Sonochemical Combined Synthesis of Nickel Ferrite and Cobalt Ferrite Magnetic Nanoparticles and Their Application in Glycan Analysis
Abstract
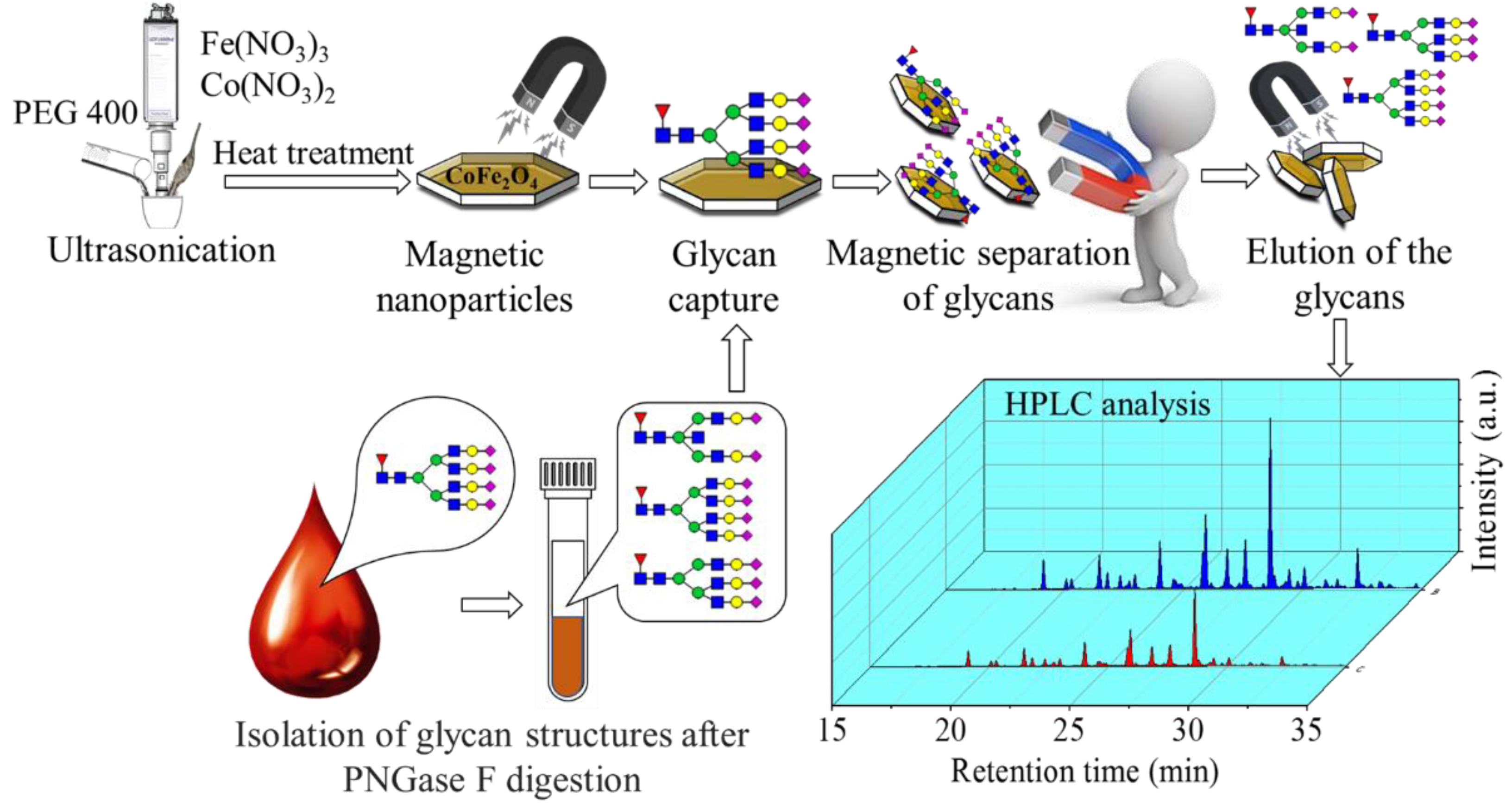
:1. Introduction
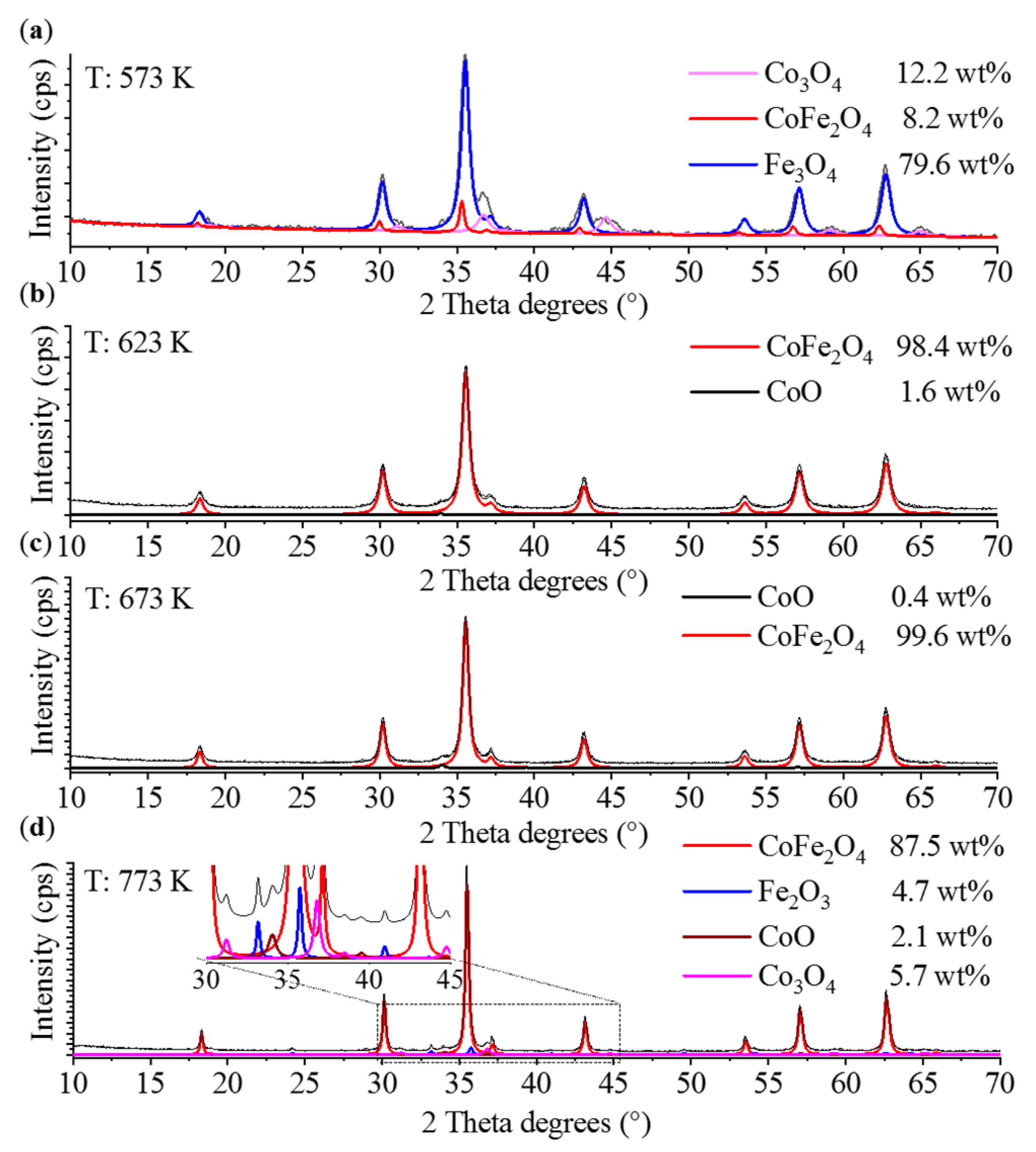
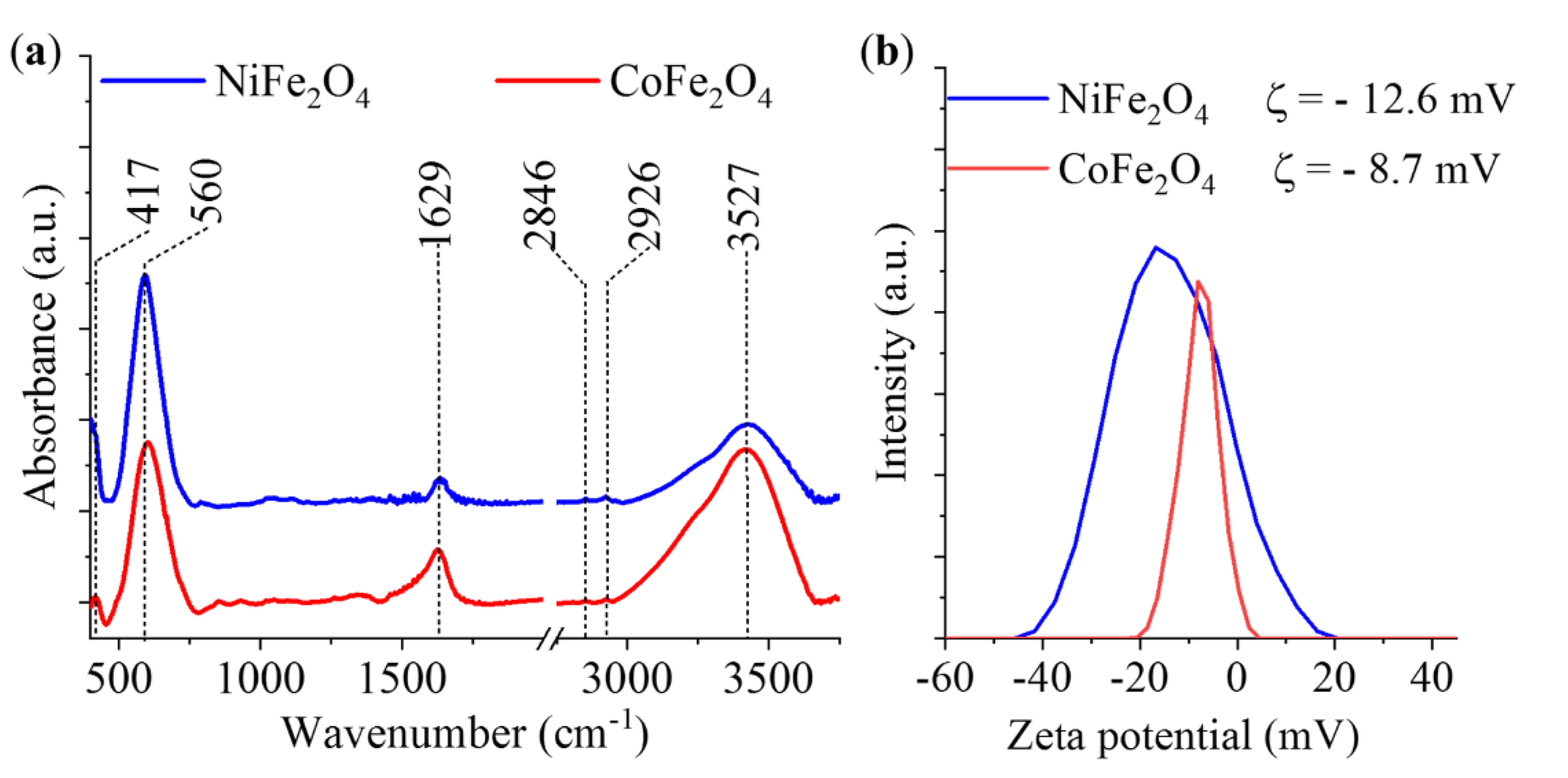
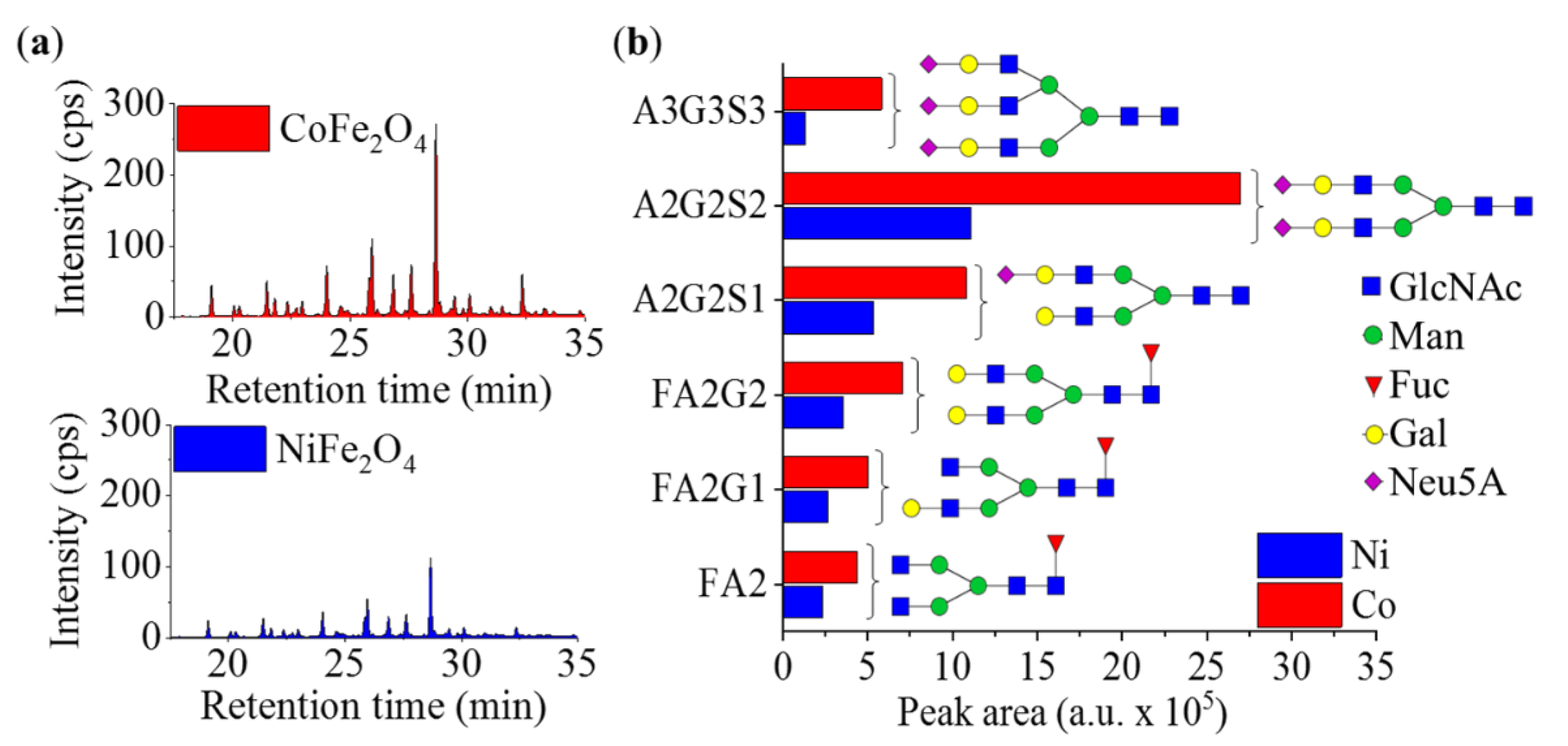
2. Results and Discussion
Comparison of the Efficacy of the Nickel Ferrite and Cobalt Ferrite Magnetic Particles in Glycan Separation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of the Magnetic Spinel Nanoparticles
3.2.2. Characterization of the Magnetic Ferrite Nanoparticles
3.2.3. Glycan Sample Preparation
3.2.4. Sample Clean-Up
3.2.5. LC-FLR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Váradi, C.; Nehéz, K.; Hornyák, O.; Viskolcz, B.; Bones, J. Serum N-Glycosylation in Parkinson’s Disease: A Novel Approach for Potential Alterations. Molecules 2019, 24, 2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhaak, L.R.; Huhn, C.; Waterreus, W.-J.; de Boer, A.R.; Neusüss, C.; Hokke, C.H.; Deelder, A.M.; Wuhrer, M. Hydrophilic Interaction Chromatography-Based High-Throughput Sample Preparation Method for N-Glycan Analysis from Total Human Plasma Glycoproteins. Anal. Chem. 2008, 80, 6119–6126. [Google Scholar] [CrossRef] [PubMed]
- Shubhakar, A.; Reiding, K.R.; Gardner, R.A.; Spencer, D.I.R.; Fernandes, D.L.; Wuhrer, M. High-Throughput Analysis and Automation for Glycomics Studies. Chromatographia 2014, 78, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Zauner, G.; Huhn, C.; Bruggink, C.; Deelder, A.M.; Wuhrer, M. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010, 397, 3457–3481. [Google Scholar] [CrossRef] [Green Version]
- Váradi, C.; Lew, C.; Guttman, A. Rapid Magnetic Bead Based Sample Preparation for Automated and High Throughput N-Glycan Analysis of Therapeutic Antibodies. Anal. Chem. 2014, 86, 5682–5687. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Váradi, C.; Sikora, E.; Vanyorek, L.; Viskolcz, B. Purification of Fluorescently Derivatized N-Glycans by Magnetic Iron Nanoparticles. Nanomaterials 2019, 9, 1480. [Google Scholar] [CrossRef] [Green Version]
- Váradi, C.; Hajdu, V.; Farkas, F.; Gilányi, I.; Oláh, C.; Viskolcz, B. The Analysis of Human Serum N-Glycosylation in Patients with Primary and Metastatic Brain Tumors. Life 2021, 11, 29. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2003, 126, 273–279. [Google Scholar] [CrossRef]
- Guardia, P.; Labarta, A.; Batlle, X. Tuning the Size, the Shape, and the Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2010, 115, 390–396. [Google Scholar] [CrossRef]
- Chou, S.-W.; Shau, Y.-H.; Wu, P.-C.; Yang, Y.-S.; Shieh, D.-B.; Chen, C.-C. In Vitro and In Vivo Studies of FePt Nanoparticles for Dual Modal CT/MRI Molecular Imaging. J. Am. Chem. Soc. 2010, 132, 13270–13278. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Kaittanis, C.; Grimm, J.; Perez, J.M. Drug/Dye-Loaded, Multifunctional Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Dual Optical/Magnetic Resonance Imaging. Small 2009, 5, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hojamberdiev, M.; Xu, Y.; Cao, B.; Wang, J.; Wu, H. Hydrothermal synthesis and magnetic properties of gadolinium-doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2011, 323, 133–137. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jang, J.-T.; Choi, J.-S.; Moon, S.H.; Noh, S.-H.; Kim, J.-W.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Kanagesan, S.; Aziz, S.; Hashim, M.; Ismail, I.; Tamilselvan, S.; Alitheen, N.; Swamy, M.; Purna Chandra Rao, B. Synthesis, Characterization and in Vitro Evaluation of Manganese Ferrite (MnFe2O4) Nanoparticles for Their Biocompatibility with Murine Breast Cancer Cells (4T1). Molecules 2016, 21, 312. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Q.; Wang, M.; Hoover, A.; Wang, X.; Zhou, F.; Towner, R.A.; Smith, N.; Saunders, D.; Song, J.; et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem. Eng. J. 2020, 396, 125239. [Google Scholar] [CrossRef]
- Bi, C.; Liang, Y.; Shen, L.; Tian, S.; Zhang, K.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Maltose-Functionalized Hydrophilic Magnetic Nanoparticles with Polymer Brushes for Highly Selective Enrichment of N-Linked Glycopeptides. ACS Omega 2018, 3, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Macher, T.; Xu, Y.; Bao, Y.; Cassady, C.J. MALDI MS In-Source Decay of Glycans Using a Glutathione-Capped Iron Oxide Nanoparticle Matrix. Anal. Chem. 2014, 86, 8496–8503. [Google Scholar] [CrossRef]
- Zalite, I.; Heidemane, G.; Kuznetsova, L.; Maiorov, M. Hydrothermal Synthesis of Cobalt Ferrite Nanosized Powders. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012011. [Google Scholar] [CrossRef]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, P.P.; Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Sonochemical Synthesis of Cobalt Ferrite Nanoparticles. Int. J. Chem. Eng. 2013, 2013, 934234. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, H.; Pol, V.G.; Bruckental, I.; Koltypin, Y.; Calderon-Moreno, J.; Nowik, I.; Gedanken, A. Sonochemical synthesis, structural and magnetic properties of air-stable Fe/Co alloy nanoparticles. New J. Chem. 2003, 27, 1194. [Google Scholar] [CrossRef]
- Gunasekaran, M.; Seenuvasakumaran, P. Structural, optical and magnetic studies of vanadium ferrite and silver doped vanadium ferrite by sol–gel method. Mater. Today Proc. 2021, 36, 797–802. [Google Scholar] [CrossRef]
- Kumar, S.J.; Prameela, P.; Rao, K.S.; Kiran, J.N.; Rao, K.H. Structural and Magnetic Properties of Copper-Substituted Nickel–Zinc Nanoparticles Prepared by Sol-Gel Method. J. Supercond. Nov. Magn. 2020, 33, 693–705. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Karolak, F.; García-Granda, S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis, structural, optical, morphological and magnetic characterization of copper substituted nickel ferrite (CuxNi1−xFe2O4) through co-precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 18480–18488. [Google Scholar] [CrossRef]
- Sagadevan, S.; Chowdhury, Z.Z.; Rafique, R.F. Preparation and Characterization of Nickel ferrite Nanoparticles via Co-precipitation Method. Mater. Res. 2018, 21, e20160533. [Google Scholar] [CrossRef] [Green Version]
- Ganure, K.A.; Dhale, L.A.; Katkar, V.T.; Lohar, K.S. Synthesis and Characterization of Lanthanum-doped Ni-Co-Zn Spinel Ferrites Nanoparticles via Normal Micro-Emulsion Method. Int. J. Nanotechnol. Appl. 2017, 11, 189–195. [Google Scholar]
- Yousuf, M.A.; Baig, M.M.; Waseem, M.; Haider, S.; Shakir, I.; Ud-Din Khan, S.; Warsi, M.F. Low cost micro-emulsion route synthesis of Cr-substituted MnFe2O4 nanoparticles. Ceram. Int. 2019, 45, 22316–22323. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Fakhrzad, M. Photocatalytic and magnetic properties of ZnFe2O4 nanoparticles synthesised by mechanical alloying. Int. J. Environ. Anal. Chem. 2020, 102, 690–706. [Google Scholar] [CrossRef]
- Moravvej-Farshi, F.; Amishi, M.; Nekouee, K.A. Influence of different milling time on synthesized Ni–Zn ferrite properties by mechanical alloying method. J. Mater. Sci. Mater. Electron. 2020, 31, 13610–13619. [Google Scholar] [CrossRef]
- Yasui, K. Numerical simulations for sonochemistry. Ultrason. Sonochemistry 2021, 78, 105728. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Guan, Y.; Zhang, Z.; Xu, G.; Yang, Y.; Guo, C. A new method of synthesis well-dispersion and dense Fe3O4@SiO2 magnetic nanoparticles for DNA extraction. Chem. Phys. Lett. 2019, 715, 7–13. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Rashad, M.M.; Haraz, F.A.; Sigmund, W. Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J. Magn. Magn. Mater. 2010, 322, 2058–2064. [Google Scholar] [CrossRef]
- Zauner, G.; Deelder, A.M.; Wuhrer, M. Recent advances in hydrophilic interaction liquid chromatography (HILIC) for structural glycomics. Electrophoresis 2011, 32, 3456–3466. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 2002, 28, 38–46. [Google Scholar] [CrossRef]
- Keser, T.; Pavić, T.; Lauc, G.; Gornik, O. Comparison of 2-Aminobenzamide, Procainamide and RapiFluor-MS as Derivatizing Agents for High-Throughput HILIC-UPLC-FLR-MS N-glycan Analysis. Front. Chem. 2018, 6, 324. [Google Scholar] [CrossRef]
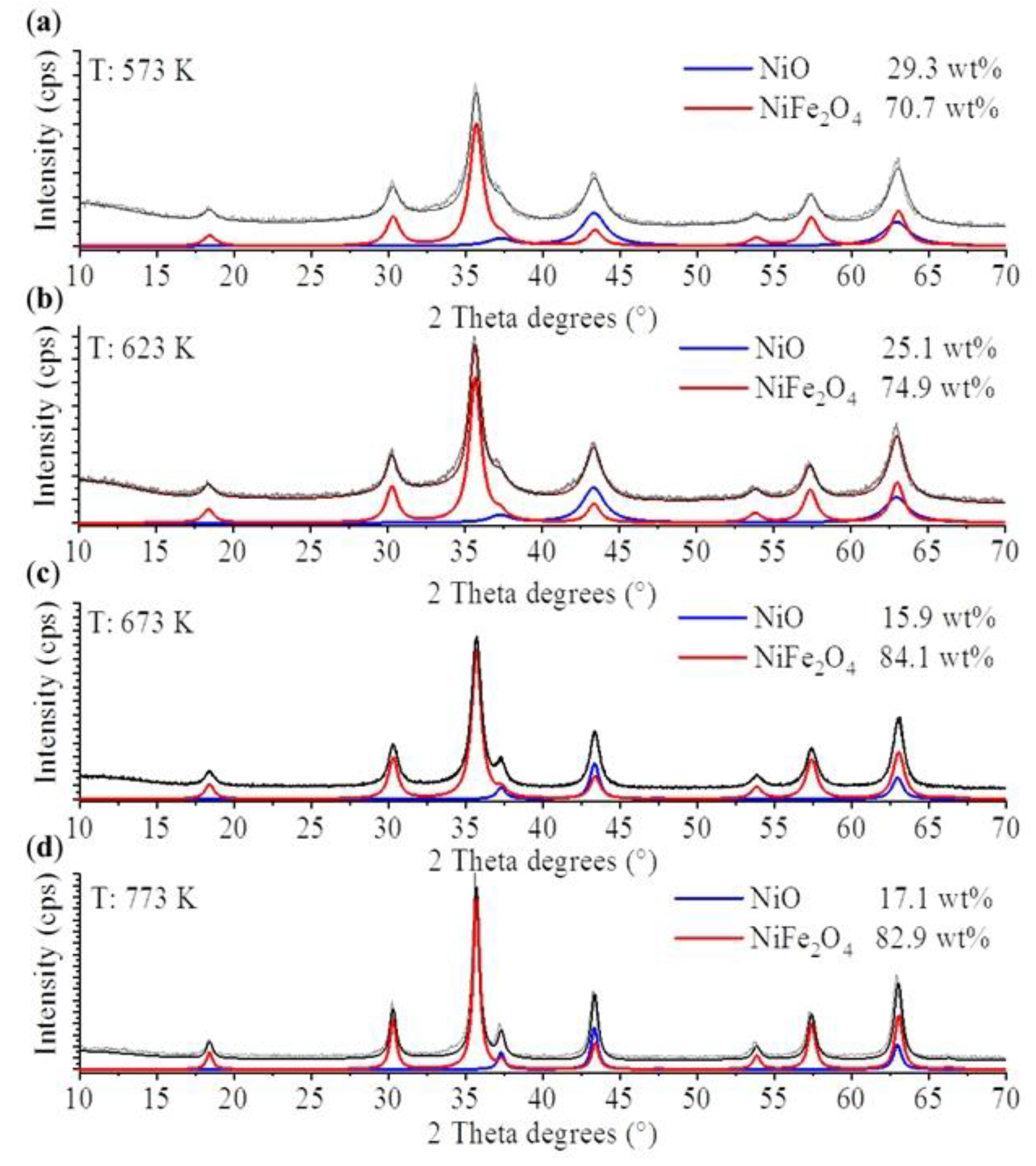

| NiFe2O4 | NiO | |
|---|---|---|
| 573 K | 7 ± 2 nm | 4 ± 1 nm |
| 623 K | 8 ± 2 nm | 5 ± 1 nm |
| 673 K | 10 ± 3 nm | 11 ± 3 nm |
| 773 K | 15 ± 3 nm | 16 ± 4 nm |
| CoFe2O4 | Fe3O4 | Co3O4 | CoO | Fe2O3 | |
|---|---|---|---|---|---|
| 573 K | 16 ± 5 nm | 13 ± 3 nm | 9 ± 2 nm | ||
| 623 K | 14 ± 3 nm | 40 ± 9 nm | |||
| 673 K | 18 ± 4 nm | 20 ± 5 nm | |||
| 773 K | 41 ± 7 nm | 21 ± 5 nm | 14 ± 4 nm | 41 ± 9 nm |
| d [nm] | Mean | SD | Min. | Max. | Q 1 | Median | Q 3 | P 90 | P 95 |
|---|---|---|---|---|---|---|---|---|---|
| NiFe2O4 (673 K) | 13.2 | 6.3 | 5.6 | 35.3 | 8.8 | 11.8 | 17.1 | 20.9 | 26.2 |
| CoFe2O4 (673 K) | 18.4 | 12.7 | 3.6 | 71.6 | 9.8 | 15.9 | 23.4 | 27.8 | 42.1 |
| FA2 | Fucosylated-bi-antennary glycan |
|---|---|
| FA2G1 | fucosylated-bi-antennary mono-galactosylated glycan |
| FA2G2 | fucosylated-bi-antennary bi-galactosylated glycan |
| A2G2S1 | bi-antennary bi-galactosylated mono-sialylated glycan |
| A2G2S2 | bi-antennary bi-galactosylated di-sialylated glycan |
| A3G3S3 | tri-antennary tri-galactosylated tri-sialylated glycan |
| Fe(NO3)3 ∙ 9H2O | Ni(NO3)2 ∙ 6H2O | Co(NO3)2 ∙ 6H2O | |
|---|---|---|---|
| NiFe2O4 | 2.78 g (6.88 mmol) | 1.00 g (3.44 mmol) | - |
| CoFe2O4 | 2.77 g (6.88 mmol) | - | 1.00 g (3.44 mmol) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilosvai, A.M.; Dojcsak, D.; Váradi, C.; Nagy, M.; Kristály, F.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Sonochemical Combined Synthesis of Nickel Ferrite and Cobalt Ferrite Magnetic Nanoparticles and Their Application in Glycan Analysis. Int. J. Mol. Sci. 2022, 23, 5081. https://doi.org/10.3390/ijms23095081
Ilosvai AM, Dojcsak D, Váradi C, Nagy M, Kristály F, Fiser B, Viskolcz B, Vanyorek L. Sonochemical Combined Synthesis of Nickel Ferrite and Cobalt Ferrite Magnetic Nanoparticles and Their Application in Glycan Analysis. International Journal of Molecular Sciences. 2022; 23(9):5081. https://doi.org/10.3390/ijms23095081
Chicago/Turabian StyleIlosvai, Agnes Maria, Dalma Dojcsak, Csaba Váradi, Miklós Nagy, Ferenc Kristály, Béla Fiser, Béla Viskolcz, and László Vanyorek. 2022. "Sonochemical Combined Synthesis of Nickel Ferrite and Cobalt Ferrite Magnetic Nanoparticles and Their Application in Glycan Analysis" International Journal of Molecular Sciences 23, no. 9: 5081. https://doi.org/10.3390/ijms23095081
APA StyleIlosvai, A. M., Dojcsak, D., Váradi, C., Nagy, M., Kristály, F., Fiser, B., Viskolcz, B., & Vanyorek, L. (2022). Sonochemical Combined Synthesis of Nickel Ferrite and Cobalt Ferrite Magnetic Nanoparticles and Their Application in Glycan Analysis. International Journal of Molecular Sciences, 23(9), 5081. https://doi.org/10.3390/ijms23095081








