlassov, A.V.; Neustroev, K.N.; Kul’minskaya, A.A.; Buneva, V.N.; Nevinsky, G.A. Multiple enzymic activities of human milk lactoferrin. Eur. J. Biochem. 2003, 270, 3353–3361. [Google Scholar] [CrossRef]
- Babina, S.E.; Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin is the major deoxyribonuclease of human milk. Biochemistry 2004, 69, 1006–1015. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Semenov, D.V.; Buneva, V.N.; Nevinsky, G.A. Human milk lactoferrin binds two DNA molecules with different affinities. FEBS Lett. 1999, 451, 235–237. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Furmanski, P.; Li, Z.P.; Fortuna, M.B.; Swamy, C.V.; Das, M.R. Multiple molecular forms of human lactoferrin. Identification of a class of lactoferrins that possess ribonuclease activity and lack iron-binding capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol 2003, 98, 1309–1314. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martin, V.; Gonzalez, P.; Gomez-Grande, A.; Llamas-Velasco, S.; Herrero-San Martin, A.; Perez-Martinez, D.; Villarejo-Galende, A.; et al. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. EBioMedicine 2020, 57, 102834. [Google Scholar] [CrossRef]
- Narayanan, S.; Redfern, R.L.; Miller, W.L.; Nichols, K.K.; McDermott, A.M. Dry eye disease and microbial keratitis: Is there a connection? Ocul. Surf. 2013, 11, 75–92. [Google Scholar] [CrossRef]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef]
- Penco, S.; Scarfi, S.; Giovine, M.; Damonte, G.; Millo, E.; Villaggio, B.; Passalacqua, M.; Pozzolini, M.; Garre, C.; Benatti, U. Identification of an import signal for, and the nuclear localization of, human lactoferrin. Biotechnol. Appl. Biochem. 2001, 34, 151–159. [Google Scholar] [CrossRef]
- Ashida, K.; Sasaki, H.; Suzuki, Y.A.; Lonnerdal, B. Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals 2004, 17, 311–315. [Google Scholar] [CrossRef]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a pleiotropic protein in health and disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Jaskiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lonnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef]
- Dhennin-Duthille, I.; Masson, M.; Damiens, E.; Fillebeen, C.; Spik, G.; Mazurier, J. Lactoferrin upregulates the expression of CD4 antigen through the stimulation of the mitogen-activated protein kinase in the human lymphoblastic T Jurkat cell line. J. Cell. Biochem. 2000, 79, 583–593. [Google Scholar] [CrossRef]
- Esaguy, N.; Aguas, A.P.; Vilanova, M.; Silva, M.T. Activation of human neutrophils by phorbol ester decreases the cytoplasm compactness and the lactoferrin content of the granulocytes. J. Leukoc. Biol. 1991, 50, 444–452. [Google Scholar] [CrossRef]
- Haversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.A.; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell. Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar]
- He, J.; Furmanski, P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature 1995, 373, 721–724. [Google Scholar] [CrossRef]
- Huang, J.M.; Wang, Z.Y.; Ju, Z.H.; Wang, C.F.; Li, Q.L.; Sun, T.; Hou, Q.L.; Hang, S.Q.; Hou, M.H.; Zhong, J.F. Two splice variants of the bovine lactoferrin gene identified in Staphylococcus aureus isolated from mastitis in dairy cattle. Genet. Mol. Res. 2011, 10, 3199–3203. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Sabater, M.; Ricart, W.; Fernandez-Real, J.M. Proadipogenic effects of lactoferrin in human subcutaneous and visceral preadipocytes. J. Nutr. Biochem. 2011, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Ikoma-Seki, K.; Nakamura, K.; Morishita, S.; Ono, T.; Sugiyama, K.; Nishino, H.; Hirano, H.; Murakoshi, M. Role of LRP1 and ERK and cAMP signaling pathways in lactoferrin-induced lipolysis in mature rat adipocytes. PLoS ONE 2015, 10, e0141378. [Google Scholar] [CrossRef]
- Miriyala, S.; Panchatcharam, M.; Ramanujam, M.; Puvanakrishnan, R. A novel tetrapeptide derivative exhibits in vitro inhibition of neutrophil-derived reactive oxygen species and lysosomal enzymes release. Oxid. Med. Cell. Longev. 2013, 2013, 853210. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, H.; Zhu, N.; Xu, Z.; Wang, Y.; Qu, Y.; Wang, J. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J. Neurochem. 2020, 152, 397–415. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Radak, Z.; Bacsi, A.; Saavedra-Molina, A.; Boldogh, I. Lactoferrin decreases LPS-induced mitochondrial dysfunction in cultured cells and in animal endotoxemia model. Innate Immun. 2010, 16, 67–79. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Ciesla, J.M.; Komisarski, M.; Kusmierek, J.T.; Tudek, B. Long-chain adducts of trans-4-hydroxy-2-nonenal to DNA bases cause recombination, base substitutions and frameshift mutations in M13 phage. Mutat. Res. 2004, 550, 33–48. [Google Scholar] [CrossRef]
- Janowska, B.; Kurpios-Piec, D.; Prorok, P.; Szparecki, G.; Komisarski, M.; Kowalczyk, P.; Janion, C.; Tudek, B. Role of damage-specific DNA polymerases in M13 phage mutagenesis induced by a major lipid peroxidation product trans-4-hydroxy-2-nonenal. Mutat. Res. 2012, 729, 41–51. [Google Scholar] [CrossRef]
- Yu, C.; Dasmahapatra, G.; Dent, P.; Grant, S. Synergistic interactions between MEK1/2 and histone deacetylase inhibitors in BCR/ABL+ human leukemia cells. Leukemia 2016, 30, 1452. [Google Scholar] [CrossRef]
- Tudek, B.; Kowalczyk, P.; Ciesla, J.M. Localization of chloroacetaldehyde-induced DNA damage in human p53 gene by DNA polymerase fingerprint analysis. IARC Sci. Publ. 1999, 279–293. Available online: https://pubmed.ncbi.nlm.nih.gov/10626228 (accessed on 25 April 2022).
- Dylewska, M.; Kusmierek, J.T.; Pilzys, T.; Poznanski, J.; Maciejewska, A.M. 1,N(6)-alpha-hydroxypropanoadenine, the acrolein adduct to adenine, is a substrate for AlkB dioxygenase. Biochem. J. 2017, 474, 1837–1852. [Google Scholar] [CrossRef]
- Graczyk, M.; Dylewska, M. Iron supplementation in chronic kidney disease. Wiad. Lek. 2017, 70, 1215–1218. [Google Scholar]
- Tudek, B.; Zdzalik-Bielecka, D.; Tudek, A.; Kosicki, K.; Fabisiewicz, A.; Speina, E. Lipid peroxidation in face of DNA damage, DNA repair and other cellular processes. Free Radic. Biol. Med. 2017, 107, 77–89. [Google Scholar] [CrossRef]
- Cunningham, F.H.; Fiebelkorn, S.; Johnson, M.; Meredith, C. A novel application of the Margin of Exposure approach: Segregation of tobacco smoke toxicants. Food Chem. Toxicol. 2011, 49, 2921–2933. [Google Scholar] [CrossRef]
- Foulkes, W.D. Inherited susceptibility to common cancers. N. Engl. J. Med. 2008, 359, 2143–2153. [Google Scholar] [CrossRef]
- Benedetti, F.; Curreli, S.; Gallo, R.C.; Zella, D. Tampering of viruses and bacteria with host DNA repair: Implications for cellular transformation. Cancers 2021, 13, 241. [Google Scholar] [CrossRef]
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef]
- Hustedt, N.; Durocher, D. The control of DNA repair by the cell cycle. Nat. Cell Biol. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Ng, P.W.; Iha, H.; Iwanaga, Y.; Bittner, M.; Chen, Y.; Jiang, Y.; Gooden, G.; Trent, J.M.; Meltzer, P.; Jeang, K.T.; et al. Genome-wide expression changes induced by HTLV-1 Tax: Evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappaB activation. Oncogene 2001, 20, 4484–4496. [Google Scholar] [CrossRef]
- Wei, L.; Gravitt, P.E.; Song, H.; Maldonado, A.M.; Ozbun, M.A. Nitric oxide induces early viral transcription coincident with increased DNA damage and mutation rates in human papillomavirus-infected cells. Cancer Res. 2009, 69, 4878–4884. [Google Scholar] [CrossRef]
- Handa, O.; Naito, Y.; Yoshikawa, T. Redox biology and gastric carcinogenesis: The role of Helicobacter pylori. Redox Rep. 2011, 16, 1–7. [Google Scholar] [CrossRef]
- Leitao, E.; Costa, A.C.; Brito, C.; Costa, L.; Pombinho, R.; Cabanes, D.; Sousa, S. Listeria monocytogenes induces host DNA damage and delays the host cell cycle to promote infection. Cell Cycle 2014, 13, 928–940. [Google Scholar] [CrossRef]
- Nougayrede, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Cocchi, F.; Latinovic, O.S.; Curreli, S.; Krishnan, S.; Munawwar, A.; Gallo, R.C.; Zella, D. Role of mycoplasma chaperone DnaK in cellular transformation. Int. J. Mol. Sci. 2020, 21, 1311. [Google Scholar] [CrossRef] [PubMed]
- Jennison, A.V.; Verma, N.K. Shigella flexneri infection: Pathogenesis and vaccine development. FEMS Microbiol. Rev. 2004, 28, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Weyler, L.; Engelbrecht, M.; Mata Forsberg, M.; Brehwens, K.; Vare, D.; Vielfort, K.; Wojcik, A.; Aro, H. Restriction endonucleases from invasive Neisseria gonorrhoeae cause double-strand breaks and distort mitosis in epithelial cells during infection. PLoS ONE 2014, 9, e114208. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Scheblyakov, D.V.; Zubkova, O.V.; Shmarov, M.M.; Rakovskaya, I.V.; Gurova, K.V.; Tararova, N.D.; Burdelya, L.G.; Naroditsky, B.S.; Ginzburg, A.L.; et al. Mycoplasma infection suppresses p53, activates NF-kappaB and cooperates with oncogenic Ras in rodent fibroblast transformation. Oncogene 2008, 27, 4521–4531. [Google Scholar] [CrossRef]
- Frisan, T. Bacterial genotoxins: The long journey to the nucleus of mammalian cells. Biochim. Biophys. Acta 2016, 1858, 567–575. [Google Scholar] [CrossRef]
- Mathiasen, S.L.; Gall-Mas, L.; Pateras, I.S.; Theodorou, S.D.P.; Namini, M.R.J.; Hansen, M.B.; Martin, O.C.B.; Vadivel, C.K.; Ntostoglou, K.; Butter, D.; et al. Bacterial genotoxins induce T cell senescence. Cell Rep. 2021, 35, 109220. [Google Scholar] [CrossRef]
- Kikusato, M.; Yoshida, H.; Furukawa, K.; Toyomizu, M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 2015, 52, 8–13. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Osko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczynska, K. Mitochondrial oxidative stress-A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 3384. [Google Scholar] [CrossRef]
- Lodato, M.A.; Rodin, R.E.; Bohrson, C.L.; Coulter, M.E.; Barton, A.R.; Kwon, M.; Sherman, M.A.; Vitzthum, C.M.; Luquette, L.J.; Yandava, C.N.; et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 2018, 359, 555–559. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- Kowalczyk, P. Sequence-Dependent Induction of Exocyclic DNA Adducts By Trans-4-hydroxy-2-nonenal and Chloroacetaldehyde, Mutagenesis and Repair in Escherichia Coli Cells. Ph.D. Thesis, Institute of Biochemistry and Biophysics, Polish Academy Sciences, Warsaw, Poland, 2005. [Google Scholar]
- Gutteridge, J.M. Hydroxyl radicals, iron, oxidative stress, and neurodegeneration. Ann. N. Y. Acad. Sci 1994, 738, 201–213. [Google Scholar] [CrossRef]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes GT and AC substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Shah, A.; Gray, K.; Figg, N.; Finigan, A.; Starks, L.; Bennett, M. Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis. Circulation 2018, 138, 1446–1462. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Konieczka, P.; Barszcz, M.; Kowalczyk, P.; Szlis, M.; Jankowski, J. The potential of acetylsalicylic acid and vitamin E in modulating inflammatory cascades in chickens under lipopolysaccharide-induced inflammation. Vet. Res. 2019, 50, 65. [Google Scholar] [CrossRef]
- Mlyczynska, E.; Myszka, M.; Kurowska, P.; Dawid, M.; Milewicz, T.; Balajewicz-Nowak, M.; Kowalczyk, P.; Rak, A. Anti-apoptotic effect of apelin in human placenta: Studies on BeWo cells and villous explants from third-trimester human pregnancy. Int. J. Mol. Sci. 2021, 22, 2760. [Google Scholar] [CrossRef]
- Soboleva, S.E.; Sedykh, S.E.; Alinovskaya, L.I.; Buneva, V.N.; Nevinsky, G.A. Cow milk lactoferrin possesses several catalytic activities. Biomolecules 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, P.; Pazdrak, B.; Kruzel, M.L. A novel human recombinant lactoferrin inhibits lung adenocarcinoma cell growth and migration with no cytotoxic effect on normal human epithelial cells. Arch. Immunol. Ther. Exp. 2021, 69, 33. [Google Scholar] [CrossRef]
- Pierce, A.; Colavizza, D.; Benaissa, M.; Maes, P.; Tartar, A.; Montreuil, J.; Spik, G. Molecular cloning and sequence analysis of bovine lactotransferrin. Eur. J. Biochem. 1991, 196, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.J.; Flanagan, B.F.; Antonopoulos, A.; Haslam, S.M.; Dell, A.; Kimber, I.; Dearman, R.J. Differential immunogenicity and allergenicity of native and recombinant human lactoferrins: Role of glycosylation. Eur. J. Immunol. 2013, 43, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lonnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell Proteomics 2012, 11, M111 015248. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montoya, I.A.; Cendon, T.S.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Rascon-Cruz, Q.; Espinoza-Sanchez, E.A.; Siqueiros-Cendon, T.S.; Nakamura-Bencomo, S.I.; Arevalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Choi, B.K.; Actor, J.K.; Rios, S.; D’Anjou, M.; Stadheim, T.A.; Warburton, S.; Giaccone, E.; Cukan, M.; Li, H.; Kull, A.; et al. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: Effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconj. J. 2008, 25, 581–593. [Google Scholar] [CrossRef]
- Nandi, S.; Yalda, D.; Lu, S.; Nikolov, Z.; Misaki, R.; Fujiyama, K.; Huang, N. Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Res. 2005, 14, 237–249. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Gong, G.; Sun, X.; Zhang, R.; Du, Z.; Liu, Y.; Li, R.; Ding, F.; Tang, B.; et al. Cattle mammary bioreactor generated by a novel procedure of transgenic cloning for large-scale production of functional human lactoferrin. PLoS ONE 2008, 3, e3453. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Fan, B.; Dai, Y.; Wang, L.; Zheng, M.; Wang, M.; Niu, H.; Xi, F.; Li, N.; et al. Differential glycosylation of rhLf expressed in the mammary gland of transgenic mice. Anim. Biotechnol. 2006, 17, 13–20. [Google Scholar] [CrossRef]
- Ward, P.P.; Cunningham, G.A.; Conneely, O.M. Commercial production of lactoferrin, a multifunctional iron-binding glycoprotein. Biotechnol. Genet. Eng. Rev. 1997, 14, 303–319. [Google Scholar] [CrossRef]
- Garre, C.; Bianchi-Scarra, G.; Sirito, M.; Musso, M.; Ravazzolo, R. Lactoferrin binding sites and nuclear localization in K562(S) cells. J. Cell Physiol. 1992, 153, 477–482. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Schonne, E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 1969, 130, 643–658. [Google Scholar] [CrossRef]
- Siebert, P.D.; Huang, B.C. Identification of an alternative form of human lactoferrin mRNA that is expressed differentially in normal tissues and tumor-derived cell lines. Proc. Natl. Acad. Sci. USA 1997, 94, 2198–2203. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, Z.; Teng, C.T. An intronic alternative promoter of the human lactoferrin gene is activated by Ets. Biochem. Biophys. Res. Commun. 2003, 301, 472–479. [Google Scholar] [CrossRef]
- Escobar-Ramirez, A.; Vercoutter-Edouart, A.S.; Mortuaire, M.; Huvent, I.; Hardiville, S.; Hoedt, E.; Lefebvre, T.; Pierce, A. Modification by SUMOylation controls both the transcriptional activity and the stability of delta-lactoferrin. PLoS ONE 2015, 10, e0129965. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xu, C.L.; An, Z.H.; Liu, J.X.; Feng, J. Effect of dietary bovine lactoferrin on performance and antioxidant status of piglets. Anim. Feed Sci. Technol. 2008, 140, 326–336. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Imase, M.; Oda, H.; Wakabayashi, H.; Ishii, K. Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: A possible role in protection against oxidative DNA damage. Int. J. Mol. Sci 2014, 15, 1003–1013. [Google Scholar] [CrossRef]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.A.; Wilk, K.M.; Bangale, Y.A.; Kruzel, M.L.; Actor, J.K. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med. Microbiol. Immunol. 2007, 196, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Aspinall, R.; Prentice, A.M.; Ngom, P.T. Interleukin 7 from maternal milk crosses the intestinal barrier and modulates T-cell development in offspring. PLoS ONE 2011, 6, e20812. [Google Scholar] [CrossRef]
- Zimecki, M.; Mazurier, J.; Machnicki, M.; Wieczorek, Z.; Montreuil, J.; Spik, G. Immunostimulatory activity of lactotransferrin and maturation of CD4- CD8- murine thymocytes. Immunol. Lett. 1991, 30, 119–123. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-kappaB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodriguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Elass-Rochard, E.; Legrand, D.; Salmon, V.; Roseanu, A.; Trif, M.; Tobias, P.S.; Mazurier, J.; Spik, G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 1998, 66, 486–491. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, M.; Luo, C.C.; Wang, J.Q.; Zheng, N. Lactoferrin Exerts antitumor effects by inhibiting angiogenesis in a HT29 human colon tumor model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef]
- Pan, S.; Weng, H.; Hu, G.; Wang, S.; Zhao, T.; Yao, X.; Liao, L.; Zhu, X.; Ge, Y. Lactoferrin may inhibit the development of cancer via its immunostimulatory and immunomodulatory activities (Review). Int. J. Oncol. 2021, 59, 85. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Febriyanti Ayuningtyas, N.; Subarnbhesaj, A.; Nguyen, P.T.; Shrestha, M.; Haing, S.; Ohta, K.; Takata, T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PLoS ONE 2018, 13, e0191683. [Google Scholar] [CrossRef]
- Freiburghaus, C.; Janicke, B.; Lindmark-Mansson, H.; Oredsson, S.M.; Paulsson, M.A. Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci. 2009, 92, 2477–2484. [Google Scholar] [CrossRef]
- Goodell, J.R.; Ougolkov, A.V.; Hiasa, H.; Kaur, H.; Remmel, R.; Billadeau, D.D.; Ferguson, D.M. Acridine-based agents with topoisomerase II activity inhibit pancreatic cancer cell proliferation and induce apoptosis. J. Med. Chem. 2008, 51, 179–182. [Google Scholar] [CrossRef]
- Xiao, Y.; Monitto, C.L.; Minhas, K.M.; Sidransky, D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Cancer Res. 2004, 10, 8683–8686. [Google Scholar] [CrossRef]
- Duarte, D.C.; Nicolau, A.; Teixeira, J.A.; Rodrigues, L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011, 94, 66–76. [Google Scholar] [CrossRef]
- Furlong, S.J.; Mader, J.S.; Hoskin, D.W. Bovine lactoferricin induces caspase-independent apoptosis in human B-lymphoma cells and extends the survival of immune-deficient mice bearing B-lymphoma xenografts. Exp. Mol. Pathol. 2010, 88, 371–375. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 2015, 15, 425. [Google Scholar] [CrossRef]
- Guedes, J.P.; Pereira, C.S.; Rodrigues, L.R.; Corte-Real, M. Bovine milk lactoferrin selectively kills highly metastatic prostate cancer PC-3 and osteosarcoma MG-63 cells in vitro. Front. Oncol. 2018, 8, 200. [Google Scholar] [CrossRef]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer Ther. 2005, 4, 612–624. [Google Scholar] [CrossRef]
- Pan, W.R.; Chen, P.W.; Chen, Y.L.S.; Hsu, H.C.; Lin, C.C.; Chen, W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef]
- Pereira, C.S.; Guedes, J.P.; Goncalves, M.; Loureiro, L.; Castro, L.; Geros, H.; Rodrigues, L.R.; Corte-Real, M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget 2016, 7, 62144–62158. [Google Scholar] [CrossRef]
- Damiens, E.; Mazurier, J.; el Yazidi, I.; Masson, M.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochim. Biophys. Acta 1998, 1402, 277–287. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Andres, M.T.; Fierro, J.F. Antimicrobial mechanism of action of transferrins: Selective inhibition of H+-ATPase. Antimicrob. Agents Chemother. 2010, 54, 4335–4342. [Google Scholar] [CrossRef]
- Miki, T.; Hardt, W.D. Outer membrane permeabilization is an essential step in the killing of gram-negative bacteria by the lectin RegIIIbeta. PLoS ONE 2013, 8, e69901. [Google Scholar] [CrossRef]
- Luna-Castro, S.; Aguilar-Romero, F.; Samaniego-Barron, L.; Godinez-Vargas, D.; de la Garza, M. Effect of bovine apo-lactoferrin on the growth and virulence of Actinobacillus pleuropneumoniae. Biometals 2014, 27, 891–903. [Google Scholar] [CrossRef]
- Angulo-Zamudio, U.A.; Vidal, J.E.; Nazmi, K.; Bolscher, J.G.M.; Leon-Sicairos, C.; Antezana, B.S.; Canizalez-Roman, A.; Leon-Sicairos, N. Lactoferrin disaggregates pneumococcal biofilms and inhibits acquisition of resistance through its DNase activity. Front. Microbiol. 2019, 10, 2386. [Google Scholar] [CrossRef]
- Berlutti, F.; Ajello, M.; Bosso, P.; Morea, C.; Andrea, P.; Giovanni, A.; Piera, V. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals 2004, 17, 271–278. [Google Scholar]
- Ciccaglione, A.F.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Cellini, L.; Marzio, L. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: An in vitro and in vivo study. J. Antimicrob. Chemother. 2019, 74, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Beljaars, L.; Bakker, H.I.; van der Strate, B.W.; Smit, C.; Duijvestijn, A.M.; Meijer, D.K.; Molema, G. The antiviral protein human lactoferrin is distributed in the body to cytomegalovirus (CMV) infection-prone cells and tissues. Pharm Res. 2002, 19, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin against SARS-CoV-2: In vitro and in silico evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.C.; Swart, P.J.; de Bethune, M.P.; Pauwels, R.; De Clercq, E.; The, T.H.; Meijer, D.K. Antiviral effects of plasma and milk proteins: Lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J. Infect. Dis. 1995, 172, 380–388. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Ye, Q.; Zhou, Y.; Xiong, W.; He, W.; Deng, M.; Zhou, M.; Guo, X.; Chen, P.; et al. Inhibition of Epstein-Barr virus infection by lactoferrin. J. Innate Immun. 2012, 4, 387–398. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. C. R. Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Frontera, L.S.; Moyano, S.; Quassollo, G.; Lanfredi-Rangel, A.; Ropolo, A.S.; Touz, M.C. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci. Rep. 2018, 8, 18020. [Google Scholar] [CrossRef]
- Diaz-Godinez, C.; Gonzalez-Galindo, X.; Meza-Menchaca, T.; Bobes, R.J.; de la Garza, M.; Leon-Sicairos, N.; Laclette, J.P.; Carrero, J.C. Synthetic bovine lactoferrin peptide Lfampin kills Entamoeba histolytica trophozoites by necrosis and resolves amoebic intracecal infection in mice. Bioscience Rep. 2019, 39, BSR20180850. [Google Scholar] [CrossRef]
- Lima, M.F.; Kierszenbaum, F. Lactoferrin effects on phagocytic cell-function.1. increased uptake and killing of an intracellular parasite by murine macrophages and human-monocytes. J. Immunol. 1985, 134, 4176–4183. [Google Scholar]
- Anand, N.; Kanwar, R.K.; Sehgal, R.; Kanwar, J.R. Antiparasitic and immunomodulatory potential of oral nanocapsules encapsulated lactoferrin protein against Plasmodium berghei. Nanomedicine 2016, 11, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Diaz, H.; Canizalez-Roman, A.; Nepomuceno-Mejia, T.; Gallardo-Vera, F.; Hornelas-Orozco, Y.; Nazmi, K.; Bolscher, J.G.M.; Carrero, J.C.; Leon-Sicairos, C.; Leon-Sicairos, N. Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 2017, 95, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, C.; Andres, M.T.; Chaves, S.R.; Fierro, J.F.; Geros, H.; Manon, S.; Rodrigues, L.R.; Corte-Real, M. Lactoferrin perturbs lipid rafts and requires integrity of Pma1p-lipid rafts association to exert its antifungal activity against Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2021, 171, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.T.; Acosta-Zaldivar, M.; Fierro, J.F. Antifungal mechanism of action of lactoferrin: Identification of H+-ATPase (P3A-type) as a new apoptotic-cell membrane receptor. Antimicrob. Agents Chemother. 2016, 60, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.T.; Viejo-Diaz, M.; Fierro, J.F. Human lactoferrin induces apoptosis-like cell death in Candida albicans: Critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008, 52, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, J.; Qin, L.; Guo, D.; Ding, H.; Deng, D. Radioprotective effect of lactoferrin in mice exposed to sublethal X-ray irradiation. Exp. Ther. Med. 2018, 16, 3143–3148. [Google Scholar] [CrossRef]
- Freiburghaus, C.; Lindmark-Mansson, H.; Paulsson, M.; Oredsson, S. Reduction of ultraviolet light-induced DNA damage in human colon cancer cells treated with a lactoferrin-derived peptide. J. Dairy Sci. 2012, 95, 5552–5560. [Google Scholar] [CrossRef]
- Guschina, T.A.; Soboleva, S.E.; Nevinsky, G.A. Recognition of specific and nonspecific DNA by human lactoferrin. J. Mol. Recognit. 2013, 26, 136–148. [Google Scholar] [CrossRef]
- Mariller, C.; Hardiville, S.; Hoedt, E.; Benaissa, M.; Mazurier, J.; Pierce, A. Proteomic approach to the identification of novel delta-lactoferrin target genes: Characterization of DcpS, an mRNA scavenger decapping enzyme. Biochimie 2009, 91, 109–122. [Google Scholar] [CrossRef]
- Oh, S.M.; Pyo, C.W.; Kim, Y.; Choi, S.Y. Neutrophil lactoferrin upregulates the human p53 gene through induction of NF-kappaB activation cascade. Oncogene 2004, 23, 8282–8291. [Google Scholar] [CrossRef]
- Pirkhezranian, Z.; Tahmoorespur, M.; Daura, X.; Monhemi, H.; Sekhavati, M.H. Interaction of camel Lactoferrin derived peptides with DNA: A molecular dynamics study. BMC Genom. 2020, 21, 60. [Google Scholar] [CrossRef]
- Xu, X.; Iwasa, H.; Hossain, S.; Sarkar, A.; Maruyama, J.; Arimoto-Matsuzaki, K.; Hata, Y. BCL-XL binds and antagonizes RASSF6 tumor suppressor to suppress p53 expression. Genes Cells 2017, 22, 993–1003. [Google Scholar] [CrossRef]
- Karade, P.G.; Jadhav, N.R. In vitro studies of the anticancer action of Tectaria cicutaria in human cancer cell lines: G0/G1 p53-associated cell cycle arrest-Part I. J Tradit. Complement. Med. 2018, 8, 459–464. [Google Scholar] [CrossRef]
- Leveillard, T.; Andera, L.; Bissonnette, N.; Schaeffer, L.; Bracco, L.; Egly, J.M.; Wasylyk, B. Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J. 1996, 15, 1615–1624. [Google Scholar] [CrossRef]
- Mariller, C.; Benaissa, M.; Hardiville, S.; Breton, M.; Pradelle, G.; Mazurier, J.; Pierce, A. Human delta-lactoferrin is a transcription factor that enhances Skp1 (S-phase kinase-associated protein) gene expression. FEBS J. 2007, 274, 2038–2053. [Google Scholar] [CrossRef]
- Hardiville, S.; Escobar-Ramirez, A.; Pina-Canceco, S.; Elass, E.; Pierce, A. Delta-lactoferrin induces cell death via the mitochondrial death signaling pathway by upregulating bax expression. Biometals 2014, 27, 875–889. [Google Scholar] [CrossRef]
- Hoedt, E.; Chaoui, K.; Huvent, I.; Mariller, C.; Monsarrat, B.; Burlet-Schiltz, O.; Pierce, A. SILAC-based proteomic profiling of the human MDA-MB-231 metastatic breast cancer cell line in response to the two antitumoral lactoferrin isoforms: The secreted lactoferrin and the intracellular delta-lactoferrin. PLoS ONE 2014, 9, e104563. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046–1060. [Google Scholar] [CrossRef]
- Malone, A.; Clark, R.F.; Hoskin, D.W.; Power Coombs, M.R. Regulation of macrophage-associated inflammatory responses by species-specific lactoferricin peptides. Front. Biosci. 2022, 27, 43. [Google Scholar] [CrossRef]
- Zimecki, M.; Artym, J.; Kocieba, M.; Duk, M.; Kruzel, M.L. The effect of carbohydrate moiety structure on the immunoregulatory activity of lactoferrin in vitro. Cell. Mol. Biol. Lett. 2014, 19, 284–296. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.A.; Olsen, M.; Zimecki, M.; Hunter, R.L., Jr.; Kruzel, M.L. Lactoferrin immunomodulation of DTH response in mice. Int. Immunopharmacol. 2002, 2, 475–486. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Siqueiros-Cendon, T.; Arevalo-Gallegos, S.; Iglesias-Figueroa, B.F.; Garcia-Montoya, I.A.; Salazar-Martinez, J.; Rascon-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M. Lactoferrin and immunologic dissonance: Clinical implications. Arch. Immunol. Ther. Exp. 2002, 50, 399–410. [Google Scholar]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008, 22, 2747–2757. [Google Scholar] [CrossRef]
- Raoof, N.A.; Adamkin, D.H.; Radmacher, P.G.; Telang, S. Comparison of lactoferrin activity in fresh and stored human milk. J. Perinatol. 2016, 36, 207–209. [Google Scholar] [CrossRef]
- Sfeir, R.M.; Dubarry, M.; Boyaka, P.N.; Rautureau, M.; Tome, D. The mode of oral bovine lactoferrin administration influences mucosal and systemic immune responses in mice. J. Nutr. 2004, 134, 403–409. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tome, D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem. Cell. Biol. 2006, 84, 303–311. [Google Scholar] [CrossRef]
- Janusz, M.; Lisowski, J. Proline-rich polypeptide (PRP)–An immunomodulatory peptide from ovine colostrum. Arch. Immunol. Ther. Exp. 1993, 41, 275–279. [Google Scholar]
- Eliassen, L.T.; Berge, G.; Sveinbjornsson, B.; Svendsen, J.S.; Vorland, L.H.; Rekdal, O. Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer Res. 2002, 22, 2703–2710. [Google Scholar]
- Tung, Y.T.; Chen, H.L.; Yen, C.C.; Lee, P.Y.; Tsai, H.C.; Lin, M.F.; Chen, C.M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rico, G.; Drago-Serrano, M.E.; Leon-Sicairos, N.; de la Garza, M. Lactoferrin: A nutraceutical with activity against Colorectal cancer. Front. Pharmacol. 2022, 13, 855852. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Ng, Y.Y.; Tarleton, K.; Tran, A.; Tran, T.; Xue, W.Y.; Youssef, P.; Yuan, P.; Zhang, D.; et al. Milk-derived proteins and peptides in head and neck carcinoma treatment. Biomolecules 2022, 12, 290. [Google Scholar] [CrossRef]
- Nakamura-Bencomo, S.; Gutierrez, D.A.; Robles-Escajeda, E.; Iglesias-Figueroa, B.; Siqueiros-Cendon, T.S.; Espinoza-Sanchez, E.A.; Arevalo-Gallegos, S.; Aguilera, R.J.; Rascon-Cruz, Q.; Varela-Ramirez, A. Recombinant human lactoferrin carrying humanized glycosylation exhibits antileukemia selective cytotoxicity, microfilament disruption, cell cycle arrest, and apoptosis activities. Investig. New Drugs 2021, 39, 400–415. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendon, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sanchez, E.A.; Arevalo-Gallegos, S.; Varela-Ramirez, A.; Rascon-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef]
- Lee, S.H.; Pyo, C.W.; Hahm, D.H.; Kim, J.; Choi, S.Y. Iron-saturated lactoferrin stimulates cell cycle progression through PI3K/Akt pathway. Mol. Cells 2009, 28, 37–42. [Google Scholar] [CrossRef]
- Baumrucker, C.R.; Gibson, C.A.; Schanbacher, F.L. Bovine lactoferrin binds to insulin-like growth factor-binding protein. Domest. Anim. Endocrinol. 2003, 24, 287–303. [Google Scholar] [CrossRef]
- Zhang, J.L.; Han, X.; Shan, Y.J.; Zhang, L.W.; Du, M.; Liu, M.; Yi, H.X.; Ma, Y. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J. Dairy Sci. 2018, 101, 1827–1833. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Scotti, M.J.; Conte, A.L.; Marazzato, M.; Zagaglia, C.; Longhi, C.; Berlutti, F.; Musci, G.; et al. Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. Int. J. Mol. Sci. 2019, 20, 5666. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Alexander, J.; Julian, L.; Roberts, M.R.; Peter, W. Apoptosis: Programmed Cell Death Eliminates Unwanted Cells. In Molecular Biology of the Cell, 5th ed.; Garland Science: New York, NY USA, 2008; p. 1115. [Google Scholar]
- Green, D.R. The Death Receptor Pathway of Apoptosis. Cold Spring Harb. Perspect. Biol. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Chen, E.Y.; Wei, S.C.; Lin, F.; Lin, Q.M.; Lan, X.H.; Xue, Y.; Wu, M. Lactoferrin inhibits apoptosis through insulin-like growth factor I in primary rat osteoblasts. Acta Pharmacol. Sin. 2014, 35, 523–530. [Google Scholar] [CrossRef]
- Kanwar, R.K.; Kanwar, J.R. Immunomodulatory lactoferrin in the regulation of apoptosis modulatory proteins in cancer. Protein Pept. Lett. 2013, 20, 450–458. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Ou, Y.; Han, Z.; Li, K.; Wang, P.; Zhou, S. Inhibition of tumor growth by recombinant adenovirus containing human lactoferrin through inducing tumor cell apoptosis in mice bearing EMT6 breast cancer. Arch. Pharm. Res. 2011, 34, 987–995. [Google Scholar] [CrossRef]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis 2004, 25, 1961–1966. [Google Scholar] [CrossRef]
- Li, D.; Sakashita, S.; Morishita, Y.; Kano, J.; Shiba, A.; Sato, T.; Noguchi, M. Binding of lactoferrin to IGBP1 triggers apoptosis in a lung adenocarcinoma cell line. Anticancer Res. 2011, 31, 529–534. [Google Scholar]
- Liu, J.L.; Fan, Y.G.; Yang, Z.S.; Wang, Z.Y.; Guo, C. Iron and Alzheimers Disease: From Pathogenesis to Therapeutic Implications. Front. Neurosci. 2018, 12, 632. [Google Scholar] [CrossRef]
- Van de Looij, Y.; Ginet, V.; Chatagner, A.; Toulotte, A.; Somm, E.; Huppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation protects the immature hypoxic-ischemic rat brain. Ann. Clin. Transl. Neurol. 2014, 1, 955–967. [Google Scholar] [CrossRef]
- Santos-Pereira, C.; Rodrigues, L.R.; Corte-Real, M. Plasmalemmal V-ATPase as a potential biomarker for lactoferrin-based anticancer therapy. Biomolecules 2022, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, C.; Andres, M.T.; Fierro, J.F.; Rodrigues, L.R.; Corte-Real, M. A review on lactoferrin as a proton pump inhibitor. Int. J. Biol. Macromol. 2022, 202, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W.; Alitheen, N.B.; Long, K.; Yeap, S.K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep. 2019, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Reisie, B.H.; Farhad, S.Z.; Sadeh, S.A. Evaluation and comparison number of gingival fibroblast and osteosarcoma cell (MG-63 cell line) adhesive to mocugraft, alloderm, and collagen membrane with or without advanced platelet-rich fibrin. Dent. Res. J. 2021, 18, 82. [Google Scholar]
- Sakai, T.; Banno, Y.; Kato, Y.; Nozawa, Y.; Kawaguchi, M. Pepsin-digested bovine lactoferrin induces apoptotic cell death with JNK/SAPK activation in oral cancer cells. J. Pharmacol. Sci. 2005, 98, 41–48. [Google Scholar] [CrossRef]
- Wang, S.S.; Tu, J.C.; Zhou, C.J.; Li, J.W.; Huang, L.; Tao, L.; Zhao, L. The effect of Lfcin-B on non-small cell lung cancer H460 cells is mediated by inhibiting VEGF expression and inducing apoptosis. Arch. Pharmacal Res. 2015, 38, 261–271. [Google Scholar] [CrossRef]
- Furlong, S.J.; Ridgway, N.D.; Hoskin, D.W. Modulation of ceramide metabolism in T-leukemia cell lines potentiates apoptosis induced by the cationic antimicrobial peptide bovine lactoferricin. Int. J. Oncol. 2008, 32, 537–544. [Google Scholar] [CrossRef][Green Version]
- Andres, M.T.; Acosta-Zaldivar, M.; Gonzalez-Seisdedos, J.; Fierro, J.F. Cytosolic acidification is the first transduction signal of lactoferrin-induced regulated cell death pathway. Int. J. Mol. Sci. 2019, 20, 5838. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef]
- Acosta-Zaldivar, M.; Andres, M.T.; Rego, A.; Pereira, C.S.; Fierro, J.F.; Corte-Real, M. Human lactoferrin triggers a mitochondrial- and caspase-dependent regulated cell death in Saccharomyces cerevisiae. Apoptosis 2016, 21, 163–173. [Google Scholar] [CrossRef]
- Francis, N.; Wong, S.H.; Hampson, P.; Wang, K.; Young, S.P.; Deigner, H.P.; Salmon, M.; Scheel-Toellner, D.; Lord, J.M. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochim. Biophys. Acta 2011, 1813, 1822–1826. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodriguez, R.; Godinez-Victoria, M.; Drago-Serrano, M.E. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective effects of lactoferrin against SARS-CoV-2 infection in vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Wang, H.; Luo, Y.; Wan, L.; Jiang, M.; Chu, Y. Lactoferrin for the treatment of COVID-19 (Review). Exp. Ther. Med. 2020, 20, 272. [Google Scholar] [CrossRef]
- Lai, X.; Yu, Y.; Xian, W.; Ye, F.; Ju, X.; Luo, Y.; Dong, H.; Zhou, Y.H.; Tan, W.; Zhuang, H.; et al. Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. iScience 2022, 25, 104136. [Google Scholar] [CrossRef]


| Form of DNA Protection | LF Properties | Mechanism | Ref. |
|---|---|---|---|
| Indirect | Iron saturation | - antioxidant activity (iron chelation) - reduction of oxidative damage (modulation of iron level) - inhibition of bacteria growth | [2,90,91] [6,31,84] [84] |
| Immune modulation | - inhibition of pro-inflammatory cytokines production; - stimulation of anti-inflammatory cytokines production; - stimulation of T and B cells maturation; - LPS binding | [24,92,93,94,95] [25,93,96], [4,22,97,98] [33,99,100,101], | |
| Antitumor | - inhibition of angiogenesis; - iron binding (necessary for cell growth); - inhibition of cell proliferation; - apoptosis induction; - stimulation of lymphocytes, leukocytes and NK activity; - increasing of surface receptors expression on neoplastic cells (identify by the immune system); | [74,102] [79] [103,104,105,106,107] [108,109,110,111,112,113,114] [103,115] [116] | |
| Antimicrobial | Antibacterial: - destruction of bacterial cells (due to: cell wall damage, LPS release or pore proteins binding); - inhibition of bacteria adhesion to the host cells (by degrading bacterial adhesins); - inhibition of biofilm formation; - iron binding (necessary for the growth of bacteria); - increasing the activity of the immune system; - increasing the sensitivity of bacteria to antibiotics; Antiviral - inhibition of host cell infection (binding to viral surface proteins or blocking receptors on host cells); - inhibition of viral replication; - stimulation of T lymphocytes to increase the antiviral activity of NK cells; Antiparasitic - cell destruction (the cell membrane damage, generation of free radicals); - stimulation of phagocytes activity; - increasing the sensitivity of pathogens to drugs; Antifungal - direct cytotoxic effect; - increasing the sensitivity of pathogens to drugs; - stimulation of leukocyte activity; | [9,117,118] [119] [120] [121] [79,93] [122] [123,124,125], [126] [125,127] [79,128] [129,130] [131,132] [133] [134] [135] [136,137] | |
| Direct | DNA binding | Gene expression modulation - DNA repair - Cell cycle regulation - Apoptosis regulation - Immune response modulation: ✓ the cytotoxic functions of NK and lymphokine-activated killer cells enhancement; ✓ cytokine synthesis modulation LF degradation by free radicals | [26,138,139,140,141,142,143] [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowska-Ośko, I.; Sulejczak, D.; Kaczyńska, K.; Kleczkowska, P.; Kramkowski, K.; Popiel, M.; Wietrak, E.; Kowalczyk, P. Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. Int. J. Mol. Sci. 2022, 23, 5248. https://doi.org/10.3390/ijms23095248
Bukowska-Ośko I, Sulejczak D, Kaczyńska K, Kleczkowska P, Kramkowski K, Popiel M, Wietrak E, Kowalczyk P. Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. International Journal of Molecular Sciences. 2022; 23(9):5248. https://doi.org/10.3390/ijms23095248
Chicago/Turabian StyleBukowska-Ośko, Iwona, Dorota Sulejczak, Katarzyna Kaczyńska, Patrycja Kleczkowska, Karol Kramkowski, Marta Popiel, Ewa Wietrak, and Paweł Kowalczyk. 2022. "Lactoferrin as a Human Genome “Guardian”—An Overall Point of View" International Journal of Molecular Sciences 23, no. 9: 5248. https://doi.org/10.3390/ijms23095248
APA StyleBukowska-Ośko, I., Sulejczak, D., Kaczyńska, K., Kleczkowska, P., Kramkowski, K., Popiel, M., Wietrak, E., & Kowalczyk, P. (2022). Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. International Journal of Molecular Sciences, 23(9), 5248. https://doi.org/10.3390/ijms23095248








