Elevated LSD1 and SNAIL Expression Indicate Poor Prognosis in Hypopharynx Carcinoma
Abstract
1. Introduction
2. Results
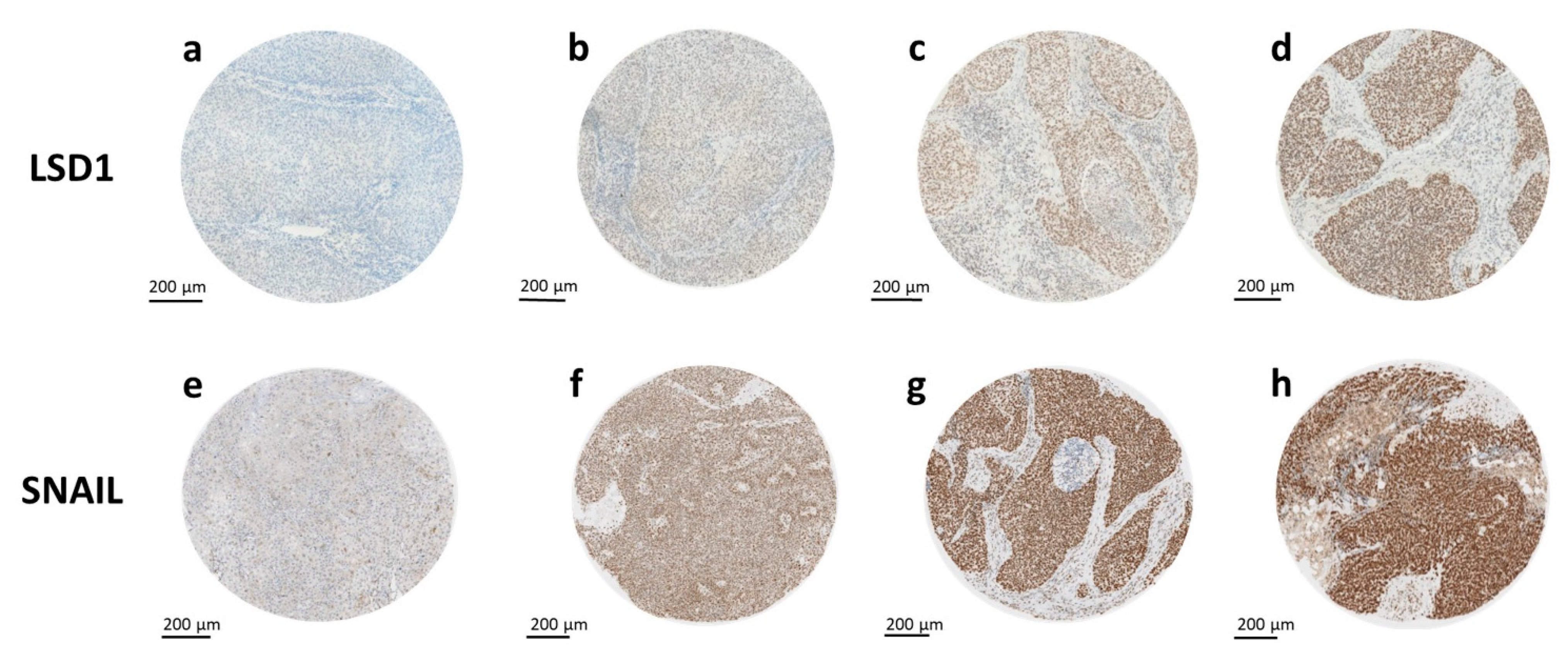
2.1. Expression of LSD1 and SNAIL
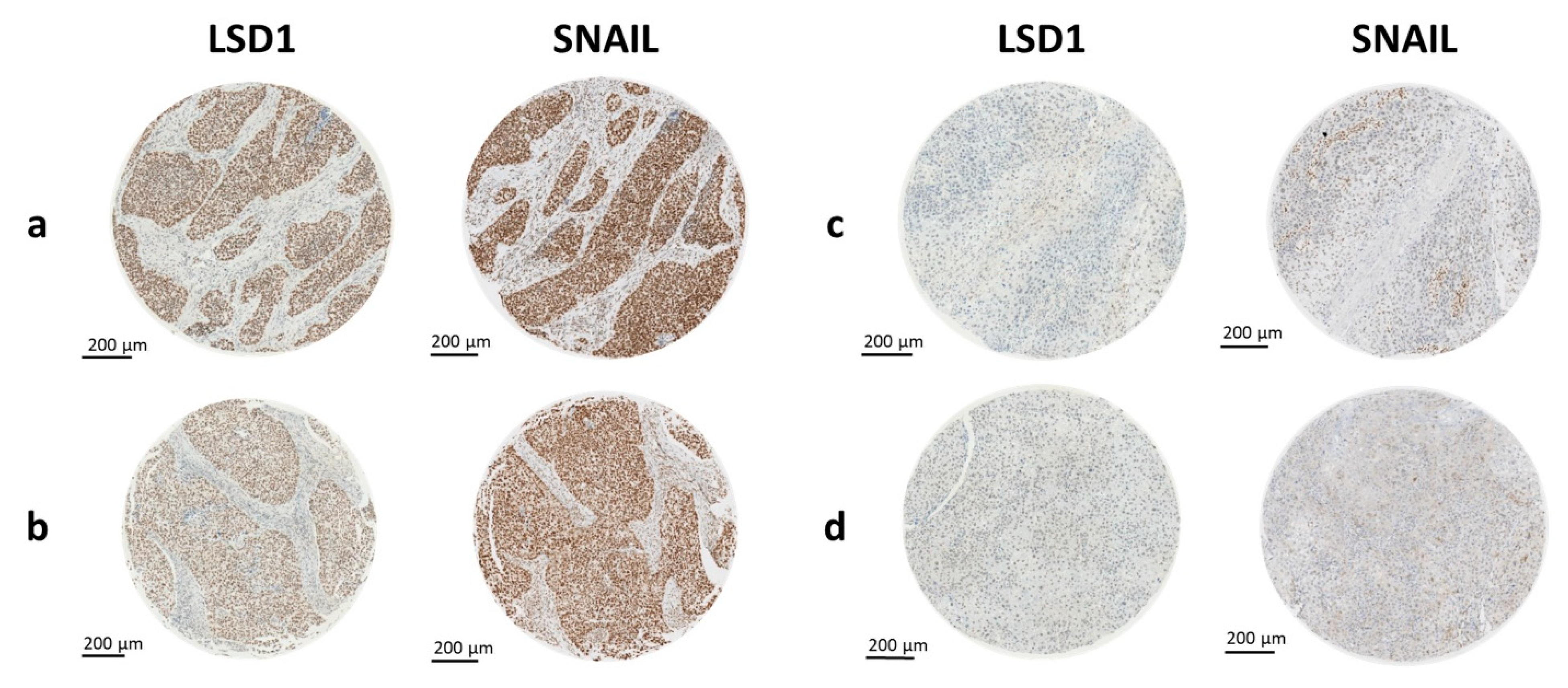
2.2. LSD1 and SNAIL Expression Differ between Tissue Types
2.3. Higher LSD1 Expression in Hypopharyngeal HNSCC
2.4. Correlation of LSD1 and SNAIL with Clinical Data and Established Risk Factors
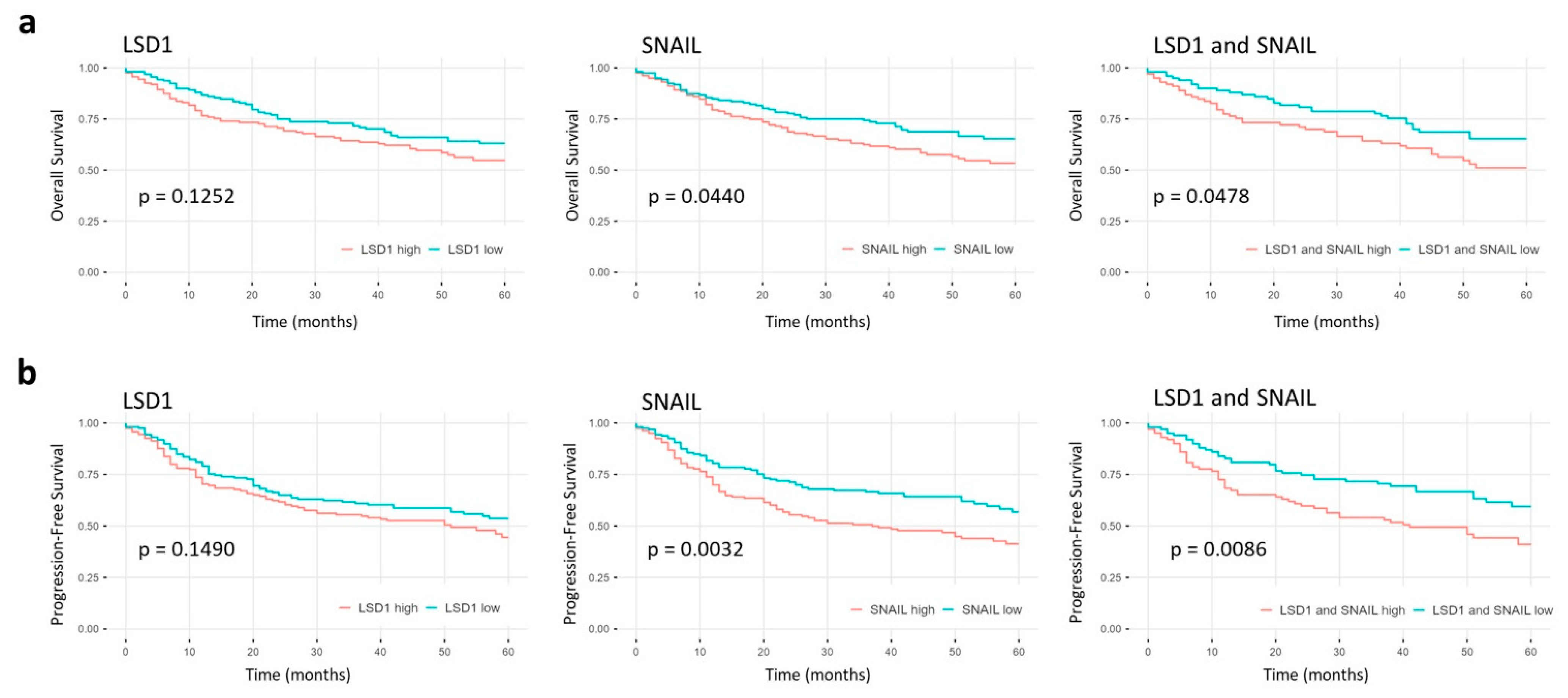
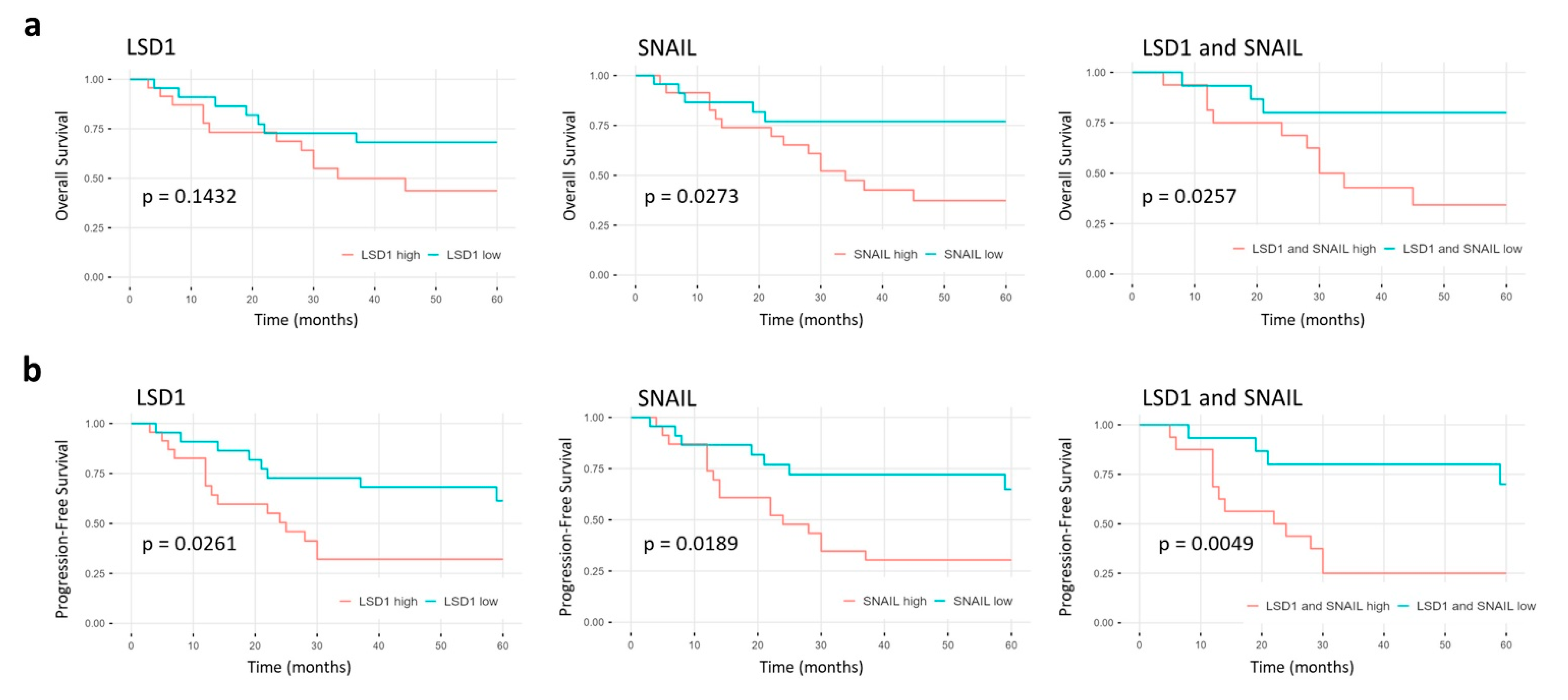
2.5. High LSD1 and SNAIL Expression Indicate Poor Prognosis
3. Discussion
4. Materials and Methods
4.1. Cohort and TMA
4.2. Immunohistochemical Staining and Evaluation
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The Molecular Biology of Head and Neck Cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/tables?cancers=1_3_5_14_4&single_unit=50000&group_cancers=1&multiple_cancers=1&years=2035&mode=population&group_populations=1&populations=903_904_905_908_909_935 (accessed on 4 September 2021).
- Agents Classified by the IARC Monographs, Volumes 1–129—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 4 September 2021).
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Westra, W.H. P16 Expression as a Surrogate Marker for HPV-Related Oropharyngeal Carcinoma: A Guide for Interpretative Relevance and Consistency. Head Neck 2012, 34, 459–461. [Google Scholar] [CrossRef]
- Bertero, L.; Massa, F.; Metovic, J.; Zanetti, R.; Castellano, I.; Ricardi, U.; Papotti, M.; Cassoni, P. Eighth Edition of the UICC Classification of Malignant Tumours: An Overview of the Changes in the Pathological TNM Classification Criteria-What Has Changed and Why? Virchows Arch. 2018, 472, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Therapie von Kopf-Hals-Tumoren|DKG. Available online: https://www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/krebsarten/andere-krebsarten/kopf-hals-tumoren/therapie.html (accessed on 14 November 2020).
- Petersen, J.F.; Timmermans, A.J.; van Dijk, B.A.C.; Overbeek, L.I.H.; Smit, L.A.; Hilgers, F.J.M.; Stuiver, M.M.; van den Brekel, M.W.M. Trends in Treatment, Incidence and Survival of Hypopharynx Cancer: A 20-Year Population-Based Study in the Netherlands. Eur. Arch. Otorhinolaryngol. 2018, 275, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Du, E.; Mazul, A.L.; Farquhar, D.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Weissler, M.C.; Hayes, D.N.; Olshan, A.F.; Zevallos, J.P. Long-Term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 2019, 129, 2506–2513. [Google Scholar] [CrossRef]
- Chin, D.; Boyle, G.M.; Porceddu, S.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Head and Neck Cancer: Past, Present and Future. Expert Rev. Anticancer Ther. 2006, 6, 1111–1118. [Google Scholar] [CrossRef]
- Rettig, E.M.; D’Souza, G. Epidemiology of Head and Neck Cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 379–396. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shen, Z.; Ye, D.; Li, Q.; Deng, H.; Liu, H.; Li, J. The Association and Clinical Significance of CDKN2A Promoter Methylation in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 50, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H.; et al. LSD1 Ablation Stimulates Anti-Tumor Immunity and Enables Checkpoint Blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, F.; Wang, J.; Yin, F.; Xu, Z.; Qi, D.; Wu, X.; Cao, Y.; Liang, W.; Liu, Y.; et al. Pluripotent Stem Cell Protein Sox2 Confers Sensitivity to LSD1 Inhibition in Cancer Cells. Cell Rep. 2013, 5, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, G.; Liu, J.; Zhang, N.; Li, L.; Ji, J.; Zhang, J.; Zhang, L.; Wang, G.; Wang, X.; et al. Phosphorylation of LSD1 at Ser112 Is Crucial for Its Function in Induction of EMT and Metastasis in Breast Cancer. Breast Cancer Res. Treat. 2016, 159, 443–456. [Google Scholar] [CrossRef]
- Huang, J.; Sengupta, R.; Espejo, A.B.; Lee, M.G.; Dorsey, J.A.; Richter, M.; Opravil, S.; Shiekhattar, R.; Bedford, M.T.; Jenuwein, T.; et al. P53 Is Regulated by the Lysine Demethylase LSD1. Nature 2007, 449, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hevi, S.; Kurash, J.K.; Lei, H.; Gay, F.; Bajko, J.; Su, H.; Sun, W.; Chang, H.; Xu, G.; et al. The Lysine Demethylase LSD1 (KDM1) Is Required for Maintenance of Global DNA Methylation. Nat. Genet. 2009, 41, 125–129. [Google Scholar] [CrossRef]
- Kontaki, H.; Talianidis, I. Lysine Methylation Regulates E2F1-Induced Cell Death. Mol. Cell 2010, 39, 152–160. [Google Scholar] [CrossRef]
- Lim, S.; Janzer, A.; Becker, A.; Zimmer, A.; Schüle, R.; Buettner, R.; Kirfel, J. Lysine-Specific Demethylase 1 (LSD1) Is Highly Expressed in ER-Negative Breast Cancers and a Biomarker Predicting Aggressive Biology. Carcinogenesis 2010, 31, 512–520. [Google Scholar] [CrossRef]
- Kahl, P.; Gullotti, L.; Heukamp, L.C.; Wolf, S.; Friedrichs, N.; Vorreuther, R.; Solleder, G.; Bastian, P.J.; Ellinger, J.; Metzger, E.; et al. Androgen Receptor Coactivators Lysine-Specific Histone Demethylase 1 and Four and a Half LIM Domain Protein 2 Predict Risk of Prostate Cancer Recurrence. Cancer Res. 2006, 66, 11341–11347. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Xu, B.; Deng, H.; Zheng, X.; Wu, C.; Jiang, J. Over-Expression of Lysine-Specific Demethylase 1 Predicts Tumor Progression and Poor Prognosis in Human Esophageal Cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 8929–8934. [Google Scholar]
- Hayami, S.; Kelly, J.D.; Cho, H.-S.; Yoshimatsu, M.; Unoki, M.; Tsunoda, T.; Field, H.I.; Neal, D.E.; Yamaue, H.; Ponder, B.A.J.; et al. Overexpression of LSD1 Contributes to Human Carcinogenesis through Chromatin Regulation in Various Cancers. Int. J. Cancer 2011, 128, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Yuan, D.; Miao, X.; Lv, Y.; Zhan, P.; Shen, X.; Song, Y. Over-Expression of LSD1 Promotes Proliferation, Migration and Invasion in Non-Small Cell Lung Cancer. PLoS ONE 2012, 7, e35065. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.H.; Lim, S.; Schramm, A.; Friedrichs, N.; Koster, J.; Versteeg, R.; Ora, I.; Pajtler, K.; Klein-Hitpass, L.; Kuhfittig-Kulle, S.; et al. Lysine-Specific Demethylase 1 Is Strongly Expressed in Poorly Differentiated Neuroblastoma: Implications for Therapy. Cancer Res. 2009, 69, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-C.; Yu, B.; Chen, Z.-S.; Liu, Y.; Liu, H.-M. TCPs: Privileged Scaffolds for Identifying Potent LSD1 Inhibitors for Cancer Therapy. Epigenomics 2016, 8, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, Y.; Li, J.; Dong, C.; Ye, X.; Chi, Y.-I.; Evers, B.M.; Zhou, B.P. The SNAG Domain of Snail1 Functions as a Molecular Hook for Recruiting Lysine-Specific Demethylase 1. EMBO J. 2010, 29, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Ballestar, E.; Esteller, M.; Cano, A. Snail Mediates E-Cadherin Repression by the Recruitment of the Sin3A/Histone Deacetylase 1 (HDAC1)/HDAC2 Complex. Mol. Cell. Biol. 2004, 24, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Pasini, D.; Díaz, V.M.; Francí, C.; Gutierrez, A.; Dave, N.; Escrivà, M.; Hernandez-Muñoz, I.; Di Croce, L.; Helin, K.; et al. Polycomb Complex 2 Is Required for E-Cadherin Repression by the Snail1 Transcription Factor. Mol. Cell. Biol. 2008, 28, 4772–4781. [Google Scholar] [CrossRef]
- Nieto, M.Á. Correlation of Snail Expression with Histological Grade and Lymph Node Status in Breast Carcinomas. Oncogene 2002, 21, 3241–3246. [Google Scholar]
- Jin, H.; Yu, Y.; Zhang, T.; Zhou, X.; Zhou, J.; Jia, L.; Wu, Y.; Zhou, B.P.; Feng, Y. Snail Is Critical for Tumor Growth and Metastasis of Ovarian Carcinoma. Int. J. Cancer 2010, 126, 2102–2111. [Google Scholar] [CrossRef]
- Blechschmidt, K.; Kremmer, E.; Hollweck, R.; Mylonas, I.; Höfler, H.; Kremer, M.; Becker, K.-F. The E-Cadherin Repressor Snail Plays a Role in Tumor Progression of Endometrioid Adenocarcinomas. Diagn. Mol. Pathol. 2007, 16, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, A.; Kitajima, Y.; Kido, S.; Shimonishi, T.; Matsuyama, S.; Kitahara, K.; Miyazaki, K. Snail Accelerates Cancer Invasion by Upregulating MMP Expression and Is Associated with Poor Prognosis of Hepatocellular Carcinoma. Br. J. Cancer 2005, 92, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, F.; Namdarian, B.; Corcoran, N.M.; Pedersen, J.; Ockrim, J.; Voelzke, B.B.; Mete, U.; Costello, A.J.; Hovens, C.M. Snail Expression Is an Independent Predictor of Tumor Recurrence in Superficial Bladder Cancers. Urol. Oncol. 2010, 28, 591–596. [Google Scholar] [CrossRef]
- Yang, M.-H.; Chang, S.-Y.; Chiou, S.-H.; Liu, C.-J.; Chi, C.-W.; Chen, P.-M.; Teng, S.-C.; Wu, K.-J. Overexpression of NBS1 Induces Epithelial-Mesenchymal Transition and Co-Expression of NBS1 and Snail Predicts Metastasis of Head and Neck Cancer. Oncogene 2007, 26, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-F.; Chen, J.-Y.; Ho, Y.-H.; Hsu, W.-H.; Wu, L.-C.; Lan, H.-Y.; Hsu, D.S.-S.; Tai, S.-K.; Chang, Y.-C.; Yang, M.-H. Snail-Induced Claudin-11 Prompts Collective Migration for Tumour Progression. Nat. Cell. Biol. 2019, 21, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The Transcription Factor Snail Controls Epithelial–Mesenchymal Transitions by Repressing E-Cadherin Expression. Nat. Cell. Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Lin, T.; Ponn, A.; Hu, X.; Law, B.K.; Lu, J. Requirement of the Histone Demethylase LSD1 in Snai1-Mediated Transcriptional Repression during Epithelial-Mesenchymal Transition. Oncogene 2010, 29, 4896–4904. [Google Scholar] [CrossRef]
- Shang, Y. Hormones and Cancer. Cell Res. 2007, 17, 277–279. [Google Scholar] [CrossRef]
- Folkerd, E.J.; Dowsett, M. Influence of Sex Hormones on Cancer Progression. J. Clin. Oncol. 2010, 28, 4038–4044. [Google Scholar] [CrossRef]
- Liang, J.; Shang, Y. Estrogen and Cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Wang, S.; Liu, Y.; Zhang, J.; Xu, Y.; Zhang, Z.; Bao, W.; Wu, S. LSD1 Sustains Estrogen-Driven Endometrial Carcinoma Cell Proliferation through the PI3K/AKT Pathway via Di-Demethylating H3K9 of Cyclin D1. Int. J. Oncol. 2017, 50, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, B.; Tang, Q.; Liu, J.; Yang, X. Bisphenol A Triggers Proliferation and Migration of Laryngeal Squamous Cell Carcinoma via GPER Mediated Upregulation of IL-6. Cell Biochem. Funct. 2017, 35, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Cheung, L.W.T.; Wong, A.S.T.; Leung, P.C.K. Estrogen Regulates Snail and Slug in the Down-Regulation of E-Cadherin and Induces Metastatic Potential of Ovarian Cancer Cells through Estrogen Receptor Alpha. Mol. Endocrinol. 2008, 22, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Grsic, K.; Opacic, I.L.; Sitic, S.; Milkovic Perisa, M.; Suton, P.; Sarcevic, B. The Prognostic Significance of Estrogen Receptor β in Head and Neck Squamous Cell Carcinoma. Oncol. Lett. 2016, 12, 3861–3865. [Google Scholar] [CrossRef] [PubMed]
- Goulioumis, A.K.; Fuxe, J.; Varakis, J.; Repanti, M.; Goumas, P.; Papadaki, H. Estrogen Receptor-Beta Expression in Human Laryngeal Carcinoma: Correlation with the Expression of Epithelial-Mesenchymal Transition Specific Biomarkers. Oncol. Rep. 2009, 22, 1063–1068. [Google Scholar] [CrossRef][Green Version]
- Nieh, S.; Jao, S.-W.; Yang, C.-Y.; Lin, Y.-S.; Tseng, Y.-H.; Liu, C.-L.; Lee, T.-Y.; Liu, T.-Y.; Chu, Y.-H.; Chen, S.-F. Regulation of Tumor Progression via the Snail-RKIP Signaling Pathway by Nicotine Exposure in Head and Neck Squamous Cell Carcinoma. Head Neck 2015, 37, 1712–1721. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chang, Y.-C. Enhancement of Cancer Stem-like and Epithelial-Mesenchymal Transdifferentiation Property in Oral Epithelial Cells with Long-Term Nicotine Exposure: Reversal by Targeting SNAIL. Toxicol. Appl. Pharmacol. 2013, 266, 459–469. [Google Scholar] [CrossRef]
- Turati, F.; Garavello, W.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; Boffetta, P.; La Vecchia, C.; et al. A Meta-Analysis of Alcohol Drinking and Oral and Pharyngeal Cancers. Part 2: Results by Subsites. Oral Oncol. 2010, 46, 720–726. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Wang, Q.; Hu, H.; Li, Z.; Wang, D.; Zhang, W.; Qi, B.; Ye, J.; Wu, H.; et al. The Histone Demethylase LSD1 Is a Novel Oncogene and Therapeutic Target in Oral Cancer. Cancer Lett. 2016, 374, 12–21. [Google Scholar] [CrossRef]
- Wakisaka, N.; Yoshida, S.; Kondo, S.; Kita, M.; Sawada-Kitamura, S.; Endo, K.; Tsuji, A.; Nakanish, Y.; Murono, S.; Yoshizaki, T. Induction of Epithelial-Mesenchymal Transition and Loss of Podoplanin Expression Are Associated with Progression of Lymph Node Metastases in Human Papillomavirus-Related Oropharyngeal Carcinoma. Histopathology 2015, 66, 771–780. [Google Scholar] [CrossRef]
- Idel, C.; Ribbat-Idel, J.; Kuppler, P.; Krupar, R.; Offermann, A.; Vogel, W.; Rades, D.; Kirfel, J.; Wollenberg, B.; Perner, S. EVI1 as a Marker for Lymph Node Metastasis in HNSCC. Int. J. Mol. Sci. 2020, 21, 854. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sánchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Hackl, M.; Zielonke, N.; Oberaigner, W.; Van Eycken, E.; et al. Prognoses and Improvement for Head and Neck Cancers Diagnosed in Europe in Early 2000s: The EUROCARE-5 Population-Based Study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Wang, Y.-Q.; Lv, J.-W.; Li, Y.-Q.; Chua, M.L.K.; Le, Q.-T.; Lee, N.; Colevas, A.D.; Seiwert, T.; Hayes, D.N.; et al. Identification and Validation of Novel Microenvironment-Based Immune Molecular Subgroups of Head and Neck Squamous Cell Carcinoma: Implications for Immunotherapy. Ann. Oncol. 2019, 30, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, S.; Ye, W.; Wang, Y.; Zhang, X.; Deng, J.; Zhang, Z.; Liu, L.; Liu, S. Targeting LSD1 Suppresses Stem Cell-like Properties and Sensitizes Head and Neck Squamous Cell Carcinoma to PD-1 Blockade. Cell Death Dis. 2021, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Li, Y.; Lu, T.; Hu, G. Silencing Snail Reverses Epithelial-Mesenchymal Transition and Increases Radiosensitivity in Hypopharyngeal Carcinoma. Onco Targets Ther. 2020, 13, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Masui, T.; Ota, I.; Yook, J.-I.; Mikami, S.; Yane, K.; Yamanaka, T.; Hosoi, H. Snail-Induced Epithelial-Mesenchymal Transition Promotes Cancer Stem Cell-like Phenotype in Head and Neck Cancer Cells. Int. J. Oncol. 2014, 44, 693–699. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yang, X.H.; Kang, T.; Zhao, Y.; Wang, C.; Evers, B.M.; Zhou, B.P. The Deubiquitinase USP28 Stabilizes LSD1 and Confers Stem-Cell-like Traits to Breast Cancer Cells. Cell Rep. 2013, 5, 224–236. [Google Scholar] [CrossRef]
- Lei, Z.-J.; Wang, J.; Xiao, H.-L.; Guo, Y.; Wang, T.; Li, Q.; Liu, L.; Luo, X.; Fan, L.-L.; Lin, L.; et al. Lysine-Specific Demethylase 1 Promotes the Stemness and Chemoresistance of Lgr5(+) Liver Cancer Initiating Cells by Suppressing Negative Regulators of β-Catenin Signaling. Oncogene 2015, 34, 3188–3198. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.; Ding, J.; Wang, Z.; Du, J.; Wu, C. Knocking down LSD1 Inhibits the Stemness Features of Colorectal Cancer Stem Cells. Braz. J. Med. Biol. Res. 2020, 53, e9230. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Chen, H.; Qiu, T.; Weng, X.-D.; Guo, J.; Wang, L.; Liu, X.-H. Effects of Cisplatin on the LSD1-Mediated Invasion and Metastasis of Prostate Cancer Cells. Mol. Med. Rep. 2016, 14, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Wan, X.; Lai, W.; Wu, C.; Jin, J.; Liu, X.; Wei, Y.; Lin, Q.; Zhang, L.; Shao, Q. Inhibition of Lysine-Specific Demethylase 1 Prevents Proliferation and Mediates Cisplatin Sensitivity in Ovarian Cancer Cells. Oncol. Lett. 2018, 15, 9025–9032. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Sui, C.-G.; Jiang, Y.-H.; Liu, Y.-P.; Meng, F.-D. Interference of Lysine-Specific Demethylase 1 Inhibits Cellular Invasion and Proliferation In Vivo in Gastric Cancer MKN-28 Cells. Biomed. Pharmacother. 2016, 82, 498–508. [Google Scholar] [CrossRef]
- Jang, T.J. Differential Membranous E-Cadherin Expression, Cell Proliferation and O-GlcNAcylation between Primary and Metastatic Nodal Lesion in Colorectal Cancer. Pathol. Res. Pract. 2016, 212, 113–119. [Google Scholar] [CrossRef]
- Chao, Y.L.; Shepard, C.R.; Wells, A. Breast Carcinoma Cells Re-Express E-Cadherin during Mesenchymal to Epithelial Reverting Transition. Mol. Cancer 2010, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or Permissive for Metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Markiewicz, A.; Wełnicka-Jaśkiewicz, M.; Seroczyńska, B.; Skokowski, J.; Majewska, H.; Szade, J.; Żaczek, A.J. Epithelial-Mesenchymal Transition Markers in Lymph Node Metastases and Primary Breast Tumors—Relation to Dissemination and Proliferation. Am. J. Transl. Res. 2014, 6, 793–808. [Google Scholar]
- Jørgensen, C.L.T.; Forsare, C.; Bendahl, P.-O.; Falck, A.-K.; Fernö, M.; Lövgren, K.; Aaltonen, K.; Rydén, L. Expression of Epithelial-Mesenchymal Transition-Related Markers and Phenotypes during Breast Cancer Progression. Breast Cancer Res. Treat. 2020, 181, 369–381. [Google Scholar] [CrossRef]
- Klapper, L.; Ribbat-Idel, J.; Kuppler, P.; Paulsen, F.-O.; Bruchhage, K.-L.; Rades, D.; Offermann, A.; Kirfel, J.; Wollenberg, B.; Idel, C.; et al. NR2F6 as a Prognostic Biomarker in HNSCC. Int. J. Mol. Sci. 2020, 21, 5527. [Google Scholar] [CrossRef] [PubMed]
- Ribbat-Idel, J.; Perner, S.; Kuppler, P.; Klapper, L.; Krupar, R.; Watermann, C.; Paulsen, F.-O.; Offermann, A.; Bruchhage, K.-L.; Wollenberg, B.; et al. Immunologic “Cold” Squamous Cell Carcinomas of the Head and Neck Are Associated with an Unfavorable Prognosis. Front. Med. 2021, 8, 622330. [Google Scholar] [CrossRef] [PubMed]
- Scheble, V.J.; Braun, M.; Beroukhim, R.; Mermel, C.H.; Ruiz, C.; Wilbertz, T.; Stiedl, A.-C.; Petersen, K.; Reischl, M.; Kuefer, R.; et al. ERG Rearrangement Is Specific to Prostate Cancer and Does Not Occur in Any Other Common Tumor. Mod. Pathol. 2010, 23, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ribbat-Idel, J.; Dressler, F.F.; Krupar, R.; Watermann, C.; Paulsen, F.-O.; Kuppler, P.; Klapper, L.; Offermann, A.; Wollenberg, B.; Rades, D.; et al. Performance of Different Diagnostic PD-L1 Clones in Head and Neck Squamous Cell Carcinoma. Front. Med. 2021, 8, 640515. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]

| HNSCC | Hypopharynx | Larynx | Oral Cavity | Oropharynx p16 pos | Oropharynx p16 neg | ||
|---|---|---|---|---|---|---|---|
| Sex | male | 112.4 | 129.6 | 104.2 ** | 110.4 | 114.4 | 126.8 |
| female | 110.2 | 119.5 | 144.5 ** | 94.9 | 98.6 | 111.6 | |
| Age | ≤61y. | 110.5 | 124.6 | 102.4 | 105.9 | 108.2 | 123.8 |
| >61y. | 112.8 | 130.5 | 113.1 | 103.2 | 110.1 | 107.3 | |
| T-Stage | T 1,2,3 | 106.8 *** | 114.3 *** | 104.9 | 103.7 | 106.8 | 111.1 |
| T 4 | 130.2 *** | 171.7 *** | 119.3 | 111.2 | 123.5 | 128.4 | |
| N-Stage | N 0,1 | 105.5 ** | 107.3 * | 109.2 | 104.0 | 97.6 ** | 109.3 |
| N 2,3 | 120.0 ** | 141.9 * | 105.5 | 106.0 | 132.1 ** | 115.5 | |
| M-Stage | M0 | 111.7 | 126.7 | 108.7 | 104.1 | 109.3 | 114.7 |
| M1 | 120.0 | 188.5 | 109.3 | 113.5 | - | 123.7 | |
| UICC | I–III | 106.6 * | 106.1 * | 111.8 | 102.5 | 103.5 | 113.3 |
| IV | 118.3 * | 142.1 * | 104.5 | 107.9 | 134.4 | 115.8 | |
| p16 | pos | 113.3 | 98.5 | 126.9 | 114.1 | 109.3 | - |
| neg | 111.4 | 130.8 | 105.5 | 103.3 | - | 114.9 | |
| Alcohol | Yes | 119.8 * | 133.0 | 108.9 | 120.0 ** | 124.8 | 119.8 |
| No | 106.7 * | 117.5 | 108.7 | 92.8 ** | 107.2 | 106.2 | |
| Nicotine | Yes | 113.0 | 128.2 | 107.5 * | 105.5 | 114.0 | 116.2 |
| No | 115.7 | 141.7 | 145.4 * | 107.7 | 100.7 | 92.8 |
| HNSCC | Hypopharynx | Larynx | Oral Cavity | Oropharynx p16 pos | Oropharynx p16 neg | ||
|---|---|---|---|---|---|---|---|
| Sex | male | 151.1 | 147.2 | 154.5 * | 157.0 | 145.0 | 150.4 |
| female | 157.4 | 130.5 | 179.3 * | 157.3 | 149.3 | 166.8 | |
| Age | ≤61y. | 151.6 | 143.9 | 154.0 | 154.8 | 149.6 | 157.6 |
| >61y. | 153.0 | 145.2 | 159.8 | 160.0 | 144.1 | 150.4 | |
| T-Stage | T 1,2 | 156.3 | 144.6 | 165.0 | 160.2 | 150.5 | 156.6 |
| T 3,4 | 148.4 | 144.8 | 151.4 | 154.0 | 137.5 | 150.1 | |
| N-Stage | N 0 | 159.5 * | 138.6 | 159.0 | 161.3 | 163.4 | 171.1 |
| N 1,2,3 | 146.6 * | 145.9 | 153.5 | 152.9 | 142.2 | 146.8 | |
| M-Stage | M0 | 152.3 | 143.6 | 157.6 | 158.7 | 146.3 | 153.7 |
| M1 | 148.8 | 192.9 | 154.6 | 134.5 | - | 162.1 | |
| UICC | I–II | 157.0 | 140.9 | 170.3 * | 165.0 | 147.5 | 157.0 |
| III–IV | 149.2 | 145.6 | 150.8 * | 153.0 | 142.5 | 152.7 | |
| p16 | pos | 150.2 | 120.8 | 181.6 * | 141.8 | 146.3 | - |
| neg | 153.2 | 147.0 | 152.9 * | 159.4 | - | 153.9 | |
| Alcohol | Yes | 152.2 | 148.8 | 160.8 | 153.1 | 155.2 | 153.6 |
| No | 151.6 | 133.2 | 156.0 | 159.2 | 144.2 | 154.4 | |
| Nicotine | Yes | 150.1 * | 139.9 | 154.2 * | 154.3 | 146.2 | 155.7 |
| No | 167.4 * | 184.8 | 188.2 * | 168.2 | 150.5 | 153.2 |
| SNAIL Low (n = 164) | SNAIL High (n = 163) | ||
|---|---|---|---|
| LSD1 low (n = 163) | 104.0 (63.4%) | 59.0 (36.2%) | p < 0.001 |
| LSD1 high (n = 164) | 60.0 (36.6%) | 104.0 (63.8%) |
| SNAIL Low (n = 285) | SNAIL High (n = 285) | ||
|---|---|---|---|
| LSD1 low (n = 288) | 177.0 (62.1%) | 111.0 (38.9%) | p < 0.001 |
| LSD1 high (n = 282) | 108.0 (37.9%) | 174.0 (61.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottner, J.; Ribbat-Idel, J.; Klapper, L.; Jagomast, T.; Lemster, A.-L.; Perner, S.; Idel, C.; Kirfel, J. Elevated LSD1 and SNAIL Expression Indicate Poor Prognosis in Hypopharynx Carcinoma. Int. J. Mol. Sci. 2022, 23, 5075. https://doi.org/10.3390/ijms23095075
Bottner J, Ribbat-Idel J, Klapper L, Jagomast T, Lemster A-L, Perner S, Idel C, Kirfel J. Elevated LSD1 and SNAIL Expression Indicate Poor Prognosis in Hypopharynx Carcinoma. International Journal of Molecular Sciences. 2022; 23(9):5075. https://doi.org/10.3390/ijms23095075
Chicago/Turabian StyleBottner, Justus, Julika Ribbat-Idel, Luise Klapper, Tobias Jagomast, Anna-Lena Lemster, Sven Perner, Christian Idel, and Jutta Kirfel. 2022. "Elevated LSD1 and SNAIL Expression Indicate Poor Prognosis in Hypopharynx Carcinoma" International Journal of Molecular Sciences 23, no. 9: 5075. https://doi.org/10.3390/ijms23095075
APA StyleBottner, J., Ribbat-Idel, J., Klapper, L., Jagomast, T., Lemster, A.-L., Perner, S., Idel, C., & Kirfel, J. (2022). Elevated LSD1 and SNAIL Expression Indicate Poor Prognosis in Hypopharynx Carcinoma. International Journal of Molecular Sciences, 23(9), 5075. https://doi.org/10.3390/ijms23095075








