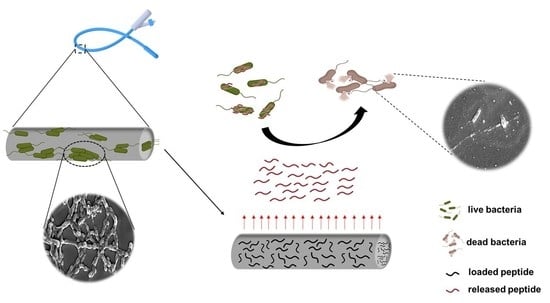
Loading of Polydimethylsiloxane with a Human ApoB-Derived Antimicrobial Peptide to Prevent Bacterial Infections
Abstract
1. Introduction
1.1. Catheter-Associated Urinary Tract Infections
1.2. Anti-Infective Biomaterials
1.3. Antimicrobial Peptides
1.4. Functionalization of PDMS with an ApoB-Derived Antimicrobial Peptide
2. Results
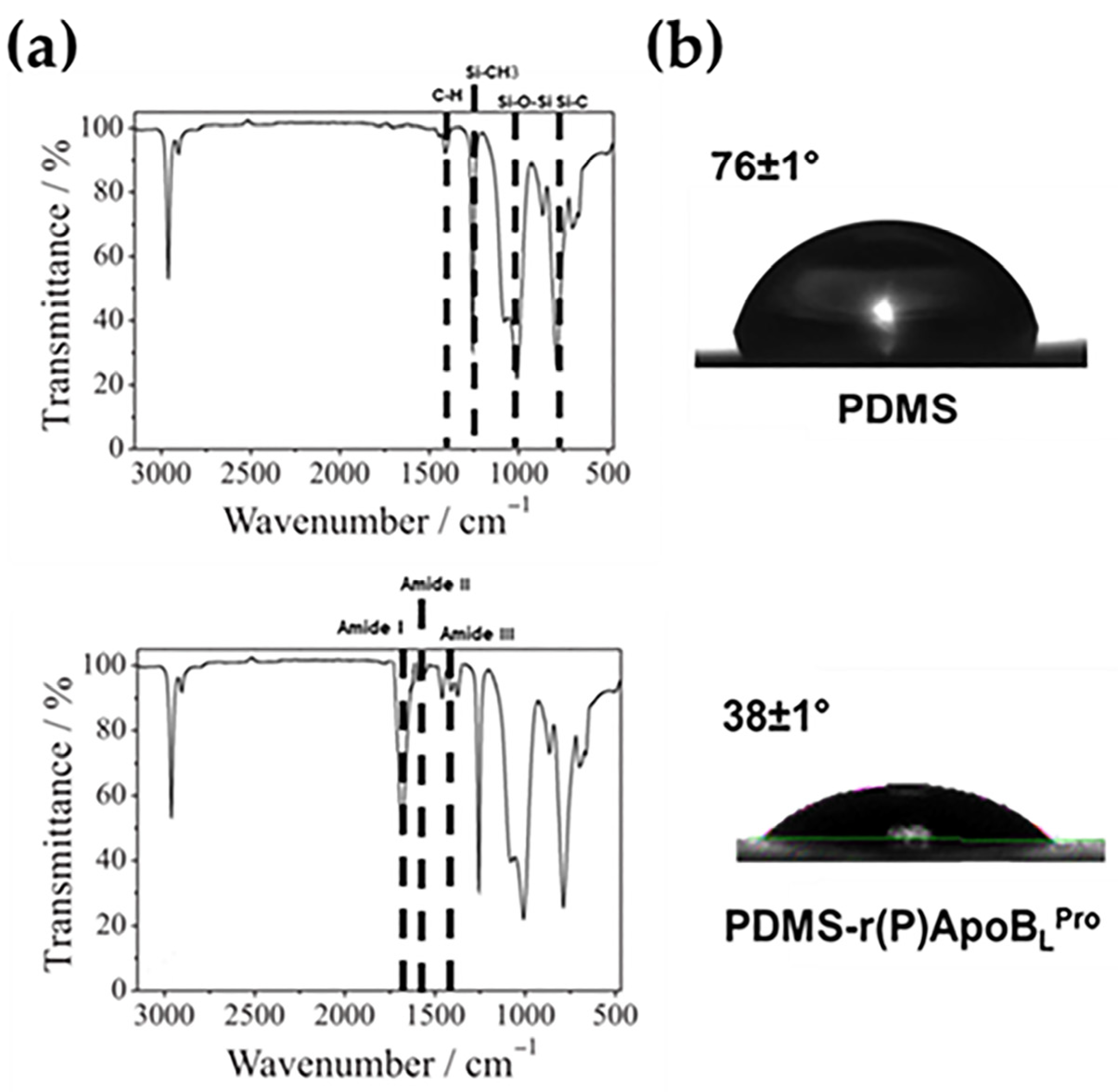
2.1. Characterization of Functionalized PDMS by Fourier-Transform Infrared (FTIR) Spectroscopy
2.2. Water Contact Angle Analyses of Functionalized PDMS
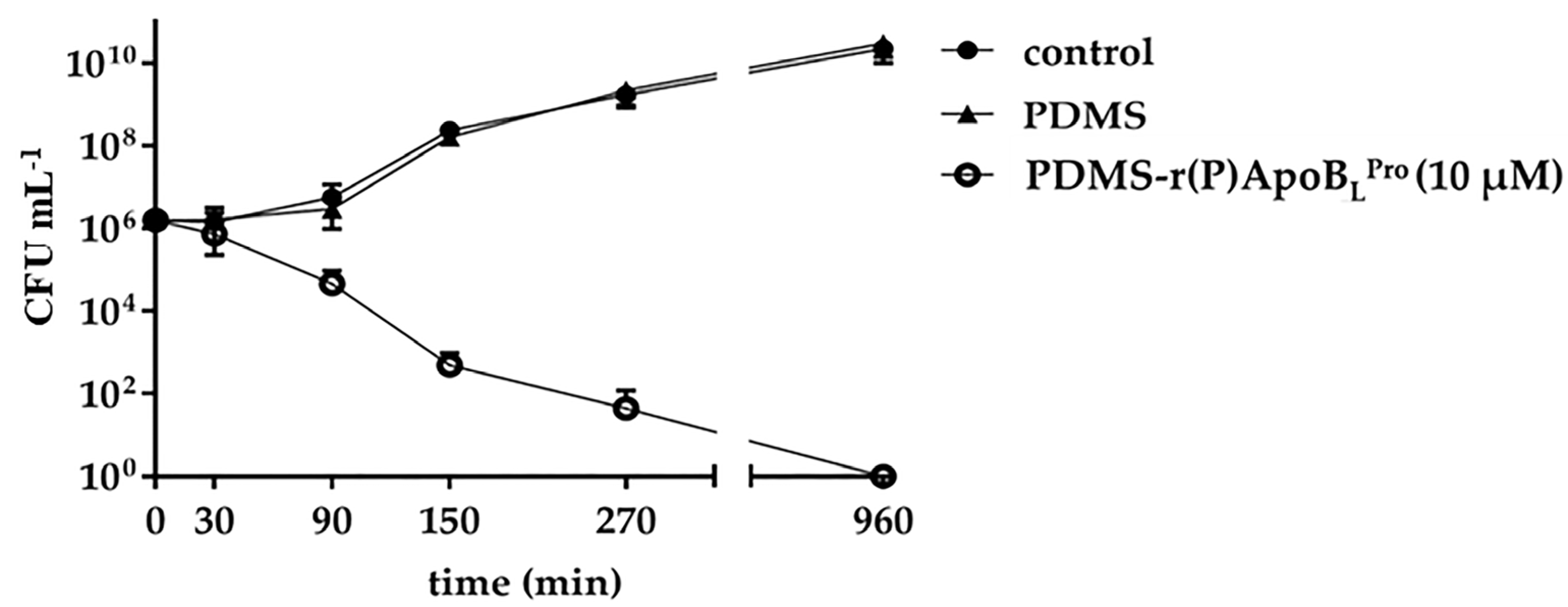
2.3. Evaluation of the Antimicrobial Activity of PDMS Functionalized with r(P)ApoBLPro
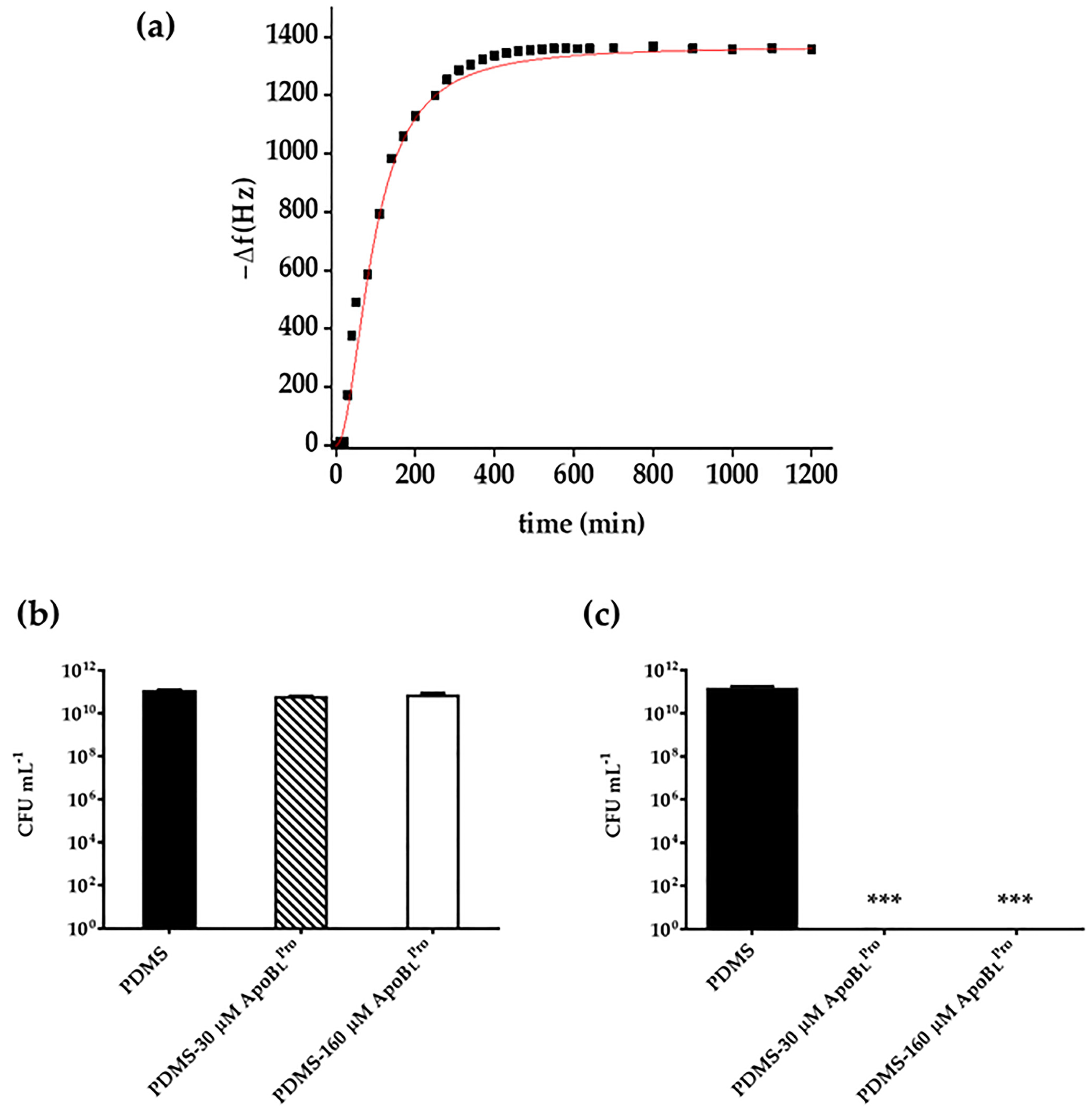
2.4. Evaluation of Peptide Release by Quartz Crystal Microbalance (QCM) Analyses
2.5. Storage Stability of PDMS Functionalized with r(P)ApoBLPro Peptide
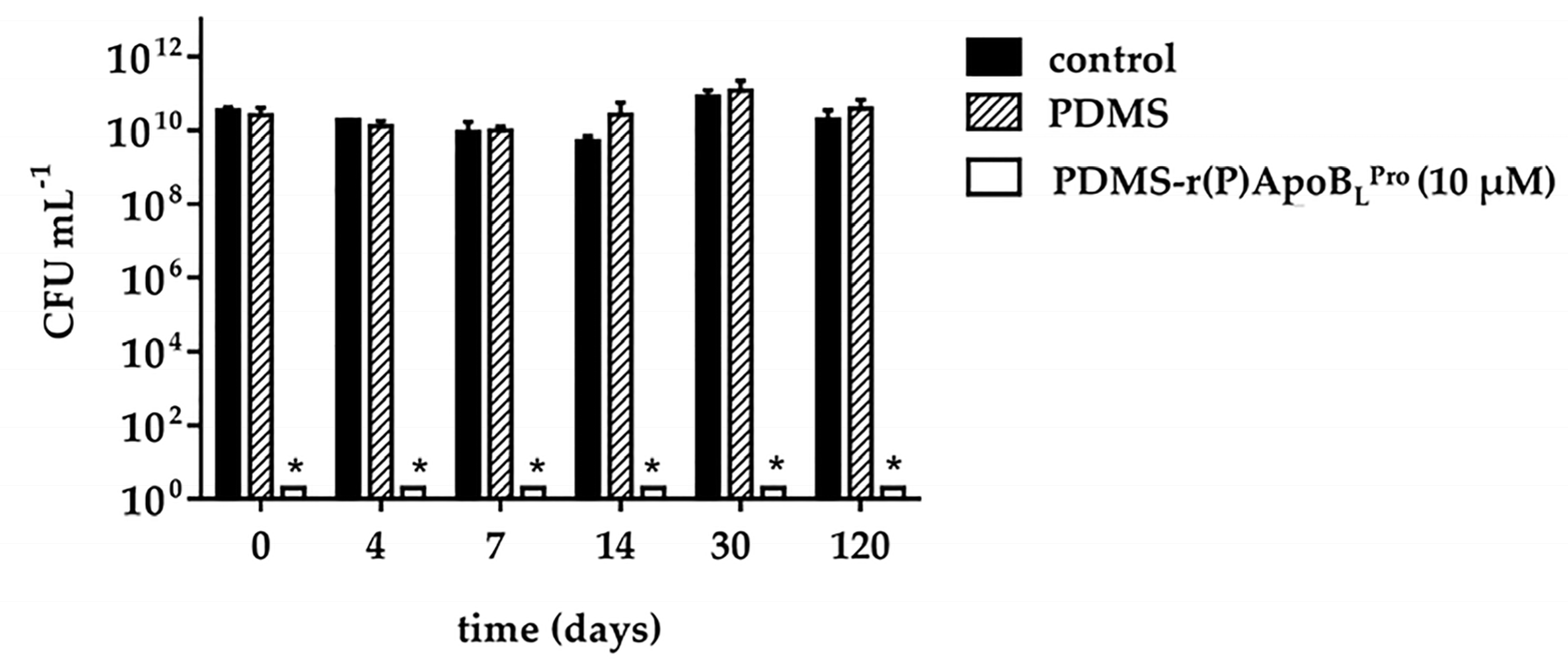
2.6. Anti-Infective Properties of PDMS Functionalized with r(P)ApoBLPro Peptide
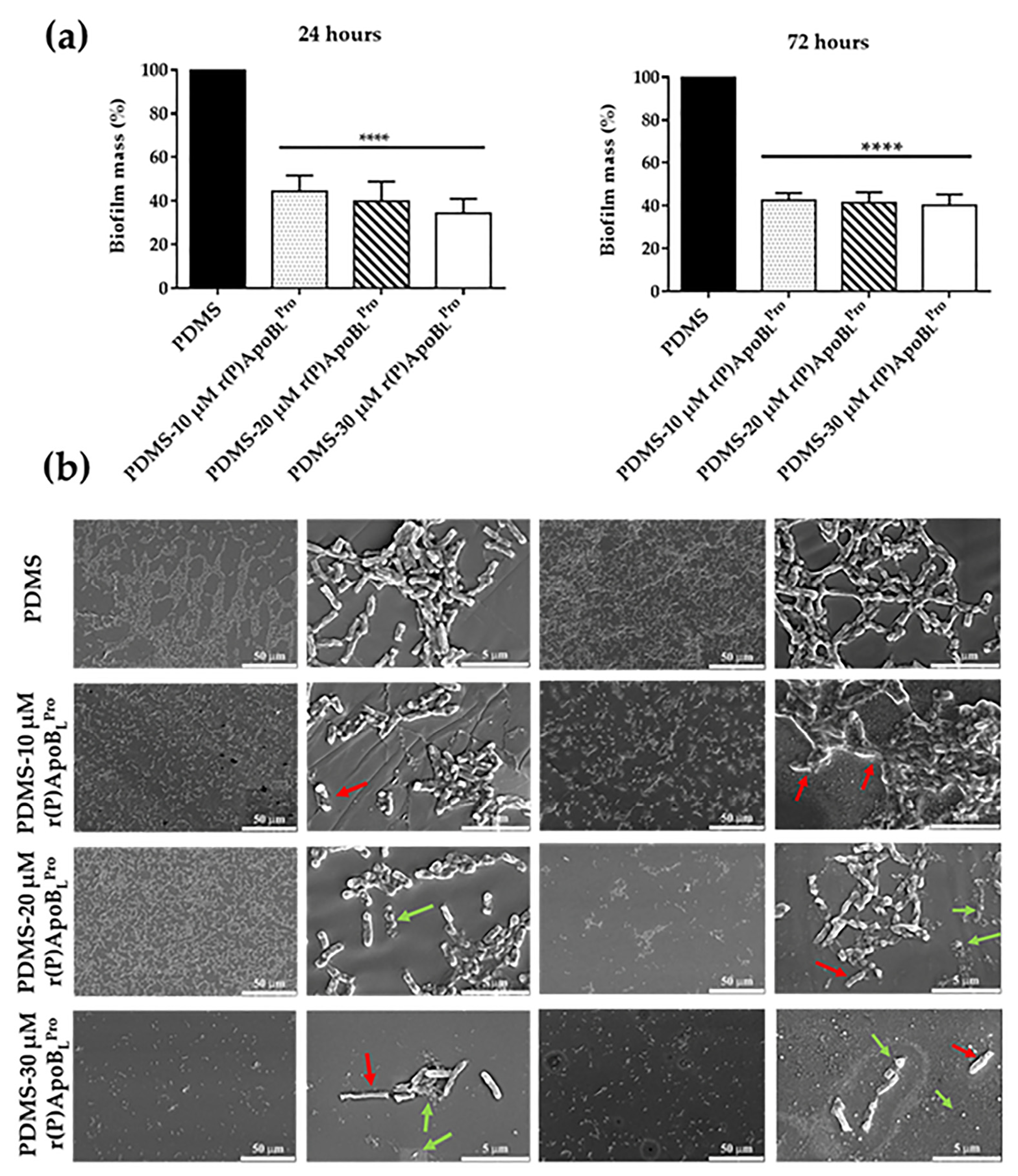
2.7. Anti-Biofilm Activity of PDMS Functionalized with r(P)ApoBLPro Peptide
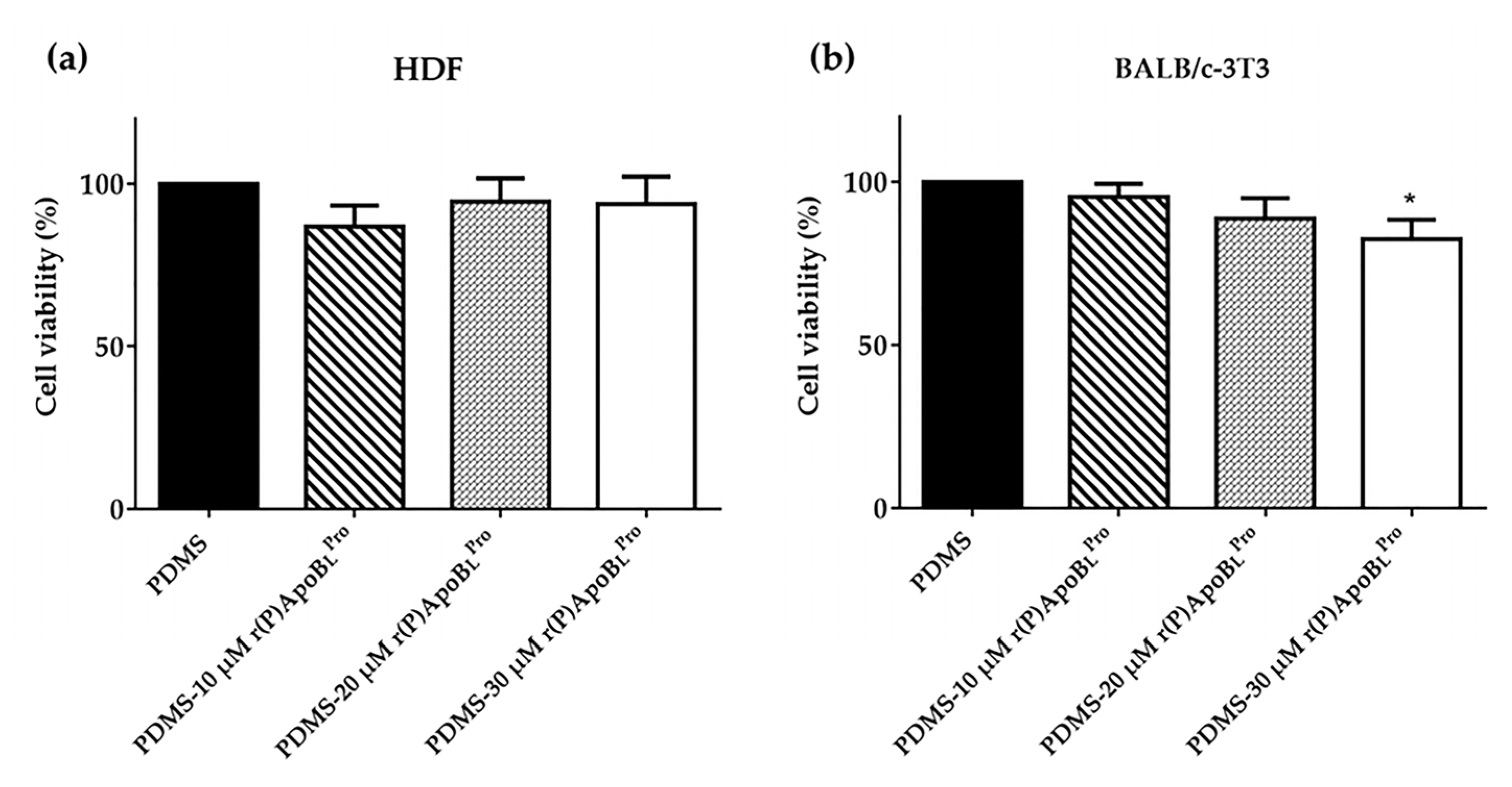
2.8. Biocompatibility of PDMS Functionalized with r(P)ApoBLPro Peptide
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains and Growth Conditions
4.3. Peptide Production
4.4. Preparation of PDMS Polymer Loaded with r(P)ApoBLPro Peptide
4.5. Characterization of PDMS Polymer Loaded with r(P)ApoBLPro Peptide by Fourier-Transform InfraTed (FTIR) Spectroscopy Analyses
4.6. Characterization of PDMS Polymer Loaded with r(P)ApoBLPro Peptide by Water Contact Angle Measurements
4.7. Antimicrobial Activity Assays
4.8. Kinetic Analysis of the Antimicrobial Activity of PDMS Polymer Loaded with r(P)ApoBLPro Peptide
4.9. Evaluation of Peptide Release from PDMS by Quartz Crystal Microbalance (QCM) Analyses
4.10. Evaluation of the Stability of PDMS Polymer Loaded with r(P)ApoBLPro Peptide over Time
4.11. Evaluation of the Anti-Infective Properties of PDMS Polymer Loaded with r(P)ApoBLPro Peptide
4.12. Evaluation of the Antimicrobial Properties of PDMS Polymer Loaded with r(P)ApoBLPro Peptide by Scanning Electron Microscopy (SEM) Analyses
4.13. Evaluation of the Antibiofilm Properties of PDMS Polymer Loaded with r(P)ApoBLPro Peptide by Crystal Violet Assay
4.14. Analysis of the Biocompatibility of PDMS Polymer Loaded with r(P)ApoBLPro Peptide
4.15. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenke, P.; Kovacs, B.; Jäckel, M.; Nagy, E. The role of biofilm infection in urology. World J. Urol. 2006, 24, 13–20. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Salmanov, A.; Litus, V.; Vdovychenko, S.; Litus, O.; Davtian, L.; Ubogov, S.; Bisyuk, Y.; Drozdova, A.; Vlasenko, I. Healthcare-associated infections in intensive care units. Wiad Lek. 2019, 72, 963–969. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef]
- Feneley, R.C.L.; Hopley, I.B.; Wells, P.N.T. Urinary catheters: History, current status, adverse events and research agenda. J. Med. Eng. Technol. 2015, 39, 459–470. [Google Scholar] [CrossRef]
- Barbadoro, P.; Labricciosa, F.M.; Recanatini, C.; Gori, G.; Tirabassi, F.; Martini, E.; Gioia, M.G.; D’Errico, M.M.; Prospero, E. Catheter-associated urinary tract infection: Role of the setting of catheter insertion. Am. J. Infect. Control 2015, 43, 707–710. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2016, 50, 20–40. [Google Scholar] [CrossRef]
- Shuman, E.K.; Chenoweth, C.E. Urinary Catheter-Associated Infections. Infect. Dis. Clin. N. Am. 2018, 32, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Urinary Catheter-Associated Infections. Infect. Dis. Clin. N. Am. 2012, 26, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Cortese, Y.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of Catheter-Associated Urinary Tract Infections and In Vitro Urinary Tract Models. J. Health Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K.D.V. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Busscher, H.J.; Van Der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef]
- Milo, S.; Hathaway, H.; Nzakizwanayo, J.; Alves, D.R.; Esteban, P.P.; Jones, B.V.; Jenkins, A.T.A. Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage. J. Mater. Chem. B 2017, 5, 5403–5411. [Google Scholar] [CrossRef]
- He, S.; Zhou, P.; Wang, L.; Xiong, X.; Zhang, Y.; Deng, Y.; Wei, S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef]
- Asri, L.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; Van Der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A Shape-Adaptive, Antibacterial-Coating of Immobilized Quaternary-Ammonium Compounds Tethered on Hyperbranched Polyurea and its Mechanism of Action. Adv. Funct. Mater. 2013, 24, 346–355. [Google Scholar] [CrossRef]
- Turner, R.J. Metal-based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef]
- Neoh, K.G.; Li, M.; Kang, E.-T.; Chiong, E.; Tambyah, P.A. Surface modification strategies for combating catheter-related complications: Recent advances and challenges. J. Mater. Chem. B 2017, 5, 2045–2067. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Ko, M.; Crowe, A.; Av-Gay, Y. Comparative efficacy of commercially available and emerging antimi-crobial urinary catheters against bacteriuria caused by E. coli in vitro. Urology 2011, 78, 334–339. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Sultana, A.; Luo, H.; Ramakrishna, S. Antimicrobial Peptides and Their Applications in Biomedical Sector. Antibiotics 2021, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Gaglione, R.; Dell’Olmo, E.; Bosso, A.; Chino, M.; Pane, K.; Ascione, F.; Itri, F.; Caserta, S.; Amoresano, A.; Lombardi, A.; et al. Novel human bioactive peptides identified in Apolipoprotein B: Evaluation of their therapeutic potential. Biochem. Pharmacol. 2017, 130, 34–50. [Google Scholar] [CrossRef]
- Gaglione, R.; Smaldone, G.; Cesaro, A.; Rumolo, M.; De Luca, M.; Di Girolamo, R.; Petraccone, L.; Del Vecchio, P.; Oliva, R.; Notomista, E.; et al. Impact of a Single Point Mutation on the Antimicrobial and Fibrillogenic Properties of Cryptides from Human Apolipoprotein B. Pharmaceuticals 2021, 14, 631. [Google Scholar] [CrossRef]
- Gaglione, R.; Pane, K.; Dell’Olmo, E.; Cafaro, V.; Pizzo, E.; Olivieri, G.; Notomista, E.; Arciello, A. Cost-effective production of recombinant peptides in Escherichia coli. New Biotechnol. 2019, 51, 39–48. [Google Scholar] [CrossRef]
- Gaglione, R.; Cesaro, A.; Dell’Olmo, E.; DELLA Ventura, B.; Casillo, A.; Di Girolamo, R.; Velotta, R.; Notomista, E.; Veldhuizen, E.J.A.; Corsaro, M.M.; et al. Effects of human antimicrobial cryptides identified in apolipoprotein B depend on specific features of bacterial strains. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Gaglione, R.; Cesaro, A.; Dell’Olmo, E.; Di Girolamo, R.; Tartaglione, L.; Pizzo, E.; Arciello, A. Cryptides Identified in Human Apolipoprotein B as New Weapons to Fight Antibiotic Resistance in Cystic Fibrosis Disease. Int. J. Mol. Sci. 2020, 21, 2049. [Google Scholar] [CrossRef]
- Gaglione, R.; Pizzo, E.; Notomista, E.; de la Fuente-Nunez, C.; Arciello, A. Host Defence Cryptides from Human Apolipo-proteins: Applications in Medicinal Chemistry. Curr. Top. Med. Chem. 2020, 20, 1324–1337. [Google Scholar] [CrossRef]
- Arciello, A. Host Defence Peptides in Medicinal Chemistry: Identification, Engineering, Characterization and Beyond. Curr. Top. Med. Chem. 2020, 20, 1235–1237. [Google Scholar] [CrossRef]
- Dell’Olmo, E.; Gaglione, R.; Cesaro, A.; Cafaro, V.; Teertstra, W.R.; de Cock, H.; Notomista, E.; Haagsman, H.P.; Veldhuizen, E.J.A.; Arciello, A. Host defence peptides identified in human apolipoprotein B as promising antifungal agents. Appl. Microbiol. Biotechnol. 2021, 105, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Dell’Olmo, E.; Gaglione, R.; Sabbah, M.; Schibeci, M.; Cesaro, A.; Di Girolamo, R.; Porta, R.; Arciello, A. Host defense peptides identified in human apolipoprotein B as novel food biopreservatives and active coating components. Food Microbiol. 2021, 99, 103804. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Torres, M.D.T.; Gaglione, R.; Dell’Olmo, E.; Di Girolamo, R.; Bosso, A.; Pizzo, E.; Haagsman, H.P.; Veldhuizen, E.J.A.; de la Fuente-Nunez, C.; et al. Synthetic Antibiotic Derived from Sequences Encrypted in a Protein from Human Plasma. ACS Nano 2022, 16, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Fisher, L.E.; Hook, A.L.; Ashraf, W.; Yousef, A.; Barrett, D.A.; Scurr, D.J.; Chen, X.; Smith, E.F.; Fay, M.; Parmenter, C.D.; et al. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J. Control. Release 2015, 202, 57–64. [Google Scholar] [CrossRef]
- Cahill, D.J.; Fry, C.H.; Foxall, P.J. Variation in urine composition in the human urinary tract: Evidence of urothelial function in situ? J. Urol. 2003, 169, 871–874. [Google Scholar] [CrossRef]
- Olmo, J.A.-D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- González-Rivera, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tinè, M.R. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. [Google Scholar] [CrossRef]
- Anjum, S.; Singh, S.; Benedicte, L.; Roger, P.; Panigrahi, M.; Gupta, B. Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances. Glob. Chall. 2017, 2, 1700068. [Google Scholar] [CrossRef]
- Andrade, V.L.; Fernandes, F.A. Prevention of catheter-associated urinary tract infection: Implementation strategies of in-ternational guidelines. Rev. Lat. Am. Enferm. 2016, 24, e2678. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Sabir, N.; Ikram, A.; Zaman, G.; Satti, L.; Gardezi, A.; Ahmed, A.; Ahmed, P. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am. J. Infect. Control 2017, 45, 1101–1105. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Francolini, I. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Armugam, A.; Teong, S.P.; Lim, D.S.W.; Chan, S.P.; Yi, G.; Yew, D.S.; Beh, C.W.; Zhang, Y. Broad spectrum antimicrobial PDMS-based biomaterial for catheter fabrication. Biomater. Res. 2021, 25, 1–13. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chow, L.N.; Mookherjee, N. Cationic host defence peptides: Multifaceted role in immune modulation and in-flammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of an-timicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef]
- Moreira, J.M.; Araújo, J.D.; Miranda, J.M.; Simões, M.; Melo, L.F.; Mergulhão, F.J. The effects of surface properties on Escherichia coli adhesion are modulated by shear stress. Colloids Surf. B Biointerfaces 2014, 123, 1–7. [Google Scholar] [CrossRef]
- Woodward, S. Use of lubricant in female urethral catheterization. Br. J. Nurs. 2005, 14, 1022–1023. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Mishra, B.; Basu, A.; Chua, R.R.Y.; Saravanan, R.; Tambyah, P.A.; Ho, B.; Chang, M.W.; Leong, S.S.J. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: Evaluation against urinary tract infection pathogens. J. Mater. Chem. B 2014, 2, 1706–1716. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S.J. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Chua, R.R.; Bow, H.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S. Development of a catheter functionalized by a poly-dopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.; Hancock, R.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Delfino, I.; Portaccio, M.B.E.; DELLA Ventura, B.; Mita, D.G.; Lepore, M. Enzyme distribution and secondary structure of sol–gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater. Sci. Eng. C 2013, 33, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. Part A 2006, 80, 333–341. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Janshoff, A.; Steinem, C.; Sieber, M.; el Bayâ, A.; Schmidt, M.A.; Galla, H.-J. Quartz crystal microbalance investigation of the interaction of bacterial toxins with ganglioside containing solid supported membranes. Eur. Biophys. J. 1997, 26, 261–270. [Google Scholar] [CrossRef]
- Fulgione, A.; Cimafonte, M.; Della Ventura, B.; Iannaccone, M.; Ambrosino, C.; Capuano, F.; Proroga, Y.T.R.; Velotta, R.; Capparelli, R. QCM-based immunosensor for rapid detection of Salmonella Typhimurium in food. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Funari, R.; Della Ventura, B.; Carrieri, R.; Morra, L.; Lahoz, E.; Gesuele, F.; Altucci, C.; Velotta, R. Detection of parathion and patulin by quartz-crystal microbalance functionalized by the photonics immobilization technique. Biosens. Bioelectron. 2015, 67, 224–229. [Google Scholar] [CrossRef]
- Salam, F.; Uludag, Y.; Tothill, I.E. Real-time and sensitive detection of Salmonella Typhimurium using an automated quartz crystal microbalance (QCM) instrument with nanoparticles amplification. Talanta 2013, 115, 761–767. [Google Scholar] [CrossRef]
- Zanfardino, A.; Bosso, A.; Gallo, G.; Pistorio, V.; Di Napoli, M.; Gaglione, R.; Dell’Olmo, E.; Varcamonti, M.; Notomista, E.; Arciello, A.; et al. Human apolipoprotein E as a reservoir of cryptic bioactive peptides: The case of ApoE 133-167. J. Pept. Sci. 2018, 24, e3095. [Google Scholar] [CrossRef] [PubMed]

| MBC (µM) | ||
|---|---|---|
| Strains | r(P)ApoBLPro | PDMS-r(P)ApoBLPro |
| Escherichia coli ATCC 25922 | 5 | 10 |
| Staphylococcus aureus ATCC 29213 | 10–20 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Gaglione, R.; Della Ventura, B.; Cesaro, A.; Di Girolamo, R.; Velotta, R.; Arciello, A. Loading of Polydimethylsiloxane with a Human ApoB-Derived Antimicrobial Peptide to Prevent Bacterial Infections. Int. J. Mol. Sci. 2022, 23, 5219. https://doi.org/10.3390/ijms23095219
De Luca M, Gaglione R, Della Ventura B, Cesaro A, Di Girolamo R, Velotta R, Arciello A. Loading of Polydimethylsiloxane with a Human ApoB-Derived Antimicrobial Peptide to Prevent Bacterial Infections. International Journal of Molecular Sciences. 2022; 23(9):5219. https://doi.org/10.3390/ijms23095219
Chicago/Turabian StyleDe Luca, Maria, Rosa Gaglione, Bartolomeo Della Ventura, Angela Cesaro, Rocco Di Girolamo, Raffaele Velotta, and Angela Arciello. 2022. "Loading of Polydimethylsiloxane with a Human ApoB-Derived Antimicrobial Peptide to Prevent Bacterial Infections" International Journal of Molecular Sciences 23, no. 9: 5219. https://doi.org/10.3390/ijms23095219
APA StyleDe Luca, M., Gaglione, R., Della Ventura, B., Cesaro, A., Di Girolamo, R., Velotta, R., & Arciello, A. (2022). Loading of Polydimethylsiloxane with a Human ApoB-Derived Antimicrobial Peptide to Prevent Bacterial Infections. International Journal of Molecular Sciences, 23(9), 5219. https://doi.org/10.3390/ijms23095219