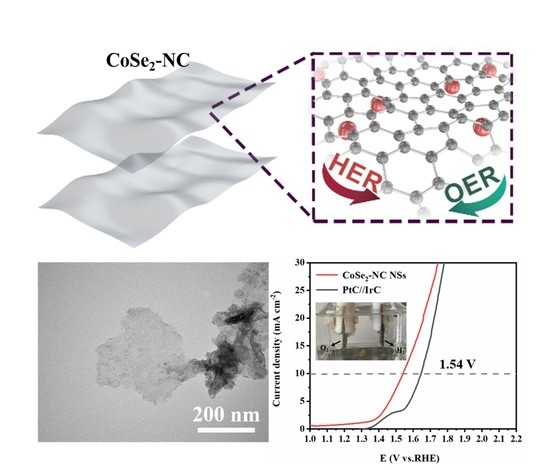
Salt-Templated Nanoarchitectonics of CoSe2-NC Nanosheets as an Efficient Bifunctional Oxygen Electrocatalyst for Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
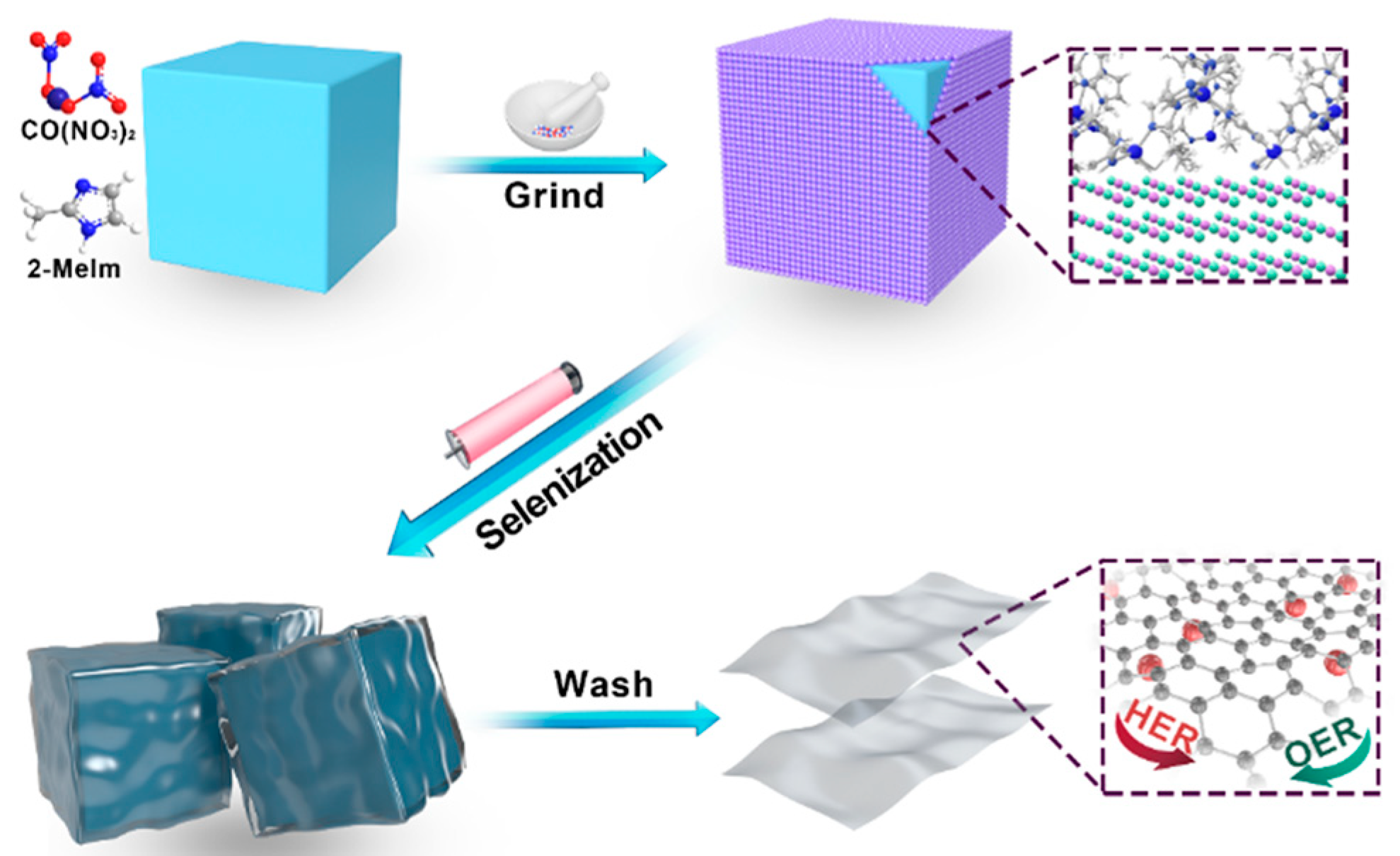
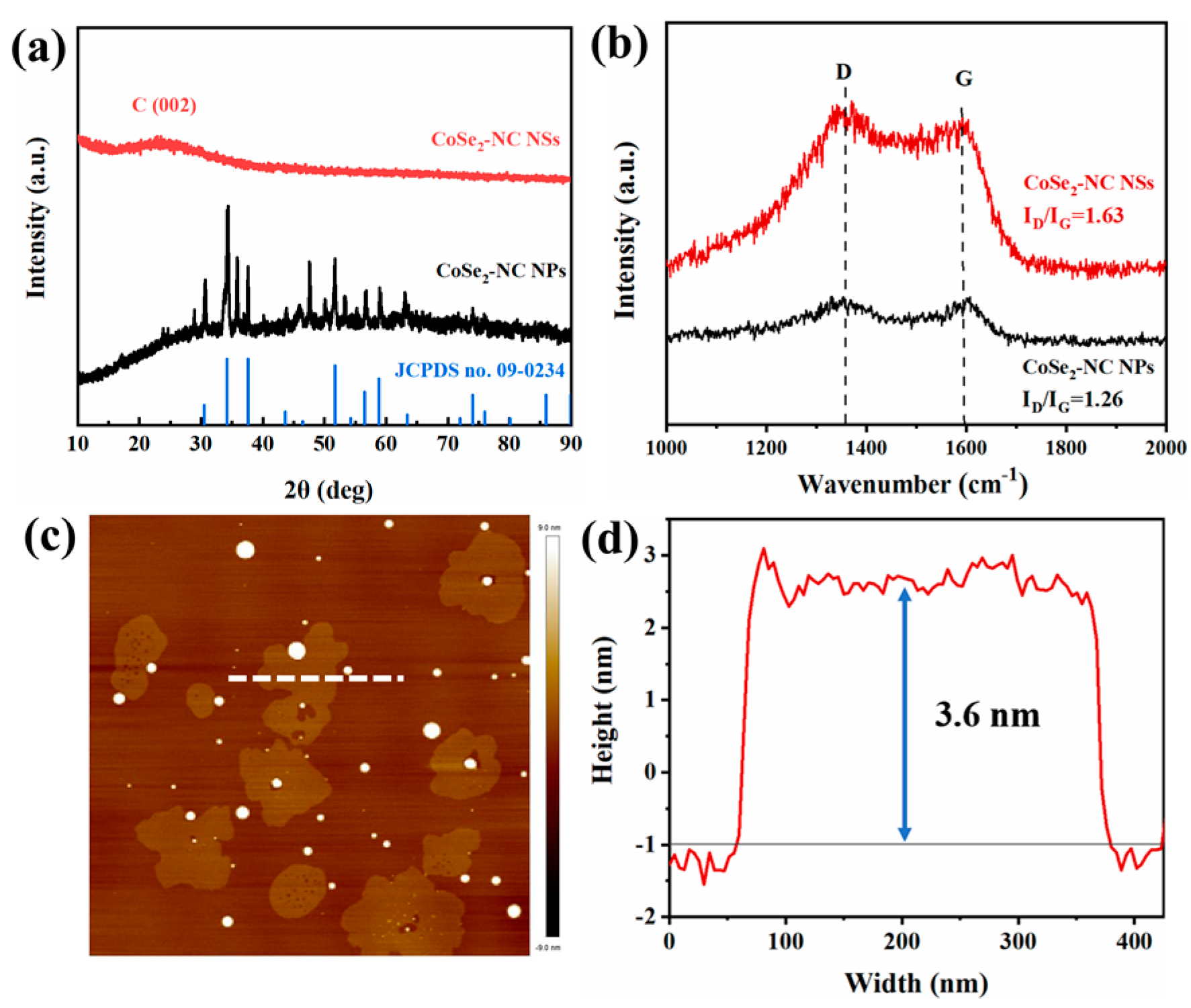
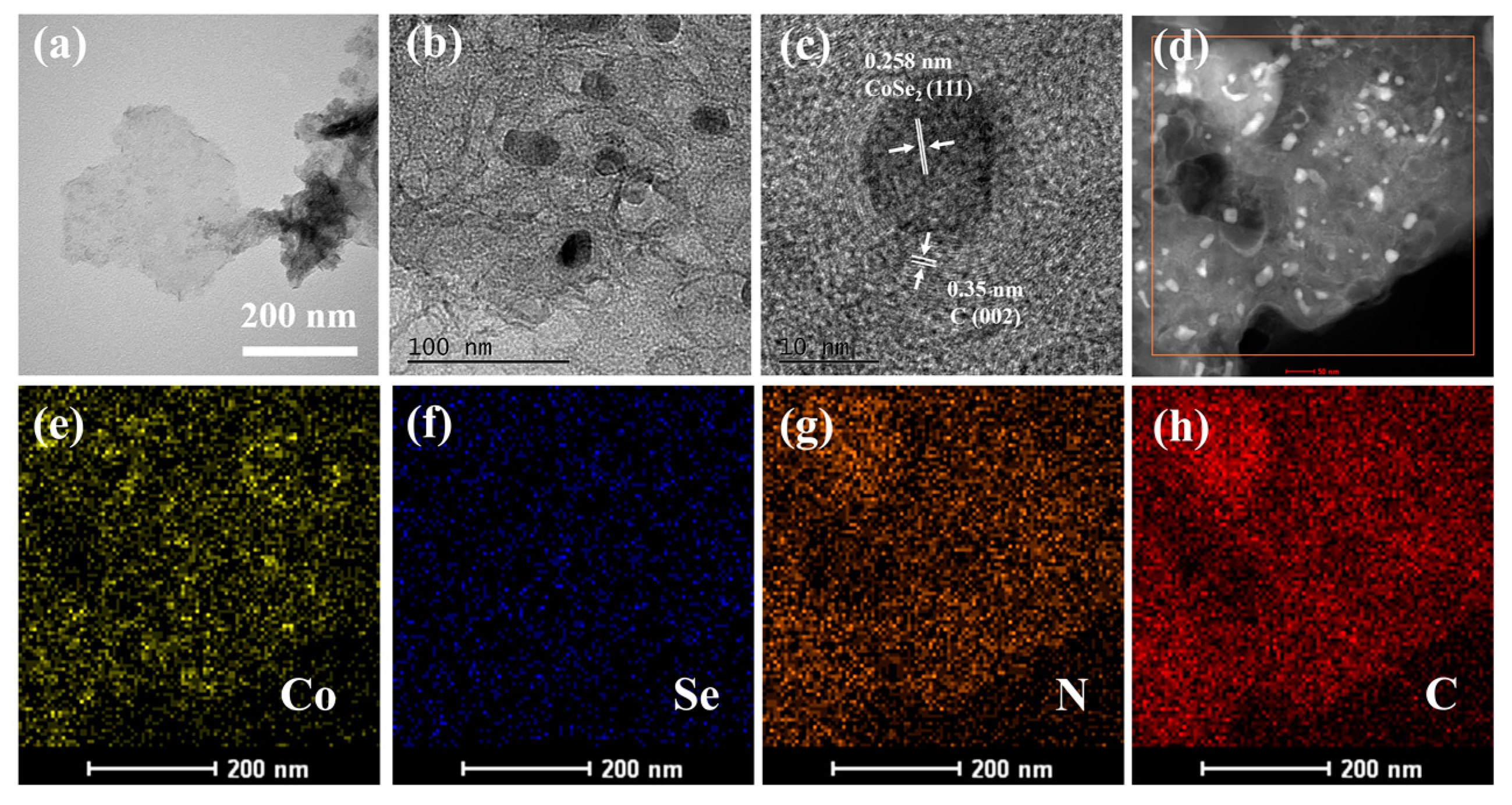
2.1. Characterization of CoSe2-NC NSs
2.2. Electronic States of CoSe2-NC NSs
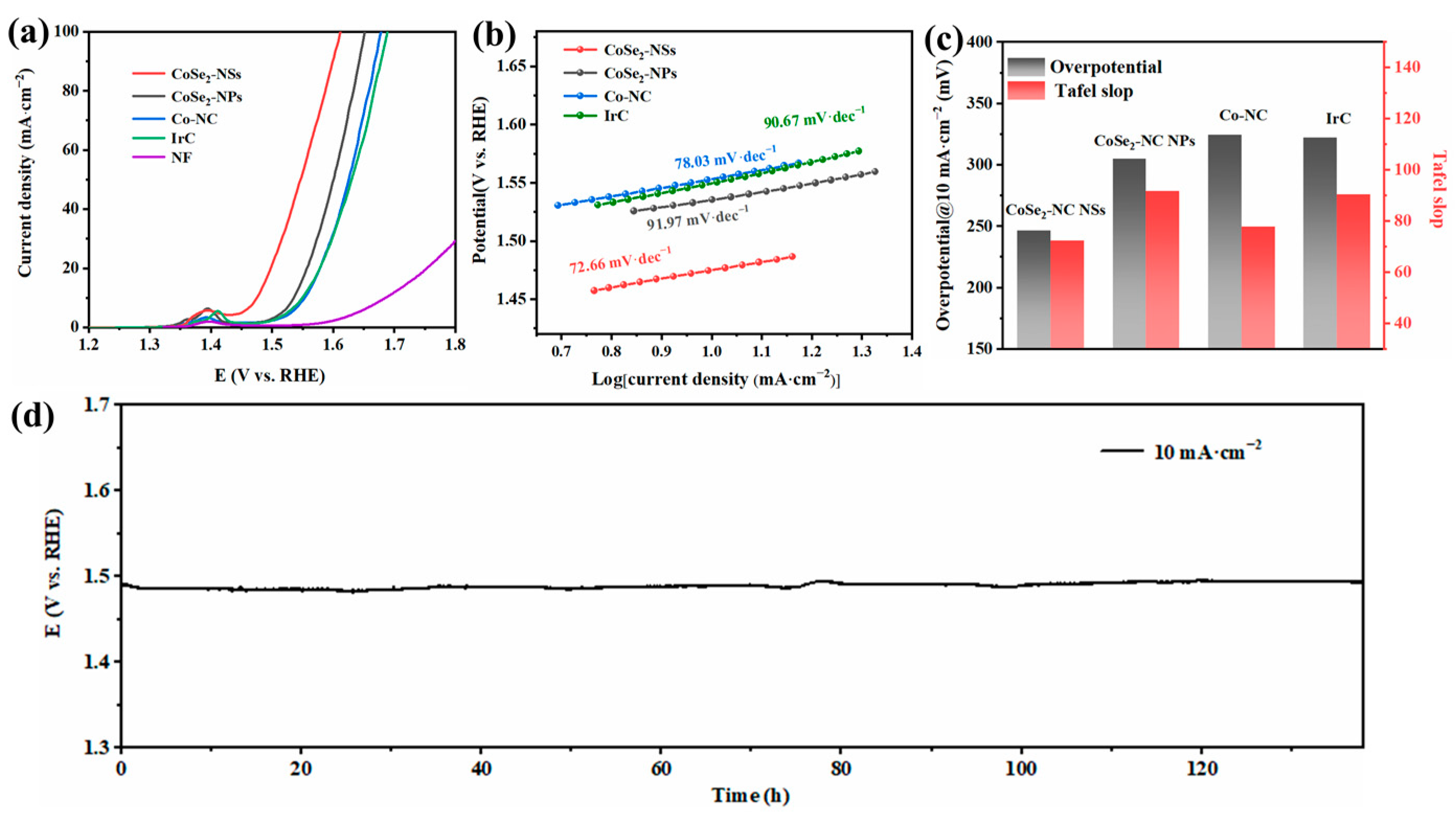
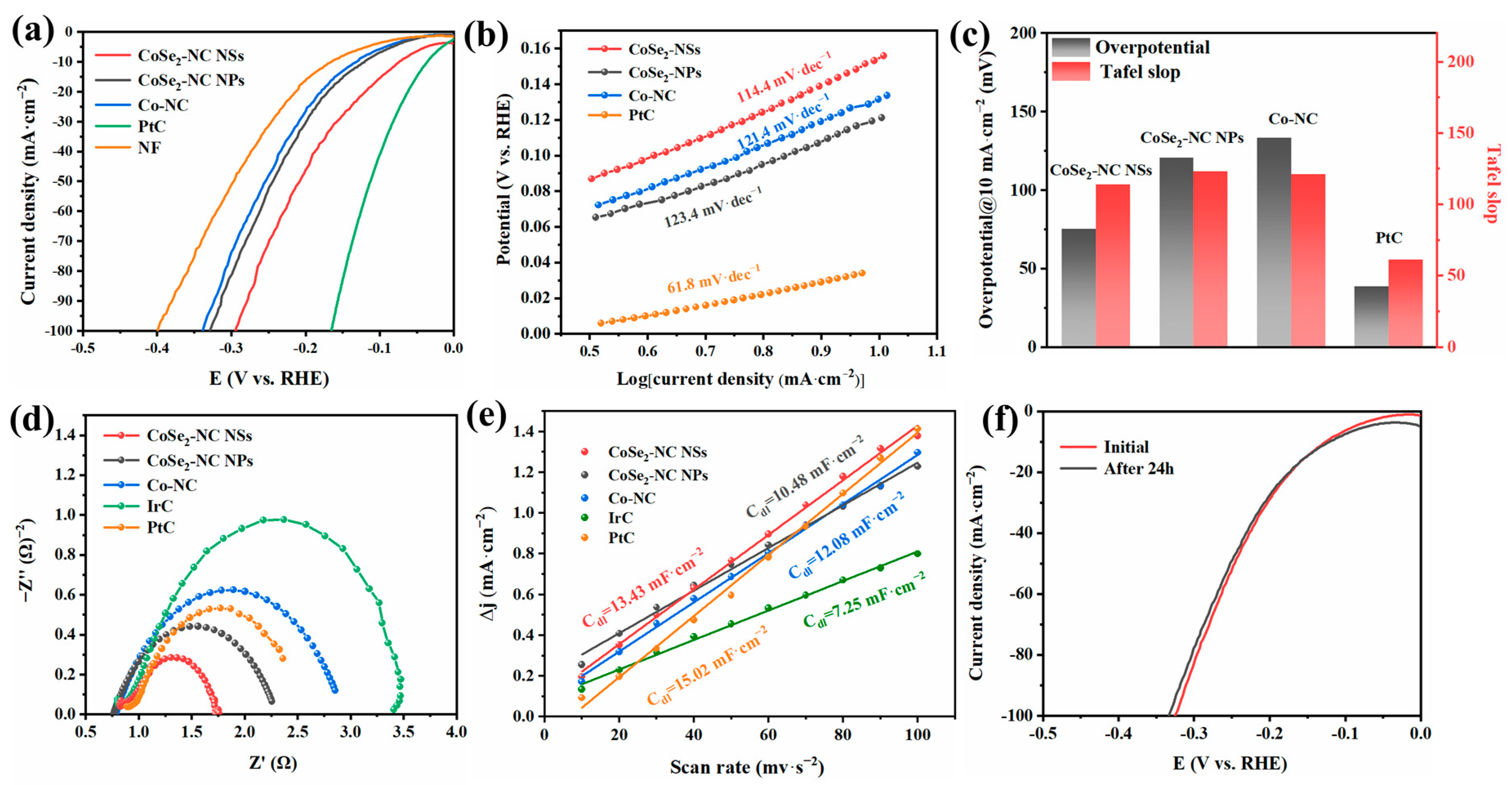
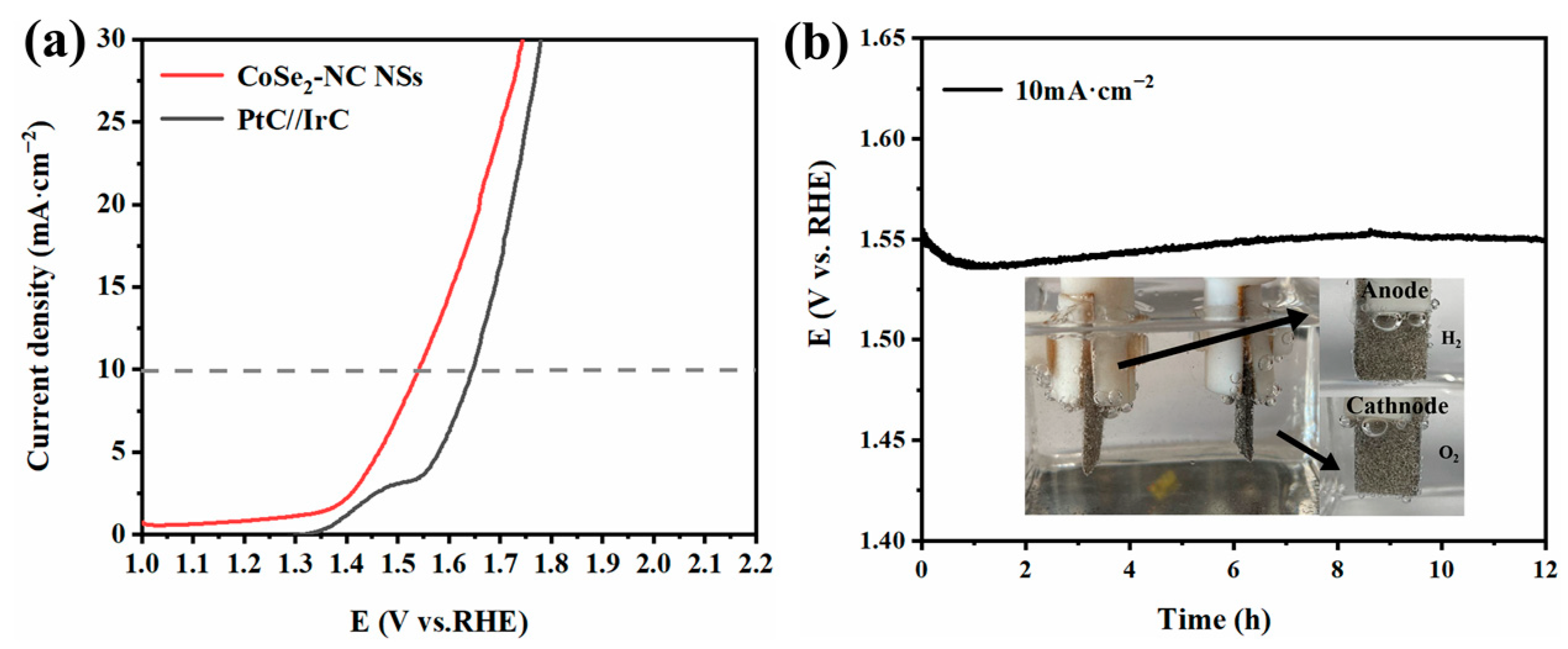
2.3. Electrochemical Performance of CoSe2-NC NSs
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of Co-NC
3.3. Synthesis of CoSe2-NC NPs
3.4. Synthesis of CoSe2-NC NSs
3.5. Material Characterization
3.6. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, L.; Jin, P.; Li, Y.; Pang, J.; Hou, J.; Peng, S.; Wang, G.; Shi, Y. NiCo2S4 microspheres grown on N, S co-doped reduced graphene oxide as an efficient bifunctional electrocatalyst for overall water splitting in alkaline and neutral pH. Nano Res. 2022, 15, 950–958. [Google Scholar] [CrossRef]
- Pang, J.X.; Fu, H.H.; Kong, W.W.; Jiang, R.; Ye, J.H.; Zhao, Z.Y.; Hou, J.; Sun, K.S.; Zheng, Y.; Chen, L. Design of NiCo2O4 nanoparticles decorated N, S co-doped reduced graphene oxide composites for electrochemical simultaneous detection of trace multiple heavy metal ions and hydrogen evolution reaction. Chem. Eng. J. 2022, 433, 133854. [Google Scholar] [CrossRef]
- Fan, C.; Zhai, X.; Chen, L.; Peng, S.; Jiang, R.; Yu, J.; Li, Y.; Zhang, Y.; Kong, W.; Ge, G.; et al. Synthesis and electrocatalytic mechanism of ultrafine MFe2O4 (M: Co, Ni, and Zn) nanocrystallites: M/Fe synergistic effects on the electrochemical detection of Cu(ii) and hydrogen evolution reaction performances. J. Mater. Chem. A 2021, 9, 22277–22290. [Google Scholar] [CrossRef]
- Ye, J.H.; Zhai, X.W.; Chen, L.; Guo, W.; Gu, T.T.; Shi, Y.L.; Hou, J.; Han, F.; Liu, Y.; Fan, C.C.; et al. Oxygen vacancies enriched nickel cobalt based nanoflower cathodes: Mechanism and application of the enhanced energy storage. J. Energy Chem. 2021, 62, 252–261. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Wang, D.; Chang, Y.-X.; Li, Y.-R.; Zhang, S.L.; Xu, S.L. Well-dispersed NiCoS2 nanoparticles/rGO composite with a large specific surface area as an oxygen evolution reaction electrocatalyst. Rare Metals 2021, 40, 3156–3165. [Google Scholar] [CrossRef]
- He, P.; Yu, X.Y.; Lou, X.W. Carbon-Incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
- Huang, T.; Chen, Y.; Lee, J.-M. Two-Dimensional Cobalt/N-Doped Carbon Hybrid Structure Derived from Metal–Organic Frameworks as Efficient Electrocatalysts for Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 5646–5650. [Google Scholar] [CrossRef]
- Sheng, H.; Janes, A.N.; Ross, R.D.; Kaiman, D.; Huang, J.; Song, B.; Schmidt, J.R.; Jin, S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Patel, M.K.; Miotello, A.; Nainesh, P. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2019, 30, 1906481–1906509. [Google Scholar] [CrossRef]
- Guo, F.; Wu, Y.; Chen, H.; Liu, Y.; Yang, L.; Ai, X.; Zou, X. High-performance oxygen evolution electrocatalysis by boronized metal sheets with self-functionalized surfaces. Energy Environ. Sci. 2019, 12, 684–692. [Google Scholar] [CrossRef]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, L.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous Cobalt Boride (Co2B) as a Highly Efficient Nonprecious Catalyst for Electrochemical Water Splitting: Oxygen and Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1502313–1502323. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, C.; Ye, F.; Pang, R.; Liu, Y.; Wu, Z.; Shao, Z.; Sun, Z.; Hu, L. Selenic Acid Etching Assisted Vacancy Engineering for Designing Highly Active Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Mater. 2021, 33, e2007523. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, J.; Wu, R.; Yu, Y.; Zhang, B. Anchoring CoO Domains on CoSe2 Nanobelts as Bifunctional Electrocatalysts for Overall Water Splitting in Neutral Media. Adv. Sci. 2016, 3, 1500426. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Wang, Z.; Gao, N.; Huang, L.; Lin, Z.; Liu, Y.; Meng, F.; Deng, J.; Jin, S.; Zhang, Q.; et al. Coupled Vacancy Pairs in Ni-Doped CoSe for Improved Electrocatalytic Hydrogen Production Through Topochemical Deintercalation. Angew. Chem. 2020, 59, 22743–22748. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Li, J.; Zhu, Y.; Gong, L.; Li, F.; Jiang, P.; Niu, F.; Li, R. Morphology engineering of CoSe2 as efficient electrocatalyst for water splitting. J. Colloid Interface Sci. 2019, 539, 646–653. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, B.; Zheng, S.; Wu, P.; Zhan, J.; Xue, H.; Xu, Q.; Pang, H. Ultrathin two-dimensional cobalt–organic framework nanosheets for high-performance electrocatalytic oxygen evolution. J. Mater. Chem. A 2018, 6, 22070–22076. [Google Scholar] [CrossRef]
- Li, C.; Zhao, D.-H.; Long, H.L.; Li, M. Recent advances in carbonized non-noble metal–organic frameworks for electrochemical catalyst of oxygen reduction reaction. Rare Met. 2021, 40, 2657–2689. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Sun, C.; Dong, Q.; Yang, J.; Dai, Z.; Lin, J.; Chen, P.; Huang, W.; Dong, X. Metal–organic framework derived CoSe2 nanoparticles anchored on carbon fibers as bifunctional electrocatalysts for efficient overall water splitting. Nano Res. 2016, 9, 2234–2243. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, R.; Wan, G.; Yang, W.; Wan, X.; Zhou, H.; Shui, J.; Yu, S.H.; Jiang, H.L. Nanocasting SiO2 into metal-organic frameworks imparts dual protection to high-loading Fe single-atom electrocatalysts. Nat. Commun. 2020, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.R.; Gao, M.R.; Gao, Q.; Li, H.H.; Xu, J.; Wu, Z.Y.; Yu, S.H. An efficient CeO2/CoSe2 Nanobelt composite for electrochemical water oxidation. Small 2015, 11, 182–188. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Fan, L.Z. MOF-derived CoSe2 microspheres with hollow interiors as high-performance electrocatalysts for the enhanced oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 15310–15314. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Guo, Y.; Liu, D.; Yu, Y.; Zhang, B. Selenium vacancy-rich CoSe2 ultrathin nanomeshes with abundant active sites for electrocatalytic oxygen evolution. J. Mater. Chem. A 2019, 7, 2536–2540. [Google Scholar] [CrossRef]
- Wurster, B.; Grumelli, D.; Hotger, D.; Gutzler, R.; Kern, K. Driving the Oxygen Evolution Reaction by Nonlinear Cooperativity in Bimetallic Coordination Catalysts. J. Am. Chem. Soc. 2016, 138, 3623–3626. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Han, Y.; Wang, Q.; Fang, Y.; Dong, S. In situ synthesis of ultrathin metal–organic framework nanosheets: A new method for 2D metal-based nanoporous carbon electrocatalysts. J. Mater. Chem. A 2017, 5, 18610–18617. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Cheng, Y.; Huang, R.; Zhang, C.; Hu, W.; Wei, G.; Yu, C. Amorphous Metal-Organic Framework-Dominated Nanocomposites with Both Compositional and Structural Heterogeneity for Oxygen Evolution. Angew. Chem. 2020, 59, 3630–3637. [Google Scholar] [CrossRef]
- Chang, S.H.; Danilovic, N.; Chang, K.C.; Subbaraman, R.; Paulikas, A.P.; Fong, D.D.; Highland, M.J.; Baldo, P.M.; Stamenkovic, V.R.; Freeland, J.W.; et al. Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat. Commun. 2014, 5, 4191. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Bu, L.; Guo, S.; Zhao, Z.; Zhu, E.; Huang, Y.; Huang, X. Morphology and Phase Controlled Construction of Pt–Ni Nanostructures for Efficient Electrocatalysis. Nano Lett. 2016, 16, 2762–2767. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, C.; Liu, Y.; Yang, M.; Li, X.; He, Y.; Li, H.; Chen, H.; Lin, Z. Dual-Shelled Multidoped Hollow Carbon Nanocages with Hierarchical Porosity for High-Performance Oxygen Reduction Reaction in Both Alkaline and Acidic Media. Nano Lett. 2020, 20, 5639–5645. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Liu, X.; Dou, Y.; Zhang, L.; Al-Mamun, M.; Jiang, L.; Yin, H.; He, C.-T.; Zhao, H. Manipulating the assembled structure of atomically thin CoSe2 nanomaterials for enhanced water oxidation catalysis. Nano Energy 2019, 57, 371–378. [Google Scholar] [CrossRef]
- Aboagye, A.; Elbohy, H.; Kelkar, A.D.; Qiao, Q.; Zai, J.; Qian, X.; Zhang, L. Electrospun carbon nanofibers with surface-attached platinum nanoparticles as cost-effective and efficient counter electrode for dye-sensitized solar cells. Nano Energy 2015, 11, 550–556. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; He, G.; Zhang, X.; Bao, S.; Hu, W. Bioinspired synthesis of nitrogen/sulfur co-doped graphene as an efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 279, 252–258. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, S.; Yin, G.; Du, C.; Wang, Z.; Wei, J. Effect of Se in Co-based selenides towards oxygen reduction electrocatalytic activity. J. Power Sources 2012, 206, 103–107. [Google Scholar] [CrossRef]
- Wu, X.; Han, S.; He, D.; Yu, C.; Lei, C.; Liu, W.; Zheng, G.; Zhang, X.; Lei, L. Metal Organic Framework Derived Fe-Doped CoSe2 Incorporated in Nitrogen-Doped Carbon Hybrid for Efficient Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 8672–8678. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, L.; Hu, H.; Li, T.; Li, X.; Guo, S.; Li, Y.; Xue, Q.; Xing, W.; Yan, Z.; et al. Stable CoSe2/carbon nanodice@reduced graphene oxide composites for high-performance rechargeable aluminum-ion batteries. Energy Environ. Sci. 2018, 11, 2341–2347. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, N.; Miotello, A.; Kothari, D. Cobalt-Boride: An efficient and robust electrocatalyst for Hydrogen Evolution Reaction. J. Power Sources 2015, 279, 620–625. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kim, S.-K.; Ahn, S.-H. Electrodeposited amorphous Co-P-B ternary catalyst for hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 6282–6288. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, J.; Zhou, K.; Yang, L.; Ke, Y.; Tang, Z.; Chen, S. CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 2016, 28, 143–150. [Google Scholar] [CrossRef]
- Liu, H.; Jin, M.; Zhan, D.; Wang, J.; Cai, X.; Qiu, Y.; Lai, L. Stacking faults triggered strain engineering of ZIF-67 derived Ni-Co bimetal phosphide for enhanced overall water splitting. Appl. Catal. B Environ. 2020, 272, 118951. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Li, H.; Liu, L.; Xue, K.; Niu, X.; Hou, J.; Chen, L. Salt-Templated Nanoarchitectonics of CoSe2-NC Nanosheets as an Efficient Bifunctional Oxygen Electrocatalyst for Water Splitting. Int. J. Mol. Sci. 2022, 23, 5239. https://doi.org/10.3390/ijms23095239
Cao H, Li H, Liu L, Xue K, Niu X, Hou J, Chen L. Salt-Templated Nanoarchitectonics of CoSe2-NC Nanosheets as an Efficient Bifunctional Oxygen Electrocatalyst for Water Splitting. International Journal of Molecular Sciences. 2022; 23(9):5239. https://doi.org/10.3390/ijms23095239
Chicago/Turabian StyleCao, Hong, Hailong Li, Linhao Liu, Kangning Xue, Xinkai Niu, Juan Hou, and Long Chen. 2022. "Salt-Templated Nanoarchitectonics of CoSe2-NC Nanosheets as an Efficient Bifunctional Oxygen Electrocatalyst for Water Splitting" International Journal of Molecular Sciences 23, no. 9: 5239. https://doi.org/10.3390/ijms23095239
APA StyleCao, H., Li, H., Liu, L., Xue, K., Niu, X., Hou, J., & Chen, L. (2022). Salt-Templated Nanoarchitectonics of CoSe2-NC Nanosheets as an Efficient Bifunctional Oxygen Electrocatalyst for Water Splitting. International Journal of Molecular Sciences, 23(9), 5239. https://doi.org/10.3390/ijms23095239