The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration
Abstract
1. Introduction
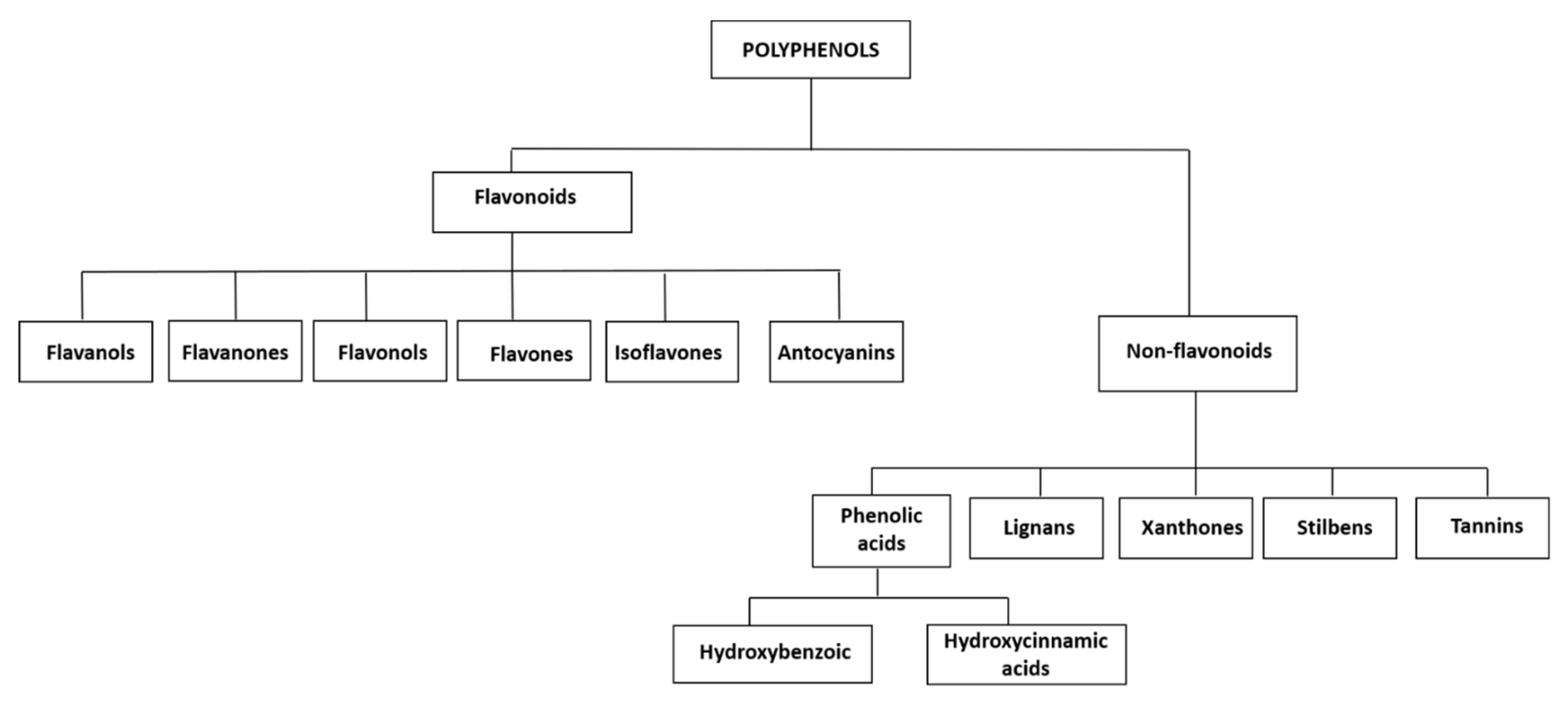
2. Polyphenols Classification
3. Flavonoids
3.1. Flavanols
3.2. Flavanones
3.3. Flavonols
3.4. Flavones
3.5. Isoflavones
4. Non-Flavonoids
4.1. Phenolic Acids: Hydroxybenzoic Derivatives
4.2. Phenolic Acids: Hydroxycinnamic Acids
4.3. Lignans
4.4. Stilbenes
4.5. Tannins
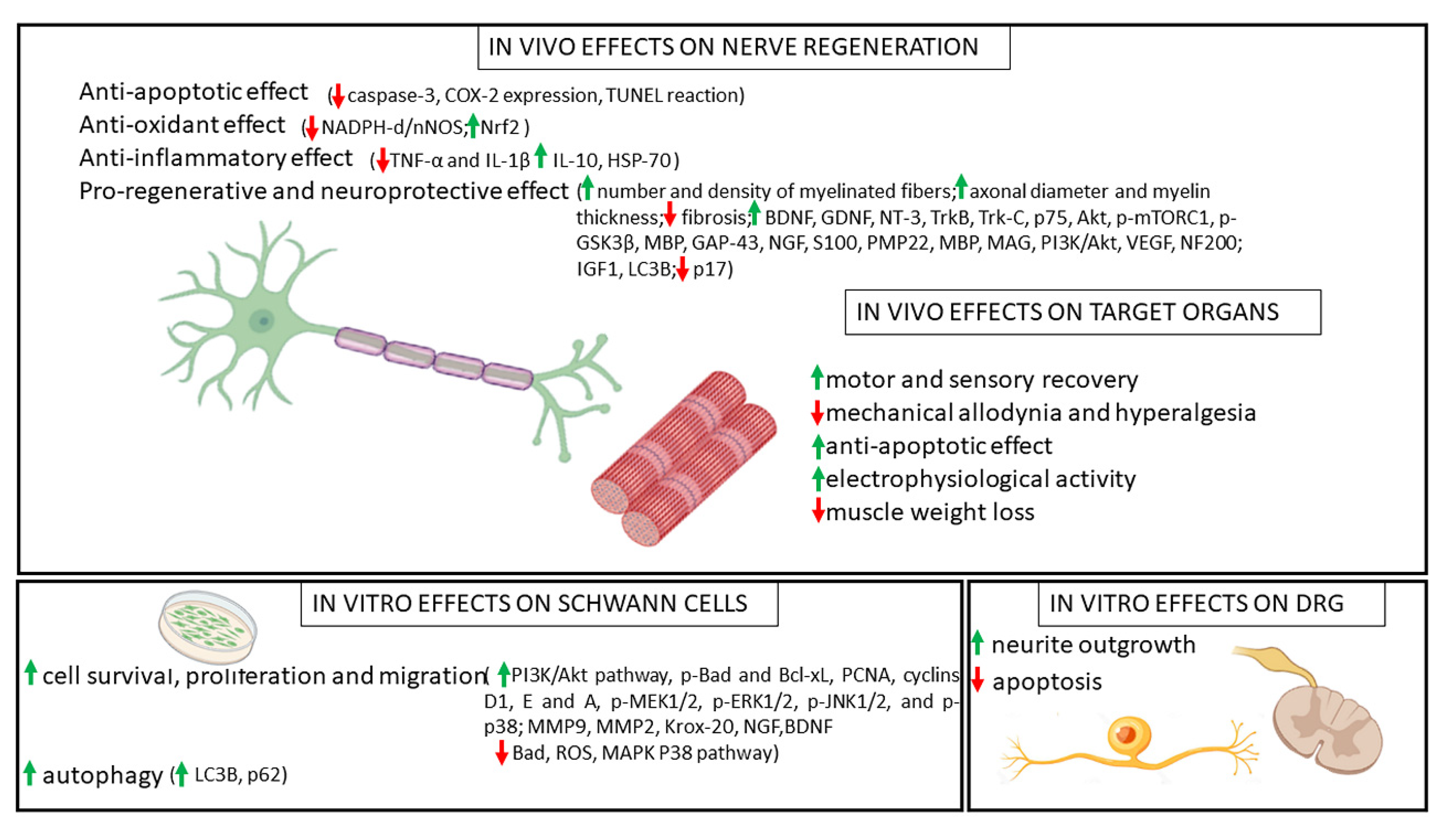
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geuna, S.; Muratori, L.; Fregnan, F.; Manfredi, M.; Bertolo, R.; Porpiglia, F. Strategies to Improve Nerve Regeneration after Radical Prostatectomy: A Narrative Review. Minerva Urol. E Nefrol. 2018, 70, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Wolfe, S. Peripheral Nerve Injury and Repair. J. Am. Acad. Orthop. Surg. 2000, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A Biomaterials Approach to Peripheral Nerve Regeneration: Bridging the Peripheral Nerve Gap and Enhancing Functional Recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral Nerve Injury, Scarring, and Recovery. Connect. Tissue Res. 2018, 60, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, K.D.; Große-Hartlage, L.; Daeschler, S.C.; Rhodius, P.; Böcker, A.; Beyersdorff, M.; Kern, A.O.; Kneser, U.; Harhaus, L. Acute and Long-Term Costs of 268 Peripheral Nerve Injuries in the Upper Extremity. PLoS ONE 2020, 15, e0229530. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.L.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- el Soury, M.; Fornasari, B.E.; Carta, G.; Zen, F.; Haastert-Talini, K.; Ronchi, G. The Role of Dietary Nutrients in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7417. [Google Scholar] [CrossRef] [PubMed]
- Yow, Y.Y.; Goh, T.K.; Nyiew, K.Y.; Lim, L.W.; Phang, S.M.; Lim, S.H.; Ratnayeke, S.; Wong, K.H. Therapeutic Potential of Complementary and Alternative Medicines in Peripheral Nerve Regeneration: A Systematic Review. Cells 2021, 10, 2194. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.T.; Larrosa, M. Polyphenol-Rich Foods for Human Health and Disease. Nutrients 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Vyas, M.; Dua, K.; Khursheed, R.; et al. Improved Neuroprotective Activity of Fisetin through SNEDDS in Ameliorating the Behavioral Alterations Produced in Rotenone-Induced Parkinson’s Model. Environ. Sci. Pollut. Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic Plants: Antioxidant Capacity and Polyphenol Characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, D.; Kocman, A.E.; Yildirim, E.; Ozatik, O.; Aydin, S.; Kose, A. Comparison of the Effects of Curcumin, Tramadol and Surgical Treatments on Neuropathic Pain Induced by Chronic Constriction Injury in Rats. Turk. Neurosurg. 2018, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Paduch, R.; Kukuła-Koch, W.; Polak-Berecka, M.; Waśko, A. Study on Biological Activity of Bread Enriched with Natural Polyphenols in Terms of Growth Inhibition of Tumor Intestine Cells. J. Med. Food 2020, 23, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kankowski, S.; Grothe, C.; Haastert-Talini, K. Neuropathic Pain: Spotlighting Anatomy, Experimental Models, Mechanisms, and Therapeutic Aspects. Eur. J. Neurosci. 2021, 54, 4475–4496. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed]
- D’Evoli, L.; Tarozzi, A.; Hrelia, P.; Lucarini, M.; Cocchiola, M.; Gabrielli, P.; Franco, F.; Morroni, F.; Cantelli-Forti, G.; Lombardi-Boccia, G. Influence of Cultivation System on Bioactive Molecules Synthesis in Strawberries: Spin-off on Antioxidant and Antiproliferative Activity. J. Food Sci. 2010, 75, C94–C99. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Zawani, M.; Fauzi, M.B. Epigallocatechin Gallate: The Emerging Wound Healing Potential of Multifunctional Biomaterials for Future Precision Medicine Treatment Strategies. Polymers 2021, 13, 3656. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-Gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.A.; Rahman, R.M.A.; Appleton, I. Mechanisms of Action of Green Tea Catechins, with a Focus on Ischemia-Induced Neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Renno, W.M.; Al-Maghrebi, M.; Al-Banaw, A. (−)-Epigallocatechin-3-Gallate (EGCG) Attenuates Functional Deficits and Morphological Alterations by Diminishing Apoptotic Gene Overexpression in Skeletal Muscles after Sciatic Nerve Crush Injury. Naunyn-Schmiedebergs Arch. Pharmacol. 2012, 385, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Renno, W.M.; Al-Maghrebi, M.; Alshammari, A.; George, P. (−)-Epigallocatechin-3-Gallate (EGCG) Attenuates Peripheral Nerve Degeneration in Rat Sciatic Nerve Crush Injury. Neurochem. Int. 2013, 62, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Renno, W.M.; Benov, L.; Khan, K.M. Possible Role of Antioxidative Capacity of (−)-Epigallocatechin-3-Gallate Treatment in Morphological and Neurobehavioral Recovery after Sciatic Nerve Crush Injury. J. Neurosurg. Spine 2017, 27, 593–613. [Google Scholar] [CrossRef]
- Yildirim, A.E.; Dalgic, A.; Divanlioglu, D.; Akdag, R.; Cetinalp, N.E.; Alagoz, F.; Helvacioglu, F.; Take, G.; Guvenc, Y.; Koksal, I.; et al. Biochemical and Histopathological Effects of Catechin on Experimental Peripheral Nerve Injuries. Turk. Neurosurg. 2015, 25, 453–460. [Google Scholar] [CrossRef]
- Renno, W.M.; Khan, K.M.; Benov, L. Is There a Role for Neurotrophic Factors and Their Receptors in Augmenting the Neuroprotective Effect of (−)-Epigallocatechin-3-Gallate Treatment of Sciatic Nerve Crush Injury? Neuropharmacology 2016, 102, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kian, K.; Khalatbary, A.R.; Ahmadvand, H.; Karimpour Malekshah, A.; Shams, Z. Neuroprotective Effects of (−)-Epigallocatechin-3-Gallate (EGCG) against Peripheral Nerve Transection-Induced Apoptosis. Nutr. Neurosci. 2019, 22, 578–586. [Google Scholar] [CrossRef]
- Wei, I.H.; Tu, H.C.; Huang, C.C.; Tsai, M.H.; Tseng, C.Y.; Shieh, J.Y. (−)-Epigallocatechin Gallate Attenuates NADPH-d/NNOS Expression in Motor Neurons of Rats Following Peripheral Nerve Injury. BMC Neurosci. 2011, 12, 52. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Cao, C.; Man, Y.; Qu, Y. Evaluation of Epigallocatechin-3-Gallate Modified Collagen Membrane and Concerns on Schwann Cells. BioMed Res. Int. 2017, 2017, 9641801. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, R.; Kakinoki, R.; Okamoto, T.; Matsumoto, T.; Hyon, S.H.; Nakamura, T. Successful Storage of Peripheral Nerve before Transplantation Using Green Tea Polyphenol: An Experimental Study in Rats. Exp. Neurol. 2003, 184, 688–696. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kakinoki, R.; Ikeguchi, R.; Hyon, S.H.; Nakamura, T. Optimal Conditions for Peripheral Nerve Storage in Green Tea Polyphenol: An Experimental Study in Animals. J. Neurosci. Methods 2005, 145, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, R.; Kakinoki, R.; Matsumoto, T.; Hyon, S.H.; Nakamura, T. Peripheral Nerve Allografts Stored in Green Tea Polyphenol Solution. Transplantation 2005, 79, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kakinoki, R.; Ikeguchi, R.; Yamakawa, T.; Ohta, S.; Fujita, S.; Noguchi, T.; Duncan, S.F.M.; Hyon, S.H.; Nakamura, T. Storage and Allogeneic Transplantation of Peripheral Nerve Using a Green Tea Polyphenol Solution in a Canine Model. J. Brachial Plex. Peripher. Nerve Inj. 2010, 5, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.H.; Zhen, P.; Li, S.S.; Liang, X.Y.; Gao, M.X.; Tian, Q.; Li, X.S. Allograft Pretreatment for the Repair of Sciatic Nerve Defects: Green Tea Polyphenols versus Radiation. Neural Regen. Res. 2015, 10, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Li, H.; Lao, J. Green Tea Polyphenols Promote Functional Recovery from Peripheral Nerve Injury in Rats. Med. Sci. Monit. 2020, 26, e923806. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Nasrolahi, M.; Azimi, M.; Salehi, M. Hesperidin Promotes Peripheral Nerve Regeneration Based on Tissue Engineering Strategy Using Alginate/Chitosan Hydrogel: In Vitro and in Vivo Study. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 299–308. [Google Scholar] [CrossRef]
- Basu, P.; Basu, A. In Vitro and In Vivo Effects of Flavonoids on Peripheral Neuropathic Pain. Molecules 2020, 25, 1171. [Google Scholar] [CrossRef]
- Rao, P.N.; Mainkar, O.; Bansal, N.; Rakesh, N.; Haffey, P.; Urits, I.; Orhurhu, V.; Kaye, A.D.; Urman, R.D.; Gulati, A.; et al. Flavonoids in the Treatment of Neuropathic Pain. Curr. Pain Headache Rep. 2021, 25, 43. [Google Scholar] [CrossRef]
- Samadian, H.; Vaez, A.; Ehterami, A.; Salehi, M.; Farzamfar, S.; Sahrapeyma, H.; Norouzi, P. Sciatic Nerve Regeneration by Using Collagen Type I Hydrogel Containing Naringin. J. Mater. Sci. Mater. Med. 2019, 30, 107. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.H.; Samadian, H.; Davani, S.T.; Kolarijani, N.R.; Mogharabian, N.; Salami, M.S.; Salehi, M. Peripheral Nerve Regeneration in Rats by Chitosan/Alginate Hydrogel Composited with Berberine and Naringin Nanoparticles: In Vitro and in Vivo Study. J. Mol. Liq. 2020, 318, 114226. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Heimfarth, L.; Passos, F.R.S.; Miguel-dos-Santos, R.; Mingori, M.R.; Moreira, J.C.F.; Lauton, S.S.; Barreto, R.S.S.; Araújo, A.A.S.; Oliveira, A.P.; et al. Naringenin Complexed with Hydroxypropyl-β-Cyclodextrin Improves the Sciatic Nerve Regeneration through Inhibition of P75NTR and JNK Pathway. Life Sci. 2020, 241, 117102. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.M. Quercetin-A Flavonoid: A Systematic Review. J. Pharm. Sci. Res. 2016, 8, 878–880. [Google Scholar]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin Suppresses NLRP3 Inflammasome Activation and Attenuates Histopathology in a Rat Model of Spinal Cord Injury. Spinal Cord 2016, 54, 592–596. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective Effect of Quercetin in Primary Neurons against Aβ(1–42): Relevance to Alzheimer’s Disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef]
- Wang, W.; Huang, C.Y.; Tsai, F.J.; Tsai, C.C.; Yao, C.H.; Chen, Y.S. Growth-Promoting Effects of Quercetin on Peripheral Nerves in Rats. Int. J. Artif. Organs 2011, 34, 1095–1105. [Google Scholar] [CrossRef]
- Chen, M.M.; Qin, J.; Chen, S.J.; Yao, L.M.; Zhang, L.Y.; Yin, Z.Q.; Liao, H. Quercetin Promotes Motor and Sensory Function Recovery Following Sciatic Nerve-Crush Injury in C57BL/6J Mice. J. Nutr. Biochem. 2017, 46, 57–67. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, X.; Wang, L.; Zhang, Q.; Ma, W.; Huang, Z.; Bao, Y.; Zhong, L.; Sun, H.; Ding, F.; et al. Isoquercitrin Promotes Peripheral Nerve Regeneration through Inhibiting Oxidative Stress Following Sciatic Crush Injury in Mice. Ann. Transl. Med. 2019, 7, 680. [Google Scholar] [CrossRef]
- Türedi, S.; Yuluğ, E.; Alver, A.; Bodur, A.; İnce, İ. A Morphological and Biochemical Evaluation of the Effects of Quercetin on Experimental Sciatic Nerve Damage in Rats. Exp. Med. 2018, 15, 3215–3224. [Google Scholar] [CrossRef]
- Thipkaew, C.; Wattanathorn, J.; Muchimapura, S. Electrospun Nanofibers Loaded with Quercetin Promote the Recovery of Focal Entrapment Neuropathy in a Rat Model of Streptozotocin-Induced Diabetes. BioMed Res. Int. 2017, 2017, 2017493. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, C.; Qi, Z.P.; Guo, W.L.; You, D.; Li, R.; Zhu, Z. Localised Delivery of Quercetin by Thermo-Sensitive PLGA-PEG-PLGA Hydrogels for the Treatment of Brachial Plexus Avulsion. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of Bioactive Compounds and Antioxidant Activity of Fruit Beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Lin, H.H.; Huang, C.Y. Characterization of Flavonol Inhibition of DnaB Helicase: Real-Time Monitoring, Structural Modeling, and Proposed Mechanism. J. Biomed. Biotechnol. 2012, 2012, 735368. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Meyer, E.; Mori, M.A.; Campos, A.C.; Andreatini, R.; Guimarães, F.S.; Milani, H.; de Oliveira, R.M.W. Myricitrin Induces Antidepressant-like Effects and Facilitates Adult Neurogenesis in Mice. Behav. Brain Res. 2017, 316, 59–65. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin Ameliorates Scopolamine-Induced Memory Impairment in Mice via Inhibiting Acetylcholinesterase and down-Regulating Brain Iron. Biochem Biophys Res. Commun 2017, 490, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ge, J.; Ye, L.; Tang, H.; Bai, X. Myricetin Promotes Peripheral Nerve Regeneration in Rat Model of Sciatic Nerve Injury via Regulation of BDNFAkt/GSK-3β/MTOR Signalling Pathway. Trop. J. Pharm. Res. 2019, 17, 2355–2363. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharm. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, J.; Jeong, N.Y.; Chung, H.J. The Natural Plant Flavonoid Apigenin Is a Strong Antioxidant That Effectively Delays Peripheral Neurodegenerative Processes. Anat. Sci. Int. 2019, 94, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and Molecular Mechanisms of Genistein in Prevention and Treatment of Diseases: An Overview. J. Food Biochem 2021, 45, e13972. [Google Scholar] [CrossRef]
- Ozbek, Z.; Aydin, H.E.; Kocman, A.E.; Ozkara, E.; Sahin, E.; Bektur, E.; Vural, M.; Kose, A.; Arslantas, A.; Baycu, C. Neuroprotective Effect of Genistein in Peripheral Nerve Injury. Turk. Neurosurg. 2017, 27, 816–822. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid. Based Complement. Altern. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Sacerdote, P.; Trovato, A.E.; Colleoni, M. Genistein, a Natural Phytoestrogen from Soy, Relieves Neuropathic Pain Following Chronic Constriction Sciatic Nerve Injury in Mice: Anti-Inflammatory and Antioxidant Activity. J. Neurochem. 2008, 107, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The Soy Isoflavone Genistein Reverses Oxidative and Inflammatory State, Neuropathic Pain, Neurotrophic and Vasculature Deficits in Diabetes Mouse Model. Eur. J. Pharm. 2011, 650, 694–702. [Google Scholar] [CrossRef]
- Yang, S.H.; Liao, C.C.; Chen, Y.; Syu, J.P.; Jeng, C.J.; Wang, S.M. Daidzein Induces Neuritogenesis in DRG Neuronal Cultures. J. Biomed. Sci. 2012, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects, Applications; Technomic Pub. Co.: Lancaster, UK, 1995; ISBN 9781566762793. [Google Scholar]
- Clifford, M.N.; Scalbert, A. Review Ellagitannins-Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharm. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.T.; Liao, H.E.; Shibu, M.A.; Ho, T.J.; Padma, V.V.; Tsai, F.J.; Chung, L.C.; Day, C.H.; Lin, C.C.; Huang, C.Y. Nerve Regeneration Potential of Protocatechuic Acid in RSC96 Schwann Cells by Induction of Cellular Proliferation and Migration through IGF-IR-PI3K-Akt Signaling. Chin. J. Physiol. 2015, 58, 412–419. [Google Scholar] [CrossRef]
- Ju, D.T.; Kuo, W.W.; Ho, T.J.; Paul, C.R.; Kuo, C.H.; Viswanadha, V.P.; Lin, C.C.; Chen, Y.S.; Chang, Y.M.; Huang, C.Y. Protocatechuic Acid from Alpinia Oxyphylla Induces Schwann Cell Migration via ERK1/2, JNK and P38 Activation. Am. J. Chin. Med. 2015, 43, 653–665. [Google Scholar] [CrossRef]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive Novel Polyphenols from the Fruit of Manilkara Zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y.; Sarkaki, A.; Bavarsad, K. Gallic Acid Prevents Memory Deficits and Oxidative Stress Induced by Intracerebroventricular Injection of Streptozotocin in Rats. Pharm. Biochem. Behav. 2013, 111, 90–96. [Google Scholar] [CrossRef]
- Latha, R.C.R.; Daisy, P. Insulin-Secretagogue, Antihyperlipidemic and Other Protective Effects of Gallic Acid Isolated from Terminalia Bellerica Roxb. in Streptozotocin-Induced Diabetic Rats. Chem. Biol. Interact. 2011, 189, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Figueira, I.; McDougall, G.J.; Vieira, H.L.A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective Effects of Digested Polyphenols from Wild Blackberry Species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Saba, A.B.; Olowu, E.R.; Dada, R.O.; Akinrinde, A.S. Gallic Acid Ameliorates Cyclophosphamide-Induced Neurotoxicity in Wistar Rats Through Free Radical Scavenging Activity and Improvement in Antioxidant Defense System. J. Diet. Suppl. 2016, 13, 402–419. [Google Scholar] [CrossRef]
- Kaur, S.; Rana, A.C.; Gangwani, S.; Sharma, R. Punica Granatum Attenuates Sciatic Nerve Ligation Induced-Neuropathic Pain. IJPSR 2012, 3, 509–518. [Google Scholar]
- Hajimoradi, M.; Fazilati, M.; Gharib-Naseri, M.K.; Sarkaki, A. Gallic Acid and Exercise Training Improve Motor Function, Nerve Conduction Velocity but Not Pain Sense Reflex after Experimental Sciatic Nerve Crush in Male Rats. Avicenna J. Phytomed. 2015, 5, 288. [Google Scholar] [PubMed]
- Gurkan, G.; Erdogan, M.A.; Yigitturk, G.; Erbas, O. The Restorative Effect of Gallic Acid on the Experimental Sciatic Nerve Damage Model. J. Korean Neurosurg. Soc. 2021, 64, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Shetty, K. Biological Functionality of Ellagic Acid: A Review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef]
- Jain, V.; Pareek, A.; Bhardwaj, Y.R.; Singh, N. Attenuating Effect of Standardized Fruit Extract of Punica Granatum L. in Rat Model of Tibial and Sural Nerve Transection Induced Neuropathic Pain. BMC Complement. Altern Med. 2013, 13, 274. [Google Scholar] [CrossRef]
- Uzar, E.; Alp, H.; Cevik, M.U.; Firat, U.; Evliyaoglu, O.; Tufek, A.; Altun, Y. Ellagic Acid Attenuates Oxidative Stress on Brain and Sciatic Nerve and Improves Histopathology of Brain in Streptozotocin-Induced Diabetic Rats. Neurol. Sci. 2012, 33, 567–574. [Google Scholar] [CrossRef]
- Shaik, M.M.; Kowshik, M. Ellagic Acid Containing Collagen-Chitosan Scaffolds as Potential Antioxidative Bio-Materials for Tissue Engineering Applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 208–215. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef] [PubMed]
- Flourat, A.L.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.H.; Allais, F. Accessing P-Hydroxycinnamic Acids: Chemical Synthesis, Biomass Recovery, or Engineered Microbial Production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio, M.; Araújo, D.; Galberto, M.; da Costa, J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Chen, Q.; Lu, J.; Rapposelli, S.; Pi, R. A Review on the Hybrids of Hydroxycinnamic Acid as Multi-Target-Directed Ligands against Alzheimer’s Disease. Bioorganic. Med. Chem. 2018, 26, 543–550. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Yu, H.; Wang, Q.; Chen, Y.; Xiang, L. Curcumin Promotes Nerve Regeneration and Functional Recovery in Rat Model of Nerve Crush Injury. Neurosci. Lett. 2013, 547, 26–31. [Google Scholar] [CrossRef]
- Liu, G.M.; Xu, K.; Li, J.; Luo, Y.G. Curcumin Upregulates S100 Expression and Improves Regeneration of the Sciatic Nerve Following Its Complete Amputation in Mice. Neural. Regen. Res. 2016, 11, 1304–1311. [Google Scholar] [CrossRef]
- Noorafshan, A.; Omidi, A.; Karbalay-Doust, S. Curcumin Protects the Dorsal Root Ganglion and Sciatic Nerve after Crush in Rat. Pathol. Res. Pract. 2011, 207, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Li, Q. Curcumin Accelerates the Repair of Sciatic Nerve Injury in Rats through Reducing Schwann Cells Apoptosis and Promoting Myelinization. Biomed. Pharmacother. 2017, 92, 1103–1110. [Google Scholar] [CrossRef]
- Moattari, M.; Moattari, F.; Kouchesfahani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M.; Mansouri, K. Curcumin and Biodegradable Membrane Promote Nerve Regeneration and Functional Recovery after Sciatic Nerve Transection in Adult Rats. Ann. Plast. Surg. 2018, 81, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Sun, D.; Chen, Z.; Zhao, W. NGF and PI3K/Akt Signaling Participate in the Ventral Motor Neuronal Protection of Curcumin in Sciatic Nerve Injury Rat Models. Biomed. Pharmacother. 2018, 103, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.M.; et al. Local Low Dose Curcumin Treatment Improves Functional Recovery and Remyelination in a Rat Model of Sciatic Nerve Crush through Inhibition of Oxidative Stress. Neuropharmacology 2018, 139, 98–116. [Google Scholar] [CrossRef]
- Kasmaie, F.M.; Jahromi, Z.; Gazor, R.; Zaminy, A. Comparison of Melatonin and Curcumin Effect at the Light and Dark Periods on Regeneration of Sciatic Nerve Crush Injury in Rats. EXCLI J. 2019, 18, 653–665. [Google Scholar] [CrossRef]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced Sciatic Nerve Regeneration by Poly-L-Lactic Acid/Multi-Wall Carbon Nanotube Neural Guidance Conduit Containing Schwann Cells and Curcumin Encapsulated Chitosan Nanoparticles in Rat. Mater. Sci. Eng. C 2020, 109, 110564. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mahmoodi, H. Improvement of Peripheral Nerve Regeneration Following Nerve Repair by Silicone Tube Filled with Curcumin: A Preliminary Study in the Rat Model. Int. J. Surg. 2013, 11, 819–825. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Guo, X.; Wang, J.; Xiang, Y. Schwann Cell Proliferation and Differentiation That Is Induced by Ferulic Acid through MEK1/ERK1/2 Signalling Promotes Peripheral Nerve Remyelination Following Crush Injury in Rats. Exp. Ther. Med. 2016, 12, 1915–1921. [Google Scholar] [CrossRef]
- Lee, S.C.; Tsai, C.C.; Yao, C.H.; Chen, Y.S.; Wu, M.C. Ferulic Acid Enhances Peripheral Nerve Regeneration across Long Gaps. Evid. Based Complementary Altern. Med. 2013, 2013, 876327. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Danial, M.; Ramli, C.; Sumari, N.A.; Selamat, N.W.; Muhammad, H.; Sanusi, J. Effectiveness of Flaxseed Oil on Peripheral Nerve Regeneration Following Crush Injury of Sciatic Nerve in Rat: Behavioural Analysis and an Electron. Microscopic Study. Malays. J. Med. Health Sci. 2020, 16 (Suppl. 1), 106–111. [Google Scholar]
- Hsu, C.C.; Huang, H.C.; Wu, P.T.; Tai, T.W.; Jou, I.M. Sesame Oil Improves Functional Recovery by Attenuating Nerve Oxidative Stress in a Mouse Model of Acute Peripheral Nerve Injury: Role of Nrf-2. J. Nutr. Biochem. 2016, 38, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural Stilbenes: An Overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chang, W.; Liu, B.; Chen, G.; Yang, Y.; Hao, Y.; Hou, Y.; Li, N. Stilbenes from the Tubers of Bletilla Striata with Potential Anti-Neuroinflammatory Activity. Bioorganic Chem. 2020, 97, 103715. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health Benefits of Resveratrol Administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Ding, Z.; Cao, J.; Shen, Y.; Zou, Y.; Yang, X.; Zhou, W.; Guo, Q.; Huang, C. Resveratrol Promotes Nerve Regeneration via Activation of P300 Acetyltransferase-Mediated VEGF Signaling in a Rat Model of Sciatic Nerve Crush Injury. Front. Neurosci. 2018, 12, 341. [Google Scholar] [CrossRef]
- Bagriyanik, H.A.; Ersoy, N.; Cetinkaya, C.; Ikizoglu, E.; Kutri, D.; Ozcana, T.; Kamanga, L.G.; Kiray, M. The Effects of Resveratrol on Chronic Constriction Injury of Sciatic Nerve in Rats. Neurosci. Lett. 2014, 561, 123–127. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann Cell Autophagy, Myelinophagy, Initiates Myelin Clearance from Injured Nerves. J. Cell Biol. 2015, 210, 153. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Repair Schwann Cell and Its Function in Regenerating Nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Gonzalez-Polo, R.; Pizarro-Estrella, E.; Yakhine-Diop, S.; Rodríguez-Arribas, M.; Gomez-Sanchez, R.; Pedro, J.M.; Fuentes, J. Is the Modulation of Autophagy the Future in the Treatment of Neurodegenerative Diseases? Curr. Top. Med. Chem. 2015, 15, 2152–2174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, J.; Liu, Y.; Huang, D.; Lu, L. Resveratrol Regulates the Recovery of Rat Sciatic Nerve Crush Injury by Promoting the Autophagy of Schwann Cells. Life Sci. 2020, 256, 117959. [Google Scholar] [CrossRef] [PubMed]
- Revin, V.V.; Pinyaev, S.I.; Parchaykina, M.V.; Revina, E.S.; Maksimov, G.V.; Kuzmenko, T.P. The Effect of Resveratrol on the Composition and State of Lipids and the Activity of Phospholipase A 2 During the Excitation and Regeneration of Somatic Nerves. Front. Physiol 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 2010, 38, 421–464. [Google Scholar] [CrossRef]
- Monfared, A.; Ghaee, A.; Ebrahimi-Barough, S. Fabrication of Tannic Acid/Poly(N-Vinylpyrrolidone) Layer-by-Layer Coating on Mg-Based Metallic Glass for Nerve Tissue Regeneration Application. Colloids Surf. B Biointerfaces 2018, 170, 617–626. [Google Scholar] [CrossRef]
- Yildiran, H.; Macit, M.S.; Özata Uyar, G. New Approach to Peripheral Nerve Injury: Nutritional Therapy. Nutr. Neurosci. 2018, 23, 744–755. [Google Scholar] [CrossRef]
- Zhang, L.; Johnson, D.; Johnson, J.A. Deletion of Nrf2 Impairs Functional Recovery, Reduces Clearance of Myelin Debris and Decreases Axonal Remyelination after Peripheral Nerve Injury. Neurobiol. Dis. 2013, 54, 329–338. [Google Scholar] [CrossRef]
- Navarro, X. Functional Evaluation of Peripheral Nerve Regeneration and Target Reinnervation in Animal Models: A Critical Overview. Eur. J. Neurosci. 2016, 43, 271–286. [Google Scholar] [CrossRef]
- Geuna, S.; Tos, P.; Battiston, B.; Guglielmone, R. Verification of the Two-Dimensional Disector, a Method for the Unbiased Estimation of Density and Number of Myelinated Nerve Fibers in Peripheral Nerves. Ann. Anat. 2000, 182, 23–34. [Google Scholar] [CrossRef]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-Film Enhanced Chitosan Nerve Guides for Long-Distance Regeneration of Peripheral Nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef]
- di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; del Bo, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]


| Epigallocatechin Gallate | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Kian et al., 2019 [29] | Sciatic nerve transection; male Sprague Dawley rats |
|
| Renno et al., 2017 [26] | Sciatic nerve crush injury; male Wistar rats |
|
| Renno et al., 2016 [28] | Sciatic nerve crush injury; male Wistar rats |
|
| Yildirim et al., 2014 [27] | Sciatic nerve crush injury; male Albino Wistar rats |
|
| Renno et al., 2013 [25] | Sciatic nerve crush injury; male Wistar rats |
|
| Renno et al., 2012 [24] | Sciatic nerve crush injury; male Wistar rats |
|
| Wei et al., 2011 [30] | Left vagus and hypoglossal nerve crush injury; male Wistar rats |
|
| Green tea extract | ||
| Chen et al., 2020 [37] | Sciatic nerve end-to-end repair; male Wistar rats |
|
| Zhou et al., 2015 [36] | 10 mm-long sciatic nerve defect repaired with allograft; male Wistar rats |
|
| Nakayama et al., 2010 [35] | 30 mm-long ulnar nerve defect repaired with allograft; male and female beagle dog | Nerve fascicles from male dog stored in DMEM containing polyphenol (1 mg/mL) for one week and then transferred to DMEM solution alone for three weeks. These nerve segments were used to repair the right female ulnar nerves. The left ones were repaired with autograft.After nerve repair, the immunosuppressant FK506 administration was started one day before the transplantation, at different doses:
|
| Ikeguchi et al., 2005 [34] | 15 mm-long sciatic nerve defect repaired with allograft; inbred Lewis rats and male Dark Agouti rats |
|
| Matsumoto et al., 2005 [33] | 15 mm-long sciatic nerve defect repaired with allograft; inbred Lewis rats |
|
| Ikeguchi et al., 2003 [32] | 15 mm-long sciatic nerve defect repaired with allograft; male (donor) and female (recipient) Lewis rats |
|
| Hesperidin | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Bagher et al., 2020 [38] | Sciatic nerve crush injury; adult male Wistar rats |
|
| Naringenin | ||
| Ebrahimi et al., 2020 [42] | Sciatic nerve crush injury; male adult Wistar rats |
|
| Oliveira et al., 2020 [43] | Sciatic nerve crush injury; male Swiss mice |
|
| Samadian et al., 2019 [41] | Sciatic nerve crush injury; male Wistar rats |
|
| Quercetin | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Chen et al., 2017 [49] | Sciatic nerve crush injury; male C57BL/6J mice |
|
| Huang et al., 2020 [53] | The C5–C7 nerve roots avulsion; C6 anterior root resection and positioning of the posterior root above the anterior root; female Sprague Dawley rats |
|
| Qiu et al., 2019 [50] | Sciatic nerve crush; male ICR mice |
|
| Turedi et al., 2018 [51] | sciatic nerve crush; Sprague Dawley rats |
|
| Thipkaew et al., 2017 [52] | Sciatic nerve crush; Wistar male rats with streptozotocin (STZ)-induced diabetes |
|
| Wang et al., 2011 [48] | 15 mm rat sciatic nerves gap; adult Sprague Dawley rats |
|
| Myricetin | ||
| Zhang et al., 2018 [62] | Sciatic nerve crush injury; Sprague Dawley rats |
|
| Genistein | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Ozbek et al., 2017 [70] | Sciatic nerve crush injury or end-to-end repair; male Sprague Dawley rats |
|
| Gallic Acid | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Gurkan et al., 2021 [87] | Sciatic nerve end-to-end repair; male albino Sprague Dawley |
|
| Hajimoradi et al., 2014 [86] | Sciatic nerve crush; male Wistar rats |
|
| Curcumin | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Jahromi et al., 2019 [108] | Sciatic nerve injury, 10 mm gap repaired PLLA tube filled with curcumin encapsulated with chitosan nanoparticles; adult male Wistar rats |
|
| Kasmaie et al., 2019 [107] | Sciatic nerve crush injury; male Wistar rats |
|
| Caillaud et al., 2018 [106] | Sciatic nerve crush injury; male Sprague Dawley rats |
|
| Moattari et al., 2018 [104] | End-to-end repair on sciatic nerve; adult male Wistar rats |
|
| Sang et al., 2018 [105] | Sciatic nerve crush injury; male Sprague Dawley rats |
|
| Zhao et al., 2017 [103] | Sciatic nerve crush injury; Male Sprague Dawley rats |
|
| Ma et al., 2013 [100] | Sciatic nerve crush injury; young adult male Sprague Dawley rats |
|
| Mohammadi., 2013 [109] | Sciatic nerve injury, 10 mm gap repaired with a silicone tube; male Wistar rats | Silicone tube filled with 10 µL curcumin (5 mg/mL) dissolved in olive oil. |
| Noorafshan et al., 2011 [102] | A 30 s crush injury induced by a serrated hemostat; adult female Sprague Dawley rats |
|
| Ferulic acid | ||
| Zhu et al., 2016 [110] | Sciatic nerve crush injury; Sprague Dawley rats |
|
| Lee et al., 2013 [111] | 15 mm sciatic nerve gap repaired with silicone rubber tube filled with ferulic acid; adult Sprague Dawley rats |
|
| Flaxseed Oil | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Danial et al., 2020 [113] | Sciatic nerve crush injury; male Sprague Dawley rats |
|
| Sesame Oil | ||
| Hsu et al., 2016 [114] | Sciatic nerve crush; male SPF C57BL/6 mice |
|
| Resveratrol | ||
|---|---|---|
| Ref. | Type of Nerve Lesion and Animal Model | Type of Administration/Experimental Groups |
| Zhang et al., 2020 [123] | Crush injury of sciatic nerve; Sprague Dawley rats |
|
| Revin et al., 2019 [124] | Cut of sciatic nerve; adult Wistar rats | First set of experiment:
|
| Ding et al., 2018 [118] | Sciatic nerve crush injury; adult male Sprague Dawley rats |
|
| Bagriyanik et al., 2014 [119] | Chronic constriction injury of sciatic nerve; male Wistar rats |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muratori, L.; Fregnan, F.; Maurina, M.; Haastert-Talini, K.; Ronchi, G. The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2022, 23, 5177. https://doi.org/10.3390/ijms23095177
Muratori L, Fregnan F, Maurina M, Haastert-Talini K, Ronchi G. The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration. International Journal of Molecular Sciences. 2022; 23(9):5177. https://doi.org/10.3390/ijms23095177
Chicago/Turabian StyleMuratori, Luisa, Federica Fregnan, Monica Maurina, Kirsten Haastert-Talini, and Giulia Ronchi. 2022. "The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration" International Journal of Molecular Sciences 23, no. 9: 5177. https://doi.org/10.3390/ijms23095177
APA StyleMuratori, L., Fregnan, F., Maurina, M., Haastert-Talini, K., & Ronchi, G. (2022). The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration. International Journal of Molecular Sciences, 23(9), 5177. https://doi.org/10.3390/ijms23095177






