Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade
Abstract
1. Introduction
2. Results
2.1. PET Radiopharmaceuticals
2.1.1. PET Radiopharmaceuticals in Oncology
[18F]-Labeled Compounds
[11C]-Labeled Compounds
[124I]-Labeled Compounds
[89Zr]-Labeled Compounds
[64Cu]-Labeled Compounds
[68Ga]-Labeled Compounds
2.1.2. PET Radiopharmaceuticals in Neurology
2.1.3. PET Radiopharmaceuticals for Cardiovascular Events
2.1.4. PET Radiopharmaceuticals for Bacteria Imaging
2.1.5. PET Radiopharmaceuticals for Infection/Inflammation
2.1.6. Novel PET Tracers in Oncology and PET Radiopharmaceuticals with Proven Use in Clinical Practice
2.2. SPECT Radiopharmaceuticals
2.2.1. SPECT Radiopharmaceuticals in Oncology
2.2.2. SPECT Radiopharmaceuticals for Cardiovascular Events
2.2.3. SPECT Radiopharmaceuticals in Neurological Disorders
3. Materials and Methods
4. Conclusions
4.1. PET Radiopharmaceuticals
4.2. SPECT Radiopharmaceuticals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuocolo, A.; Petretta, M. PET and SPECT Specialty Grand Challenge. When Knowledge Travels at the Speed of Light, Photons Take to the Field. Front. Nucl. Med. 2021, 1, 671914. [Google Scholar] [CrossRef]
- Van der Meulen, N.P.; Strobel, K.; Lima, T.V.M. New Radionuclides and Technological Advances in SPECT and PET Scanners. Cancers 2021, 13, 6183. [Google Scholar] [CrossRef] [PubMed]
- Wadsak, W.; Mitterhauser, M. Basics and principles of radiopharmaceuticals for PET/CT. Eur. J. Radiol. 2010, 73, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Rousseau, E.; Kwon, D.; Lin, K.S.; Bénard, F.; Chen, X. Insight into the Development of PET Radiopharmaceuticals for Oncology. Cancers 2020, 12, 1312. [Google Scholar] [CrossRef]
- Lee, Y.S. Radiopharmaceuticals for Molecular Imaging. Open Nucl. Med. J. 2010, 2, 178–185. [Google Scholar] [CrossRef]
- Kuna, M.; Mahdi, F.; Chade, A.R.; Bidwell, G.L., III. Molecular Size Modulates Pharmacokinetics, Biodistribution, and Renal Deposition of the Drug Delivery Biopolymer Elastin-like Polypeptide. Sci. Rep. 2018, 8, 7923. [Google Scholar] [CrossRef]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef]
- Kratochwil, N.A.; Huber, W.; Muller, F.; Kansy, M.; Gerber, P.R. Predicting plasma protein binding of drugs: A new approach. Biochem. Pharmacol. 2002, 64, 1355–1374. [Google Scholar] [CrossRef]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; la Fougère, C.; Mariani, G.; Massalha, S.; et al. Two decades of SPECT/CT—The coming of age of a technology: An updated review of literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99m Tc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef]
- Fahey, F.; Stabin, M. Dose optimization in nuclear medicine. Semin. Nucl. Med. 2014, 44, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A., Jr.; Huda, W.; Yoshizumi, T.T.; Mahesh, M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 2008, 248, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, M.; Rizzo, A.; Rovera, G.; Indovina, L.; Schillaci, O. Radiation dose in nuclear medicine: The hybrid imaging. Radiol. Med. 2019, 124, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends. Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for Positron Emission Tomography: Recent Advances in 11C-, 18F-, 13N-, and 15O-Labeling Reactions. Angew. Chemie. Int. Ed. 2019, 58, 2580–2605. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, X.; Li, J.; Yao, H.; Yuan, S. Fluorine-18 labeled amino acids for tumor PET/CT imaging. Oncotarget 2017, 8, 60581–60588. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Alexiou, G.A.; Fotopoulos, A.D.; Jabbour, P.; Kyritsis, A.P.; Sioka, C. Performance of 18F-FDG,11C-Methionine, and 18F-FET PET for Glioma Grading: A Meta-analysis. Clin. Nucl. Med. 2019, 44, 864–869. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Chen, K.; Shao, Y.; Kiesewetter, D.O.; Niu, G.; Chen, X. Boramino acid as a marker for amino acid transporters. Sci. Adv. 2015, 1, e1500694. [Google Scholar] [CrossRef]
- Nodwell, M.B.; Yang, H.; Čolović, M.; Yuan, Z.; Merkens, H.; Martin, R.E.; Bénard, F.; Schaffer, P.; Britton, R. 18F-Fluorination of Unactivated C-H Bonds in Branched Aliphatic Amino Acids: Direct Synthesis of Oncological Positron Emission Tomography Imaging Agents. J. Am. Chem. Soc. 2017, 139, 3595–3598. [Google Scholar] [CrossRef]
- Nodwell, M.B.; Yang, H.; Merkens, H.; Malik, N.; Čolović, M.; Wagner, B.; Martin, R.E.; Bénard, F.; Schaffer, P.; Britton, R. 18F-branched-chain amino acids: Structure-activity relationships and PET imaging potential. J. Nucl. Med. 2019, 60, 1003–1009. [Google Scholar] [CrossRef]
- Paquette, M.; Lavallée, É.; Phoenix, S.; Ouellet, R.; Senta, H.; Van Lier, J.E.; Guérin, B.; Lecomte, R.; Turcotte, É.E. Improved estrogen receptor assessment by PET using the novel radiotracer 18F-4FMFES in estrogen receptor–positive breast cancer patients: An ongoing phase II clinical trial. J. Nucl. Med. 2018, 59, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, Y. Molecular Targeted Therapy of Lung Cancer; Springer: Singapore, 2017. [Google Scholar]
- Huang, G. Nuclear Medicine in Oncology; Springer: Singapore, 2019. [Google Scholar]
- Cai, J.; Chang, J.; Yin, F. Principles and Practice of Image-Guided Radiation Therapy of Lung Cancer; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kandathil, A.; Kay, F.U.; Butt, Y.M.; Wachsmann, J.W.; Subramaniam, R.M. Role of FDG PET/CT in the eighth edition of TNM staging of non–small cell lung cancer. Radiographics 2018, 38, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Telo, S.; Calderoni, L.; Vichi, S.; Zagni, F.; Castellucci, P.; Fanti, S. Review: Alternative and new radiopharmaceutical agents for lung cancer. Curr. Radiopharm. 2019, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.J.; Mittal, B.R.; Maturu, V.N.; Agarwal, R.; Bal, A.; Dey, P.; Shukla, J.; Gupta, D. (18)F-FDG and (18)F-FLT PET/CT imaging in the characterization of mediastinal lymph nodes. Ann. Nucl. Med. 2016, 30, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Saga, T.; Inubushi, M.; Koizumi, M.; Yoshikawa, K.; Zhang, M.R.; Tanimoto, K.; Horiike, A.; Yanagitani, N.; Ohyanagi, F.; Nishio, M. Prognostic value of (18) F-fluoroazomycin arabinoside PET/CT in patients with advanced non-small-cell lung cancer. Cancer Sci. 2015, 106, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Szyszko, T.A.; Yip, C.; Szlosarek, P.; Goh, V.; Cook, G.J. The role of new PET tracers for lung cancer. Lung Cancer 2016, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, A.S.; Gkiozos, I.; Kontopyrgias, G.; Charpidou, A.; Kotteas, E.; Kyrgias, G.; Tolia, M. Modern radiopharmaceuticals for lung cancer imaging with positron emission tomography/computed tomography scan: A systematic review. SAGE Open Med. 2020, 8, 2050312120961594. [Google Scholar] [CrossRef]
- Araz, M.; Aras, G.; Küçük, Ö.N. The role of 18F–NaF PET/CT in metastatic bone disease. J. Bone Oncol. 2015, 4, 92–97. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer with Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef]
- Sørensen, M.; Mikkelsen, K.S.; Frisch, K.; Villadsen, G.E.; Keiding, S. Regional metabolic liver function measured by 2-[18F]fluoro-2-deoxy-galactose PET/CT in patients with cirrhosis. J. Hepatol. 2013, 58, 1119–1124. [Google Scholar] [CrossRef]
- Horsager, J.; Munk, O.L.; Sørensen, M. Metabolic liver function measured in vivo by dynamic (18)F-FDGal PET/CT without arterial blood sampling. EJNMMI Res. 2015, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Bak-Fredslund, K.P.; Keiding, S.; Villadsen, G.E.; Kramer, S.; Schlander, S.; Sørensen, M. [18F]-Fluoro-2-deoxy-D-galactose positron emission tomography/computed tomography as complementary imaging tool in patients with hepatocellular carcinoma. Liver Int. 2020, 40, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Pak, K.; Kim, K. Diagnostic accuracy of F-18 FDG PET or PET/CT for detection of lymph node metastasis in clinically node negative head and neck cancer patients: A systematic review and meta-analysis. Am. J. Otolaryngol. 2019, 40, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.; Leccisotti, L.; Bussu, F.; Miccichè, F.; Rufini, V. Role of (18)F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2013, 33, 1–8. [Google Scholar] [PubMed]
- Helsen, N.; Roothans, D.; Van Den Heuvel, B.; Van den Wyngaert, T.; Van den Weyngaert, D.; Carp, L.; Stroobants, S. 18F-FDG-PET/CT for the detection of disease in patients with head and neck cancer treated with radiotherapy. PLoS ONE 2017, 12, e0182350. [Google Scholar] [CrossRef] [PubMed]
- Schöder, H.; França, P.; Nakajima, R.; Burnazi, E.; Roberts, S.; Brand, C.; Grkovski, M.; Mauguen, A.; Dunphy, M.P.; Ghossein, R.A.; et al. Safety and Feasibility of PARP1/2 Imaging with 18F-PARPi in Patients with Head and Neck Cancer. Clin. Cancer Res. 2020, 26, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.; Kossatz, S.; Reiner, T. Molecular imaging of PARP. J. Nucl. Med. 2017, 58, 1025–1030. [Google Scholar] [CrossRef]
- Wilson, T.C.; Xavier, M.A.; Knight, J.; Verhoog, S.; Torres, J.B.; Mosley, M.; Hopkins, S.L.; Wallington, S.; Allen, P.D.; Kersemans, V.; et al. PET imaging of PARP expression using (18)F-Olaparib. J. Nucl. Med. 2019, 60, 504–510. [Google Scholar] [CrossRef]
- Guru, N.; De Souza França, P.D.; Pirovano, G.; Huang, C.; Patel, G.S.; Reiner, T. [18F]PARPi Imaging Is Not Affected by HPV Status In Vitro. Mol. Imaging 2021, 2021, 6641397. [Google Scholar] [CrossRef]
- Young, R.J.; Demétrio De Souza França, P.; Pirovano, G.; Piotrowski, A.F.; Nicklin, P.J.; Riedl, C.C.; Schwartz, J.; Bale, T.A.; Donabedian, P.L.; Kossatz, S.; et al. Preclinical and first-in-human-brain-cancer applications of [18F]poly (ADP-ribose) polymerase inhibitor PET/MR. Neurooncol. Adv. 2020, 2, vdaa119. [Google Scholar] [CrossRef]
- Ramos-Álvarez, I.; Moreno, P.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moody, T.W.; Coy, D.H.; Jensen, R.T. Insights into bombesin receptors and ligands: Highlighting recent advances. Peptides 2015, 72, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Mantey, S.A.; Nuche-Berenguer, B.; Reitman, M.L.; González, N.; Coy, D.H.; Jensen, R.T. Comparative pharmacology of bombesin receptor subtype-3, nonpeptide agonist MK-5046, a universal peptide agonist, and peptide antagonist Bantag-1 for human bombesin receptors. J. Pharmacol. Exp. Ther. 2013, 347, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.R.; Burger, I.A.; Schibli, R.; Friebe, M.; Dinkelborg, L.; Graham, K.; Borkowski, S.; Bacher-Stier, C.; Valencia, R.; Srinivasan, A.; et al. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J. Nucl. Med. 2015, 56, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Chitkara, A.; Saklani, M.; Kaur, A.; Mathur, R.; Tiwari, A.; Singh, B.; Mishra, A.K. Targeting Bombesin Peptide Receptors for Cancer Imaging: Perspective in Prostate, Lung and Breast Cancer. Nov. Appro. Can. Study 2020, 5, 483–491. [Google Scholar] [CrossRef]
- Taddei, C.; Pike, V.W. [11C]Carbon monoxide: Advances in production and application to PET radiotracer development over the past 15 years. EJNMMI Radiopharm. Chem. 2019, 4, 25. [Google Scholar] [CrossRef]
- Qu, W.; Hu, B.; Babich, J.W.; Waterhouse, N.; Dooley, M.; Ponnala, S.; Urgiles, J. A general 11C-labeling approach enabled by fluoride-mediated desilylation of organosilanes. Nat. Commun. 2020, 11, 1736. [Google Scholar] [CrossRef]
- Yang, L.; Scott, P.J.H.; Shao, X. [11C]Carbon Dioxide: Starting Point for Labeling PET Radiopharmaceuticals. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Karamé, I., Shaya, J., Srour, H., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Moldovean, S.N.; Timaru, D.G.; Chiş, V. All-Atom Molecular Dynamics Investigations on the Interactions between D2 Subunit Dopamine Receptors and Three 11C-Labeled Radiopharmaceutical Ligands. Int. J. Mol. Sci. 2022, 23, 2005. [Google Scholar] [CrossRef]
- Dahl, K.; Halldin, C.; Schou, M. New methodologies for the preparation of carbon-11 labeled radiopharmaceuticals. Clin. Transl. Imaging 2017, 5, 275–289. [Google Scholar] [CrossRef]
- Gómez-Vallejo, V.; Gaja, V.; Koziorowski, J.; Llop, J. Specific activity of 11 C-labeled radiotracers: A big challenge for PET chemists. In Positron Emission Tomography—Current Clinical and Research Aspects; Hsieh, C.-H., Ed.; IntechOpen: London, UK, 2012; pp. 183–209. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Liang, S.H.; Holland, J.P.; Collier, T.L.; Hooker, J.M.; Wilson, A.A.; Vasdev, N. 11CO2 Fixation: A renaissance in PET radiochemistry. Chem. Comm. 2013, 49, 5621–5629. [Google Scholar] [CrossRef]
- Taddei, C.; Gee, A.D. Recent progress in [11C]carbon dioxide ([11C]CO2) and [11C]carbon monoxide ([11C]CO) chemistry. J. Label. Comp. Radiopharm. 2018, 61, 237–251. [Google Scholar] [CrossRef]
- Haywood, T.; Kealey, S.; Sánchez-Cabezas, S.; Hall, J.J.; Allott, L.; Smith, G.; Plisson, C.; Miller, P.W. Carbon-11 radiolabelling of organosulfur compounds: (11)C synthesis of the progesterone receptor agonist Tanaproget. Chem. Eur. J. 2015, 21, 9034–9038. [Google Scholar] [CrossRef] [PubMed]
- Haywood, T.; Cesarec, S.; Kealey, S.; Plisson, C.; Miller, P.W. Ammonium [11C]thiocyanate: Revised preparation and reactivity studies of a versatile nucleophile for carbon-11 radiolabelling. MedChemComm 2018, 9, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Haskali, M.B.; Pike, V.W. [11C]Fluoroform, a breakthrough for versatile labeling of PET radiotracer trifluoromethyl groups in high molar activity. Chem. Eur. J. 2017, 23, 8156–8160. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.; Castellucci, P. Clinical indications of 11C-choline PET/CT in prostate cancer patients with biochemical relapse. Theranostics 2012, 2, 313–317. [Google Scholar] [CrossRef]
- Grassi, I.; Nanni, C.; Allegri, V.; Morigi, J.J.; Montini, G.C.; Castellucci, P.; Fanti, S. The clinical use of PET with (11)C-acetate. Am. J. Nucl. Med. Mol. Imaging. 2012, 2, 33–47. [Google Scholar]
- Chen, M.; Zhu, W.; Du, J.; Yang, C.; Han, B.; Zhou, D.; Huo, L.; Zhuang, J. 11C-acetate positron emission tomography is more precise than 18F-fluorodeoxyglucose positron emission tomography in evaluating tumor burden and predicting disease risk of multiple myeloma. Sci. Rep. 2021, 11, 22188. [Google Scholar] [CrossRef]
- Spick, C.; Herrmann, K.; Czernin, J. Evaluation of Prostate Cancer with 11C-Acetate PET/CT. J. Nucl. Med. 2016, 57 (Suppl. 3), 30S–37S. [Google Scholar] [CrossRef]
- Jambor, I.; Borra, R.; Kemppainen, J.; Lepomäki, V.; Parkkola, R.; Dean, K.; Alanen, K.; Arponen, E.; Nurmi, M.; Aronen, H.J.; et al. Improved detection of localized prostate cancer using co-registered MRI and 11 C-acetate PET/CT. Eur. J. Radiol. 2012, 81, 2966–2972. [Google Scholar] [CrossRef]
- Mena, E.; Turkbey, B.; Mani, H.; Adler, S.; Valera, V.A.; Bernardo, M.; Shah, V.; Pohida, T.; McKinney, Y.; Kwarteng, G.; et al. 11 C-acetate PET/CT in localized prostate cancer: A study with MRI and histopathologic correlation. J. Nucl. Med. 2012, 53, 538–545. [Google Scholar] [CrossRef]
- Schöder, H.; Ong, S.C.; Reuter, V.E.; Cai, S.; Burnazi, E.; Dalbagni, G.; Larson, S.M.; Bochner, B.H. Initial results with (11)C-acetate positron emission tomography/computed tomography (PET/CT) in the staging of urinary bladder cancer. Mol. Imaging. Biol. 2012, 14, 245–251. [Google Scholar] [CrossRef]
- Haseebuddin, M.; Dehdashti, F.; Siegel, B.A.; Liu, J.; Roth, E.B.; Nepple, K.G.; Siegel, C.L.; Fischer, K.C.; Kibel, A.S.; Andriole, G.L.; et al. 11C-acetate PET/CT before radical prostatectomy: Nodal staging and treatment failure prediction. J. Nucl. Med. 2013, 54, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Daouacher, G.; von Below, C.; Gestblom, C.; Ahlström, H.; Grzegorek, R.; Wassberg, C.; Sörensen, J.; Waldén, M. Laparoscopic extended pelvic lymph node (LN) dissection as validation of the performance of [(11)C]-acetate positron emission tomography/computer tomography in the detection of LN metastasis in intermediate- and high-risk prostate cancer. BJU Int. 2016, 118, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, S.; Karlsson, C.T.; Sundström, T.; Ögren, M.; Ögren, M.; Axelsson, J.; Riklund, K. (11)C-acetate PET/CT in pre-therapeutic lymph node staging in high-risk prostate cancer patients and its influence on disease management: A retrospective study. EJNMMI Res. 2014, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.C.; Radecka, E.; Hellström, M.; Jacobsson, H.; Sundin, A. [11C]-acetate positron emission tomography–computed tomography imaging of prostate cancer lymph-node metastases correlated with histopathological findings after extended lymphadenectomy. Scand. J. Urol. 2015, 49, 35–42. [Google Scholar] [CrossRef]
- Leisser, A.; Pruscha, K.; Ubl, P.; Wadsak, W.; Mayerhöfer, M.; Mitterhauser, M.; Hacker, M.; Kramer, G.; Shariat, S.; Karanikas, G.; et al. Evaluation of fatty acid synthase in prostate cancer recurrence: SUV of [(11)C]-acetate PET as a prognostic marker. Prostate 2015, 75, 1760–1767. [Google Scholar] [CrossRef]
- Spick, C.; Polanec, S.H.; Mitterhauser, M.; Wadsak, W.; Anner, P.; Reiterits, B.; Haug, A.R.; Hacker, M.; Beheshti, M.; Karanikas, G. Detection of bone metastases using 11C-acetate PET in patients with prostate cancer with biochemical recurrence. Anticancer Res. 2015, 35, 6787–6791. [Google Scholar]
- Bahce, I.; Yaqub, M.; Errami, H.; Schuit, R.C.; Schober, P.; Thunnissen, E.; Windhorst, A.D.; Lammertsma, A.A.; Smit, E.F.; Hendrikse, N.H. Effects of erlotinib therapy on [(11)C]erlotinib uptake in EGFR mutated, advanced NSCLC. EJNMMI Res. 2016, 6, 10. [Google Scholar] [CrossRef]
- Petrulli, J.R.; Zheng, M.; Huang, Y.; Nabulsi, N.B.; Goldberg, S.B.; Contessa, J.N.; Morris, E.D. Evaluation of quantitative modeling methods in whole-body, dynamic [11C]-erlotinib PET. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 143–153. [Google Scholar]
- Yaqub, M.; Bahce, I.; Voorhoeve, C.; Schuit, R.C.; Windhorst, A.D.; Hoekstra, O.S.; Boellaard, R.; Hendrikse, N.H.; Smit, E.F.; Lammertsma, A.A. Quantitative and simplified analysis of 11C-erlotinib studies. J. Nucl. Med. 2016, 57, 861–866. [Google Scholar] [CrossRef][Green Version]
- Kumar, K.; Ghosh, A. Radiochemistry, Production Processes, Labeling Methods, and ImmunoPET Imaging Pharmaceuticals of Iodine-124. Molecules 2021, 26, 414. [Google Scholar] [CrossRef]
- Salodkin, S.S.; Golovkov, V.M. Cyclotron Production of Iodine-124. Russ. Phys. J. 2020, 62, 2347–2353. [Google Scholar] [CrossRef]
- Dubost, E.; McErlain, H.; Babin, V.; Sutherland, A.; Cailly, T. Recent Advances in Synthetic Methods for Radioiodination. J. Org. Chem. 2020, 85, 8300–8310. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Takeda, T.; Yokokawa, M.; Yu, J.; Makino, A.; Kiyono, Y.; Shiba, K.; Kinuya, S.; Odani, A. Comparison of Radioiodine-or Radiobromine-labeled RGD Peptides between Direct and Indirect Labeling Methods. Chem. Pharm. Bull. 2018, 66, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Rangger, C.; Haubner, R. Radiolabelled Peptides for Positron Emission Tomography and Endoradiotherapy in Oncology. Pharmaceuticals 2020, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.D.; Lapi, S.E. Designing the Magic Bullet? The Advancement of Immuno-PET into Clinical Use. J. Nucl. Med. 2013, 54, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Samnick, S.; Al-Momani, E.; Schmid, J.S.; Mottok, A.; Buck, A.K.; Lapa, C. Initial Clinical Investigation of [18F]Tetrafluoroborate PET/CT in Comparison to [124I]iodine PET/CT for Imaging Thyroid Cancer. Clin. Nucl. Med. 2018, 43, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Gustafson, W.C.; Dannoon, S.F.; Nekritz, E.A.; Lee, C.L.; Murphy, S.T.; VanBrocklin, H.F.; Hernandez-Pampaloni, M.; Haas-Kogan, D.A.; Weiss, W.A.; et al. Tumor dosimetry using [124I]m-iodobenzylguanidine microPET/CT for [131I]m-iodobenzylguanidine treatment of neuroblastoma in a murine xenograft model. Mol. Imaging Biol. 2012, 14, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B. Radioiodine: The classic theranostic agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Cascini, G.L.; Niccoli Asabella, A.; Notaristefano, A.; Restuccia, A.; Ferrari, C.; Rubini, D.; Altini, C.; Rubini, G. 124 Iodine: A Longer-Life Positron Emitter Isotope—New Opportunities in Molecular Imaging. Biomed. Res. Int. 2014, 2014, 672094. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, N.; Chen, Z.; Liu, T.; Xu, X.; Lei, X.; Shen, L.; Gao, J.; Yang, Z.; Zhu, H. Construction of 124I-trastuzumab for noninvasive PET imaging of HER2 expression: From patient-derived xenograft models to gastric cancer patients. Gastric Cancer 2020, 23, 614–626. [Google Scholar] [CrossRef]
- Zettlitz, K.A.; Tavaré, R.; Knowles, S.M.; Steward, K.K.; Timmerman, J.M.; Wu, A.M. ImmunoPET of Malignant and Normal B Cells with 89Zr- and 124I-Labeled Obinutuzumab Antibody Fragments Reveals Differential CD20 Internalization In Vivo. Clin. Cancer Res. 2017, 23, 7242–7252. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Zhu, H.; Li, G.H.; Xie, Q.; Yang, X.T.; Xu, X.X.; Tian, X.B.; Wan, Y.K.; Yang, Z. Construction of Anti-hPD-L1 HCAb Nb6 and in Situ 124I Labeling for Noninvasive Detection of PD-L1 Expression in Human Bone Sarcoma. Bioconj. Chem. 2019, 30, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhu, H.; Xie, Q.; Tian, X.; Yang, X.; Feng, F.; Jiang, Q.; Sheng, X.; Yang, Z. Evaluation of 124I-JS001 for hPD1 immuno-PET imaging using sarcoma cell homografts in humanized mice. Acta Pharm. Sin. B 2020, 10, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Escorcia, F.E.; Steckler, J.M.; Abdel-Atti, D.; Price, E.W.; Carlin, S.D.; Scholz, W.W.; Lewis, J.S.; Houghton, J.L. Tumor-Specific Zr-89 Immuno-PET Imaging in a Human Bladder Cancer Model. Mol. Imaging Biol. 2018, 20, 808–815. [Google Scholar] [CrossRef]
- Houghton, J.L.; Abdel-Atti, D.; Scholz, W.W.; Lewis, J.S. Preloading with Unlabeled CA19.9 Targeted Human Monoclonal Antibody Leads to Improved PET Imaging with 89Zr-5B1. Mol. Pharm. 2017, 14, 908–915. [Google Scholar] [CrossRef]
- Lohrmann, C.; O′Reilly, E.M.; O′Donoghue, J.A.; Pandit-Taskar, N.; Carrasquillo, J.A.; Lyashchenko, S.K.; Ruan, S.; Teng, R.; Scholz, W.; Maffuid, P.W.; et al. Retooling a Blood-Based Biomarker: Phase I Assessment of the High-Affinity CA19-9 Antibody HuMab-5B1 for Immuno-PET Imaging of Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 7014–7023. [Google Scholar] [CrossRef]
- Zanzonico, P.; Carrasquillo, J.A.; Pandit-Taskar, N.; O′Donoghue, J.A.; Humm, J.L.; Smith-Jones, P.; Ruan, S.; Divgi, C.; Scott, A.M.; Kemeny, N.E.; et al. PET-based compartmental modeling of (124)I-A33 antibody: Quantitative characterization of patient-specific tumor targeting in colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1700–1706. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Franssen, G.M.; Mulders, P.F.; Oyen, W.J.; van Dongen, G.A.; Laverman, P.; Oosterwijk, E.; Boerman, O.C. ImmunoPET Imaging of Renal Cell Carcinoma with (124)I- and (89)Zr-Labeled Anti-CAIX Monoclonal Antibody cG250 in Mice. Cancer Biother. Radiopharm. 2013, 28, 510–515. [Google Scholar] [CrossRef]
- Cheal, S.M.; Punzalan, B.; Doran, M.G.; Evans, M.J.; Osborne, J.R.; Lewis, J.S.; Zanzonico, P.; Larson, S.M. Pairwise comparison of 89Zr- and 124I-labeled cG250 based on positron emission tomography imaging and nonlinear immunokinetic modeling: In vivo carbonic anhydrase IX receptor binding and internalization in mouse xenografts of clear-cell renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 985–994. [Google Scholar] [CrossRef]
- Divgi, C.R.; Uzzo, R.G.; Gatsonis, C.; Bartz, R.; Treutner, S.; Yu, J.Q.; Chen, D.; Carrasquillo, J.A.; Larson, S.M.; Bevan, P.; et al. Positron Emission Tomography/Computed Tomography Identification of Clear Cell Renal Cell Carcinoma: Results from the REDECT Trial. J. Clin. Oncol. 2013, 31, 187–194. [Google Scholar] [CrossRef]
- Povoski, S.P.; Hall, N.C.; Murrey, D.A., Jr.; Sharp, D.S.; Hitchcock, C.L.; Mojzisik, C.M.; Bahnson, E.E.; Knopp, M.V.; Martin, E.W., Jr.; Bahnson, R.R. Multimodal Imaging and Detection Strategy With 124I-Labeled Chimeric Monoclonal Antibody cG250 for Accurate Localization and Confirmation of Extent of Disease During Laparoscopic and Open Surgical Resection of Clear Cell Renal Cell Carcinoma. Surg. Innov. 2013, 20, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, M.C.; Chen, D.Y.T.; Yu, J.Q.; Pilmack, E.R. Potential role of (124)I-girentuximab in the presurgical diagnosis of clear-cell renal cell cancer. Biologics 2012, 6, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Lin, K.S.; Benard, F. Past, Present, and Future: Development of Theranostic Agents Targeting Carbonic Anhydrase IX. Theranostics 2017, 7, 4322–4339. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Malmberg, J.; Estrada, S.; Eriksson, O.; Orlova, A. Development of a 124I-labeled version of the anti-PSMA monoclonal antibody capromab for immunoPET staging of prostate cancer: Aspects of labeling chemistry and biodistribution. Int. J. Oncol. 2014, 44, 1998–2008. [Google Scholar] [CrossRef]
- Frigerio, B.; Morlino, S.; Luison, E.; Seregni, E.; Lorenzoni, A.; Satta, A.; Valdagni, R.; Bogni, A.; Chiesa, C. Anti-PSMA 124I-scFvD2B as a new immuno-PET tool for prostate cancer: Preclinical proof of principle. J. Exp. Clin. Cancer Res. 2019, 38, 326–334. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II Study of Lutetium-177-Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O′Donoghue, J.A.; Beylergil, V.; Lyashchenko, S.; Ruan, S.; Solomon, S.B.; Durack, J.C.; Carrasquillo, J.A.; Lefkowitz, R.A.; Gonen, M.; et al. 89Zr-huJ591 Immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2093–2105. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Durack, J.C.; Lyashchenko, S.K.; Cheal, S.M.; Beylergil, V.; Lefkowitz, R.A.; Carrasquillo, J.A.; Martinez, D.F.; Fung, A.M.; et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin. Cancer Res. 2015, 21, 5277–5285. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O′Donoghue, J.A.; Divgi, C.R.; Wills, E.A.; Schwartz, L.; Gönen, M.; Smith-Jones, P.; Bander, N.H.; Scher, H.I.; Larson, S.M.; et al. Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res. 2015, 5, 28. [Google Scholar] [CrossRef]
- Fung, E.K.; Cheal, S.M.; Fareedy, S.B.; Punzalan, B.; Beylergil, V.; Amir, J.; Chalasani, S.; Weber, W.A.; Spratt, D.E.; Veach, D.R.; et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: Optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.M.; Zettlitz, K.J.; Tavare, R.; Rochefortl, M.M.; Salazar, F.B.; Stout, D.B.; Yazaki, P.J.; Reiter, R.E.; Wu, A.M. Quantitative ImmunoPET of Prostate Cancer Xenografts with 89 Zr- and 124 I-Labeled Anti-PSCA A11 Minibody. J. Nucl. Med. 2014, 55, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.M.; Tavare, R.; Zettlitz, K.J.; Rochefortl, M.M.; Salazar, F.B.; Jiang, Z.K.; Reiter, R.E.; Wu, A.M. Applications of ImmunoPET: Using 124 I-Anti-PSCA A11 Minibody for Imaging Disease Progression and Response to Therapy in Mouse Xenograft Models of Prostate Cancer. Clin. Cancer Res. 2014, 20, 6367–6378. [Google Scholar] [CrossRef]
- Tsai, W.K.; Zettlitz, K.A.; Tavare, R.; Kobayashi, N.; Reiter, R.E.; Wy, A.M. Dual-Modality ImmunoPET/Fluorescence Imaging of Prostate Cancer with an Anti-PSCA Cys-Minibody. Theranostics 2018, 8, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Zettlitz, K.A.; Wen-Ting, K.T.; Knowles, S.M.; Kobayashi, N.; Donahue, T.R.; Reiter, R.E.; Wu, A.M. Dual-Modality Immuno-PET and Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using an Anti–Prostate Stem Cell Antigen Cys-Diabody. J. Nucl. Med. 2018, 59, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, J.A.; O′Donoghue, J.A.; Beylergil, V.; Ruan, S.; Pandit-Taskar, N.; Larson, S.M.; Smith-Jones, P.M.; Lyashchenko, S.K.; Ohishi, N.; Ohtomo, T.; et al. I-124 codrituzumab imaging and biodistribution in patients with hepatocellular carcinoma. EJNMMI Res. 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Mikulová, M.B.; Mikuš, P. Advances in Development of Radiometal Labeled Amino Acid-Based Compounds for Cancer Imaging and Diagnostics. Pharmaceuticals 2021, 14, 167. [Google Scholar] [CrossRef]
- Yoon, J.K.; Park, B.N.; Ryu, E.K.; An, Y.S.; Lee, S.J. Current Perspectives on 89Zr-PET Imaging. Int. J. Mol. Sci. 2020, 21, 4309. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Viola-Villegas, N.; Holland, J.P.; Divilov, V.; Carlin, S.D.; Gomes-DaGama, E.M.; Chiosis, G.; Carbonetti, G.; de Stanchina, E.; Lewis, J.S. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J. Nucl. Med. 2013, 54, 936–943. [Google Scholar] [CrossRef]
- Gaykema, S.B.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J.; et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, S.J.; Oosting, S.F.; Brouwers, A.H.; Bongaerts, A.H.; de Jong, J.R.; Lub-de Hooge, M.N.; Oude Munnink, T.H.; Fiebrich, H.B.; Sluiter, W.J.; Links, T.P.; et al. Everolimus reduces (89)Zr-bevacizumab tumor uptake in patients with neuroendocrine tumors. J. Nucl. Med. 2014, 55, 1087–1092. [Google Scholar] [CrossRef]
- Oosting, S.F.; Brouwers, A.H.; van Es, S.C.; Nagengast, W.B.; Oude Munnink, T.H.; Lub-de Hooge, M.N.; Hollema, H.; de Jong, J.R.; de Jong, I.J.; de Haas, S.; et al. 89Zr-Bevacizumab PET Visualizes Heterogeneous Tracer Accumulation in Tumor Lesions of Renal Cell Carcinoma Patients and Differential Effects of Antiangiogenic Treatment. J. Nucl. Med. 2015, 56, 63–69. [Google Scholar] [CrossRef]
- Bahce, I.; Huisman, M.C.; Verwer, E.E.; Ooijevaar, R.; Boutkourt, F.; Vugts, D.J.; van Dongen, G.A.; Boellaard, R.; Smit, E.F. Pilot study of (89)Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res. 2014, 4, 35–42. [Google Scholar] [CrossRef]
- Jansen, M.H.; Veldhuijzen van Zanten, S.E.M.; van Vuurden, D.G.; Huisman, M.C.; Vugts, D.J.; Hoekstra, O.S.; van Dongen, G.A.; Kaspers, G.L. Molecular Drug Imaging: 89Zr-bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2017, 58, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen van Zanten, S.E.M.; Sewing, A.C.P.; van Lingen, A.; Hoekstra, O.S.; Wesseling, P.; Meel, M.H.; van Vuurden, D.G.; Kaspers, G.J.L.; Hulleman, E.; Bugiani, M. Multiregional tumor drug-uptake imaging by PET and microvascular morphology in end-stage diffuse intrinsic pontine glioma. J. Nucl. Med. 2018, 59, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Oosting, S.F.; van Asselt, S.J.; Brouwers, A.H.; Bongaerts, A.H.; Steinberg, J.D.; de Jong, J.R.; Lub-de Hooge, M.N.; van der Horst-Schrivers, A.N.; Walenkamp, A.M.; Hoving, E.W.; et al. 89Zr-Bevacizumab PET Visualizes Disease Manifestations in Patients with von Hippel-Lindau Disease. J. Nucl. Med. 2016, 57, 1244–1250. [Google Scholar] [CrossRef]
- Golestani, R.; Zeebregts, C.J.; Terwisscha van Scheltinga, A.G.; Lub-de Hooge, M.N.; van Dam, G.M.; Glaudemans, A.W.; Dierckx, R.A.; Tio, R.A.; Suurmeijer, A.J.; Boersma, H.H.; et al. Feasibility of vascular endothelial growth factor imaging in human atherosclerotic plaque using (89)Zr-bevacizumab positron emission tomography. Mol. Imaging 2013, 12, 235–243. [Google Scholar] [CrossRef]
- Even, A.J.; Hamming-Vrieze, O.; van Elmpt, W.; Winnepenninckx, V.J.; Heukelom, J.; Tesselaar, M.E.; Vogel, W.V.; Hoeben, A.; Zegers, C.M.; Vugts, D.J.; et al. Quantitative assessment of Zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: A theragnostic approach. Oncotarget 2017, 8, 3870–3880. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; Gootjes, E.C.; Huisman, M.C.; Vugts, D.J.; Roth, C.; Luik, A.M.; Mulder, E.R.; Schuit, R.C.; Boellaard, R.; Hoekstra, O.S.; et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015, 6, 30384–30393. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.E.; Boellaard, R.; van Lingen, A.; Lammertsma, A.A.; van Dongen, G.A.; Verheul, H.M.; Menke, C.W.; Huisman, M.C. PET/CT-derived whole-body and bone marrow dosimetry of 89Zr-cetuximab. J. Nucl. Med. 2015, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Jauw, Y.W.; Zijlstra, J.M.; de Jong, D.; Vugts, D.J.; Zweegman, S.; Hoekstra, O.S.; van Dongen, G.A.; Huisman, M.C. Performance of 89Zr-Labeled-Rituximab-PET as an Imaging Biomarker to Assess CD20 Targeting: A Pilot Study in Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma. PLoS ONE 2017, 12, e0169828. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, S.; Tsang, A.S.M.; Raterman, H.; Ramwadhdoebe, T.; Vugts, D.; van Dongen, G.; Huisman, M.; Hoekstra, O.; Tak, P.P.; Voskuyl, A.; et al. B-cell imaging with zirconium-89 labeled rituximab PET-CT at baseline is associated with therapeutic response 24 weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res. Ther. 2016, 18, 266. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Fine, B.M.; Pandit-Taskar, N.; Larson, S.M.; Fleming, S.E.; Fox, J.J.; Cheal, S.M.; O’Donoghue, J.A.; Ruan, S.; Ragupathi, G.; et al. Imaging patients with metastatic castration-resistant prostate cancer using 89Zr-DFO-MSTP2109A anti-STEAP1 antibody. J. Nucl. Med. 2019, 60, 1517–1523. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. ClinicalTrials. Phase 1 Imaging Study of 89Zr-DFO-HuMab-5B1 with HuMab-5B1—Full Text View. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02687230 (accessed on 7 February 2022).
- Lamberts, L.E.; Menke-van der Houven van Oordt, C.W.; ter Weele, E.J.; Bensch, F.; Smeenk, M.M.; Voortman, J.; Hoekstra, O.S.; Williams, S.P.; Fine, B.M.; Maslyar, D.; et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin. Cancer Res. 2016, 22, 1642–1652. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- O’Donoghue, J.A.; Lewis, J.S.; Pandit-Taskar, N.; Fleming, S.E.; Schöder, H.; Larson, S.M.; Beylergil, V.; Ruan, S.; Lyashchenko, S.K.; Zanzonico, P.B.; et al. Pharmacokinetics, biodistribution, and radiation dosimetry for (89)zr-trastuzumab in patients with esophagogastric cancer. J. Nucl. Med. 2018, 59, 161–166. [Google Scholar] [CrossRef]
- Sanchez-Vega, F.; Hechtman, J.F.; Castel, P.; Ku, G.Y.; Tuvy, Y.; Won, H.; Fong, C.J.; Bouvier, N.; Nanjangud, G.J.; Soong, J.; et al. EGFR and MET amplifications determine response to HER2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 2019, 9, 199–209. [Google Scholar] [CrossRef]
- Moek, K.L.; Waaijer, S.; Kok, I.C.; Suurs, F.V.; Brouwers, A.H.; Menke-van der Houven van Oordt, C.W.; Wind, T.T.; Gietema, J.A.; Schröder, C.P.; Mahesh, S.; et al. 89Zr-labeled bi-specific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, heterogeneous tumor uptake. Clin. Cancer Res. 2019, 25, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, L.; Adler, S.; Turkbey, I.B.; Mertan, F.; Ton, A.; Do, K.; Kummar, S.; Gonzalez, E.M.; Bhattacharyya, S.; Jacobs, P.M.; et al. Dosimetry and first human experience with 89Zr-panitumumab. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 195–203. [Google Scholar] [PubMed]
- van Helden, E.J.; Elias, S.G.; Gerritse, S.L.; van Es, S.C.; Boon, E.; Huisman, M.C.; van Grieken, N.; Dekker, H.; van Dongen, G.; Vugts, D.J.; et al. [89Zr]Zr-cetuximab PET/CT as biomarker for cetuximab mono- therapy in patients with RAS wild-type advanced colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2481. [Google Scholar] [CrossRef] [PubMed]
- Hekman, M.C.H.; Rijpkema, M.; Aarntzen, E.H.; Mulder, S.F.; Langenhuijsen, J.F.; Oosterwijk, E.; Boerman, O.C.; Oyen, W.J.G.; Mulders, P.F.A. Positron Emission Tomography/Computed Tomography with 89Zr-girentuximab Can Aid in Diagnostic Dilemmas of Clear Cell Renal Cell Carcinoma Suspicion. Eur. Urol. 2018, 74, 257–260. [Google Scholar] [CrossRef]
- Joraku, A.; Hatano, K.; Kawai, K.; Kandori, S.; Kojima, T.; Fukumitsu, N.; Isobe, T.; Mori, Y.; Sakata, M.; Hara, T.; et al. Phase I/IIa PET imaging study with 89zirconium labeled anti-PSMA minibody for urological malignancies. Ann. Nucl. Med. 2019, 33, 119–127. [Google Scholar] [CrossRef]
- van Es, S.C.; Brouwers, A.H.; Mahesh, S.; Leliveld-Kors, A.M.; de Jong, I.J.; Lub-de Hooge, M.N.; de Vries, E.; Gietema, J.A.; Oosting, S.F. 89Zr-Bevacizumab PET: Potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. J. Nucl. Med. 2017, 58, 905–910. [Google Scholar] [CrossRef]
- Verhoeff, S.R.; van Es, S.C.; Boon, E.; van Helden, E.; Angus, L.; Elias, S.G.; Oosting, S.F.; Aarntzen, E.H.; Brouwers, A.H.; Kwee, T.C.; et al. Lesion detection by [89Zr]Zr-DFO-girentuximab and [18F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1931–1939. [Google Scholar] [CrossRef]
- Gaykema, S.B.; Schröder, C.P.; Vitfell-Rasmussen, J.; Chua, S.; Oude Munnink, T.H.; Brouwers, A.H.; Bongaerts, A.H.; Akimov, M.; Fernandez-Ibarra, C.; Lub-de Hooge, M.N.; et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 2014, 20, 3945–3954. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-PET imaging to assess target engagement: Experience from 89Zr-anti-HER3 mAb (GSK2849330) in patients with solid tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef]
- Bensch, F.; Lamberts, L.E.; Smeenk, M.M.; Jorritsma-Smit, A.; Lub-de Hooge, M.N.; Terwisscha van Scheltinga, A.; de Jong, J.R.; Gietema, J.A.; Schröder, C.P.; Thomas, M.; et al. 89Zr-lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin. Cancer Res. 2017, 23, 6128–6137. [Google Scholar] [CrossRef]
- Verhoeff, S.R.; van den Heuvel, M.M.; van Herpen, C.M.L.; Piet, B.; Aarntzen, E.H.J.G.; Heskamp, S. Programmed Cell Death-1/Ligand-1 PET Imaging: A Novel Tool to Optimize Immunotherapy? PET Clin. 2020, 15, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.R.; Green, D.A.; Spratt, D.E.; Lyashchenko, S.; Fareedy, S.B.; Robinson, B.D.; Beattie, B.J.; Jain, M.; Lewis, J.S.; Christos, P.; et al. A prospective pilot study of (89)Zr-J591/prostate specific membrane antigen positron emission tomography in men with localized prostate cancer undergoing radical prostatectomy. J. Urol. 2014, 191, 1439–1445. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Ruan, S.; Lyashchenko, S.K.; Carrasquillo, J.A.; Heller, G.; Martinez, D.F.; Cheal, S.M.; Lewis, J.S.; Fleisher, M.; et al. First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: Pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J. Nucl. Med. 2016, 57, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-humans imaging with 89Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: Preliminary pharmacokinetics, biodistribution, and lesion targeting. J. Nucl. Med. 2020, 61, 512–519. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.A.; Danila, D.C.; Pandit-Taskar, N.; Beylergil, V.; Cheal, S.M.; Fleming, S.E.; Fox, J.J.; Ruan, S.; Zanzonico, P.B.; Ragupathi, G.; et al. Pharmacokinetics and biodistribution of a [89Zr]Zr-DFO-MSTP2109A Anti-STEAP1 antibody in metastatic castration-resistant prostate cancer patients. Mol. Pharm. 2019, 16, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.; Schröder, C.P. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.V.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [89Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Carrasquillo, J.A.; Riedl, C.C.; Yeh, R.; Hatzoglou, V.; Ross, D.S.; Jhaveri, K.; Chandarlapaty, S.; Hyman, D.M.; Zeglis, B.M.; et al. Identification of HER2-positive metastases in patients with HER2-negative primary breast cancer by using HER2-targeted 89Zr-pertuzumab PET/CT. Radiology 2020, 296, 370–378. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O′Donoghue, J.A. First-in-human human epidermal growth factor receptor 2-targeted imaging using 89Zr-pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef]
- Heukelom, J.; Hamming, O.; Bartelink, H.; Hoebers, F.; Giralt, J.; Herlestam, T.; Verheij, M.; van den Brekel, M.; Vogel, W.; Slevin, N.; et al. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer 2013, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- De Feo, M.S.; Pontico, M.; Frantellizzi, V.; Corica, F.; De Cristofaro, F.; De Vincentis, G. 89Zr-PET imaging in humans: A systematic review. Clin. Transl. Imaging 2022, 10, 23–36. [Google Scholar] [CrossRef]
- van Loon, J.; Even, A.; Aerts, H.; Öllers, M.; Hoebers, F.; van Elmpt, W.; Dubois, L.; Dingemans, A.C.; Lalisang, R.I.; Kempers, P.; et al. PET imaging of zirconium-89 labeled cetuximab: A phase I trial in patients with head and neck and lung cancer. Radiother. Oncol. 2017, 122, 267–273. [Google Scholar] [CrossRef] [PubMed]
- van Brummelen, E.; Huisman, M.C.; de Wit-van der Veen, L.J.; Nayak, T.K.; Stokkel, M.; Mulder, E.R.; Hoekstra, O.S.; Vugts, D.J.; Van Dongen, G.; Verheul, H.M.; et al. 89Zr-labeled CEA-targeted IL-2 variant immunocytokine in patients with solid tumors: CEA-mediated tumor accumulation and role of IL-2 receptor-binding. Oncotarget 2018, 9, 24737–24749. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, A.R.; Osso, J., Jr. The current status and future of theranostic Copper-64 radiopharmaceuticals. Iran. J. Nucl. Med. 2017, 25, 1–10. [Google Scholar]
- Pyo, A.; Yun, M.; Kim, H.S.; Kim, T.Y.; Lee, J.J.; Kim, J.Y.; Lee, S.; Kwon, S.Y.; Bom, H.S.; Kim, H.S.; et al. 64Cu-Labeled Repebody Molecules for Imaging of Epidermal Growth Factor Receptor-Expressing Tumors. J. Nucl. Med. 2018, 59, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Xu, X.; Zhao, M.; Zhang, B.; Deng, S.; Wu, Y. 64Cu-based Radiopharmaceuticals in Molecular Imaging. Technol. Cancer Res. Treat. 2019, 18, 1533033819830758. [Google Scholar] [CrossRef]
- Mccall, K.C.; Humm, J.L.; Bartlett, R.; Reese, M.; Carlin, S. Copper-64-diacetyl-bis (N(4)- methylthiosemicarbazone) pharmacokinetics in FaDu xenograft tumors and correlation with microscopic markers of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e393–e399. [Google Scholar] [CrossRef]
- Lopci, E.; Grassi, I.; Rubello, D.; Colletti, P.M.; Cambioli, S.; Gamboni, A.; Salvi, F.; Cicoria, G.; Lodi, F.; Dazzi, C.; et al. Prognostic evaluation of disease outcome in solid tumors investigated with 64Cu-ATSM PET/CT. Clin. Nucl. Med. 2016, 41, e87–e92. [Google Scholar] [CrossRef] [PubMed]
- Grassi, I.; Nanni, C.; Cicoria, G.; Blasi, C.; Bunkheila, F.; Lopci, E.; Colletti, P.M.; Rubello, D.; Fanti, S. Usefulness of 64Cu-ATSM in head and neck cancer: A preliminary prospective study. Clin. Nucl. Med. 2014, 39, e59–e63. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. PET radiopharmaceuticals for imaging of tumor hypoxia: A review of the evidence. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 365–384. [Google Scholar]
- Vázquez, M.C.; Martínez, P.; Alvarez, A.R.; González, M.; Zanlungo, S. Increased copper levels in in vitro and in vivo models of Niemann-Pick, C. disease. Biometals 2012, 25, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Smpokou, P.; Samanta, M.; Berry, G.T.; Hecht, L.; Engle, E.C.; Lichter-Konecki, U. Menkes disease in affected females: The clinical disease spectrum. Am. J. Med. Genet. A 2015, 167A, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Yoshimoto, M.; Matsumoto, H.; Furukawa, T.; Zhang, M.R.; Inubushi, M.; Tsuji, A.B.; Fujibayashi, Y.; Higashi, T.; Saga, T. 64Cu-ATSM internal radiotherapy to treat tumors with bevacizumab-induced vascular decrease and hypoxia in human colon carcinoma xenografts. Oncotarget 2017, 8, 88815–88826. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Furukawa, T.; Matsumoto, H.; Yoshimoto, M.; Kiyono, Y.; Zhang, M.R.; Fujibayashi, Y.; Saga, T. (64)Cu-ATSM therapy targets regions with activated DNA repair and enrichment of CD133 (+) cells in an HT-29 tumor model: Sensitization with a nucleic acid antimetabolite. Cancer Lett. 2016, 376, 74–82. [Google Scholar] [CrossRef]
- Yoshii, Y.; Furukawa, T.; Kiyono, Y.; Watanabe, R.; Mori, T.; Yoshii, H.; Asai, T.; Okazawa, H.; Welch, M.J.; Fujibayashi, Y. Internal radiotherapy with copper-64-diacetyl-bis(N4 -methylthiosemicarbazone) reduces CD133+ highly tumorigenic cells and metastatic ability of mouse colon carcinoma. Nucl. Med. Biol. 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In Medical Isotopes; Naqvi, S.A.R., Imrani, M.B., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/70578 (accessed on 7 February 2022).
- Jalilian, A.R. An overview on Ga-68 radiopharmaceuticals for positron emission tomography applications. Iran. J. Nucl. Med. 2016, 24, 1–10. [Google Scholar]
- Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of Gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics 2018, 8, 16. [Google Scholar] [CrossRef]
- Raj, N.; Reidy-Lagunes, D. The role of 68Ga-DOTATATE positron emission tomography/computed tomography in well-differentiated neuroendocrine tumors: A case-based approach illustrates potential benefits and challenges. Pancreas 2018, 47, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; van Lith, S.; Boss, M.; Brom, M.; Joosten, L.; Béhé, M.; Buitinga, M.; Gotthardt, M. Exendin-4 analogs in insulinoma theranostics. J. Label. Comp. Radiopharm. 2019, 62, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mao, F.; Niu, G.; Peng, L.; Lang, L.; Li, F.; Ying, H.; Wu, H.; Pan, B.; Zhu, Z.; et al. 68Ga-BBN-RGD PET/CT for GRPR and integrin αvβ3 imaging in patients with breast cancer. Theranostics 2018, 8, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, D.; Lang, L.; Zhu, Z.; Wang, L.; Wu, P.; Niu, G.; Li, F.; Chen, X. 68Ga-NOTA-Aca-BBN(7-14) PET/CT in Healthy Volunteers and Glioma Patients. J. Nucl. Med. 2016, 57, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Schweighöfer, P.; Feldwisch, J.; Seemann, J.; Frejd, F.Y.; Lindman, H.; Sörensen, J. Diagnostic HER2-binding radiopharmaceutical, [68Ga]Ga-ABY-025, for routine clinical use in breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 12–23. [Google Scholar]
- Djekidel, M.; Das, J.M. Nuclear Medicine Neuro PET Assessment, Protocols, and Interpretation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570591 (accessed on 28 March 2022).
- Cybulska, K.; Perk, L.; Booij, J.; Laverman, P.; Rijpkema, M. Huntington’s Disease: A Review of the Known PET Imaging Biomarkers and Targeting Radiotracers. Molecules 2020, 25, 482. [Google Scholar] [CrossRef]
- Delva, A.; Van Weehaeghe, D.; Koole, M.; Van Laere, K.; Vandenberghe, W. Loss of presynaptic terminal integrity in the substantia nigra in early parkinson’s disease. Mov. Disord. 2020, 35, 1977–1986. [Google Scholar] [CrossRef]
- Xiong, M.; Roshanbin, S.; Rokka, J.; Schlein, E.; Ingelsson, M.; Sehlin, D.; Eriksson, J.; Syvänen, S. In vivo imaging of synaptic density with [11C]UCB-J PET in two mouse models of neurodegenerative disease. Neuroimage 2021, 239, 118302. [Google Scholar] [CrossRef]
- Borja, A.J.; Hancin, E.C.; Raynor, W.Y.; Ayubcha, C.; Detchou, D.K.; Werner, T.J.; Revheim, M.E.; Alavi, A. A Critical Review of PET Tracers Used for Brain Tumor Imaging. PET Clin. 2021, 16, 219–231. [Google Scholar] [CrossRef]
- Minoshima, S.; Mosci, K.; Cross, D.; Thientunyakit, T. Brain [F-18]FDG PET for Clinical Dementia Workup: Differential Diagnosis of Alzheimer’s Disease and Other Types of Dementing Disorders. Semin. Nucl. Med. 2021, 51, 230–240. [Google Scholar] [CrossRef]
- Chételat, G.; Arbizu, J.; Barthel, H.; Garibotto, V.; Law, I.; Morbelli, S.; van de Giessen, E.; Agosta, F.; Barkhof, F.; Brooks, D.J.; et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020, 19, 951–962. [Google Scholar] [CrossRef]
- Blazhenets, G.; Frings, L.; Ma, Y.; Sörensen, A.; Eidelberg, D.; Wiltfang, J.; Meyer, P.T. Alzheimer’s Disease Neuroimaging Initiative. Validation of the Alzheimer Disease Dementia Conversion-Related Pattern as an ATN Biomarker of Neurodegeneration. Neurology 2021, 96, e1358–e1368. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, S.; Drzezga, A.E.; Barthel, H.; Bohnen, N.; Djekidel, M.; Lewis, D.H.; Mathis, C.A.; McConathy, J.; Nordberg, A.; Sabri, O.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0. J. Nucl. Med. 2016, 57, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.Y.; Frias, B.; Wren, M.C.; Schöll, M.; Fox, N.C.; Årstad, E.; Lashley, T.; Sander, K. Discriminatory ability of next-generation tau PET tracers for Alzheimer’s disease. Brain 2021, 144, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Gatsonis, C.; Apgar, C.; Chaudhary, K.; Gareen, I.; Hanna, L.; Hendrix, J.; Hillner, B.E.; Olson, C.; Lesman-Segev, O.H.; et al. Association of Amyloid Positron Emission Tomography with Subsequent Change in Clinical Management Among Medicare Beneficiaries with Mild Cognitive Impairment or Dementia. JAMA 2019, 321, 1286–1294. [Google Scholar] [CrossRef]
- Sood, A.; Shukla, J.; Shree, R.; Vatsa, R.; Modi, M.; Mittal, B.R. Comparative Performance of 99mTc-TRODAT-1 SPECT/CT and 18F-FDOPA PET/CT Imaging in Patients with Parkinson’s Disease, Parkinson-Plus Syndrome, and Essential Tremor. Clin. Nucl. Med. 2021, 46, 95–102. [Google Scholar] [CrossRef]
- Ni, R.; Nitsch, R.M. Recent Developments in Positron Emission Tomography Tracers for Proteinopathies Imaging in Dementia. Front. Aging Neurosci. 2022, 13, 751897. [Google Scholar] [CrossRef]
- Pietroboni, A.M.; Colombi, A.; Carandini, T.; Sacchi, L.; Fenoglio, C.; Marotta, G.; Arighi, A.; De Riz, M.A.; Fumagalli, G.G.; Castellani, M.; et al. Amyloid PET imaging and dementias: Potential applications in detecting and quantifying early white matter damage. Alzheimers Res. Ther. 2022, 14, 33. [Google Scholar] [CrossRef]
- Cselényi, Z.; Jönhagen, M.E.; Forsberg, A.; Halldin, C.; Julin, P.; Schou, M.; Johnström, P.; Varnäs, K.; Svensson, S.; Farde, L. Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J. Nucl. Med. 2012, 53, 415–424. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Ni, R.; Gulyas, B.; Toth, M.; Haggkvist, J.; Halldin, C.; Voytenko, L.; Marutle, A.; Nordberg, A. Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: A correlative positron emission tomography and in vitro imaging study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Sehlin, D.; Fang, X.T.; Cato, L.; Antoni, G.; Lannfelt, L.; Syvanen, S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat. Commun. 2016, 7, 10759. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, T.; Shi, K.; Diehl-Schmid, J.; Natale, B.; Drzezga, A.; Förster, S.; Förstl, H.; Schwaiger, M.; Yakushev, I.; Wester, H.J.; et al. 18F-FIBT may expand PET for β-amyloid imaging in neurodegenerative diseases. Mol. Psychiatry 2020, 25, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.R.; Sehlin, D.; Roshanbin, S.; Falk, V.L.; Saito, T.; Saido, T.C.; Neumann, U.; Rokka, J.; Eriksson, J.; Syvänen, S. 11C-PIB and 124I-antibody PET provide differing estimates of brain amyloid-beta after therapeutic intervention. J. Nucl. Med. 2021, 63, 302–309. [Google Scholar] [CrossRef]
- Ni, R. Positron emission tomography in animal models of Alzheimer’s disease amyloidosis: Translational implications. Pharmaceuticals 2021, 14, 1179. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Gamez, J.E.; Singh, U.; Sadowsky, C.H.; Villena, T.; Sabbagh, M.N.; Beach, T.G.; Duara, R.; Fleisher, A.S.; Frey, K.A.; et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015, 72, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Sabri, O.; Sabbagh, M.N.; Seibyl, J.; Barthel, H.; Akatsu, H.; Ouchi, Y.; Senda, K.; Murayama, S.; Ishii, K.; Takao, M.; et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: Phase 3 study. Alzheimers Dement. 2015, 11, 964–974. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Wolk, D.A.; Sadowsky, C.; Safirstein, B.; Rinne, J.O.; Duara, R.; Perry, R.; Agronin, M.; Gamez, J.; Shi, J.; Ivanoiu, A.; et al. Use of flutemetamol F 18-labeled positron emission tomography and other biomarkers to assess risk of clinical progression in patients with amnestic mild cognitive impairment. JAMA Neurol. 2018, 75, 1114–1123. [Google Scholar] [CrossRef]
- Cotta Ramusino, M.; Perini, G.; Altomare, D.; Barbarino, P.; Weidner, W.; Salvini Porro, G.; Barkhof, F.; Rabinovici, G.D.; van der Flier, W.M.; Frisoni, G.B.; et al. Outcomes of clinical utility in amyloid-PET studies: State of art and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2157–2168. [Google Scholar] [CrossRef]
- Ito, H.; Shinotoh, H.; Shimada, H.; Miyoshi, M.; Yanai, K.; Okamura, N.; Takano, H.; Takahashi, H.; Arakawa, R.; Kodaka, F.; et al. Imaging of amyloid deposition in human brain using positron emission tomography and [18F]FACT: Comparison with [11C]PIB. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Choi, S.R.; Zhao, R.; Ploessl, K.; Alexoff, D.; Zhu, L.; Zha, Z.; Kung, H.F. A new highly deuterated [18F]AV-45, [18F]D15FSP, for imaging β-Amyloid plaques in the brain. ACS Med. Chem. Lett. 2021, 12, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, G.S.; Dhavale, D.D.; Prior, J.L.; Yan, P.; Cirrito, J.; Rath, N.P.; Laforest, R.; Cairns, N.J.; Lee, J.M.; Kotzbauer, P.T.; et al. Fluselenamyl: A novel benzoselenazole derivative for PET detection of amyloid plaques (Aβ) in Alzheimer’s disease. Sci. Rep. 2016, 6, 35636. [Google Scholar] [CrossRef]
- Hooker, J.M.; Carson, R.E. Human Positron Emission Tomography Neuroimaging. Annu. Rev. Biomed. Eng. 2019, 21, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A.; Gunn, R.N.; Rabiner, E.A.; Slifstein, M.; Comley, R.; Salinas, C.; Johnson, C.N.; Jakobsen, S.; Houle, S.; Laruelle, M.; et al. Radiosynthesis and characterization of 11C-GSK215083 as a PET radioligand for the 5-HT 6 receptor. J. Nucl. Med. 2012, 53, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A.; Rabiner, E.A.; Gunn, R.N.; Searle, G.; Martarello, L.; Comley, R.A.; Davy, M.; Wilson, A.A.; Houle, S.; Mizrahi, R.; et al. Human kinetic modeling of the 5HT6 PET radioligand 11C-GSK215083 and its utility for determining occupancy at both 5HT6 and 5HT2A receptors by SB742457 as a potential therapeutic mechanism of action in Alzheimer disease. J. Nucl. Med. 2015, 56, 1901–1909. [Google Scholar] [CrossRef][Green Version]
- Quiroz, Y.T.; Sperling, R.A.; Norton, D.J.; Baena, A.; Arboleda-Velasquez, J.F.; Cosio, D.; Schultz, A.; Lapoint, M.; Guzman-Velez, E.; Miller, J.B.; et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol. 2018, 75, 548–556. [Google Scholar] [CrossRef]
- Harada, R.; Okamura, N.; Furumoto, S.; Yanai, K. Imaging Protein Misfolding in the Brain Using β-Sheet Ligands. Front. Neurosci. 2018, 12, 585. [Google Scholar] [CrossRef]
- Saint-Aubert, L.; Lemoine, L.; Chiotis, K.; Leuzy, A.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging: Present and future directions. Mol. Neurodegener. 2017, 12, 19. [Google Scholar] [CrossRef]
- Chien, D.T.; Szardenings, A.K.; Bahri, S.; Walsh, J.C.; Mu, F.; Xia, C.; Shankle, W.R.; Lerner, A.J.; Su, M.Y.; Elizarov, A.; et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 2013, 34, 457–468. [Google Scholar] [CrossRef]
- Hostetler, E.D.; Walji, A.M.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Haley, H.; Holahan, M.; et al. Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J. Nucl. Med. 2016, 57, 1599–1606. [Google Scholar] [CrossRef]
- Devous, M.D., Sr.; Joshi, A.D.; Navitsky, M.; Southekal, S.; Pontecorvo, M.J.; Shen, H.; Lu, M.; Shankle, W.R.; Seibyl, J.P.; Marek, K.; et al. Test-retest reproducibility for the tau PET imaging agent flortaucipir F 18. J. Nucl. Med. 2017, 59, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Wooten, D.W.; Guehl, N.J.; Verwer, E.E.; Shoup, T.M.; Yokell, D.L.; Zubcevik, N.; Vasdev, N.; Zafonte, R.D.; Johnson, K.A.; El Fakhri, G.; et al. Pharmacokinetic evaluation of the tau PET radiotracer 18F-T807 (18F-AV-1451) in human subjects. J. Nucl. Med. 2017, 58, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Schultz, A.; Betensky, R.A.; Becker, J.A.; Sepulcre, J.; Rentz, D.; Mormino, E.; Chhatwal, J.; Amariglio, R.; Papp, K.; et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016, 79, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Arteaga, J.; Cashion, D.K.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Kasi, D.; Lam, C.; Liang, Q.; Liu, C.; et al. A highly selective and specific PET tracer for imaging of tau pathologies. J. Alzheimers Dis. 2012, 31, 601–612. [Google Scholar] [CrossRef]
- Schonhaut, D.R.; McMillan, C.T.; Spina, S.; Dickerson, B.C.; Siderowf, A.; Devous, M.D., Sr.; Tsai, R.; Winer, J.; Russell, D.S.; Litvan, I.; et al. 18F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study. Ann. Neurol. 2017, 82, 622–634. [Google Scholar] [CrossRef]
- Smith, R.; Schöll, M.; Londos, E.; Ohlsson, T.; Hansson, O. 18F-AV-1451 in Parkinson’s disease with and without dementia and in dementia with Lewy bodies. Sci. Rep. 2018, 8, 4717. [Google Scholar] [CrossRef]
- Schöll, M.; Lockhart, S.N.; Schonhaut, D.R.; O′Neil, J.P.; Janabi, M.; Ossenkoppele, R.; Baker, S.L.; Vogel, J.W.; Faria, J.; Schwimmer, H.D.; et al. PET imaging of tau deposition in the aging human brain. Neuron 2016, 89, 971–982. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Schonhaut, D.R.; Schöll, M.; Lockhart, S.N.; Ayakta, N.; Baker, S.L.; O′Neil, J.P.; Janabi, M.; Lazaris, A.; Cantwell, A.; et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 2016, 139, 1551–1567. [Google Scholar] [CrossRef]
- Cho, H.; Choi, J.Y.; Hwang, M.S.; Lee, J.H.; Kim, Y.J.; Lee, H.M.; Lyoo, C.H.; Ryu, Y.H.; Lee, M.S. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 2016, 87, 375–383. [Google Scholar] [CrossRef]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Normandin, M.D.; Meltzer, A.C.; Siao Tick Chong, M.; Andrea, N.V.; Antón-Fernández, A.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.; et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann. Neurol. 2017, 81, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Cho, H.; Ahn, S.J.; Lee, J.H.; Ryu, Y.H.; Lee, M.S.; Lyoo, C.H. Off-target 18F-AV-1451 binding in the basal ganglia correlates with age-related iron accumulation. J. Nucl. Med. 2018, 59, 117–120. [Google Scholar] [CrossRef]
- Vermeiren, C.; Motte, P.; Viot, D.; Mairet-Coello, G.; Courade, J.P.; Citron, M.; Mercier, J.; Hannestad, J.; Gillard, M. The tau positron-emission tomography tracer AV-1451 binds with similar affinities to tau fibrils and monoamine oxidases. Mov. Disord. 2018, 33, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Schöll, M.; Honer, M.; Nilsson, C.F.; Englund, E.; Hansson, O. Tau neuropathology correlates with FDG-PET, but not AV-1451-PET, in progressive supranuclear palsy. Acta Neuropathol. 2017, 133, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Lowe, V.J.; Curran, G.; Fang, P.; Liesinger, A.M.; Josephs, K.A.; Parisi, J.E.; Kantarci, K.; Boeve, B.F.; Pandey, M.K.; Bruinsma, T.; et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol. Commun. 2016, 4, 58. [Google Scholar] [CrossRef]
- Sander, K.; Lashley, T.; Gami, P.; Gendron, T.; Lythgoe, M.F.; Rohrer, J.D.; Schott, J.M.; Revesz, T.; Fox, N.C.; Årstad, E. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016, 12, 1116–1124. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Furumoto, S.; Fodero-Tavoletti, M.T.; Mulligan, R.S.; Hodges, J.; Harada, R.; Yates, P.; Piguet, O.; Pejoska, S.; Doré, V.; et al. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 816–826. [Google Scholar] [CrossRef]
- Li, Y.; Tsui, W.; Rusinek, H.; Butler, T.; Mosconi, L.; Pirraglia, E.; Mozley, D.; Vallabhajosula, S.; Harada, R.; Furumoto, S.; et al. Cortical laminar binding of PET amyloid and tau tracers in Alzheimer disease. J. Nucl. Med. 2015, 56, 270–273. [Google Scholar] [CrossRef]
- Jonasson, M.; Wall, A.; Chiotis, K.; Saint-Aubert, L.; Wilking, H.; Sprycha, M.; Borg, B.; Thibblin, A.; Eriksson, J.; Sörensen, J.; et al. Tracer kinetic analysis of (S)-18F-THK5117 as a PET tracer for assessing tau pathology. J. Nucl. Med. 2016, 57, 574–581. [Google Scholar] [CrossRef]
- Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Tago, T.; Hiraoka, K.; Watanuki, S.; Shidahara, M.; et al. 18F-THK5351: A novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J. Nucl. Med. 2016, 57, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, A.; Okamura, N.; Furukawa, K.; Furumoto, S.; Harada, R.; Tomita, N.; Hiraoka, K.; Watanuki, S.; Ishikawa, Y.; Tago, T.; et al. Longitudinal assessment of tau pathology in patients with Alzheimer’s disease using [18F]THK-5117 positron emission tomography. PLoS ONE 2015, 10, e0140311. [Google Scholar] [CrossRef] [PubMed]
- Chiotis, K.; Saint-Aubert, L.; Savitcheva, I.; Jelic, V.; Andersen, P.; Jonasson, M.; Eriksson, J.; Lubberink, M.; Almkvist, O.; Wall, A.; et al. Imaging in-vivo tau pathology in Alzheimer’s disease with THK5317 PET in a multimodal paradigm. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1686–1699. [Google Scholar] [CrossRef]
- Sone, D.; Imabayashi, E.; Maikusa, N.; Okamura, N.; Furumoto, S.; Kudo, Y.; Ogawa, M.; Takano, H.; Yokoi, Y.; Sakata, M.; et al. Regional tau deposition and subregion atrophy of medial temporal structures in early Alzheimer’s disease: A combined positron emission tomography/magnetic resonance imaging study. Alzheimer’s Dement. 2017, 9, 35–40. [Google Scholar] [CrossRef]
- Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R.S.; Villemagne, V.L.; Akatsu, H.; Yamamoto, T.; et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J. Nucl. Med. 2013, 54, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Honer, M.; Gobbi, L.; Knust, H.; Kuwabara, H.; Muri, D.; Koerner, M.; Valentine, H.; Dannals, R.F.; Wong, D.F.; Borroni, E. Preclinical evaluation of 18F-RO6958948, 11C-RO6931643, and 11C-RO6924963 as novel PET radiotracers for imaging tau aggregates in Alzheimer disease. J. Nucl. Med. 2018, 59, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Ishiki, A.; Kai, H.; Sato, N.; Furukawa, K.; Furumoto, S.; Tago, T.; Tomita, N.; Watanuki, S.; Hiraoka, K.; et al. Correlations of 18F-THK5351 PET with post-mortem burden of tau and astrogliosis in Alzheimer’s disease. J. Nucl. Med. 2017, 59, 671–674. [Google Scholar] [CrossRef]
- Wong, D.F.; Comley, R.A.; Kuwabara, H.; Rosenberg, P.B.; Resnick, S.M.; Ostrowitzki, S.; Vozzi, C.; Boess, F.; Oh, E.; Lyketsos, C.G.; et al. First in-human PET study of 3 novel tau radiopharmaceuticals: [11C]RO6924963, [11C]RO6931643, and [18F]RO6958948. J. Nucl. Med. 2018, 59, 1869–1876. [Google Scholar] [CrossRef]
- Xia, C.F.; Arteaga, J.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Kasi, D.; Lam, C.; Liang, Q.; Liu, C.; Mocharla, V.P.; et al. [(18F)]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013, 9, 666–676. [Google Scholar] [CrossRef]
- Betthauser, T.J.; Lao, P.J.; Murali, D.; Barnhart, T.E.; Furumoto, S.; Okamura, N.; Stone, C.K.; Johnson, S.C.; Christian, B.T. In vivo comparison of tau radioligands 18F-THK-5351 and 18F-THK-5317. J Nucl. Med. 2017, 58, 996–1002. [Google Scholar] [CrossRef]
- Dupont, A.C.; Largeau, B.; Guilloteau, D.; Santiago Ribeiro, M.J.; Arlicot, N. The Place of PET to assess new therapeutic effectiveness in neurodegenerative diseases. Contrast Media Mol. Imaging 2018, 2018, 7043578. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Niccolini, F.; Pagano, G.; Politis, M. Cholinergic imaging in dementia spectrum disorders. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Kroth, H.; Berndt, M.; Capotosti, F.; Molette, J.; Schieferstein, H.; Oden, F.; Juergens, T.; Darmency, V.; Schmitt-Willich, H.; et al. Characterization of the novel PET tracer PI-2620 for the assessment of Tau pathology in Alzheimer’s disease and other tauopathies. J. Nucl. Med. 2017, 58, 847. [Google Scholar]
- Li, C.; Götz, J. Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat. Rev. Drug Discov. 2017, 16, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.L.T.M.; Bohnen, N.I. Cholinergic dysfunction in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 377. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Spreng, R.N. Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. 2016, 7, 13249. [Google Scholar] [CrossRef]
- Butler, T.; Harvey, P.; Deshpande, A.; Tanzi, E.; Li, Y.; Tsui, W.; Silver, C.; Fischer, E.; Wang, X.; Chen, J.; et al. Basal forebrain septal nuclei are enlarged in healthy subjects prior to the development of Alzheimer’s disease. Neurobiol. Aging 2018, 65, 201–205. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Grothe, M.J.; Ray, N.J.; Müller, M.; Teipel, S.J. Recent advances in cholinergic imaging and cognitive decline—Revisiting the cholinergic hypothesis of dementia. Curr. Geriatr. Rep. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Mazère, J.; Lamare, F.; Allard, M.; Fernandez, P.; Mayo, W. 123I-Iodobenzovesamicol SPECT imaging of cholinergic systems in dementia with Lewy bodies. J. Nucl. Med. 2017, 58, 123–128. [Google Scholar] [CrossRef]
- Horti, A.G. Development of [(18)F]ASEM, a specific radiotracer for quantification of the α7-nAChR with positron-emission tomography. Biochem. Pharmacol. 2015, 97, 566–575. [Google Scholar] [CrossRef]
- Horti, A.G.; Gao, Y.; Kuwabara, H.; Wang, Y.; Abazyan, S.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Sahibzada, N.; Holt, D.P.; et al. 18F-ASEM, a radiolabeled antagonist for imaging the 7-nicotinic acetylcholine receptor with PET. J. Nucl. Med. 2014, 55, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Petrou, M.; Frey, K.A.; Kilbourn, M.R.; Scott, P.J.; Raffel, D.M.; Bohnen, N.I.; Müller, M.L.; Albin, R.L.; Koeppe, R.A. In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: Biodistribution, dosimetry, and tracer kinetic analyses. J. Nucl. Med. 2014, 55, 396–404. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Li, S.; Zheng, M.Q.; Scheunemann, M.; Lin, S.F.; Nabulsi, N.; Holden, D.; Pracitto, R.; Labaree, D.; Ropchan, J.; et al. PET imaging of α7 nicotinic acetylcholine receptors: A comparative study of [18F]ASEM and [18F]DBT-10 in nonhuman primates, and further evaluation of [18F]ASEM in humans. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kellar, K.J.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Dannals, R.F.; Horti, A.G. Derivatives of dibenzothiophene for positron emission tomography imaging of α7-nicotinic acetylcholine receptors. J. Med. Chem. 2013, 56, 7574–7589. [Google Scholar] [CrossRef]
- Tu, Z.; Zhang, X.; Jin, H.; Yue, X.; Padakanti, P.K.; Yu, L.; Liu, H.; Flores, H.P.; Kaneshige, K.; Parsons, S.M.; et al. Synthesis and biological characterization of a promising F-18 PET tracer for vesicular acetylcholine transporter. Bioorg. Med. Chem. 2015, 23, 4699–4709. [Google Scholar] [CrossRef]
- Jin, H.; Yue, X.; Liu, H.; Han, J.; Flores, H.; Su, Y.; Parsons, S.M.; Perlmutter, J.S.; Tu, Z. Kinetic modeling of [18F]VAT, a novel radioligand for positron emission tomography imaging vesicular acetylcholine transporter in non-human primate brain. J. Neurochem. 2018, 144, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Shiba, K. In vivo and in vitro characteristics of radiolabeled vesamicol analogs as the vesicular acetylcholine transporter imaging agents. Contrast Media Mol. Imaging 2018, 2018, 4535476. [Google Scholar] [CrossRef] [PubMed]
- Aghourian, M.; Legault-Denis, C.; Soucy, J.P.; Rosa-Neto, P.; Gauthier, S.; Kostikov, A.; Gravel, P.; Bédard, M.A. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [18F]-FEOBV. Mol. Psychiatry 2017, 22, 1531–1538. [Google Scholar] [CrossRef]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Roberts, J.M.; Nandi, A.; Cascella, N.; Brasic, J.; Weerts, E.M.; Kitzmiller, K.; Phan, J.A.; et al. Brain PET imaging of α7-nAChR with [18F]ASEM:reproducibility, occupancy, receptor density, and changes in schizophrenia. Int. J. Neuropsychopharmacol. 2018, 21, 656–667. [Google Scholar] [CrossRef]
- Van Waarde, A.; Dierckx, R.; Zhou, X.; Khanapur, S.; Tsukada, H.; Ishiwata, K.; Luurtsema, G.; de Vries, E.; Elsinga, P.H. Potential therapeutic applications of adenosine A2A receptor ligands and opportunities for A2A receptor imaging. Med. Res. Rev. 2018, 38, 5–56. [Google Scholar] [CrossRef]
- Takata, K.; Kato, H.; Shimosegawa, E.; Okuno, T.; Koda, T.; Sugimoto, T.; Mochizuki, H.; Hatazawa, J.; Nakatsuji, Y. 11C-Acetate PET Imaging in Patients with Multiple Sclerosis. PLoS ONE 2014, 9, e111598. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, S.P.; Plisson, C.; Rabiner, E.A.; Howes, O. Advances in CNS PET: The state-of-the-art for new imaging targets for pathophysiology and drug development. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 451–489. [Google Scholar] [CrossRef] [PubMed]
- Barret, O.; Hannestad, J.; Vala, C.; Alagille, D.; Tavares, A.; Laruelle, M.; Jennings, D.; Marek, K.; Russell, D.; Seibyl, J.; et al. Characterization in humans of 18F-MNI-444, a PET radiotracer for brain adenosine 2A receptors. J. Nucl. Med. 2015, 56, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Barret, O.; Hannestad, J.; Alagille, D.; Vala, C.; Tavares, A.; Papin, C.; Morley, T.; Fowles, K.; Lee, H.; Seibyl, J.; et al. Adenosine 2A receptor occupancy by tozadenant and preladenant in rhesus monkeys. J. Nucl. Med. 2014, 55, 1712–1718. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Skosnik, P.D.; Ranganathan, M.; Naganawa, M.; Toyonaga, T.; Finnema, S.; Hillmer, A.T.; Esterlis, I.; Huang, Y.; Nabulsi, N.; et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol. Psychiatry 2021, 26, 7690–7698. [Google Scholar] [CrossRef]
- Mercier, J.; Archen, L.; Bollu, V.; Carré, S.; Evrard, Y.; Jnoff, E.; Kenda, B.; Lallemand, B.; Michel, P.; Montel, F.; et al. Discovery of heterocyclic nonacetamide synaptic vesicle protein 2A (SV2A) ligands with single-digit nanomolar potency: Opening avenues towards the first SV2A positron emission tomography (PET) ligands. ChemMedChem 2014, 9, 693–698. [Google Scholar] [CrossRef]
- Koole, M.; van Aalst, J.; Devrome, M.; Mertens, N.; Serdons, K.; Lacroix, B.; Mercier, J.; Sciberras, D.; Maguire, P.; Van Laere, K. Quantifying SV2A density and drug occupancy in the human brain using [11C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 396–406. [Google Scholar] [CrossRef]
- Mercier, J.; Provins, L.; Valade, A. Discovery and development of SV2A PET tracers: Potential for imaging synaptic density and clinical applications. Drug Discov. Today Technol. 2017, 25, 45–52. [Google Scholar] [CrossRef]
- Finnema, S.J.; Nabulsi, N.B.; Eid, T.; Detyniecki, K.; Lin, S.F.; Chen, M.K.; Dhaher, R.; Matuskey, D.; Baum, E.; Holden, D.; et al. Imaging synaptic density in the living human brain. Sci. Transl. Med. 2016, 8, 348ra96. [Google Scholar] [CrossRef]
- Finnema, S.J.; Nabulsi, N.B.; Mercier, J.; Lin, S.F.; Chen, M.K.; Matuskey, D.; Gallezot, J.D.; Henry, S.; Hannestad, J.; Huang, Y.; et al. Kinetic evaluation and test–retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J. Cereb. Blood Flow Metab. 2017, 38, 2041–2052. [Google Scholar] [CrossRef]
- Kawamura, K.; Shimoda, Y.; Yui, J.; Zhang, Y.; Yamasaki, T.; Wakizaka, H.; Hatori, A.; Xie, L.; Kumata, K.; Fujinaga, M.; et al. A useful PET probe [11C]BU99008 with ultra-high specific radioactivity for small animal PET imaging of I2-imidazoline receptors in the hypothalamus. Nucl. Med. Biol. 2017, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tyacke, R.J.; Myers, J.; Venkataraman, A.; Mick, I.; Turton, S.; Passchier, J.; Husbands, S.M.; Rabiner, E.A.; Gunn, R.N.; Murphy, P.S.; et al. Evaluation of 11C-BU99008, a positron emission tomography ligand for the Imidazoline 2 binding site in human brain. J. Nucl. Med. 2018, 59, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Tyacke, R.J.; Fisher, A.; Robinson, E.S.; Grundt, P.; Turner, E.M.; Husbands, S.M.; Hudson, A.L.; Parker, C.A.; Nutt, D.J. Evaluation and initial in vitro and ex vivo characterization of the potential positron emission tomography ligand, BU99008 (2-(4,5-Dihydro-1H-imidazol-2-yl)-1-methyl-1H-indole), for the imidazoline2 binding site. Synapse 2012, 66, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A.; Nabulsi, N.; Holden, D.; Lin, S.F.; Cass, T.; Labaree, D.; Kealey, S.; Gee, A.D.; Husbands, S.M.; Quelch, D.; et al. Evaluation of 11C-BU99008, a PET ligand for the imidazoline2 binding sites in rhesus brain. J. Nucl. Med. 2014, 55, 838–844. [Google Scholar] [CrossRef]
- Pillai, R.L.I.; Tipre, D.N. Metabotropic glutamate receptor 5—A promising target in drug development and neuroimaging. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1151–1170. [Google Scholar] [CrossRef]
- Yamasaki, T.; Fujinaga, M.; Yui, J.; Wakizaka, H.; Ohya, T.; Nengaki, N.; Ogawa, M.; Ikoma, Y.; Hatori, A.; Xie, L.; et al. Improved visualization and specific binding for metabotropic glutamate receptor subtype 1 (mGluR1) using [11C]ITMM with ultra-high specific activity in small-animal PET. PLoS ONE 2015, 10, e0130006. [Google Scholar] [CrossRef]
- Fujinaga, M.; Yamasaki, T.; Yui, J.; Hatori, A.; Xie, L.; Kawamura, K.; Asagawa, C.; Kumata, K.; Yoshida, Y.; Ogawa, M.; et al. Synthesis and evaluation of novel radioligands for positron emission tomography imaging of metabotropic glutamate receptor subtype 1 (mGluR1) in rodent brain. J. Med. Chem. 2012, 55, 2342–2352. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Ishikawa, K.; Ishii, K.; Ishiwata, K. Decreased metabotropic glutamate receptor type 1 availability in a patient with spinocerebellar ataxia type 6: A 11C-ITMM PET study. J. Neurol. Sci. 2015, 355, 202–205. [Google Scholar] [CrossRef]
- Sakata, M.; Toyohara, J.; Ishibashi, K.; Wagatsuma, K.; Ishii, K.; Zhang, M.R.; Ishiwata, K. Age and gender effects of 11C-ITMM binding to metabotropic glutamate receptor type 1 in healthy human participants. Neurobiol. Aging 2017, 55, 72–77. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Toyohara, J.; Ishii, K.; Ishiwata, K. Comparison of imaging using 11C-ITMM and 18F-FDG for the detection of cerebellar ataxia. J. Neurol. Sci. 2017, 375, 97–102. [Google Scholar] [CrossRef]
- Toyohara, J.; Sakata, M.; Oda, K.; Ishii, K.; Ito, K.; Hiura, M.; Fujinaga, M.; Yamasaki, T.; Zhang, M.R.; Ishiwata, K. Initial human PET studies of metabotropic glutamate receptor type 1 ligand 11C-ITMM. J. Nucl. Med. 2013, 54, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Ghitza, U.E. Human brain imaging of opioid receptors. In Imaging of the Human Brain Health and Disease; Academic Press: Cambridge, MA, USA, 2014; pp. 81–98. [Google Scholar] [CrossRef]
- Lohith, T.G.; Zoghbi, S.S.; Morse, C.L.; Araneta, M.F.; Barth, V.N.; Goebl, N.A.; Tauscher, J.T.; Pike, V.W.; Innis, R.B.; Fujita, M. Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J. Nucl. Med. 2012, 53, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, S.; Pracitto, R.; Navarro, A.; Shirali, A.; Ropchan, J.; Huang, Y. Fluorine-18-labeled antagonist for PET imaging of kappa opioid receptors. ACS Chem. Neurosci. 2017, 8, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Hazari, P.P.; Pandey, A.; Chaturvedi, S.; Mishra, A.K. New trends and current status of positron-emission tomography and single-photon-emission computerized tomography radioligands for neuronal serotonin receptors and serotonin transporter. Bioconjug. Chem. 2017, 28, 2647–2672. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Stepanov, V.; Martinsson, S.; Ettrup, A.; Takano, A.; Knudsen, G.M.; Halldin, C.; Farde, L.; Finnema, S.J. Fenfluramine reduces [11C]Cimbi-36 binding to the 5-HT2A receptor in the nonhuman primate brain. Int. J. Neuropsychopharmacol. 2017, 20, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Paterson, L.M.; Kornum, B.R.; Nutt, D.J.; Pike, V.W.; Knudsen, G.M. 5-HT radioligands for human brain imaging with PET and SPECT. Med. Res. Rev. 2013, 33, 54–111. [Google Scholar] [CrossRef]
- Finnema, S.J.; Stepanov, V.; Ettrup, A.; Nakao, R.; Amini, N.; Svedberg, M.; Lehmann, C.; Hansen, M.; Knudsen, G.M.; Halldin, C. Characterization of [(11)C]Cimbi-36 as an agonist PET radioligand for the 5-HT2A and 5-HT2C receptors in the nonhuman primate brain. Neuroimage 2014, 84, 342–353. [Google Scholar] [CrossRef]
- Henry, K.; Kim, M.J.; Shrestha, S.; Cortes, M.; Singh, P.; Morse, C.; Liow, J.S.; Gladding, R.; Brower, C.; Gallager, E.; et al. S173. Evaluation of a potent and selective PET radioligand to image COX-1 in human and nonhuman primates. Biol. Psychiatry 2018, 83, S415. [Google Scholar] [CrossRef]
- Singh, P.; Shrestha, S.; Cortes-Salva, M.Y.; Jenko, K.J.; Zoghbi, S.S.; Morse, C.L.; Innis, R.B.; Pike, V.W. 3-Substituted 1,5-Diaryl-1 H -1,2,4-triazoles as prospective PET radioligands for imaging brain COX-1 in monkey. Part 1: Synthesis and pharmacology. ACS Chem. Neurosci. 2018, 9, 2610–2619. [Google Scholar] [CrossRef]
- Mansur, A.; Comley, R.; Lewis, Y.; Middleton, L.; Huiban, M.; Guo, Q.; Passchier, J.; Tsukada, H.; Gunn, R.; Rabiner, E. Imaging of mitochondrial complex 1 with 18F-BCPP-EF in the healthy human brain. J. Nucl. Med. 2018, 59, 1709. [Google Scholar]
- Tsukada, H.; Nishiyama, S.; Fukumoto, D.; Kanazawa, M.; Harada, N. Novel PET probes 18F-BCPP-EF and 18F-BCPP-BF for mitochondrial complex I: A PET study in comparison with 18F-BMS-747158-02 in rat brain. J. Nucl. Med. 2014, 55, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Ohba, H.; Kanazawa, M.; Kakiuchi, T.; Harada, N. Evaluation of 18F-BCPP-EF for mitochondrial complex 1 imaging in the brain of conscious monkeys using PET. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Nishiyama, S.; Kanazawa, M.; Tsukada, H. Development of novel PET probes, [18F]BCPP-EF, [18F]BCPP-BF, and [11C]BCPP-EM for mitochondrial complex 1 imaging in the living brain. J. Label. Compd. Radiopharm. 2013, 56, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Asai, T.; Ishii, T.; Koide, H.; Kiyokawa, C.; Hashimoto, M.; Kikuchi, T.; Shimizu, K.; Harada, N.; Tsukada, H.; et al. Non-invasive evaluation of neuroprotective drug candidates for cerebral infarction by PET imaging of mitochondrial complex-I activity. Sci. Rep. 2016, 6, 30127. [Google Scholar] [CrossRef]
- Hideo, T.; Shingo, N.; Hiroyuki, O.; Masakatsu, K.; Takeharu, K.; Norihiro, H. Comparing amyloid-β deposition, neuroinflammation, glucose metabolism, and mitochondrial complex I activity in brain: A PET study in aged monkeys. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2127–2136. [Google Scholar] [CrossRef]
- Tsukada, H.; Kanazawa, M.; Ohba, H.; Nishiyama, S.; Harada, N.; Kakiuchi, T. PET imaging of mitochondrial complex I with 18F-BCPP-EF in the brains of MPTP-treated monkeys. J. Nucl. Med. 2016, 57, 950–953. [Google Scholar] [CrossRef]
- Wey, H.Y.; Gilbert, T.M.; Zürcher, N.R.; She, A.; Bhanot, A.; Taillon, B.D.; Schroeder, F.A.; Wang, C.; Haggarty, S.J.; Hooker, J.M. Insights into neuroepigenetics through human histone deacetylase PET imaging. Sci. Transl. Med. 2016, 8, 351ra106. [Google Scholar] [CrossRef]
- Wang, C.; Schroeder, F.A.; Wey, H.Y.; Borra, R.; Wagner, F.F.; Reis, S.; Kim, S.W.; Holson, E.B.; Haggarty, S.J.; Hooker, J.M. In vivo imaging of histone deacetylases (HDACs) in the central nervous system and major peripheral organs. J. Med. Chem. 2014, 57, 7999–8009. [Google Scholar] [CrossRef]
- Wey, H.Y.; Wang, C.; Schroeder, F.A.; Logan, J.; Price, J.C.; Hooker, J.M. Kinetic analysis and quantification of [11C]Martinostat for in vivo HDAC imaging of the brain. ACS Chem. Neurosci. 2015, 6, 708–715. [Google Scholar] [CrossRef]
- Barret, O.; Thomae, D.; Tavares, A.; Alagille, D.; Papin, C.; Waterhouse, R.; McCarthy, T.; Jennings, D.; Marek, K.; Russell, D.; et al. In vivo assessment and dosimetry of 2 novel PDE10A PET radiotracers in humans: 18F-MNI-659 and 18F-MNI-654. J. Nucl. Med. 2014, 55, 1297–1304. [Google Scholar] [CrossRef]
- Postnov, A.; Schmidt, M.E.; Pemberton, D.J.; de Hoon, J.; van Hecken, A.; van den Boer, M.; Zannikos, P.; van der Ark, P.; Palmer, J.A.; Rassnick, S.; et al. Fatty acid amide hydrolase inhibition by JNJ-42165279: A multiple-ascending dose and a positron emission tomography study in healthy volunteers. Clin. Transl. Sci. 2018, 11, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hamill, T.G.; Chioda, M.; Chobanian, H.; Fung, S.; Guo, Y.; Chang, L.; Bakshi, R.; Hong, Q.; Dellureficio, J.; et al. Discovery of MK-3168: A PET tracer for imaging brain fatty acid amide hydrolase. ACS Med. Chem. Lett. 2013, 4, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Li, W.; Sanabria, S.; Holahan, M.; Purcell, M.; Declercq, R.; Depre, M.; Bormans, G.; van Laere, K.; Hamill, T. Translational studies with [11C]MK-3168, a PET tracer for fatty acid amide hydrolase (FAAH). J. Nucl. Med. 2012, 53, 397. [Google Scholar]
- Lu, S.; Haskali, M.B.; Ruley, K.M.; Dreyfus, N.J.; DuBois, S.L.; Paul, S.; Liow, J.S.; Morse, C.L.; Kowalski, A.; Gladding, R.L.; et al. PET ligands [18F]LSN3316612 and [11C]LSN3316612 quantify O-linked-β- N-acetyl-glucosamine hydrolase in the brain. Sci. Transl. Med. 2020, 12, eaau2939. [Google Scholar] [CrossRef]
- Lee, J.H.; Liow, J.S.; Paul, S.; Morse, C.L.; Haskali, M.B.; Manly, L.; Shcherbinin, S.; Ruble, J.C.; Kant, N.; Collins, E.C.; et al. PET quantification of brain O-GlcNAcase with [18F]LSN3316612 in healthy human volunteers. EJNMMI Res. 2020, 10, 20. [Google Scholar] [CrossRef]
- Lindberg, A.; Nag, S.; Schou, M.; Arakawa, R.; Nogami, T.; Moein, M.M.; Elmore, C.S.; Pike, V.W.; Halldin, C. Development of a 18F-labeled PET radioligand for imaging 5-HT1B receptors: [18F]AZ10419096. Nucl. Med. Biol. 2019, 79, 11–16. [Google Scholar] [CrossRef]
- Koole, M.; Lohith, T.G.; Valentine, J.L.; Bennacef, I.; Declercq, R.; Reynders, T.; Riffel, K.; Celen, S.; Serdons, K.; Bormans, G.; et al. Preclinical safety evaluation and human dosimetry of [18F]MK-6240, a novel pet tracer for imaging neurofibrillary tangles. Mol. Imaging Biol. 2020, 22, 173–180. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, M.J.; Eldridge, M.; Lehmann, M.L.; Frankland, M.; Liow, J.S.; Yu, Z.X.; Cortes-Salva, M.; Telu, S.; Henter, I.D.; et al. PET measurement of cyclooxygenase-2 using a novel radioligand: Upregulation in primate neuroinflammation and first-in-human study. J. Neuroinflamm. 2020, 17, 140. [Google Scholar] [CrossRef]
- Yan, X.; Telu, S.; Dick, R.M.; Liow, J.S.; Zanotti-Fregonara, P.; Morse, C.L.; Manly, L.S.; Gladding, R.L.; Shrestha, S.; Lerchner, W.; et al. [11C]deschloroclozapine is an improved PET radioligand for quantifying a human muscarinic DREADD expressed in monkey brain. J. Cereb. Blood. Flow. Metab. 2021, 41, 2571–2582. [Google Scholar] [CrossRef]
- Roth, B.L. DREADDs for neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.H.; Juarez Anaya, F.; Hong, J.; Miller, W.; Telu, S.; Singh, P.; Cortes, M.Y.; Henry, K.; Tye, G.L.; et al. First-in-human evaluation of [11C]PS13, a novel PET radioligand, to quantify cyclooxygenase-1 in the brain. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Tsai, C.L.; Huang, Y.Y.; Shiue, C.Y.; Tzen, K.Y.; Yen, R.F.; Hsin, L.W. A novel potential positron emission tomography imaging agent for vesicular monoamine transporter type 2. PLoS ONE 2016, 11, e0161295. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Liu, S.; Tang, X.; Pan, Q.; Zhang, Z.; Ma, H.; Nie, D.; Tang, C.; Tang, G. N-(2–18F-fluoropropionyl)-l-glutamate as a potential oncology tracer for PET imaging of glioma. Appl. Radiat. Isot. 2021, 168, 109530. [Google Scholar] [CrossRef] [PubMed]
- Taillefer, R.; Harel, F. Radiopharmaceuticals for cardiac imaging: Current status and future trends. J. Nucl. Cardiol. 2018, 25, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Sogbein, O.O.; Pelletier-Galarneau, M.; Schindler, T.H.; Wei, L.; Wells, R.G.; Ruddy, T.D. New SPECT and PET Radiopharmaceuticals for Imaging Cardiovascular Disease. BioMed. Res. Int. 2014, 2014, 942960. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Taillefer, R. Journey in evolution of nuclear cardiology. State-of-the-art paper: Will there be another quantum leap with the F-18-labeled myocardial perfusion radiotracers. JACC Cardiovasc. Imaging 2012, 5, 1269–1284. [Google Scholar] [CrossRef]
- Taillefer, R.; Wackers, F.J.T. Kinetics of conventional and new cardiac radiotracers. In Nuclear Cardiac Imaging: Principles and Applications, 5th ed.; Iskandrian, A., Garcia, E.V., Eds.; Oxford University Press: New York, NY, USA, 2016; pp. 58–80. Available online: https://oxfordmedicine.com/view/10.1093/med/9780199392094.001.0001/med-9780199392094 (accessed on 28 March 2022).
- Li, Y.; Zhang, W.; Wu, H.; Liu, G. Advanced Tracers in PET Imaging of Cardiovascular Disease. BioMed. Res. Int. 2014, 2014, 504532. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Deshayes, E.; Sciagrà, R.; Settimo, L.; Declerck, J.M.; Pan, X.B.; Yoshinaga, K.; Katoh, C.; Slomka, P.J.; Germano, G.; et al. Quantification of myocardial blood flow in absolute terms using 82Rb PET imaging: The RUBY-10 Study. JACC Cardiovasc. Imaging 2014, 7, 1119–1127. [Google Scholar] [CrossRef]
- Renaud, J.M.; Yip, K.; Guimond, J.; Trottier, M.; Pibarot, P.; Turcotte, E.; Maguire, C.; Lalonde, L.; Gulenchyn, K.; Farncombe, T.; et al. Characterization of 3-dimensional PET systems for accurate quantification of myocardial blood flow. J. Nucl. Med. 2017, 58, 103–109. [Google Scholar] [CrossRef]
- Guehl, N.J.; Pelletier-Galarneau, M.; Wooten, D.W.; Guerrero, J.L.; Kas, A.; Normandin, M.D.; Fakhri, G.E.; Alpert, N.M. Preclinical validation of a single-scan rest/stress imaging technique for 13N-ammonia positron emission tomography cardiac perfusion studies. Circ. Cardiovasc. Imaging 2020, 13, e009407. [Google Scholar] [CrossRef]
- Juneau, D.; Ruddy, T.D.; Beanlands, R.; deKemp, R.A. False-positive 13N-ammonia positron emission tomography perfusion scan caused by misalignment of adjacent lung activity during attenuation correction. J. Nucl. Cardiol. 2018, 25, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Fathala, A.; Aboulkheir, M.; Shoukri, M.M.; Alsergani, H. Diagnostic accuracy of 13N-ammonia myocardial perfusion imaging with PET-CT in the detection of coronary artery disease. Cardiovasc. Diagn. Ther. 2019, 9, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; deKemp, R.A. 82Rb is the best flow tracer for high-volume sites. Ann. Nucl. Cardiol. 2019, 5, 53–62. [Google Scholar] [CrossRef]
- Kaster, T.S.; Dwivedi, G.; Susser, L.; Renaud, J.M.; Beanlands, R.S.; Chow, B.J.; deKemp, R.A. Single low-dose CT scan optimized for rest-stress PET attenuation correction and quantification of coronary artery calcium. J. Nucl. Cardiol. 2015, 22, 419–428. [Google Scholar] [CrossRef]
- Mc Ardle, B.A.; Dowsley, T.F.; deKemp, R.A.; Wells, G.A.; Beanlands, R.S. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 60, 1828–1837. [Google Scholar] [CrossRef]
- Dorbala, S.; Di Carli, M.F.; Beanlands, R.S.; Merhige, M.E.; Williams, B.A.; Veledar, E.; Chow, B.J.; Min, J.K.; Pencina, M.J.; Berman, D.S.; et al. Prognostic value of stress myocardial perfusion positron emission tomography: Results from a multicenter observational registry. J. Am. Coll. Cardiol. 2013, 61, 176–184. [Google Scholar] [CrossRef]
- Dekemp, R.A.; Declerck, J.; Klein, R.; Pan, X.B.; Nakazato, R.; Tonge, C.; Arumugam, P.; Berman, D.S.; Germano, G.; Beanlands, R.S.; et al. Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J. Nucl. Med. 2013, 54, 571–577. [Google Scholar] [CrossRef]
- Ziadi, M.C.; Dekemp, R.A.; Williams, K.; Guo, A.; Renaud, J.M.; Chow, B.J.; Klein, R.; Ruddy, T.D.; Aung, M.; Garrard, L.; et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J. Nucl. Cardiol. 2012, 19, 670–680. [Google Scholar] [CrossRef]
- Prior, J.O.; Allenbach, G.; Valenta, I.; Kosinski, M.; Burger, C.; Verdun, F.R.; Bischof Delaloye, A.; Kaufmann, P.A. Quantification of myocardial blood flow with Rb-82 positron emission tomography: Clinical validation with O-15 water. Eur. J. Nucl. Mol. Imaging 2012, 39, 1037–1047. [Google Scholar] [CrossRef]
- Maddahi, J.; Czernin, J.; Lazewatsky, J.; Huang, S.C.; Dahlbom, M.; Schelbert, H.; Sparks, R.; Ehlgen, A.; Crane, P.; Zhu, Q.; et al. Phase I, first-human study of BMS747158, a novel 18F-labeled tracer for myocardial perfusion PET: Dosimetry, biodistribution, safety and imaging characteristics after a single injection at rest. J. Nucl. Med. 2011, 52, 1490–1498. [Google Scholar] [CrossRef]
- Berman, D.S.; Maddahi, J.; Tamarappoo, B.K.; Czernin, J.; Taillefer, R.; Udelson, J.E.; Gibson, C.M.; Devine, M.; Lazewatsky, J.; Bhat, G.; et al. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease. J. Am. Coll. Cardiol. 2013, 61, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Manabe, O.; Kikuchi, T.; Scholte, A.; El Mahdiui, M.; Nishii, R.; Zhang, M.R.; Suzuki, E.; Yoshinaga, K. Radiopharmaceutical tracers for cardiac imaging. J. Nucl. Cardiol. 2018, 25, 1204–1236. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Uusitalo, V.; Kero, T.; Saraste, A.; Raijmakers, P.G.; Lammertsma, A.A.; Heymans, M.W.; Kajander, S.A.; Pietilä, M.; James, S.; et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: Cut-off values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J. Am. Coll. Cardiol. 2014, 64, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Bateman, T. Current Status of myocardial perfusion PET in the United States. Ann. Nucl. Cardiol. 2017, 3, 157–162. [Google Scholar] [CrossRef]
- Dweck, M.R.; Jones, C.; Joshi, N.V.; Fletcher, A.M.; Richardson, H.; White, A.; Marsden, M.; Pessotto, R.; Clark, J.C.; Wallace, W.A.; et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012, 125, 76–86. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Bengel, F.M. PET imaging of the autonomic nervous system. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 362–382. [Google Scholar]
- Cuhlmann, S.; Gsell, W.; Van der Heiden, K.; Habib, J.; Tremoleda, J.L.; Khalil, M.; Turkheimer, F.; Meens, M.J.; Kwak, B.R.; Bird, J.; et al. In Vivo Mapping of Vascular Inflammation Using the Translocator Protein Tracer 18F-FEDAA1106. Mol. Imaging 2014, 13, 1–11. [Google Scholar] [CrossRef]
- Guilarte, T.R. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol. Ther. 2019, 194, 44–58. [Google Scholar] [CrossRef]
- Vivash, L.; O’Brien, T.J. Imaging Microglial Activation with TSPO PET: Lighting Up Neurologic Diseases? J. Nucl. Med. 2016, 57, 165–168. [Google Scholar] [CrossRef]
- Largeau, B.; Dupont, A.C.; Guilloteau, D.; Santiago-Ribeiro, M.J.; Arlicot, N. TSPO PET Imaging: From Microglial Activation to Peripheral Sterile Inflammatory Diseases? Contrast Media Mol. Imaging 2017, 2017, 6592139. [Google Scholar] [CrossRef]
- Hatori, A.; Yui, J.; Xie, L.; Yamasaki, T.; Kumata, K.; Fujinaga, M.; Wakizaka, H.; Ogawa, M.; Nengaki, N.; Kawamura, K.; et al. Visualization of acute liver damage induced by cycloheximide in rats using PET with [18F]FEDAC, a radiotracer for translocator protein (18 kDa). PLoS ONE 2014, 9, e86625. [Google Scholar] [CrossRef]
- Gaemperli, O.; Shalhoub, J.; Owen, D.R.; Lamare, F.; Johansson, S.; Fouladi, N.; Davies, A.H.; Rimoldi, O.E.; Camici, P.G. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur. Heart J. 2012, 33, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, S.; Silvola, J.; Kiugel, M.; Liljenbäck, H.; Savisto, N.; Li, X.G.; Thiele, A.; Lehmann, L.; Heinrich, T.; Vollmer, S.; et al. 18-kDa translocator protein ligand 18F-FEMPA: Biodistribution and uptake into atherosclerotic plaques in mice. J. Nucl. Cardiol. 2017, 24, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.; Patel, V.N.; Escolero, S.; Nelson, J.R.; Poitrasson-Rivière, A.; Shreves, C.K.; Freiburger, N.; Hubers, D.; Rothley, J.; Corbett, J.R.; et al. Initial clinical experience of N13-ammonia myocardial perfusion PET/CT using a compact superconducting production system. J. Nucl. Cardiol. 2019, 28, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Mäki, M.; Underwood, R.S.; Min, J.K.; Elmore, K.; Stuijfzand, W.J.; van Royen, N.; et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2017, 2, 1100–1107. [Google Scholar] [CrossRef]
- Driessen, R.S.; Danad, I.; Stuijfzand, W.J.; Schumacher, S.P.; Knuuti, J.; Mäki, M.; Lammertsma, A.A.; van Rossum, A.C.; van Royen, N.; Raijmakers, P.G.; et al. Impact of revascularization on absolute myocardial blood flow as assessed by serial [15O]H2O positron emission tomography imaging. Circ. Cardiovasc. Imaging 2018, 11, e007417. [Google Scholar] [CrossRef]
- Klein, R.; Celiker-Guler, E.; Rotstein, B.H.; deKemp, R.A. PET and SPECT Tracers for Myocardial Perfusion Imaging. Semin. Nucl. Med. 2020, 50, 208–218. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Zhou, Y. Mitochondrial-targeted molecular imaging in cardiac disease. BioMed. Res. Int. 2017, 2017, 5246853. [Google Scholar] [CrossRef]
- Mou, T.; Zhang, X. Research progress on 18F-labeled agents for imaging of myocardial perfusion with positron emission tomography. Molecules 2017, 22, 562. [Google Scholar] [CrossRef]
- Maddahi, J.; Lazewatsky, J.; Udelson, J.E.; Berman, D.S.; Beanlands, R.; Heller, G.V.; Bateman, T.M.; Knuuti, J.; Orlandi, C. Phase-III Clinical Trial of Fluorine-18 Flurpiridaz Positron Emission Tomography for Evaluation of Coronary Artery Disease. J. Am. Coll Cardiol. 2020, 76, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Sherif, H.M.; Nekolla, S.G.; Saraste, A.; Reder, S.; Yu, M.; Robinson, S.; Schwaiger, M. Simplified quantification of myocardial flow reserve with flurpiridaz F 18: Validation with micro-spheres in a pig model. J. Nucl. Med. 2011, 52, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Mou, T.; Zhao, Z.; You, L.; Li, Y.; Wang, Q.; Fang, W.; Lu, J.; Peng, C.; Zhang, X. Synthesis and evaluation of 18F-labeled pyridaben analogues for myocardial perfusion imaging in mice, rats and Chinese mini-swine. Sci. Rep. 2016, 6, 33450. [Google Scholar] [CrossRef] [PubMed]
- Mou, T.; Zhao, Z.; Fang, W.; Peng, C.; Guo, F.; Liu, B.; Ma, Y.; Zhang, X. Synthesis and preliminary evaluation of 18F-labeled pyridaben analogues for myocardial perfusion imaging with PET. J. Nucl. Med. 2012, 53, 472–479. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, H.S.; Le, U.N.; Jiang, S.N.; Kim, H.J.; Lee, K.C.; Woo, S.K.; Chung, J.; Kim, H.S.; Bom, H.S.; et al. Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl)triphenylphosphonium cation, in a rat myocardial infarction model. J. Nucl. Med. 2012, 53, 1779–1785. [Google Scholar] [CrossRef]
- Heo, G.S.; Sultan, D.; Liu, Y. Current and Novel Radiopharmaceuticals for Imaging Cardiovascular Inflammation. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 4–20. [Google Scholar] [CrossRef]
- LaForest, R.; Woodard, P.K.; Gropler, R.J. Cardiovascular PET/MRI: Challenges and Opportunities. Cardiol. Clin. 2016, 34, 25–35. [Google Scholar] [CrossRef]
- Amsallem, M.; Saito, T.; Tada, Y.; Dash, R.; McConnell, M.V. Magnetic Resonance Imaging and Positron Emission Tomography Approaches to Imaging Vascular and Cardiac Inflammation. Circ. J. 2016, 80, 1269–1277. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Joseph, P.; Tawakol, A. Imaging atherosclerosis with positron emission tomography. Eur. Heart J. 2016, 37, 2974–2980. [Google Scholar] [CrossRef]
- Van der Vorst, E.P.C.; Peters, L.J.F.; Müller, M.; Gencer, S.; Yan, Y.; Weber, C.; Döring, Y. G-Protein Coupled Receptor Targeting on Myeloid Cells in Atherosclerosis. Front. Pharmacol. 2019, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Pelisek, J.; Laitinen, I.; Schottelius, M.; Mohring, M.; Döring, Y.; van der Vorst, E.P.; Kallmayer, M.; Steiger, K.; Poschenrieder, A.; et al. Imaging the Cytokine Receptor CXCR4 in Atherosclerotic Plaques with the Radiotracer 68Ga-Pentixafor for PET. J. Nucl. Med. 2017, 58, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, D.; Thackeray, J.T.; Daum, G.; Sohns, J.M.; Kropf, S.; Wester, H.J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Clinical Molecular Imaging of Chemokine Receptor CXCR4 Expression in Atherosclerotic Plaque Using 68Ga-Pentixafor PET: Correlation with Cardiovascular Risk Factors and Calcified Plaque Burden. J. Nucl. Med. 2018, 59, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Sedding, D.G.; Dutzmann, J.; Haghikia, A.; König, T.; Napp, L.C.; Schütze, C.; Owsianski-Hille, N.; Wester, H.J.; Kropf, S.; et al. Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [68Ga]pentixafor PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, W.; Wollenweber, T.; Lu, X.; Wei, Y.; Beitzke, D.; Wadsak, W.; Kropf, S.; Wester, H.J.; Haug, A.R.; et al. [68Ga]Pentixafor PET/MR imaging of chemokine receptor 4 expression in the human carotid artery. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Derlin, T.; Haghikia, A.; Napp, L.C.; Wang, Y.; Ross, T.L.; Schäfer, A.; Tillmanns, J.; Wester, H.J.; Wollert, K.C.; et al. Molecular Imaging of the Chemokine Receptor CXCR4 After Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Luehmann, H.P.; Zhao, Y.; Detering, L.; Sultan, D.H.; Hsiao, H.M.; Krupnick, A.S.; Gelman, A.E.; Combadiere, C.; et al. Noninvasive Imaging of CCR2+ Cells in Ischemia-Reperfusion Injury After Lung Transplantation. Am. J. Transpl. 2016, 16, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gunsten, S.P.; Sultan, D.H.; Luehmann, H.P.; Zhao, Y.; Blackwell, T.S.; Bollermann-Nowlis, Z.; Pan, J.H.; Byers, D.E.; Atkinson, J.J.; et al. PET-based Imaging of Chemokine Receptor 2 in Experimental and Disease-related Lung Inflammation. Radiology 2017, 283, 758–768. [Google Scholar] [CrossRef]
- Li, W.; Luehmann, H.P.; Hsiao, H.M.; Tanaka, S.; Higashikubo, R.; Gauthier, J.M.; Sultan, D.; Lavine, K.J.; Brody, S.L.; Gelman, A.E.; et al. Visualization of Monocytic Cells in Regressing Atherosclerotic Plaques by Intravital 2-Photon and Positron Emission Tomography-Based Imaging-Brief Report. Arter. Thromb. Vasc Biol. 2018, 38, 1030–1036. [Google Scholar] [CrossRef]
- Heo, G.S.; Kopecky, B.; Sultan, D.; Ou, M.; Feng, G.; Bajpai, G.; Zhang, X.; Luehmann, H.; Detering, L.; Su, Y.; et al. Molecular Imaging Visualizes Recruitment of Inflammatory Monocytes and Macrophages to the Injured Heart. Circ. Res. 2019, 124, 881–890. [Google Scholar] [CrossRef]
- Luehmann, H.P.; Pressly, E.D.; Detering, L.; Wang, C.; Pierce, R.; Woodard, P.K.; Gropler, R.J.; Hawker, C.J.; Liu, Y. PET/CT Imaging of Chemokine Receptor CCR5 in Vascular Injury Model Using Targeted Nanoparticle. J. Nucl. Med. 2014, 55, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pierce, R.; Luehmann, H.P.; Sharp, T.L.; Welch, M.J. PET Imaging of Chemokine Receptors in Vascular Injury–Accelerated Atherosclerosis. J. Nucl. Med. 2013, 54, 1135–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bauer, W.; Kreissl, M.C.; Weirather, J.; Bauer, E.; Israel, I.; Richter, D.; Riehl, G.; Buck, A.; Samnick, S. Specific somatostatin receptor II expression in arterial plaque: 68Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis 2013, 230, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.; Hellberg, S.; Kiugel, M.; Virta, J.; Li, X.G.; Käkelä, M.; Helariutta, K.; Luoto, P.; Liljenbäck, H.; Hakovirta, H.; et al. Comparison of Somatostatin Receptor 2-Targeting PET Tracers in the Detection of Mouse Atherosclerotic Plaques. Mol. Imaging Biol. 2016, 18, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, A.; Alavi, A.; Thamake, S.; Amerinia, R.; Ranganathan, D.; Tworowska, I.; Delpassand, E.S. Assessment of vulnerable atherosclerotic and fibrotic plaques in coronary arteries using 68Ga-DOTATATE PET/CT. Am. J. Nucl. Med. Mol. Imaging 2014, 5, 65–71. [Google Scholar] [PubMed]
- Li, X.; Samnick, S.; Lapa, C.; Israel, I.; Buck, A.K.; Kreissl, M.C.; Bauer, W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: Correlation with 18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012, 2, 52. [Google Scholar] [CrossRef]
- Malmberg, C.; Ripa, R.S.; Johnbeck, C.B.; Knigge, U.; Langer, S.W.; Mortensen, J.; Oturai, P.S.; Loft, A.; Hag, A.M.; Kjær, A. 64Cu-DOTATATE for Noninvasive Assessment of Atherosclerosis in Large Arteries and Its Correlation with Risk Factors: Head-to-Head Comparison with 68Ga-DOTATOC in 60 Patients. J. Nucl. Med. 2015, 56, 1895–1900. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Sandholt, B.V.; Keller, S.H.; Hansen, A.E.; Clemmensen, A.E.; Sillesen, H.; Højgaard, L.; Ripa, R.S.; Kjær, A. 64Cu-DOTATATE PET/MRI for Detection of Activated Macrophages in Carotid Atherosclerotic Plaques. Arter. Thromb. Vasc. Biol. 2015, 35, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Schatka, I.; Wollenweber, T.; Haense, C.; Brunz, F.; Gratz, K.F.; Bengel, F.M. Peptide Receptor–Targeted Radionuclide Therapy Alters Inflammation in Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2013, 62, 2344–2345. [Google Scholar] [CrossRef]
- Tahara, N.; Mukherjee, J.; de Haas, H.J.; Petrov, A.D.; Tawakol, A.; Haider, N.; Tahara, A.; Constantinescu, C.C.; Zhou, J.; Boersma, H.H.; et al. 2- deoxy-2-[18F]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nat. Med. 2014, 20, 215–219. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, S.; Seo, H.S.; Lee, Y.J.; Eo, J.S.; Jeong, J.M.; Lee, B.; Kim, J.Y.; Park, Y.M.; Jeong, M. Novel PET Imaging of Atherosclerosis with 68Ga-Labeled NOTA-Neomannosylated Human Serum Albumin. J. Nucl. Med. 2016, 57, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Senders, M.L.; Hernot, S.; Carlucci, G.; van de Voort, J.C.; Fay, F.; Calcagno, C.; Tang, J.; Alaarg, A.; Zhao, Y.; Ishino, S.; et al. Nanobody- Facilitated Multiparametric PET/MRI Phenotyping of Atherosclerosis. JACC Cardiovasc. Imaging 2018, 12, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, Z.; Hyafil, F.; Anizan, N.; Diallo, D.; Aid-Launais, R.; Mohanta, S.; Li, Y.; Braeuer, M.; Steiger, K.; Vigne, J.; et al. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using 111In-tilmanocept. EJNMMI Res. 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, S.; Silvola, J.M.U.; Kiugel, M.; Liljenbäck, H.; Metsälä, O.; Viljanen, T.; Metso, J.; Jautihiainen, M.; Saukko, P.; Nuutila, P.; et al. Type 2 diabetes enhances arterial uptake of choline in atherosclerotic mice: An imaging study with positron emission tomography tracer 18F-fluoromethylcholine. Cardiovasc. Diabetol. 2016, 15, 26. [Google Scholar] [CrossRef]
- Vöö, S.; Kwee, R.M.; Sluimer, J.C.; Schreuder, F.H.; Wierts, R.; Bauwens, M.; Heeneman, S.; Cleutjens, J.P.; van Oostenbrugge, R.J.; Daemen, J.W.; et al. Imaging Intraplaque Inflammation in Carotid Atherosclerosis With 18F-Fluorocholine Positron Emission Tomography-Computed Tomography. Circ. Cardiovasc. Imaging 2016, 9, e004467. [Google Scholar] [CrossRef]
- Ye, Y.X.; Calcagno, C.; Binderup, T.; Courties, G.; Keliher, E.J.; Wojtkiewicz, G.R.; Iwamoto, Y.; Tang, J.; Pérez-Medina, C.; Mani, V.; et al. Imaging Macrophage and Hematopoietic Progenitor Proliferation in Atherosclerosis. Circ. Res. 2015, 117, 835–845. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; Di Carli, M.F.; et al. Polymeric Nanoparticle PET/MR Imaging Allows Macrophage Detection in Atherosclerotic Plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef]
- Keliher, E.J.; Ye, Y.X.; Wojtkiewicz, G.R.; Aguirre, A.D.; Tricot, B.; Senders, M.L.; Groenen, H.; Fay, F.; Perez-Medina, C.; Calcagno, C.; et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischemic heart disease. Nat. Commun. 2017, 8, 14064. [Google Scholar] [CrossRef]
- Meester, E.J.; Krenning, B.J.; de Blois, R.H.; Norenberg, J.P.; de Jong, M.; Bernsen, M.R.; Van der Heiden, K. Imaging of atherosclerosis, targeting LFA-1 on inflammatory cells with 111In-DANBIRT. J. Nucl. Cardiol. 2018, 26, 1697–1704. [Google Scholar] [CrossRef]
- Mota, R.; Campen, M.J.; Cuellar, M.E.; Garver, W.S.; Hesterman, J.; Qutaish, M.; Daniels, T.; Nysus, M.; Wagner, C.R.; Norenberg, J.P. 111In-DANBIRT In Vivo Molecular Imaging of Inflammatory Cells in Atherosclerosis. Contrast Media Mol. Imaging 2018, 6508724. [Google Scholar] [CrossRef]
- Di Gialleonardo, V.; Signore, A.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; De Vries, E.F.J. N-(4–18F- Fluorobenzoyl)Interleukin-2 for PET of Human-Activated T Lymphocytes. J. Nucl. Med. 2012, 53, 679–686. [Google Scholar] [CrossRef]
- Hermann, S.; Starsichova, A.; Waschkau, B.; Kuhlmann, M.; Wenning, C.; Schober, O.; Schäfers, M. Non-FDG imaging of atherosclerosis: Will imaging of MMPs assess plaque vulnerability? J. Nucl. Cardiol. 2012, 19, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, N.; van Waarde, A.; Bischoff, R.; Oltenfreiter, R.; van de Wiele, C.; Dierckx, R.A.; Elsinga, P.H. Probes for Non-invasive Matrix Metalloproteinase-targeted Imaging with PET and SPECT. Curr. Pharm. Des. 2013, 19, 4647–4672. [Google Scholar] [CrossRef] [PubMed]
- Malm, B.J.; Sadeghi, M.M. Multi-modality molecular imaging of aortic aneurysms. J. Nucl. Cardiol. 2017, 24, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Toczek, J.; Ye, Y.; Gona, K.; Kim, H.Y.; Han, J.; Razavian, M.; Golestani, R.; Zhang, J.; Wu, T.L.; Jung, J.J.; et al. Preclinical Evaluation of RYM1, a Matrix Metalloproteinase-Targeted Tracer for Imaging Aneurysm. J. Nucl. Med. 2017, 58, 1318–1323. [Google Scholar] [CrossRef]
- Toczek, J.; Bordenave, T.; Gona, K.; Kim, H.Y.; Beau, F.; Georgiadis, D.; Correia, I.; Ye, Y.; Razavian, M.; Jung, J.J.; et al. Novel Matrix Metalloproteinase 12 selective radiotracers for vascular molecular imaging. J. Med. Chem. 2019, 62, 9743–9752. [Google Scholar] [CrossRef]
- Butsch, V.; Börgel, F.; Galla, F.; Schwegmann, K.; Hermann, S.; Schäfers, M.; Riemann, B.; Wünsch, B.; Wagner, S. Design, (Radio)Synthesis, and in Vitro and in Vivo Evaluation of Highly Selective and Potent Matrix Metalloproteinase 12 (MMP-12) Inhibitors as Radiotracers for Positron Emission Tomography. J. Med. Chem. 2018, 61, 4115–4134. [Google Scholar] [CrossRef]
- Hugenberg, V.; Wagner, S.; Kopka, K.; Schäfers, M.; Schuit, R.C.; Windhorst, A.D.; Hermann, S. Radiolabeled Selective Matrix Metalloproteinase 13 (MMP-13) Inhibitors: (Radio)Syntheses and in Vitro and First in Vivo Evaluation. J. Med. Chem. 2017, 60, 307–321. [Google Scholar] [CrossRef]
- Auletta, S.; Varani, M.; Horvat, R.; Galli, F.; Signore, A.; Hess, S. PET Radiopharmaceuticals for Specific Bacteria Imaging: A Systematic Review. J. Clin. Med. 2019, 8, 197. [Google Scholar] [CrossRef]
- Bhatt, J.; Mukherjee, A.; Shinto, A.; Koramadai Karuppusamy, K.; Korde, A.; Kumar, M.; Sarma, H.D.; Repaka, K.; Dash, A. Gallium-68 labeled Ubiquicidin derived octapeptide as a potential infection imaging agent. Nucl. Med. Biol. 2018, 62–63, 47–53. [Google Scholar] [CrossRef]
- Ebenhan, T.; Sathekge, M.M.; Lengana, T.; Koole, M.; Gheysens, O.; Govender, T.; Zeevaart, J.R. 68Ga-NOTA-Functionalized Ubiquicidin: Cytotoxicity, Biodistribution, Radiation Dosimetry, and First-in-Human PET/CT Imaging of Infections. J. Nucl. Med. 2018, 59, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Vilche, M.; Reyes, A.L.; Vasilskis, E.; Oliver, P.; Balter, H.; Engler, H. 68Ga-NOTA-UBI-29-41 as a PET tracer for detection of bacterial infection. J. Nucl. Med. 2016, 57, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Zeevaart, J.R.; Venter, J.D.; Govender, T.; Kruger, G.H.; Jarvis, N.V.; Sathekge, M.M. Preclinical Evaluation of 68Ga-Labeled 1,4,7-triazacyclononane-1,4,7-triacetic acid-ubiquicidin as a radioligand for PET infection imaging. J. Nucl. Med. 2014, 55, 308–314. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, H.H.; McLeroth, P.; Berkowitz, R.D.; Mont, M.A.; Stabin, M.G.; Siegel, B.A.; Alavi, A.; Barnett, T.M.; Gelb, J.; et al. [124I]FIAU: Human dosimetry and infection imaging in patients with suspected prosthetic joint infection. Nucl. Med. Biol. 2016, 43, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.; Awais, R.O.; Luckett, J.; Turton, D.; Williams, P.; Perkins, A.C.; Hill, P.J. [18F]FDG-6-P as a novel in vivo tool for imaging staphylococcal infections. EJNMMI Res. 2015, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D.; Arjun, C.; Krishnamohan, R.; Samuel, G.; Banerjee, S. 68Ga-labeled ciprofloxacin conjugates as radiotracers for targeting bacterial infection. Chem. Biol. Drug. Des. 2016, 87, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.M.; Jørgensen, N.P.; Kyneb, M.H.; Borghammer, P.; Meyer, R.L.; Thomsen, T.R.; Bender, D.; Jensen, S.B.; Nielsen, O.L.; Alstrup, A. Preclinical evaluation of potential infection-imaging probe [68Ga]Ga-DOTA-K-A9 in sterile and infectious inflammation. J. Label. Compd. Radiopharm. 2018, 61, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Fodah, R.; Warawa, J.M.; Ng, C.K. Validation of 2- 18F-Fluorodeoxysorbitol as a potential radiopharmaceutical for imaging bacterial infection in the lung. J. Nucl. Med. 2018, 59, 134–139. [Google Scholar] [CrossRef]
- Yao, S.; Xing, H.; Zhu, W.; Wu, Z.; Zhang, Y.; Ma, Y.; Liu, Y.; Zhu, Z.; Li, Z.; Fang, L. Infection imaging with 18F-FDS and first-in-human evaluation. Nucl. Med. Biol. 2016, 43, 206–214. [Google Scholar] [CrossRef]
- Weinstein, E.A.; Ordonez, A.A.; DeMarco, V.P.; Murawski, A.M.; Pokkali, S.; MacDonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef]
- Ning, X.; Seo, W.; Lee, S.; Takemiya, K.; Rafi, M.; Feng, X.; Weiss, D.; Wang, X.; Williams, L.; Camp, V.M.; et al. Fluorine-18 labeled maltohexaose images bacterial infections by PET. Angew. Chem. Int. Ed. Engl. 2014, 53, 14096–14101. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, G.; Namavari, M.; Jouannot, E.B.; Hoehne, A.; Reeves, R.; Hardy, J.; Gambhir, S.S. Investigation of 6-[18F]-fluoromaltose as a novel PET tracer for imaging bacterial infection. PLoS ONE 2014, 9, e107951. [Google Scholar] [CrossRef]
- Sellmyer, M.A.; Lee, I.; Hou, C.; Weng, C.C.; Li, S.; Lieberman, B.P.; Zeng, C.; Mankoff, D.A.; Mach, R.H. Bacterial infection imaging with [18F]fluoropropyl-trimethoprim. Proc. Natl. Acad. Sci. USA 2017, 114, 8372–8377. [Google Scholar] [CrossRef]
- Gowrishankar, G.; Hardy, J.; Wardak, M.; Namavari, M.; Reeves, R.E.; Neofytou, E.; Srinivasan, A.; Wu, J.C.; Contag, C.H.; Gambhir, S.S. Specific imaging of bacterial infection using 6’’-18F fluoromaltotriose: A second-generation PET tracer targeting the maltodextrin transporter in bacteria. J. Nucl. Med. 2017, 58, 1679–1684. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Weinstein, E.A.; Bambarger, L.E.; Saini, V.; Chang, Y.S.; DeMarco, V.P.; Klunk, M.H.; Urbanowski, M.E.; Moulton, K.L.; Murawski, A.M.; et al. A systematic approach for developing bacteria-specific imaging tracers. J. Nucl. Med. 2017, 58, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Mokaleng, B.B.; Ebenhan, T.; Ramesh, S.; Govender, T.; Kruger, H.G.; Parboosing, R.; Hazari, P.P.; Mishra, A.K.; Marjanovic-Painter, B.; Zeevaart, J.R.; et al. Synthesis, 68Ga-radiolabeling, and preliminary in vivo assessment of a depsipeptide-derived compound as a potential PET/CT infection imaging agent. BioMed. Res. Int. 2015, 2015, 284354. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Mokaleng, B.B.; Venter, J.D.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M. Preclinical assessment of a 68Ga-DOTA functionalized depsipeptide as a radiodiagnostic infection imaging agent. Molecules 2017, 22, 1403. [Google Scholar] [CrossRef]
- Dunlap, J.B.; Fan, G.; Leeborg, N.; Braziel, R.M. B-Cell Malignancies. In Molecular Pathology in Clinical; Leonard, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 579–602. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B cells in rheumatoid arthritis: Pathogenic mechanisms and treatment prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar] [CrossRef]
- Wekerle, H. B cells in multiple sclerosis. Autoimmunity 2017, 50, 57–60. [Google Scholar] [CrossRef]
- Sospedra, M. B cells in multiple sclerosis. Curr. Opin. Neurol. 2018, 31, 256–262. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Mattoscio, M.; Magliozzi, R.; Bar-Or, A.; Muraro, P.A. B cells in multiple sclerosis—From targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 2021, 17, 399–414. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Simmons, K.M.; Cambier, J.C. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat. Rev. Nephrol. 2017, 13, 712–720. [Google Scholar] [CrossRef] [PubMed]
- McManigle, W.; Youssef, A.; Sarantopoulos, S. B cells in chronic graft-versus-host disease. Hum. Immunol. 2019, 80, 393–399. [Google Scholar] [CrossRef]
- Pal Singh, S.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef]
- Wen, T.; Wang, J.; Shi, Y.; Quin, H.; Liu, P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia 2021, 35, 312–332. [Google Scholar] [CrossRef]
- Zhang, D.; Gong, H.; Meng, F. Recent Advances in BTK Inhibitors for the Treatment of Inflammatory and Autoimmune Diseases. Molecules 2021, 26, 4907. [Google Scholar] [CrossRef]
- Arneson, L.C.; Carroll, K.J.; Ruderman, E.M. Bruton’s Tyrosine Kinase Inhibition for the Treatment of Rheumatoid Arthritis. Immunotargets Ther. 2021, 10, 333–342. [Google Scholar] [CrossRef]
- Contentti, E.C.; Correale, J. Bruton’s tyrosine kinase inhibitors: A promising emerging treatment option for multiple sclerosis. Expert Opin. Emerg. Drugs 2020, 25, 377–381. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Preshlock, S.; Kaur, T.; Tran, T.; Wilson, T.C.; Mhanna, K.; Henderson, B.D.; Batalla, D.; Scott, P.J.H.; Shao, X. Synthesis of Radiopharmaceuticals via “In-Loop” 11C-Carbonylation as Exemplified by the Radiolabeling of Inhibitors of Bruton’s Tyrosine Kinase. Front. Nucl. Med. 2022, 1, 820235. [Google Scholar] [CrossRef]
- Hatori, A.; Yui, J.; Yamasaki, T.; Xie, L.; Kumata, K.; Fujinaga, M.; Yoshida, Y.; Ogawa, M.; Nengaki, N.; Kawamura, K.; et al. PET imaging of lung inflammation with [18F]FEDAC, a radioligand for translocator protein (18 kDa). PLoS ONE 2012, 7, e45065. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; Gallezot, J.D.; Schafbauer, T.; Lim, K.; Kloczynski, T.; Morris, E.D.; Carson, R.E.; Ding, Y.S.; Cosgrove, K.P. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. NeuroImage 2012, 63, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.S.; Kuhnast, B.; Damont, A.; Roeda, D.; Tavitian, B.; Dolle, F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 2012, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerdere, S.; Callaghan, P.D.; Pham, T.; Rahardjo, G.L.; Amhaoul, H.; Berghofer, P.; Quinlivan, M.; Mattner, F.; Loc′h, C.; Katsifis, A.; et al. PET imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy. EJNMMI Res. 2012, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Maecke, H.R.; Reubi, J.C. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J. Nucl. Med. 2011, 52, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Di Gialleonardo, V.; Signore, A.; Willemsen, A.T.; Sijbesma, J.W.; Dierckx, R.A.; de Vries, E.F. Pharmacokinetic modeling of N-(4-[18F]fluorobenzoyl)interleukin-2 binding to activated lymphocytes in an xenograft model of inflammation. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Fotis, L.; Agrogiannis, G.; Vlachos, I.S.; Pantopoulou, A.; Margoni, A.; Kostaki, M.; Verikokos, C.; Tzivras, D.; Mikhailidis, D.P.; Perrea, D. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early stages of atherosclerosis in a rat model. In Vivo 2012, 26, 243–250. [Google Scholar]
- Douglas, A.P.; Thursky, K.A.; Worth, L.J.; Drummond, E.; Hogg, A.; Hicks, R.J.; Slavin, M.A. FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: A retrospective comparison to conventional CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 166–173. [Google Scholar] [CrossRef]
- Leroy-Freschini, B.; Treglia, G.; Argemi, X.; Bund, C.; Kessler, R.; Herbrecht, R.; Imperiale, A. 18 F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM Int. J. Med. 2018, 111, 613–622. [Google Scholar] [CrossRef]
- Ankrah, O.A.; Span, L.F.R.; Klein, H.C.; de Jong, P.A.; Dierckx, R.A.J.O.; Kwee, T.C.; Sathekge, M.M.; Glaudemans, A.W.J.M. Role of FDG PET/CT in monitoring treatment response in patients with invasive fungal infections. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 174–183. [Google Scholar] [CrossRef]
- Fuchs, M.; Briel, M.; Daikeler, T.; Walker, U.A.; Rasch, H.; Berg, S.; Ng, Q.K.; Raatz, H.; Jayne, D.; Kötter, I.; et al. The impact of 18 F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, I.; Thürmel, K.; Pyka, T.; Eiber, M.; Wolfram, S.; Moog, P.; Reeps, C.; Essler, M. Imaging large vessel vasculitis with fully integrated PET/MRI: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Pijl, J.P.; Kwee, T.C.; Slart, R.H.J.A.; Glaudemans, A.W.J.M. PET/CT Imaging for Personalized Management of Infectious Diseases. J. Pers. Med. 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Bier, G.; Hoffmann, V.; Kloth, C.; Othman, A.E.; Eigentler, T.; Garbe, C.; La Fougère, C.; Pfannenberg, C.; Nikolaou, K.; Klumpp, B. CT imaging of bone and bone marrow infiltration in malignant melanoma—Challenges and limitations for clinical staging in comparison to 18 FDG-PET/CT. Eur. J. Radiol. 2016, 85, 732–738. [Google Scholar] [CrossRef]
- Kong, B.Y.; Menzies, A.M.; Saunders, C.A.; Liniker, E.; Ramanujam, S.; Guminski, A.; Kefford, R.F.; Long, G.V.; Carlino, M.S. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res. 2016, 29, 572–577. [Google Scholar] [CrossRef]
- Hueting, R. Radiocopper for the imaging of copper metabolism. J. Labelled Comp. Radiopharm. 2014, 57, 231–238. [Google Scholar] [CrossRef]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J. and Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef]
- Narayanaswami, V.; Dahl, K.; Bernard-Gauthier, V.; Josephson, L.; Cumming, P.; Vasdev, N. Emerging PET Radiotracers and Targets for Imaging of Neuroinflammation in Neurodegenerative Diseases: Outlook Beyond TSPO. Mol. Imaging 2018, 17, 1536012118792317. [Google Scholar] [CrossRef]
- Fujita, M.; Kobayashi, M.; Ikawa, M.; Gunn, R.N.; Rabiner, E.A.; Owen, D.R.; Zoghbi, S.S.; Haskali, M.B.; Telu, S.; Pike, V.W.; et al. Comparison of four 11C-labeled PET ligands to quantify translocator protein 18kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176-based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res. 2017, 7, 84. [Google Scholar] [CrossRef]
- Boutin, H.; Murray, K.; Pradillo, J.; Maroy, R.; Smigova, A.; Gerhard, A.; Jones, P.A.; Trigg, W. 18F-GE-180: A novel TSPO radiotracer compared to 11C-R-PK11195 in a preclinical model of stroke. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 503–511. [Google Scholar] [CrossRef]
- Ikawa, M.; Lohith, T.G.; Shrestha, S.; Telu, S.; Zoghbi, S.S.; Castellano, S.; Taliani, S.; Da Settimo, F.; Fujita, M.; Pike, V.W.; et al. 11C-ER176, a Radioligand for 18-kDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J. Nucl. Med. 2017, 58, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Holton, P.; Reiser, V.; Jones, P.A.; et al. In Vivo Detection of Age- and Disease-Related Increases in Neuroinflammation by 18F-GE180 TSPO MicroPET Imaging in Wild-Type and Alzheimer’s Transgenic Mice. J. Neurosci. 2015, 35, 15716–15730. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Picon, F.R.; Snellman, A.; Eskola, O.; Helin, S.; Solin, O.; Haaparanta-Solin, M.; Rinne, J.O. Neuroinflammation Appears Early on PET Imaging and Then Plateaus in a Mouse Model of Alzheimer Disease. J. Nucl. Med. 2018, 59, 509–515. [Google Scholar] [CrossRef]
- Chaney, A.; Cropper, H.C.; Johnson, E.M.; Lechtenberg, K.J.; Peterson, T.C.; Stevens, M.Y.; Buckwalter, M.S.; James, M.L. 11 C-DPA-713 Versus 18F-GE-180: A Preclinical Comparison of Translocator Protein 18 kDa PET Tracers to Visualize Acute and Chronic Neuroinflammation in a Mouse Model of Ischemic Stroke. J. Nucl. Med. 2019, 60, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Mahler, C.; Vomacka, L.; Lindner, S.; Havla, J.; Brendel, M.; Boning, G.; Ertl-Wagner, B.; Kumpfel, T.; Milenkovic, V.M.; et al. TSPO PET with [18F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Raffel, J.; Nandoskar, A.; Record, C.; Brooks, D.J.; Owen, D.; Sharp, D.; Muraro, P.A.; Gunn, R.; Nicholas, R. Confirmation of Specific Binding of the 18-kDa Translocator Protein (TSPO) Radioligand [18F]GE-180: A Blocking Study Using XBD173 in Multiple Sclerosis Normal Appearing White and Grey Matter. Mol. Imaging Biol. 2019, 21, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Fregonara, P.; Veronese, M.; Pascual, B.; Rostomily, R.C.; Turkheimer, F.; Masdeu, J.C. The validity of 18F-GE180 as a TSPO imaging agent. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1205–1207. [Google Scholar] [CrossRef]
- Tremoleda, J.L.; Thau-Zuchman, O.; Davies, M.; Foster, J.; Khan, I.; Vadivelu, K.C.; Yip, P.K.; Sosabowski, J.; Trigg, W.; Michael-Titus, A.T. In vivo PET imaging of the neuroinflammatory response in rat spinal cord injury using the TSPO tracer [18F]GE-180 and effect of docosahexaenoic acid. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1710–1722. [Google Scholar] [CrossRef]
- James, M.L.; Belichenko, N.P.; Shuhendler, A.J.; Hoehne, A.; Andrews, L.E.; Condon, C.; Nguyen, T.V.; Reiser, V.; Jones, P.; Trigg, W.; et al. [18F]GE-180 PET Detects Reduced Microglia Activation After LM11A-31 Therapy in a Mouse Model of Alzheimer’s Disease. Theranostics 2017, 7, 1422–1436. [Google Scholar] [CrossRef]
- Sridharan, S.; Lepelletier, F.X.; Trigg, W.; Banister, S.; Reekie, T.; Kassiou, M.; Gerhard, A.; Hinz, R.; Boutin, H. Comparative Evaluation of Three TSPO PET Radiotracers in a LPS-Induced Model of Mild Neuroinflammation in Rats. Mol. Imaging Biol. 2017, 19, 77–89. [Google Scholar] [CrossRef]
- Yoder, K.K.; Nho, K.; Risacher, S.L.; Kim, S.; Shen, L.; Saykin, A.J. Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J. Nucl. Med. 2013, 54, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, N.R.; Loggia, M.L.; Lawson, R.; Chonde, D.B.; Izquierdo-Garcia, D.; Yasek, J.E.; Akeju, O.; Catana, C.; Rosen, B.R.; Cudkowicz, M.E.; et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: Assessed with [11C]-PBR28. Neuroimage Clin. 2015, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, W.C.; Lyoo, C.H.; Liow, J.S.; Wei, M.; Snow, J.; Page, E.; Jenko, K.J.; Morse, C.L.; Zoghbi, S.S.; Pike, V.W.; et al. 11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol. Aging 2016, 44, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Datta, G.; Colasanti, A.; Kalk, N.; Owen, D.; Scott, G.; Rabiner, E.A.; Gunn, R.N.; Lingford-Hughes, A.; Malik, O.; Ciccarelli, O.; et al. 11C-PBR28 and 18F-PBR111 Detect White Matter Inflammatory Heterogeneity in Multiple Sclerosis. J. Nucl. Med. 2017, 58, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Middleton, R.J.; Chan, R.; Hatty, C.R.; Kam, W.W.; Quin, C.; Graeber, M.B.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014, 5, 5452. [Google Scholar] [CrossRef]
- Mirzaei, N.; Tang, S.P.; Ashworth, S.; Coello, C.; Plisson, C.; Passchier, J.; Selvaraj, V.; Tyacke, R.J.; Nutt, D.J.; Sastre, M. In vivo imaging of microglial activation by positron emission tomography with [11C]PBR28 in the 5XFAD model of Alzheimer’s disease. Glia 2016, 64, 993–1006. [Google Scholar] [CrossRef]
- Simmons, D.A.; James, M.L.; Belichenko, N.P.; Semaan, S.; Condon, C.; Kuan, J.; Shuhendler, A.J.; Miao, Z.; Chin, F.T.; Longo, F.M. TSPO-PET imaging using [18F]PBR06 is a potential translatable biomarker for treatment response in Huntington’s disease: Preclinical evidence with the p75NTR ligand LM11A-31. Hum. Mol. Genet. 2018, 27, 2893–2912. [Google Scholar] [CrossRef]
- Owen, D.R.; Yeo, A.J.; Gunn, R.N.; Song, K.; Wadsworth, G.; Lewis, A.; Rhodes, C.; Pulford, D.J.; Bennacef, I.; Parker, C.A.; et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 2012, 32, 1–5. [Google Scholar] [CrossRef]
- Guo, Q.; Colasanti, A.; Owen, D.R.; Onega, M.; Kamalakaran, A.; Bennacef, I.; Matthews, P.M.; Rabiner, E.A.; Turkheimer, F.E.; Gunn, R.N. Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: A genetic polymorphism effect on in vivo binding. J. Nucl. Med. 2013, 54, 1915–1923. [Google Scholar] [CrossRef]
- Colasanti, A.; Guo, Q.; Muhlert, N.; Giannetti, P.; Onega, M.; Newbould, R.D.; Ciccarelli, O.; Rison, S.; Thomas, C.; Nicholas, R.; et al. In Vivo Assessment of Brain White Matter Inflammation in Multiple Sclerosis with 18 F-PBR111 PET. J. Nucl. Med. 2014, 55, 1112–1118. [Google Scholar] [CrossRef]
- Takano, A.; Piehl, F.; Hillert, J.; Varrone, A.; Nag, S.; Gulyas, B.; Stenkrona, P.; Villemagne, V.L.; Rowe, C.C.; Macdonell, R.; et al. In vivo TSPO imaging in patients with multiple sclerosis: A brain PET study with [18F]FEDAA1106. EJNMMI Res. 2013, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Mattsson, P.; Forsberg, A.; Takano, A.; Nag, S.; Gulyas, B.; Borg, J.; Boellaard, R.; Al-Tawil, N.; Eriksdotter, M.; et al. In vivo imaging of the 18-kDa translocator protein (TSPO) with [18F]FEDAA1106 and PET does not show increased binding in Alzheimer’s disease patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, F.; Kosaka, J.; Ota, M.; Higuchi, M.; Ito, H.; Fujimura, Y.; Nozaki, S.; Takahashi, S.; Mizukami, K.; Asada, T.; et al. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [11C]DAA1106. Psychiatry Res. 2012, 203, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Gauthier, V.; Mossine, A.V.; Mahringer, A.; Aliaga, A.; Bailey, J.J.; Shao, X.; Stauff, J.; Arteaga, J.; Sherman, P.; Grand’Maison, M.; et al. Identification of [18F]TRACK, a fluorine-18-labeled tropomyosin receptor kinase (Trk) inhibitor for PET imaging. J. Med. Chem. 2018, 61, 1737–1743. [Google Scholar] [CrossRef]
- Mossine, A.V.; Brooks, A.F.; Makaravage, K.J.; Miller, J.M.; Ichiishi, N.; Sanford, M.S.; Scott, P.J.H. Synthesis of [18F]Arenes via the copper-mediated [18F]Fluorination of boronic acids. Org. Lett. 2015, 17, 5780–5783. [Google Scholar] [CrossRef]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. BioMed. Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef]
- Song, Y.; Xiao, Z.; Wang, K.; Wang, X.; Zhang, C.; Fang, F.; Sun, X.; Shen, B. Development and evaluation of 18F-IRS for molecular imaging mutant EGF receptors in NSCLC. Sci. Rep. 2017, 7, 3121. [Google Scholar] [CrossRef]
- Su, Z.; Herholz, K.; Gerhard, A.; Roncaroli, F.; Du Plessis, D.; Jackson, A.; Turkheimer, F.; Hinz, R. [11C]-(R)PK11195 tracer kinetics in the brain of glioma patients and a comparison of two referencing approaches. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1406–1419. [Google Scholar] [CrossRef]
- Sellmyer, M.A.; Lee, I.; Hou, C.; Lieberman, B.P.; Zeng, C.; Mankoff, D.A.; Mach, R.H. Quantitative PET reporter gene imaging with [11C]Trimethoprim. Mol. Therapy 2017, 25, 120–126. [Google Scholar] [CrossRef]
- Nepal, P.; Rodrigue, P.; Olsavsky, T. 18F-fluciclovine (Axumin) PET/CT detecting occult bone metastasis. Egypt J. Radiol. Nucl. Med. 2020, 51, 142. [Google Scholar] [CrossRef]
- Strebl, M.G.; Wang, C.; Schroeder, F.A.; Placzek, M.S.; Wey, H.-Y.; Van de Bittner, G.C.; Neelamegam, R.; Hooker, J.M. Development of a fluorinated class-I HDAC radiotracer reveals key chemical determinants of brain penetrance. ACS Chem. Neurosci. 2016, 7, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Tegnebratt, T.; Lu, L.; Eksborg, S.; Chireh, A.; Damberg, P.; Nikkhou-Aski, S.; Foukakis, T.; Rundqvist, H.; Holmin, S.; Kuiper, R.V. Treatment response assessment with (R)-[11CPAQ PET in the MMTV-PyMT mouse model of breast cancer. EJNMMI Res. 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J. Evaluation of DNA synthesis with carbon-11-labeled 4′-thiothymidine. World. J. Radiol. 2016, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- Okochi, Y.; Nihashi, T.; Fujii, M.; Kato, K.; Okada, Y.; Ando, Y.; Maesawa, S.; Takebayashi, S.; Wakabayashi, T.; Naganawa, S. Clinical use of 11C-methionine and 18F-FDG-PET for germinoma in central nervous system. Ann. Nucl. Med. 2014, 28, 94–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef]
- Papagiannopoulou, D. Technetium-99m radiochemistry for pharmaceutical applications. J. Labelled Comp. Radiopharm. 2017, 60, 502–520. [Google Scholar] [CrossRef]
- Imbert, L.; Poussier, S.; Franken, P.R.; Songy, B.; Verger, A.; Morel, O.; Wolf, D.; Noel, A.; Karcher, G.; Marie, P.-Y. Compared performance of high-sensitivity cameras dedicated to myocardial perfusion SPECT: A comprehensive analysis of phantoms and human images. J. Nucl. Med. 2012, 53, 1897–1903. [Google Scholar] [CrossRef]
- Goshen, E.; Beilin, L.; Stern, E.; Kenig, T.; Goldkorn, R.; Ben-Haim, S. Feasibility study of a novel general purpose CZT-based digital SPECT camera: Initial clinical results. EJNMMI Phys. 2018, 5, 6. [Google Scholar] [CrossRef]
- Hutton, B.F.; Erlandsson, K.; Thielemans, K. Advances in clinical molecular imaging instrumentation. Clin. Translat. Imaging 2018, 6, 31–45. [Google Scholar] [CrossRef]
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An update on technological developments and clinical applications. Br. J. Radiol. 2018, 91, 20160402. [Google Scholar] [CrossRef]
- Bordonne, M.; Chawki, M.B.; Marie, P.-Y.; Zaragori, T.; Roch, V.; Grignon, R.; Imbert, L. High-quality brain perfusion SPECT images may be achieved with a high-speed recording using CZT camera. EJNMMI Phys. 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, V.; Green, R.; Ricciardi, C.; Assante, R.; Zampella, E.; Nappi, C.; Gaudieri, V.; Mannarino, T.; Genova, A.; De Simini, G.; et al. A machine learning-based approach to directly compare the diagnostic accuracy of myocardial perfusion imaging by conventional and cadmium zinc telluride SPECT. J. Nucl. Cardiol. 2022, 29, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, F.; Sadeghzadeh, N. Tumor targeting with 99mTc radiolabeled peptides: Clinical application and recent development. Chem. Biol. Drug Des. 2018, 93, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Mansi, R.; Nicolas, G.P.; Wild, D. Radiolabeled Somatostatin Analogs—A Continuously Evolving Class of Radiopharmaceuticals. Cancers 2022, 14, 1172. [Google Scholar] [CrossRef] [PubMed]
- Brabander, T.; Kwekkeboom, D.J.; Feelders, R.A.; Brouwers, A.H.; Teunissen, J.A.M. Nuclear Medicine Imaging of Neuroendocrine Tumors. Front. Horm. Res. 2015, 44, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Makris, G.; Radford, L.L.; Kuchuk, M.; Gallazzi, F.; Jurisson, S.S.; Smith, C.J.; Hennkens, H.M. NOTA and NODAGA [99mTc]Tc- and [186Re]Re-Tricarbonyl Complexes: Radiochemistry and First Example of a [99mTc]Tc-NODAGA Somatostatin Receptor-Targeting Bioconjugate. Bioconjug. Chem. 2018, 29, 4040–4049. [Google Scholar] [CrossRef] [PubMed]
- Makris, G.; Kuchuk, M.; Gallazzi, F.; Jurisson, S.S.; Smith, C.J.; Hennkens, H.M. Somatostatin receptor targeting with hydrophilic [99mTc/186Re]Tc/Re-tricarbonyl NODAGA and NOTA complexes. Nucl. Med. Biol. 2019, 71, 39–46. [Google Scholar] [CrossRef]
- Abiraj, K.; Ursillo, S.; Tamma, M.L.; Rylova, S.N.; Waser, B.; Constable, E.C.; Fani, M.; Nicolas, G.P.; Reubi, J.C.; Maecke, H.R. The tetraamine chelator outperforms HYNIC in a new technetium HYNIC in a new technetium-99m-labelled somatostatin receptor 2 antagonist. EJNMMI Res. 2018, 8, 75. [Google Scholar] [CrossRef]
- Fani, M.; Weingaertner, V.; Peitl, P.K.; Mansi, R.; Gaonkar, R.H.; Garnuszek, P.; Mikolajczak, R.; Novak, D.; Simoncic, U.; Hubalewska-Dydejczyk, A.; et al. Selection of the First 99mTc-Labelled Somatostatin Receptor Subtype 2 Antagonist for Clinical Translation—Preclinical Assessment of Two Optimized Candidates. Pharmaceuticals 2021, 14, 19. [Google Scholar] [CrossRef]
- Gaonkar, R.H.; Wiesmann, F.; Del Pozzo, L.; Mcdougall, L.; Zanger, S.; Mikolajczak, R.; Mansi, R.; Fani, M. SPECT Imaging of SST2-Expressing Tumors with 99mTc-Based Somatostatin Receptor Antagonists: The Role of Tetraamine, HYNIC, and Spacers. Pharmaceuticals 2021, 14, 300. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The Rise of PSMA Ligands for Diagnosis and Therapy of Prostate Cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef] [PubMed]
- Wester, H.J.; Schottelius, M. PSMA-Targeted Radiopharmaceuticals for Imaging and Therapy. Semin. Nucl. Med. 2019, 49, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Barret, J.A.; Coleman, E.; Goldsmith, S.J.; Vallabhajosula, S.; Petry, N.A.; Cho, S.; Armor, T.; Stubbs, J.B.; Maresca, K.P.; Stabin, M.G.; et al. First-in-Man Evaluation of 2 High-Affinity PSMA-Avid Small Molecules for Imaging Prostate Cancer. J. Nucl. Med. 2013, 54, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Haberkorn, U.; Zechmann, C.; Armor, T.; Mier, W.; Spohn, F.; Debus, N.; Holland-Letz, T.; Babich, J.; Kratochwill, C. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 950–959. [Google Scholar] [CrossRef]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen for Molecular Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef]
- Lu, G.; Maresca, K.P.; Hillier, S.H.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.J.; Babich, J.W. Synthesis and SAR of 99mTc/Re-labeled small molecule prostate specific membrane antigen inhibitors with novel polar chelates. Bioorg. Med. Chem. Lett. 2013, 23, 1557–1563. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Hu, S.; He, S.; Bao, X.; Ma, G.; Luo, J.; Cheng, J.; Zhang, Y. 99mTc-labeling and evaluation of a HYNIC modified small-molecular inhibitor of prostate specific membrane antigen. Nucl. Med. Biol. 2017, 48, 69–75. [Google Scholar] [CrossRef]
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cueves, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef]
- Santos-Cuevas, C.; Davazo, J.; Ferro-Flores, G.; García-Pérez, F.O.; Ocampo-García, B.; Ignacio-Alvarez, E.; Gómez-Argumosa, E.; Pedraza-López, M. 99mTc-labeled PSMA inhibitor: Biokinetics and radiation dosimetry in healthy subjects and imaging of prostate cancer tumors in patients. Nucl. Med. Biol. 2017, 52, 1–6. [Google Scholar] [CrossRef]
- García-Pérez, F.O.; Davazo, J.; López-Buenrostro, S.; Santos-Cuevas, C.; Ferro-Flores, G.; Jímenez-Ríos, A.; Scavuzzo, A.; Santana-Ríos, Z.; Medina-Ornelas, S. Head to head comparison of performance of 99m Tc-EDDA/HYNICiPSMA SPECT/CT and 68 Ga-PSMA-11 PET/CT a prospective study in biochemical recurrence prostate cancer patients. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 332–340. [Google Scholar] [PubMed]
- Lawal, I.O.; Ankrah, A.O.; Mokgoro, N.; Vorster, M.; Maes, A.; Satheke, M.M. Diagnostic sensitivity of Tc-99m HYNIC PSMA SPECT/CT in prostate carcinoma: A comparative analysis with Ga-68 PSMA PET/CT. Prostate 2017, 77, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, M.; Wester, H.-J. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Tilki, D.; Mansholt, J.; Berliner, C.; Bernreuther, C.; Steuber, T.; Maurer, T.; Graefen, M. Metastases-yield and Prostate-specific Antigen Kinetics Following Salvage Lymph Node Dissection for Prostate Cancer: A Comparison between Conventional Surgical Approach and Prostate-specific Membrane Antigen-radioguided Surgery. Eur. Urol. Focus 2019, 5, 50–53. [Google Scholar] [CrossRef]
- Cimadamore, A.; Cheng, M.; Santoni, M.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Galosi, A.B.; Scarpelli, M.; Montironi, R. New Prostate Cancer Targets for Diagnosis, Imaging, and Therapy: Focus on Prostate-Specific Membrane Antigen. Front. Oncol. 2018, 8, 653. [Google Scholar] [CrossRef]
- Evazalipour, M.; D′Huyvetter, M.; Tehrani, B.S.; Abolhassani, M.; Omidfar, K.; Abdoli, S.; Arezumand, R.; Morovvati, H.; Lahoutte, T.; Muyldermans, S.; et al. Generation and characterization of nanobodies targeting PSMA for molecular imaging of prostate cancer. Contrast Media Mol. Imaging 2014, 9, 211–220. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Azad, G.K.; Taylor, B.P.; Lee, E.; Morrison, M.S.; Hughes, S.; Morris, S.; Rudman, S.; Chowdhury, S.; Goh, V. Imaging ανβ3 integrin expression in skeletal metastases with 99mTc-maraciclatide single-photon emission computed tomography: Detection and therapy response assessment. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 898–903. [Google Scholar] [CrossRef]
- Zhu, Z.; Miao, W.; Li, Q.; Dai, H.; Ma, Q.; Wang, F.; Yang, A.; Jia, B.; Jing, X.; Liu, S.; et al. 99mTc-3PRGD2 for integrin receptor imaging of lung cancer: A multicenter study. J. Nucl. Med. 2012, 53, 716–722. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, B.; Gao, S.; Ji, T.; Wen, Q.; Song, Y.; Zhu, L.; Xu, Z.; Liu, L. 99mTc-3P4-RGD2 Scintimammography in the Assessment of Breast Lesions: Comparative Study with 99mTc-MIBI. PLoS ONE 2014, 9, e108349. [Google Scholar] [CrossRef]
- Liu, L.; Song, Y.; Gao, S. 99mTc-3PRGD2 Scintimammography in Palpable and Non-palpable Breast Lesions. Mol. Imaging 2014, 13, 5. [Google Scholar] [CrossRef]
- Zhao, D.; Jin, X.; Li, F.; Liang, J.; Lin, Y. Integrin ανβ3 Imaging of Radioactive Iodine-Refractory Thyroid Cancer Using 99mTc-3PRGD2. J. Nucl. Med. 2012, 53, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zheng, S.; Dai, H.; Wang, F.; Jin, X.; Zhu, Z.; Jia, B. Comparison of 99mTc-3prgd2 integrin receptor imaging with 99mTc-MDP bone scan in diagnosis of bone metastasis in patients with lung cancer: A multicenter study. PLoS ONE 2014, 22, e111221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fu, F.; Li, F.; Zhu, Z.; Yang, Y.; Chen, X.; Jia, B.; Zheng, S.; Huang, C.; Miao, W. Comparison of [99mTc]3PRGD2 Imaging and [18F]FDG PET/CT in Breast Cancer and Expression of Integrin ανβ3 in Breast cancer Vascular Endothelial Cells. Mol. Imaging Biol. 2018, 20, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, Z.; Huang, C.; Chen, Y.; Miao, W. [99mTc]3PRGD2 for integrin receptor imaging of esophageal cancer: A comparative study with [18F]FDG PET/CT. Ann. Nucl. Med. 2019, 33, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.D.; Glover, D.K. Chapter—1 Overview of Tracer Kinetics and Cellular Mechanism of Uptake. In Clinical Nuclear Cardiology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–13. ISBN 9780323057967. [Google Scholar]
- Crișan, G.; Macea, A.M.; Andrieș, G.; Chiș, V. Experimental and computational Raman spectroscopies applied to 2-methoxy-2-methylpropylisonitrile (MIBI) ligand of the 99mTc-sestamibi radiopharmaceutical. J. Mol. Struct. 2021, 1246, 131159. [Google Scholar] [CrossRef]
- Duvall, W.L.; Case, J.; Lundbye, J.; Cerqueira, M. Efficiency of tetrofosmin versus sestamibi achieved through shorter injection-to-imaging times: A systematic review of literature. J. Nucl. Cardiol. 2021, 28, 1381–1394. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 27, 1188. [Google Scholar] [CrossRef]
- Bolzati, C.; Dolmella, A. Nitrido Technetium-99m Core in Radiopharmaceutical Applications: Four Decades of Research. Inorganics 2020, 8, 3. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, G.; Wen, Q.; Bai, L.; Chen, B.; Ji, T.; Ji, B.; Ma, Q. Pharmacokinetics and Biodistribution of 99mTc N-MPO in Healthy Human Volunteers. Clin. Nucl. Med. 2014, 39, e14–e19. [Google Scholar] [CrossRef]
- Salvarese, N.; Carta, D.; Mazano, C.; Gerardi, G.; Melendez-Alafort, L.; Bolzati, C. [99mTc][Tc(N)(DASD)(PNPn)]+ (DASD=1,4-Dioxa-8-azaspiro[4,5]decandithiocarbamate, PNPn=Bisphosphioamine) for Myocadial Imaging: Synthesis, Pharmacological and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 1114–11126. [Google Scholar] [CrossRef]
- Johnson, L.L.; Seldin, D.W. Clinical experience with technetium-99m teboroxime, a neutral, lipophilic myocardial perfusion imaging agent. Am. J. Cardiol. 1990, 66, E63–E67. [Google Scholar] [CrossRef]
- Beanlands, R.S.; DeKemp, R.A.; Harmsen, E.; Veinot, J.P.; Hartman, N.G.; Ruddy, T.D. Myocardial kinetics of technetium-99m teboroxime in the presence of post-ischemic injury, necrosis and low flow re-perfusion. J. Am. Coll. Cardiol. 1996, 28, 487–494. [Google Scholar] [CrossRef]
- Okada, D.R.; Johnson, G.; Okada, R.D. Myocardial clearance of technetium-99m-teboroxime in re-perfused injured canine myocardium. EJNMMI Res. 2014, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fang, W.; Liu, S. Novel 99mTc(III) Complexes [99mTcCl9CDO)(CDOH)2B-R] (CDOH2= Cyclohexadinone Dioxime) Useful as Radiotracers for Heart Imaging. Bioconjug. Chem. 2016, 27, 2770–2779. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S. 99mTc-3Cboroxime: A novel 99mTc(III) complex [99mTcCl(CDO)(CDOH2)B-3C] (CDOH2 = cyclohexanedione dioxime; 3C-B(OH)2 = 3-(carbamoylphenyl)boronic acid) with high heart uptake and long myocardial retention. Dalton Trans. 2017, 46, 14509–14518. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Liu, M.; Fang, W.; Liu, S. Sulfonyl-Containing Boronate Caps for Optimization of Biological Properties of 99mTc(III) Radiotracers [99mTcCl(CDO)(CDOH2)B-R] (CDOH2 = Cyclohexanedione Dioxime). J. Med. Chem. 2018, 68, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.Y.; Wang, L.; Hsu, B.; Zhao, Z.-Q.; Liu, S.; Fang, W. 99mTc-3SPboroxime: A neutral 99mTc(III) radiotracer with high heart uptake and long myocardial retention. J. Nucl. Cardiol. 2021, 28, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Bhalla, R.; Vijaya Raj, K.K.; Mandal, S.; Pickett, R.; Luthra, S.K. Radiotracers for SPECT imaging: Current scenario and future prospects. Radiochim. Acta 2012, 100, 95–107. [Google Scholar] [CrossRef]
- Valotassiou, V.; Malamitsi, J.; Papatrintafyllou, J.; Dardiotis, E.; Tsougos, I.; Psimadas, D.; Alexiou, S.; Hadjigeorgiou, G.; Georgoulias, P. SPECT and PET imaging in Alzheimer’s disease. Ann. Nucl. Med. 2018, 32, 583–593. [Google Scholar] [CrossRef]
- Chen, C.-J.; Bando, K.; Ashino, H.; Taguchi, K.; Shiraishi, H.; Shima, K.; Fujimoto, O.; Kitamura, C.; Matsushima, S.; Uchida, K.; et al. In Vivo SPECT Imaging of Amyloid-β Deposition with Radioiodinated Imidazo[1,2-a]Pyridine Derivative DRM106 in a Mouse Model of Alzheimer’s Disease. J. Nucl. Med. 2015, 56, 120–126. [Google Scholar] [CrossRef]
- Maya, Y.; Okumura, Y.; Kobayashi, R.; Onishi, T.; Shoyama, Y.; Barret, O.; Alagille, D.; Jennings, D.; Marek, K.; Seibyl, O.; et al. Preclinical properties and human in vivo assessment of 123I-ABC577 as a novel SPECT agent for imaging amyloid-β. Brain 2016, 139, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.P.; Noor, A.; Hickey, J.L.; McLean, C.A.; White, J.M.; Donnelly, P.S. Rhenium and technetium complexes of thioamide derivatives of pyridylhydrazine that bind to amyloid-β plaques. J. Biol. Inorg. Chem. 2018, 23, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Sagnou, M.; Mavroidi, B.; Shegani, A.; Paravatou-Petsotas, M.; Raptopoulou, C.; Psycharis, V.; Pirmettis, I.; Papadopoulos, M.S.; Pelecanou, M. Remarkable Brain Penetration of Cyclopentadienyl M(CO)3+ (M = 99mTc, Re) Derivatives of Benzothiazole and Benzimidazole Paves the Way for Their Application as Diagnostic, with Single-Photon-Emission Computed Tomography (SPECT), and Therapeutic Agents for Alzheimer’s Disease. J. Med. Chem. 2019, 62, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
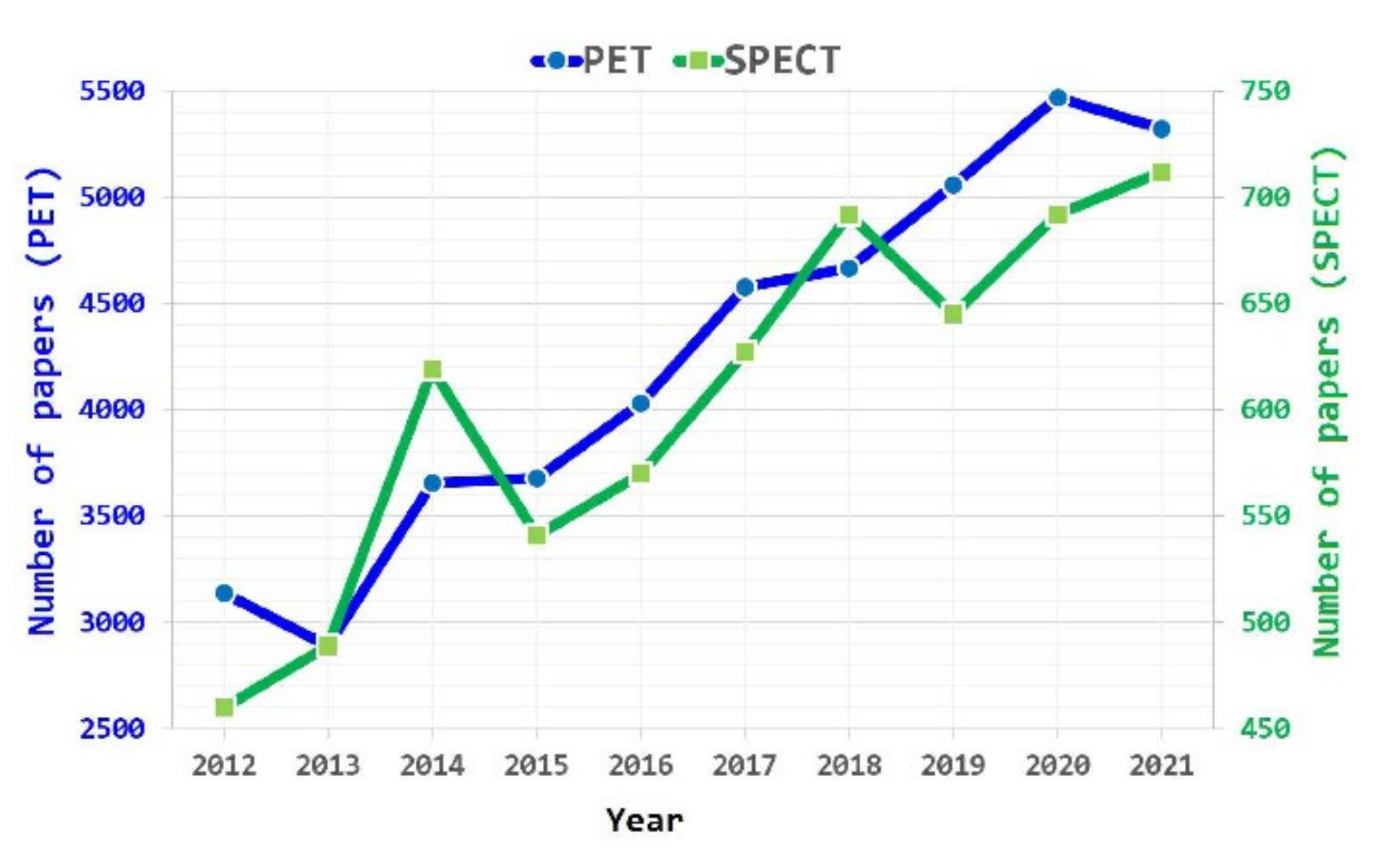
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. https://doi.org/10.3390/ijms23095023
Crișan G, Moldovean-Cioroianu NS, Timaru D-G, Andrieș G, Căinap C, Chiș V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. International Journal of Molecular Sciences. 2022; 23(9):5023. https://doi.org/10.3390/ijms23095023
Chicago/Turabian StyleCrișan, George, Nastasia Sanda Moldovean-Cioroianu, Diana-Gabriela Timaru, Gabriel Andrieș, Călin Căinap, and Vasile Chiș. 2022. "Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade" International Journal of Molecular Sciences 23, no. 9: 5023. https://doi.org/10.3390/ijms23095023
APA StyleCrișan, G., Moldovean-Cioroianu, N. S., Timaru, D.-G., Andrieș, G., Căinap, C., & Chiș, V. (2022). Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. International Journal of Molecular Sciences, 23(9), 5023. https://doi.org/10.3390/ijms23095023







