Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70
Abstract
1. Introduction
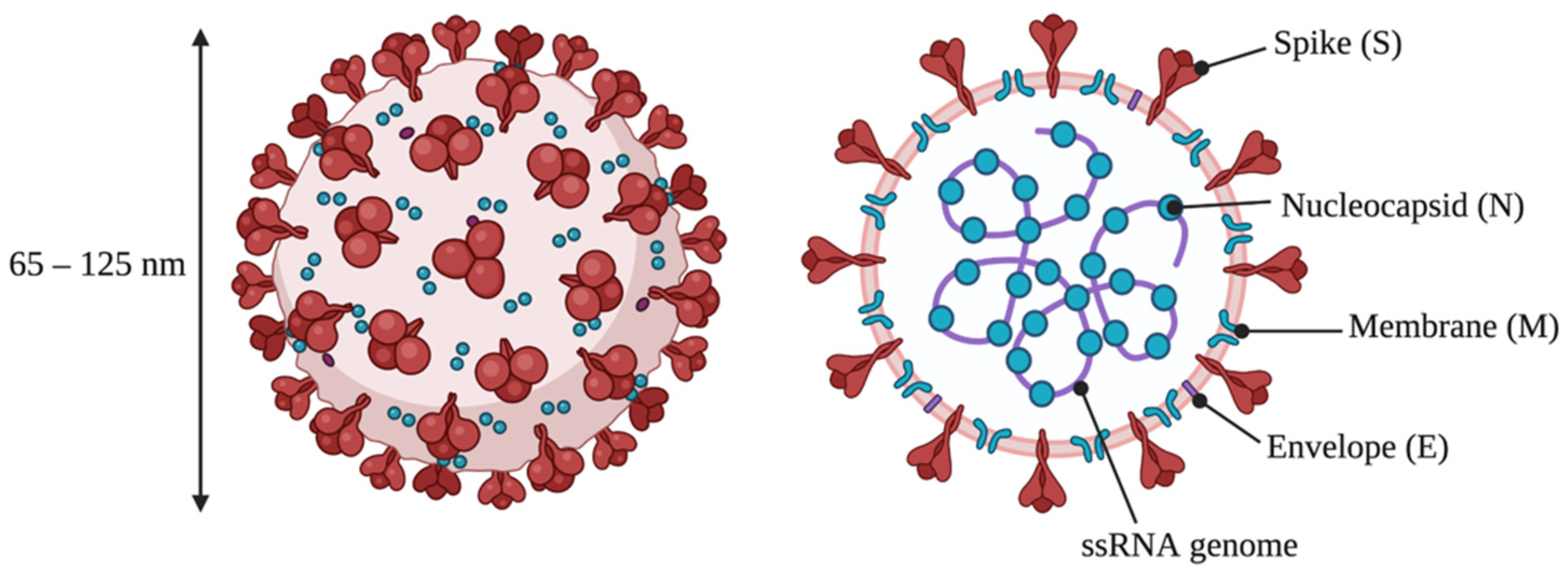
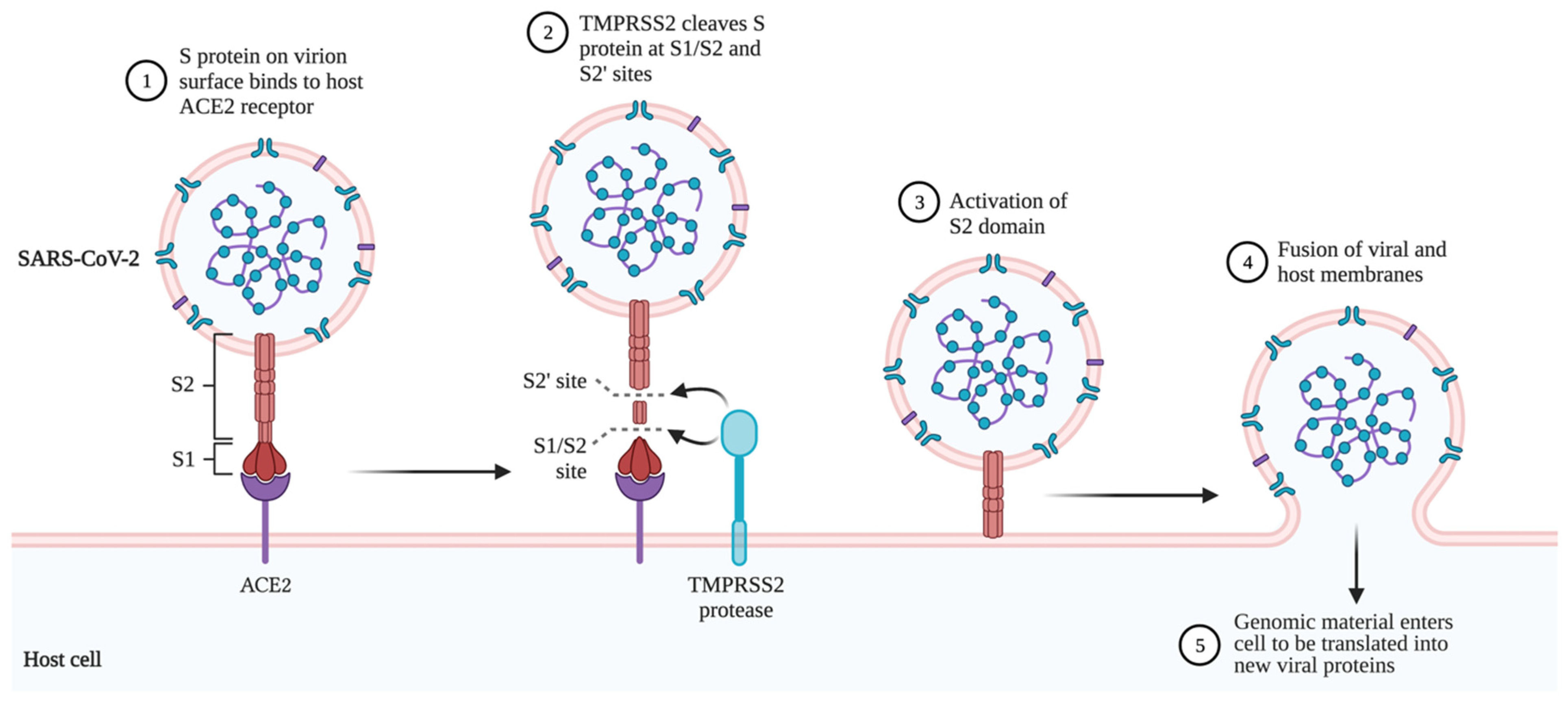
2. Structure and Replication of SARS-CoV-2
3. COVID-19 Symptoms and Complications
3.1. Neurological Symptoms
3.2. Long COVID
4. Photobiomodulation
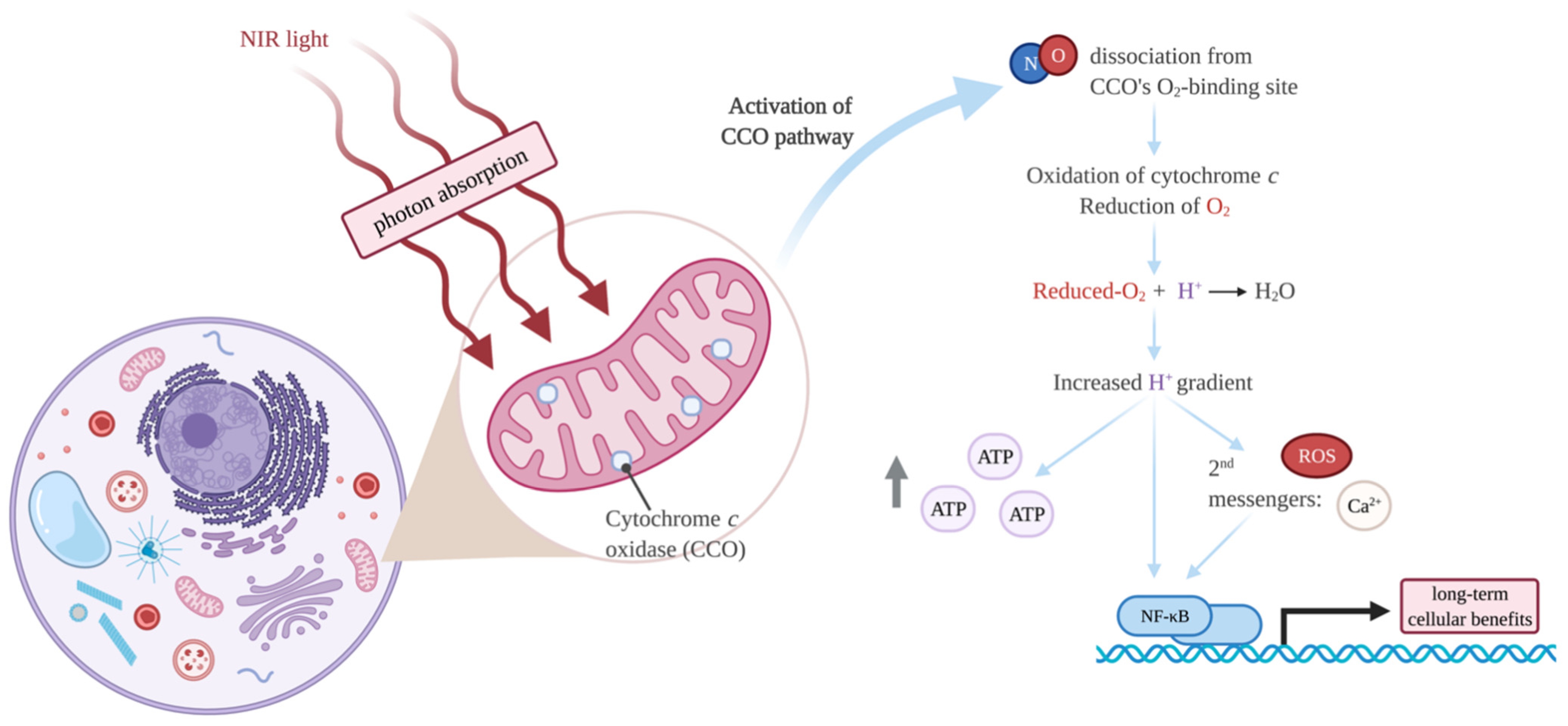
Molecular Mechanisms of PBMT
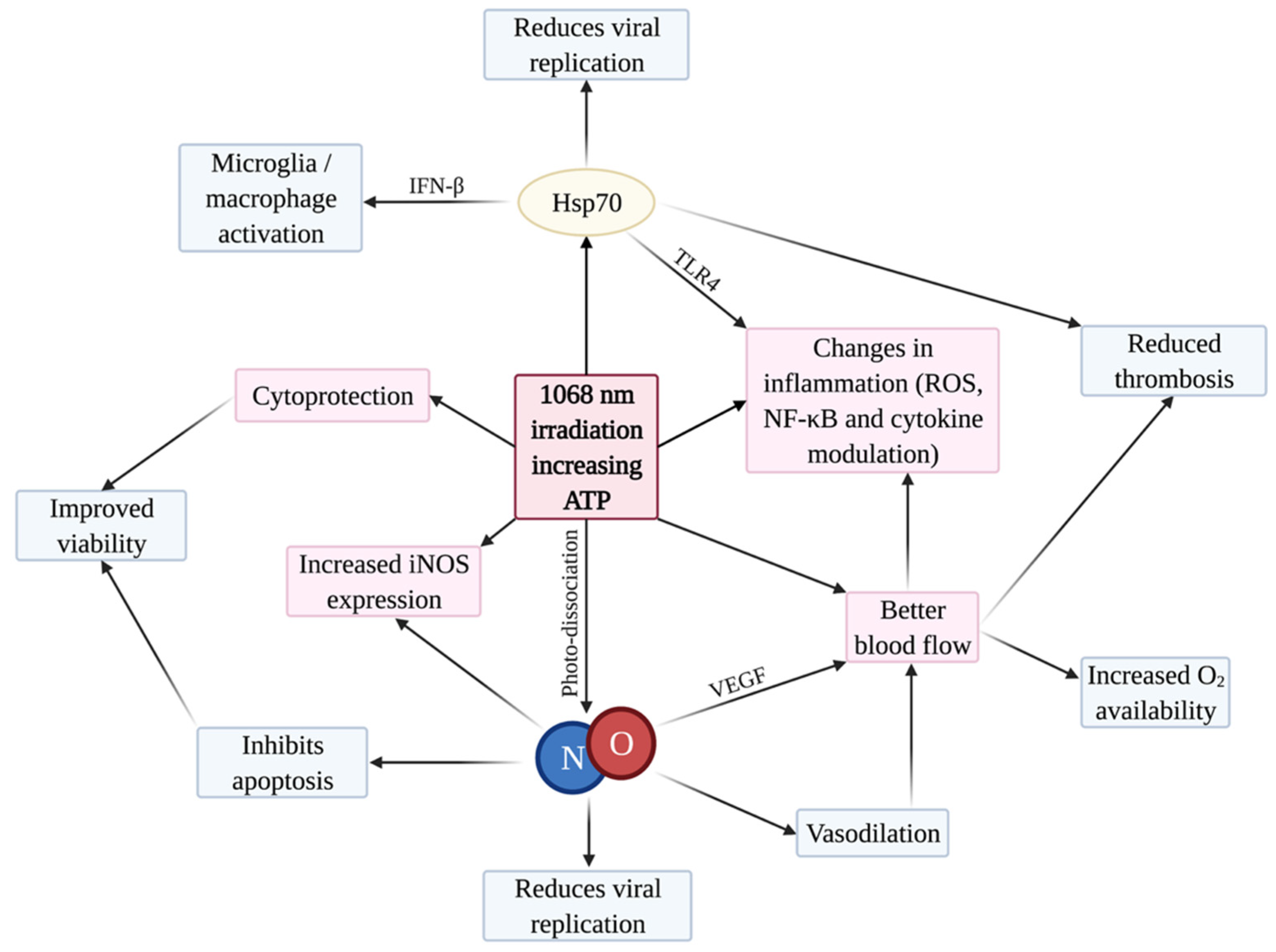
5. Rationale for PBMT to Treat COVID-19
5.1. Cytoprotection
5.2. iNOS and NO
5.3. Inflammation
5.4. Blood Flow and Thrombosis
5.5. Photo-Preconditioning by Heat Shock Proteins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Astuti, I.; Ysrafil. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Sabino, C.P.; Ball, A.R.; Baptista, M.S.; Dai, T.; Hamblin, M.R.; Ribeiro, M.S.; Santos, A.L.; Sellera, F.P.; Tegos, G.P.; Wainwright, M. Light-based technologies for management of COVID-19 pandemic crisis. J. Photochem. Photobiol. B Biol. 2020, 212, 111999. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Keidar, S.; Kaplan, M.; Gamliel-Lazarovich, A. ACE2 of the heart: From angiotensin I to angiotensin (1–7). Cardiovasc. Res. 2007, 73, 463–469. [Google Scholar] [CrossRef]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 23–29. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kang, A.; Luo, X.; Jeyanathan, M.; Gillgrass, A.; Afkhami, S.; Xing, Z. COVID-19: Current knowledge in clinical features, immunological responses, and vaccine development. FASEB J. 2021, 35, e21409. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Prasad, A.; Prasad, M. Single virus targeting multiple organs: What we know and where we are heading? Front. Med. 2020, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.I.; Abdelmoneim, A.H.; Mahmoud, E.M.; Makhawi, A.M. Cytokine Storm in COVID-19 Patients, Its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediat. Inflamm. 2020, 2020, 8198963. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; What we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Fekrazad, R. Photobiomodulation and antiviral photodynamic therapy as a possible novel approach in COVID-19 Management. Photobiomodulation Photomed. Laser Surg. 2020, 38, 255–257. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Zhao, M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents 2020, 55, 105982. [Google Scholar] [CrossRef] [PubMed]
- Vetrici, M.A.; Mokmeli, S.; Bohm, A.R.; Monici, M.; Sigman, S.A. Evaluation of adjunctive photobiomodulation (PBMT) for COVID-19 pneumonia via clinical status and pulmonary severity indices in a preliminary trial. J. Inflamm. Res. 2021, 14, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.B.; Kelly, P.A.; Gonzalez, E.; Arya, R.; Roberts, L.N. Pulmonary embolism in hospitalised patients with COVID-19. Thromb. Res. 2020, 195, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–443. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef]
- Mishra, R.; Banerjea, A.C. Neurological damage by coronaviruses: A catastrophe in the queue! Front. Immunol. 2020, 11, 565521. [Google Scholar] [CrossRef]
- Koralnik, I.J.; Tyler, K.L. COVID-19: A global threat to the nervous system. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Guo, Q.; Zheng, Y.; Shi, J.; Wang, J.; Li, G.; Li, C.; Fromson, J.A.; Xu, Y.; Liu, X.; Xu, H.; et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: A mixed-method study. Brain. Behav. Immun. 2020, 88, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.; McGavern, D. Portals of viral entry into the central nervous system. In The Blood-Brain Barrier in Health and Disease; CRC Press: Boca Raton, FL, USA, 2015; Volume 2, pp. 23–47. [Google Scholar]
- Murta, V.; Villarreal, A.; Ramos, A.J. Severe acute respiratory syndrome coronavirus 2 impact on the central nervous system: Are astrocytes and microglia main players or merely bystanders? ASN Neuro 2020, 12, 1–17. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef]
- Matsuda, K.; Park, C.H.; Sunden, Y.; Kimura, T.; Ochiai, K.; Kida, H.; Umemura, T. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza A virus in mice. Vet. Pathol. 2004, 41, 101–107. [Google Scholar] [CrossRef]
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Ait Benali, S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020, 41, 2657–2669. [Google Scholar] [CrossRef]
- Alam, S.B.; Willows, S.; Kulka, M.; Sandhu, J.K. Severe acute respiratory syndrome coronavirus 2 may be an underappreciated pathogen of the central nervous system. Eur. J. Neurol. 2020, 27, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446. [Google Scholar] [CrossRef]
- Mori, I.; Nishiyama, Y.; Yokochi, T.; Kimura, Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.E.; Chappell, M.C.; Ferrario, C.M.; Tallant, E.A. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol. Cell Physiol. 2006, 290, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Gowrisankar, Y.V.; Clark, M.A. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 2016, 138, 74–85. [Google Scholar] [CrossRef]
- Xu, J.; Lazartigues, E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell. Mol. Neurobiol. 2020, 2020, 305–309. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimer’s Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID: A meta-analysis and systematic review. medRxiv 2021. [Google Scholar] [CrossRef]
- Maiman, T.H. Stimulated optical radiation in Ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Mester, E.; Szende, B.; Gärtner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Mester, E.; Mester, A.F.; Mester, A. The biomedical effects of laser application. Lasers Surg. Med. 1985, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Spiry, T.; Szende, B.; Tota, J.G. Effect of laser rays on wound healing. Am. J. Surg. 1971, 122, 532–535. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Huang, Y.Y.; Heiskanen, V. Non-mammalian hosts and photobiomodulation: Do all life-forms respond to light? Photochem. Photobiol. 2019, 95, 126–139. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (Light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Pruitt, T.; Carter, C.; Wang, X.; Wu, A.; Liu, H. Photobiomodulation at different wavelengths boosts mitochondrial redox metabolism and hemoglobin oxygenation: Lasers vs. Light-Emitting Diodes In Vivo. Metabolites 2022, 12, 103. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of low level light therapy. Int. Soc. Opt. Eng. 2006, 6140, 614001. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, A.C.H.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Karu, T.I. Molecular mechanism of the therapeutic effect of low-intensity laser radiation. Lasers Life Sci. 1988, 2, 53–74. [Google Scholar]
- Karu, T.I. Multiple roles of cytochrome C oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Afanas’eva, N.I. Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light. Dokl. Akad. Nauk 1995, 342, 693–695. [Google Scholar] [PubMed]
- Moncada, S.; Erusalimsky, J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994, 356, 295–298. [Google Scholar] [CrossRef]
- Lane, N. Cell biology: Power games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Duggett, N.A.; Chazot, P.L. Low-intensity light therapy (1068 nm) protects CAD neuroblastoma cells from β-amyloid-mediated cell death. Biol. Med. 2014, 6, 1000210. [Google Scholar] [CrossRef]
- Dougal, G.; Lee, S.Y. Evaluation of the efficacy of low-level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin. Exp. Dermatol. 2013, 38, 713–718. [Google Scholar] [CrossRef]
- Celine Lee, S.Y.; Seong, I.W.; Kim, J.S.; Cheon, K.A.; Gu, S.H.; Kim, H.H.; Park, K.H. Enhancement of cutaneous immune response to bacterial infection after low-level light therapy with 1072 nm infrared light: A preliminary study. J. Photochem. Photobiol. B Biol. 2011, 105, 175–182. [Google Scholar] [CrossRef]
- Bradford, A.; Barlow, A.; Chazot, P.L. Probing the differential effects of infrared light sources IR1072 and IR880 on human lymphocytes: Evidence of selective cytoprotection by IR1072. J. Photochem. Photobiol. B Biol. 2005, 81, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain photobiomodulation therapy: A narrative review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Chen, J.C.; Wang, W.J. Near-infrared absorption property of biological soft tissue constituents. J. Med. Biol. Eng. 2001, 21, 7–14. [Google Scholar]
- Oliveira, M.C.; Greiffo, F.R.; Rigonato-Oliveira, N.C.; Custódio, R.W.A.; Silva, V.R.; Damaceno-Rodrigues, N.R.; Almeida, F.M.; Albertini, R.; Lopes-Martins, R.Á.B.; De Oliveira, L.V.F.; et al. Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J. Photochem. Photobiol. B Biol. 2014, 134, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aimbire, F.; De Oliveira, A.P.L.; Albertini, R.; Corrêa, J.C.; De Campos, C.B.L.; Lyon, J.P.; Silva, J.A.; Costa, M.S. Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1β levels in airway and lung from rat subjected to LPS-induced inflammation. Inflammation 2008, 31, 189–197. [Google Scholar] [CrossRef]
- Moraes, G.D.C.; Vitoretti, L.B.; De Brito, A.A.; Alves, C.E.; De Oliveira, N.C.R.; Dias, A.D.S.; Matos, Y.S.T.; Oliveira, M.C.; Oliveira, L.V.F.; Da Palma, R.K.; et al. Low-level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving P2X7 receptor. Oxid. Med. Cell. Longev. 2018, 2018, 6798238. [Google Scholar] [CrossRef]
- Vatankhah, Z.; Mokmeli, S.; Boshbishe, S. Evaluation The effect of Low Level Laser Therapy (LLLT) in treatment of asthma, added to conventional drug therapy (crossover, case control clinical trial). Photodiagnosis Photodyn. Ther. 2008, 5, S22. [Google Scholar] [CrossRef]
- de Souza, G.H.M.; Ferraresi, C.; Moreno, M.A.; Pessoa, B.V.; Damiani, A.P.M.; Filho, V.G.; dos Santos, G.V.; Zamunér, A.R. Acute effects of photobiomodulation therapy applied to respiratory muscles of chronic obstructive pulmonary disease patients: A double-blind, randomized, placebo-controlled crossover trial. Lasers Med. Sci. 2020, 35, 1055–1063. [Google Scholar] [CrossRef]
- De Marchi, T.; Frâncio, F.; Ferlito, J.V.; Weigert, R.; de Oliveira, C.; Merlo, A.P.; Pandini, D.L.; Pasqual-Júnior, B.A.; Giovanella, D.; Tomazoni, S.S.; et al. Effects of photobiomodulation therapy combined with static magnetic field in severe COVID-19 patients requiring intubation: A pragmatic randomized placebo-controlled trial. J. Inflamm. Res. 2021, 14, 3569–3585. [Google Scholar] [CrossRef] [PubMed]
- Sigman, S.A.; Mokmeli, S.; Monici, M.; Vetrici, M.A. A 57-year-old african american man with severe COVID-19 pneumonia who responded to supportive photobiomodulation therapy (PBMT): First use of PBMT in COVID-19. Am. J. Case Rep. 2020, 21, e926779. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med. Sci. 2016, 31, 1151–1160. [Google Scholar] [CrossRef]
- Nizmutdinov, D.; Xiaoming, Q.; Berman, M.H.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.S.; Stevens, A.B.; Huang, J. Transcranial Near Infrared light stimulation improves cognition in patients with Dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef]
- Michalikova, S.; Ennaceur, A.; van Rensburg, R.; Chazot, P.L. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: Effects of low infrared light. Neurobiol. Learn. Mem. 2008, 89, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Talanian, R.V.; Billiar, T.R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3- like activity via two distinct mechanisms. J. Biol. Chem. 1997, 272, 31138–31148. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Haendeler, J.; Nehls, M.; Zeiher, A.M. Suppression of apoptosis by nitric oxide via inhibition of interleukin- 1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 1997, 185, 601–607. [Google Scholar] [CrossRef]
- Melino, G.; Bernassola, F.; Catani, M.V.; Rossi, A.; Corazzari, M.; Sabatini, S.; Vilbois, F.; Green, D.R. Nitric oxide inhibits apoptosis via AP-1-dependent CD95L transactivation. Cancer Res. 2000, 60, 2377–2383. [Google Scholar]
- Jones, M.L.; Ganopolsky, J.G.; Labbé, A.; Wahl, C.; Prakash, S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88, 401–407. [Google Scholar] [CrossRef]
- Croen, K.D. Evidence for an antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J. Clin. Investig. 1993, 91, 2446–2452. [Google Scholar] [CrossRef]
- Karupiah, G.; Xie, Q.W.; Buller, R.M.L.; Nathan, C.; Duarte, C.; MacMicking, J.D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science 1993, 261, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Järhult, J.D.; Lennerstrand, J.; Lundkvist, Å. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020, 37, 101734. [Google Scholar] [CrossRef]
- de Matos, B.; Buchaim, D.; Pomini, K.; Barbalho, S.; Guiguer, E.; Reis, C.; Bueno, C.; Cunha, M.; Pereira, E.; Buchaim, R. Photobiomodulation therapy as a possible new approach in covid-19: A systematic review. Life 2021, 11, 580. [Google Scholar] [CrossRef]
- Wajih, N.; Alipour, E.; Rigal, F.; Zhu, J.; Perlegas, A.; Caudell, D.L.; Kim-Shapiro, D. Effects of nitrite and far-red light on coagulation. Nitric Oxide Biol. Chem. 2021, 107, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nejatifard, M.; Asefi, S.; Jamali, R.; Hamblin, M.R.; Fekrazad, R. Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: A systematic review. Cytokine 2021, 137, 155312. [Google Scholar] [CrossRef] [PubMed]
- Bathini, M.; Raghushaker, C.R.; Mahato, K.K. The Molecular Mechanisms of Action of Photobiomodulation against neurodegenerative diseases: A systematic review. Cell. Mol. Neurobiol. 2020, 42, 955–971. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.L.C.; Silva, J.A.; Serra, A.J.; Pallotta, R.C.; Da Silva, E.A.P.; Marques, A.C.D.F.; Feliciano, R.D.S.; Marcos, R.L.; Leal-Junior, E.C.; Carvalho, P.D.T.C.D. Photobiomodulation therapy in the modulation of inflammatory mediators and bradykinin receptors in an experimental model of acute osteoarthritis. Lasers Med. Sci. 2017, 32, 87–94. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Nagata, K.; Tedford, C.E.; Mccarthy, T.; Hamblin, M.R. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics 2013, 6, 829–838. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-Level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- Chen, A.C.H.; Huang, Y.Y.; Sharma, S.K.; Hamblin, M.R. Effects of 810-nm laser on murine bone-marrow-derived dendritic cells. Photomed. Laser Surg. 2011, 29, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. The role of nitric oxide in low level light therapy. Mech. Low-Light Ther. III 2008, 6846, 684602. [Google Scholar] [CrossRef]
- Skobelkin, O.K.; Michailov, V.A.; Zakharov, S.D. Preoperative activation of the immune system by Low reactive Level Laser Therapy (LLLT) in oncologic patients: A preliminary report. LASER Ther. 1991, 3, 169–175. [Google Scholar] [CrossRef]
- Drohomirecka, A.; Iwaszko, A.; Walski, T.; Pliszczak-Król, A.; Wąż, G.; Graczyk, S.; Gałecka, K.; Czerski, A.; Bujok, J.; Komorowska, M. Low-level light therapy reduces platelet destruction during extracorporeal circulation. Sci. Rep. 2018, 8, 16963. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Han, M.K.; Salgado, K.F.; Streeter, J.; Zivin, J.A. Safety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbits. Stroke 2008, 39, 3073–3078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grillo, S.L.; Duggett, N.A.; Ennaceur, A.; Chazot, P.L. Non-invasive infra-red therapy (1072 nm) reduces β-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J. Photochem. Photobiol. B Biol. 2013, 123, 13–22. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Perfetto, B.; Guerrera, L.P.; Oliviero, G.; Baroni, A. Q-switched 1064 nm Nd-Yag nanosecond laser effects on skin barrier function and on molecular rejuvenation markers in keratinocyte-fibroblasts interaction. Lasers Med. Sci. 2019, 34, 595–605. [Google Scholar] [CrossRef]
- Kakimura, J.-I.; Kitamura, Y.; Takata, K.; Umeki, M.; Suzuki, S.; Shibagaki, K.; Taniguchi, T.; Nomura, Y.; Gebicke-Haerter, P.J.; Smith, M.A.; et al. Microglial activation and amyloid-β clearance induced by exogenous heat-shock proteins. FASEB J. 2002, 16, 601–603. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Baré, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70. Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Pica, F.; Palamara, A.T.; Rossi, A.; De Marco, A.; Amici, C.; Santoro, M.G. Δ12-prostaglandin J2 is a potent inhibitor of influenza A virus replication. Antimicrob. Agents Chemother. 2000, 44, 200–204. [Google Scholar] [CrossRef]
- Conti, C.; De Marco, A.; Mastromarino, P.; Tomao, P.; Santoro, M.G. Antiviral effect of hyperthermic treatment in rhinovirus infection. Antimicrob. Agents Chemother. 1999, 43, 822–829. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Carattoli, A.; Rozera, C.; Fortini, D.; Giorgi, C.; Belardo, G.; Amici, C.; Santoro, M.G. Induction of the heat-shock response by antiviral prostaglandins in human cells infected with human immunodeficiency virus type 1. Eur. J. Biochem. 1998, 256, 334–341. [Google Scholar] [CrossRef]
- Mastromarino, P.; Conti, C.; Petruzziello, R.; De Marco, A.; Pica, F.; Santoro, M.G. Inhibition of Sindbis virus replication by cyclopentenone prostaglandins: A cell-mediated event associated with heat-shock protein synthesis. Antiviral Res. 1993, 20, 209–222. [Google Scholar] [CrossRef]
- Kim, M.Y.; Shu, Y.; Carsillo, T.; Zhang, J.; Yu, L.; Peterson, C.; Longhi, S.; Girod, S.; Niewiesk, S.; Oglesbee, M. hsp70 and a novel axis of type i interferon-dependent antiviral immunity in the measles virus-infected brain. J. Virol. 2013, 87, 998–1009. [Google Scholar] [CrossRef]
- Kim, M.Y.; Oglesbee, M. Virus-heat shock protein interaction and a novel axis for innate antiviral immunity. Cells 2012, 1, 646–666. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef]
- Hulina, A.; Rajković, M.G.; Despot, D.J.; Jelić, D.; Dojder, A.; Čepelak, I.; Rumora, L. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells. Cell Stress Chaperones 2018, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Toshchakov, V.; Jones, B.W.; Perera, P.Y.; Thomas, K.; Cody, M.J.; Zhang, S.; Williams, B.R.G.; Major, J.; Hamilton, T.A.; Fenton, M.J.; et al. TLR4, but not TLR2, mediates IFN-β-induced STATIα/β-dependent gene expression in macrophages. Nat. Immunol. 2002, 3, 392–398. [Google Scholar] [CrossRef]
- Nagarajan, U.M. Induction and function of IFNβ during viral and bacterial infection. Crit. Rev. Immunol. 2011, 31, 459–474. [Google Scholar] [CrossRef]
- Allende, M.; Molina, E.; Guruceaga, E.; Tamayo, I.; González-Porras, J.R.; Gonzalez-López, T.J.; Toledo, E.; Rabal, O.; Ugarte, A.; Roldán, V.; et al. Hsp70 protects from stroke in atrial fibrillation patients by preventing thrombosis without increased bleeding risk. Cardiovasc. Res. 2016, 110, 309–318. [Google Scholar] [CrossRef]
- Soheilifar, S.; Fathi, H.; Naghdi, N. Photobiomodulation therapy as a high potential treatment modality for COVID-19. Lasers Med. Sci. 2020, 2020, 935–938. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitchen, L.C.; Berman, M.; Halper, J.; Chazot, P. Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70. Int. J. Mol. Sci. 2022, 23, 5221. https://doi.org/10.3390/ijms23095221
Kitchen LC, Berman M, Halper J, Chazot P. Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70. International Journal of Molecular Sciences. 2022; 23(9):5221. https://doi.org/10.3390/ijms23095221
Chicago/Turabian StyleKitchen, Lydia C., Marvin Berman, James Halper, and Paul Chazot. 2022. "Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70" International Journal of Molecular Sciences 23, no. 9: 5221. https://doi.org/10.3390/ijms23095221
APA StyleKitchen, L. C., Berman, M., Halper, J., & Chazot, P. (2022). Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70. International Journal of Molecular Sciences, 23(9), 5221. https://doi.org/10.3390/ijms23095221






