Allopregnanolone Promotes Migration and Invasion of Human Glioblastoma Cells through the Protein Tyrosine Kinase c-Src Activation
Abstract
1. Introduction
2. Results
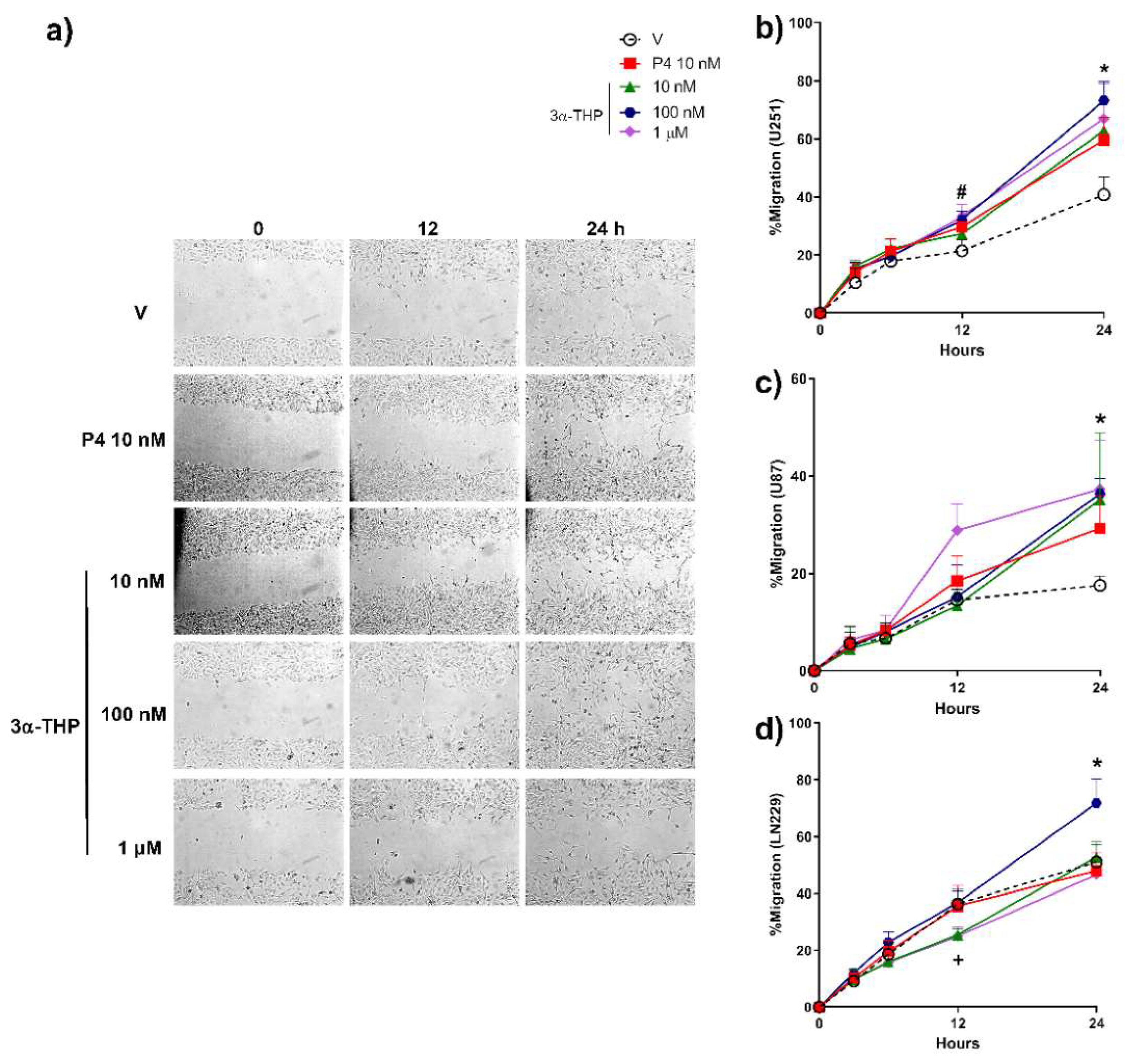
2.1. 3α-THP Promotes Migration and Invasion of Human GB Cells
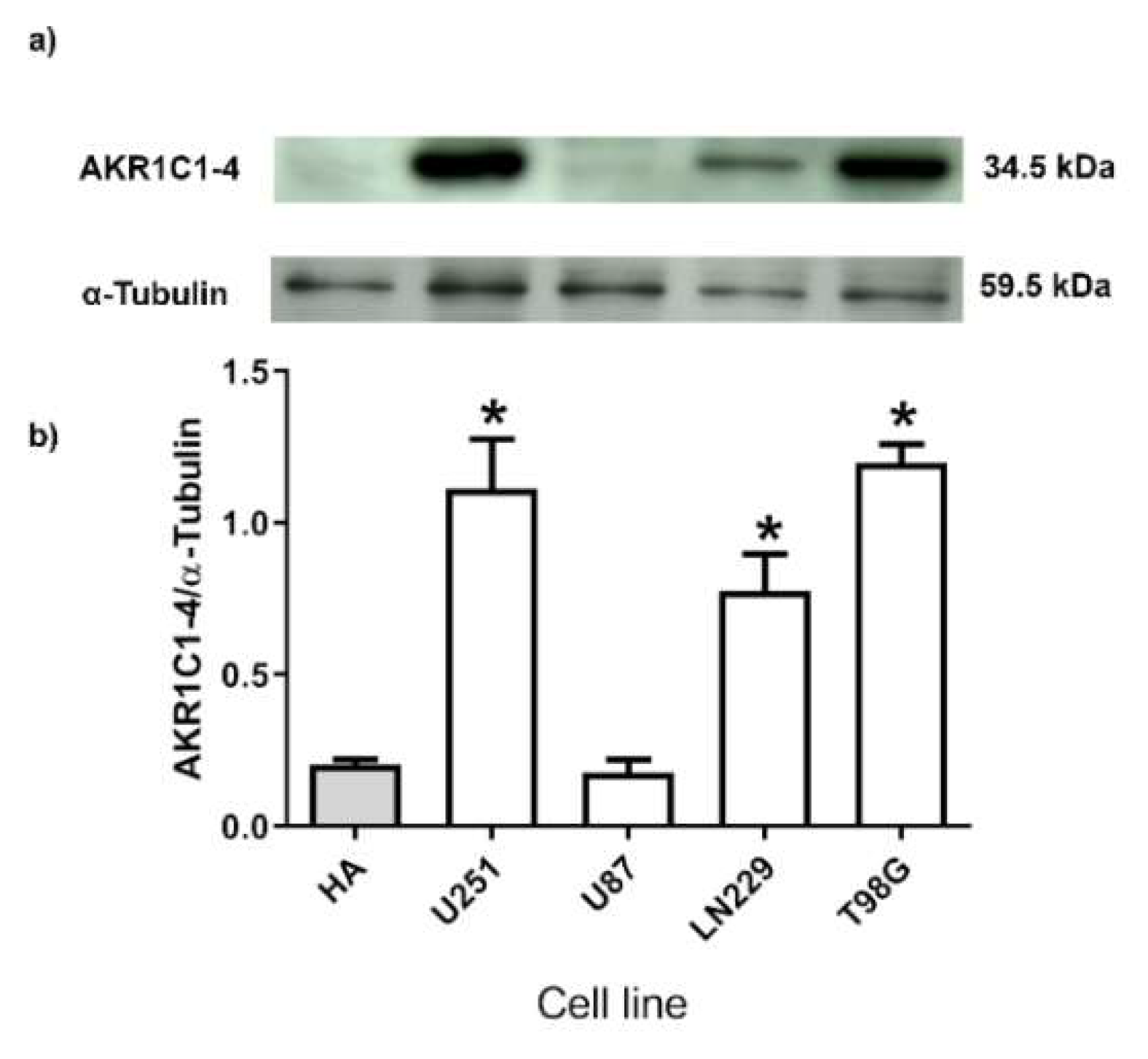
2.2. Human GB Cell Lines Express 3α-THP Metabolism Enzymes
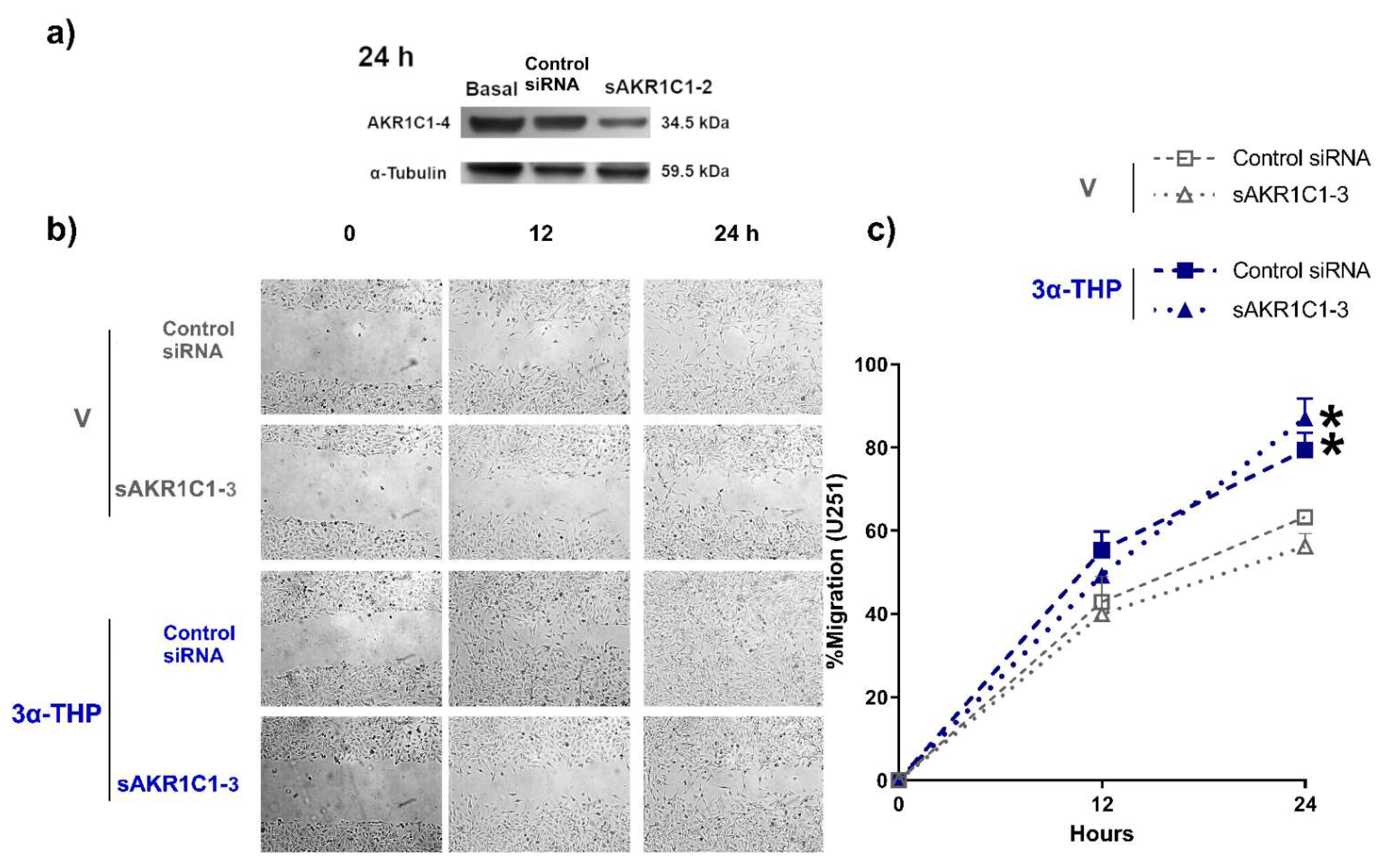
2.3. Role of AKR1C1-3 Silencing in the Effect of 3α-THP on GB Cell Migration and Invasion
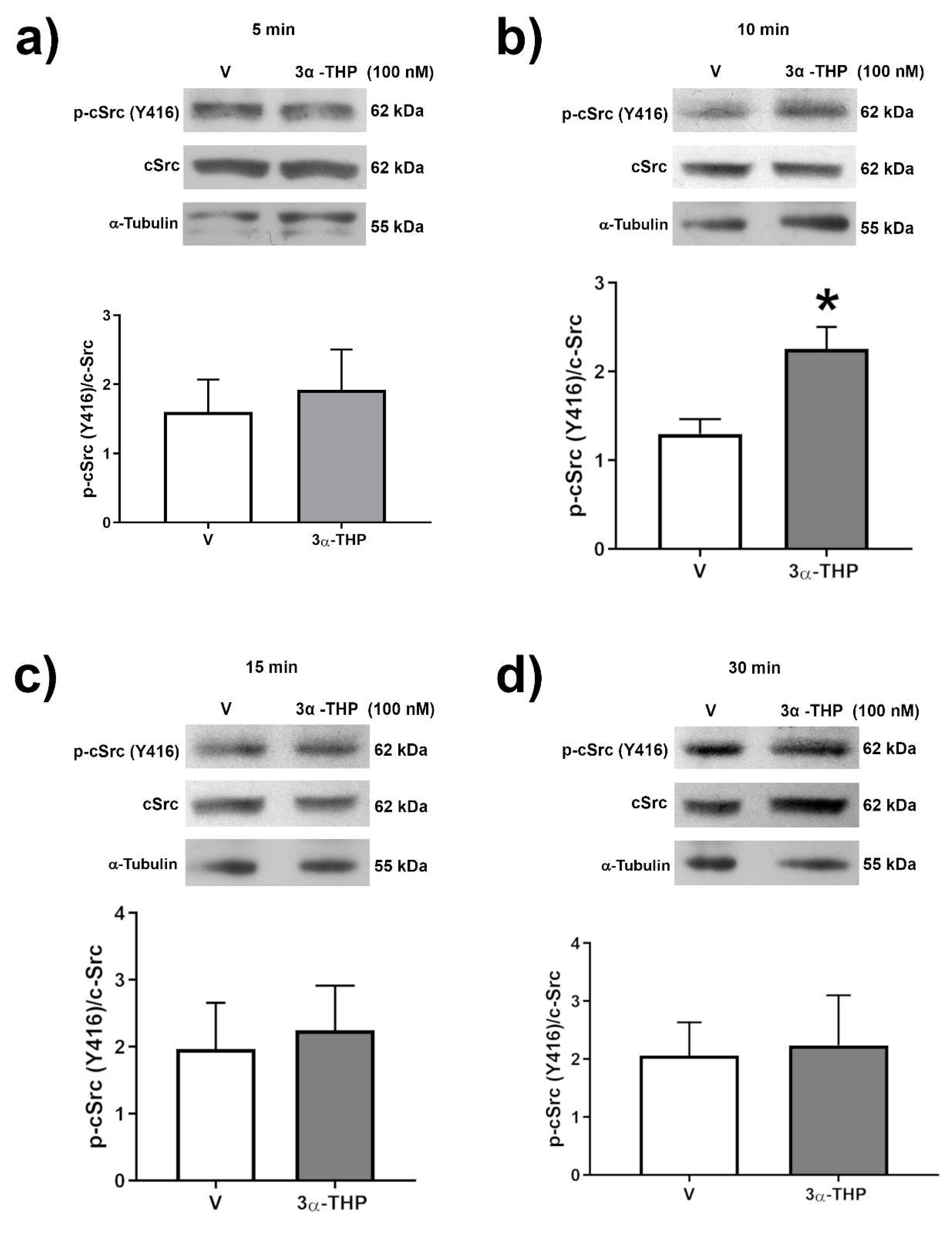
2.4. 3α-THP Promotes c-Src Activation in Human GB Cell Lines
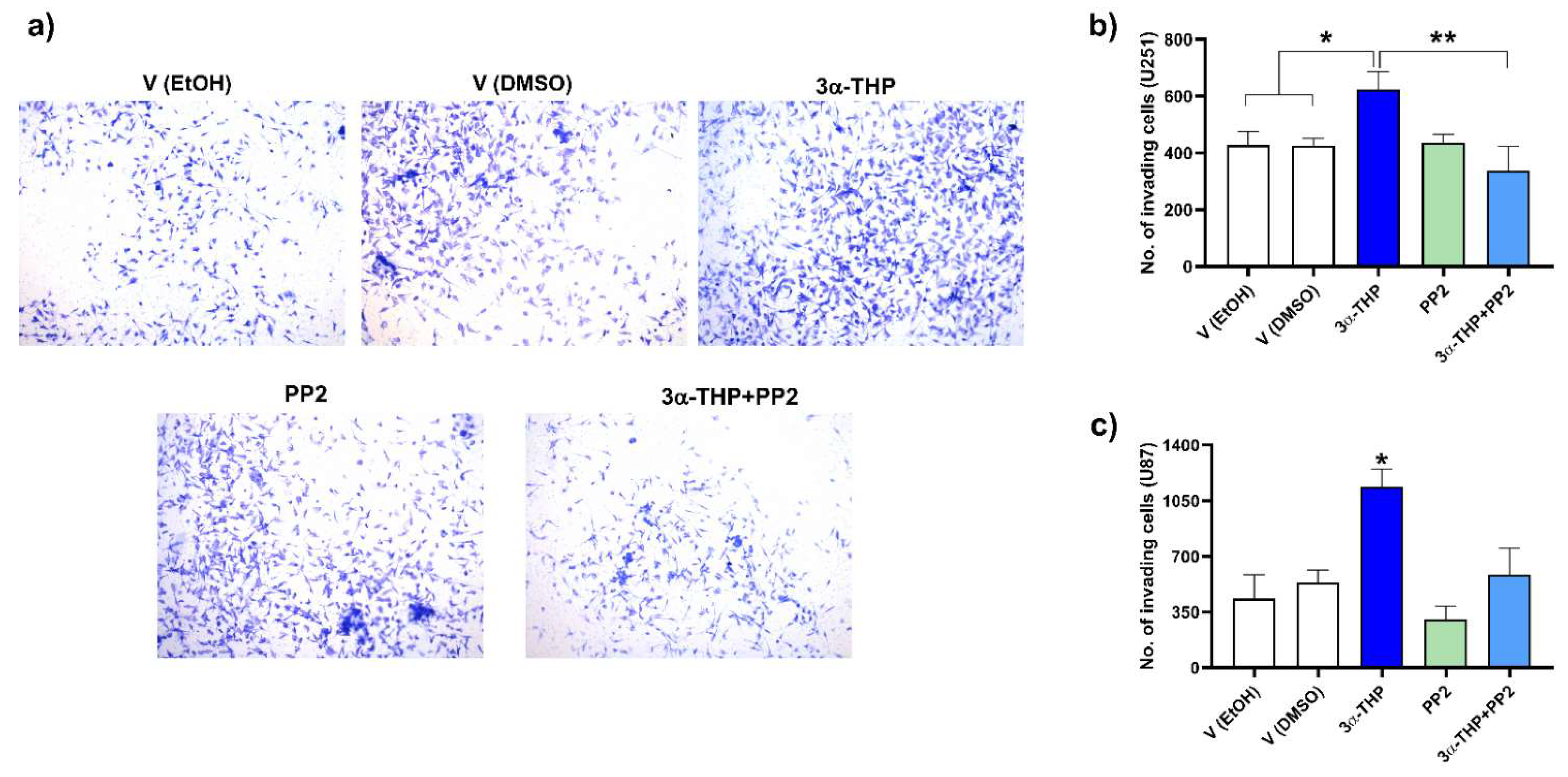
2.5. Influence of c-Src Inhibition in the Effect of 3α-THP on Cell Invasion
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Migration Assays
4.3. Invasion Assays
4.4. siRNA Silencing of AKR1C1-3
4.5. Protein Extraction and Western Blotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3α-HSD | 3α-Hydroxysteroid dehydrogenase |
| 20α-HSD | 20α-Hydroxysteroid dehydrogenase |
| 3α-THP | Allopregnanolone |
| 5α-DHP | Dihydroprogesterone |
| 5αR | 5α-Reductase |
| AKR1C1 | Aldo-keto reductase family 1 member C1 (also 20α(3α)-HSD) |
| AKR1C2 | Aldo-keto reductase family 1 member C2 (also 3α-HSD type 3) |
| AKR1C3 | Aldo-keto reductase family 1 member C3 (also 3α(17β)-HSD type 2) |
| AKR1C4 | Aldo-keto reductase family 1 member C4 (also 3α-HSD type 1) |
| AraC | cytosine β-D-arabinofuranoside |
| c-Src | SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase |
| ERK | Mitogen-activated protein kinase |
| FAK | Focal adhesion kinase |
| GABA | γ-aminobutyric acid |
| GABAAR | γ-aminobutyric acid type A receptor |
| GABABR | γ-aminobutyric acid type B receptor |
| GB | Glioblastoma |
| HA | Human astrocytes |
| mPRα | Membrane progesterone receptor α (PAQR-7) |
| mPRβ | Membrane progesterone receptor β (PAQR-8) |
| mPRγ | Membrane progesterone receptor γ (PAQR-5) |
| mPRδ | Membrane progesterone receptor δ (PAQR-6) |
| mPRε | Membrane progesterone receptor ε (PAQR-9) |
| P4 | Progesterone |
| PR | Progesterone receptor |
| sAKR1C1-3 | GB cells with silenced expression of the Aldo-keto reductase family 1 member C1-3 |
| V | Vehicle, ethanol 0.1% in the medium |
References
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.G.; Smith, T.R.; Gittleman, H.R.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995–2015. Neuro-Oncology 2020, 22, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Simińska, D.; Korbecki, J.; Kojder, K.; Kapczuk, P.; Fabiańska, M.; Gutowska, I.; Machoy-Mokrzyńska, A.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of anthropometric factors in glioblastoma multiforme—Literature review. Brain Sci. 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Ohgaki, H. Epidemiology of brain tumors. Methods Mol. Biol. 2009, 472, 323–342. [Google Scholar]
- Bello-Alvarez, C.; Camacho-Arroyo, I. Impact of sex in the prevalence and progression of glioblastomas: The role of gonadal steroid hormones. Biol. Sex Differ. 2021, 12, 28. [Google Scholar] [CrossRef]
- Germán-Castelán, L.; Manjarrez-Marmolejo, J.; González-Arenas, A.; Camacho-Arroyo, I. Intracellular Progesterone Receptor Mediates the Increase in Glioblastoma Growth Induced by Progesterone in the Rat Brain. Arch. Med. Res. 2016, 47, 419–426. [Google Scholar] [CrossRef]
- Piña-Medina, A.G.; Hansberg-Pastor, V.; González-Arenas, A.; Cerbón, M.; Camacho-Arroyo, I. Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids 2016, 105, 19–25. [Google Scholar] [CrossRef]
- Del Moral-Morales, A.; González-Orozco, J.C.; Capetillo-Velázquez, J.M.; Piña-Medina, A.G.; Camacho-Arroyo, I. The Role of mPRδ and mPRε in Human Glioblastoma Cells: Expression, Hormonal Regulation, and Possible Clinical Outcome. Horm. Cancer 2020, 11, 117–127. [Google Scholar] [CrossRef]
- Piña-Medina, A.G.; Díaz, N.F.; Molina-Hernández, A.; Mancilla-Herrera, I.; Camacho-Arroyo, I. Effects of progesterone on the cell number of gliomaspheres derived from human glioblastoma cell lines. Life Sci. 2020, 249, 117536. [Google Scholar] [CrossRef]
- Bello-Alvarez, C.; Moral-Morales, A.D.; González-Arenas, A.; Camacho-Arroyo, I. Intracellular Progesterone Receptor and cSrc Protein Working Together to Regulate the Activity of Proteins Involved in Migration and Invasion of Human Glioblastoma Cells. Front. Endocrinol. 2021, 12, 286. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Cavarretta, I.; Magnaghi, V.; Ballabio, M.; Martini, L.; Motta, M. Crosstalk between normal and tumoral brain cells. Effect on sex steroid metabolism. Endocrine 1998, 8, 65–71. [Google Scholar] [CrossRef]
- Garcia, L.M.P.; Valdez, R.A.; Navarrete, A.; Cabeza, M.; Segovia, J.; Romano, M.C. Cell line derived from glioblastoma synthesizes steroid hormone. Effect of enzyme inhibitors. Endocr. Abstr. 2018, 56, 136. [Google Scholar] [CrossRef]
- Pinacho-Garcia, L.M.; Valdez, R.A.; Navarrete, A.; Cabeza, M.; Segovia, J.; Romano, M.C. The effect of finasteride and dutasteride on the synthesis of neurosteroids by glioblastoma cells. Steroids 2020, 155, 108556. [Google Scholar] [CrossRef]
- Wiebe, J.P.; Muzia, D.; Hu, J.; Szwajcer, D.; Hill, S.A.; Seachrist, J.L. The 4-pregnene and 5α-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res. 2000, 60, 936–943. [Google Scholar]
- Wiebe, J.P.; Zhang, G.; Welch, I.; Cadieux-Pitre, H.-A.T. Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors. Breast Cancer Res. 2013, 15, R38. [Google Scholar] [CrossRef]
- Pelegrina, L.T.; de los Ángeles Sanhueza, M.; Ramona Cáceres, A.R.; Cuello-Carrión, D.; Rodriguez, C.E.; Laconi, M.R. Effect of progesterone and first evidence about allopregnanolone action on the progression of epithelial human ovarian cancer cell lines. J. Steroid Biochem. Mol. Biol. 2020, 196, 105492. [Google Scholar] [CrossRef]
- Zamora-Sánchez, C.J.; Hansberg-Pastor, V.; Salido-Guadarrama, I.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone promotes proliferation and differential gene expression in human glioblastoma cells. Steroids 2017, 119, 36–42. [Google Scholar] [CrossRef]
- Gago, N.; Akwa, Y.; Sananès, N.; Guennoun, R.; Baulieu, E.E.; El-Etr, M.; Schumacher, M. Progesterone and the oligodendroglial lineage: Stage-dependent biosynthesis and metabolism. Glia 2001, 36, 295–308. [Google Scholar] [CrossRef]
- Penning, T.M. Human hydroxysteroid dehydrogenases and pre-receptor regulation: Insights into inhibitor design and evaluation. J. Steroid Biochem. Mol. Biol. 2011, 125, 46–56. [Google Scholar] [CrossRef]
- Bixo, M.; Andersson, A.; Winblad, B.; Purdy, R.H.; Bäckström, T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997, 764, 173–178. [Google Scholar] [CrossRef]
- Agis-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Poletti, A.; Cavarretta, I.; Celotti, F.; Colciago, A.; Magnaghi, V.; Motta, M.; Negri-Cesi, P.; Martini, L. The 5α-reductase in the central nervous system: Expression and modes of control. J. Steroid Biochem. Mol. Biol. 1998, 65, 295–299. [Google Scholar] [CrossRef]
- Wang, J.M.; Johnston, P.B.; Ball, B.G.; Brinton, R.D. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J. Neurosci. 2005, 25, 4706–4718. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Ellestad, K.K.; Maingat, F.; Warren, K.G.; Han, M.H.; Steinman, L.; Baker, G.B.; Power, C. Impaired neurosteroid synthesis in multiple sclerosis. Brain 2011, 134, 2703–2721. [Google Scholar] [CrossRef]
- Melfi, S.; Guevara, M.; Bonalume, V.; Ruscica, M.; Colciago, A.; Simoncini, T.; Magnaghi, V. Src and phospho-FAK kinases are activated by allopregnanolone promoting Schwann cell motility, morphology and myelination. J. Neurochem. 2017, 141, 165–178. [Google Scholar] [CrossRef]
- Zamora-Sánchez, C.; del Moral-Morales, A.; Hernández-Vega, A.; Hansberg-Pastor, V.; Salido-Guadarrama, I.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone Alters the Gene Expression Profile of Human Glioblastoma Cells. Int. J. Mol. Sci. 2018, 19, 864. [Google Scholar] [CrossRef]
- Zamora-Sánchez, C.J.; Hernández-Vega, A.M.; Gaona-Domínguez, S.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. 5alpha-dihydroprogesterone promotes proliferation and migration of human glioblastoma cells. Steroids 2020, 163, 108708. [Google Scholar] [CrossRef]
- Torrisi, F.; Vicario, N.; Spitale, F.M.; Cammarata, F.P.; Minafra, L.; Salvatorelli, L.; Russo, G.; Cuttone, G.; Valable, S.; Gulino, R.; et al. The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance. Cancers 2020, 12, 2860. [Google Scholar] [CrossRef]
- Li, Y.; Min, W.; Li, M.; Han, G.; Dai, D.; Zhang, L.; Chen, X.; Wang, X.; Zhang, Y.; Yue, Z.; et al. Identification of hub genes and regulatory factors of glioblastoma multiforme subgroups by RNA-seq data analysis. Int. J. Mol. Med. 2016, 38, 1170. [Google Scholar] [CrossRef]
- Cirotti, C.; Contadini, C.; Barilà, D. SRC Kinase in Glioblastoma: News from an Old Acquaintance. Cancers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Penning, T.M.; Burczynski, M.E.; Jez, J.M.; Hung, C.F.; Lin, H.K.; Ma, H.; Moore, M.; Palackal, N.; Ratnam, K. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000, 351, 67–77. [Google Scholar] [CrossRef]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo–keto reductase superfamily. Biochem. J. 1997, 326, 625–636. [Google Scholar] [CrossRef]
- Zeng, C.-M.; Chang, L.-L.; Ying, M.-D.; Cao, J.; He, Q.-J.; Zhu, H.; Yang, B. Aldo-Keto Reductase AKR1C1-AKR1C4: Functions, Regulation, and Intervention for Anti-cancer Therapy. Front. Pharmacol. 2017, 8, 119. [Google Scholar] [CrossRef]
- Carrano, A.; Juarez, J.J.; Incontri, D.; Ibarra, A.; Guerrero Cazares, H. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef]
- Zwain, I.H.; Yen, S.S.C. Neurosteroidogenesis in Astrocytes, Oligodendrocytes, and Neurons of Cerebral Cortex of Rat Brain. Endocrinology 1999, 140, 3843–3852. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Jenson, A.V.; Baskin, A.M. Hijacking Sexual Immuno-Privilege in GBM—An Immuno-Evasion Strategy. Int. J. Mol. Sci. 2021, 22, 10983. [Google Scholar] [CrossRef]
- Wiebe, J.P.; Beausoleil, M.; Zhang, G.; Cialacu, V. Opposing actions of the progesterone metabolites, 5α-dihydroprogesterone (5αP) and 3α-dihydroprogesterone (3αHP) on mitosis, apoptosis, and expression of Bcl-2, Bax and p21 in human breast cell lines. J. Steroid Biochem. Mol. Biol. 2010, 118, 125–132. [Google Scholar] [CrossRef]
- Wiebe, J.P.; Rivas, M.A.; Mercogliano, M.F.; Elizalde, P.V.; Schillaci, R. Progesterone-induced stimulation of mammary tumorigenesis is due to the progesterone metabolite, 5α-dihydroprogesterone (5αP) and can be suppressed by the 5α-reductase inhibitor, finasteride. J. Steroid Biochem. Mol. Biol. 2015, 149, 27–34. [Google Scholar] [CrossRef]
- Germán-Castelán, L.; Manjarrez-Marmolejo, J.; González-Arenas, A.; González-Morán, M.G.; Camacho-Arroyo, I. Progesterone induces the growth and infiltration of human astrocytoma cells implanted in the cerebral cortex of the rat. BioMed Res. Int. 2014, 2014, 393174. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Lim, S.-W.; Lin, H.-Y.; Wang, S.-A.; Hsu, S.-P.; Kao, T.-J.; Ko, C.-Y.; Hsu, T.-I. Allopregnanolone suppresses glioblastoma survival through decreasing DPYSL3 and S100A11 expression. J. Steroid Biochem. Mol. Biol. 2022, 219, 106067. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.; Yousuf, S.; Stein, D.G. Anti-tumor effects of progesterone in human glioblastoma multiforme: Role of PI3K/Akt/mTOR signaling. J. Steroid Biochem. Mol. Biol. 2015, 146, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Celotti, F.; Castano, P.; Martini, L. Differential localization of the 5 alpha-reductase and the 3 alpha-hydroxysteroid dehydrogenase in neuronal and glial cultures. Endocrinology 1993, 132, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Pal, J.; Somasundaram, K. Elucidating the cancer-specific genetic alteration spectrum of glioblastoma derived cell lines from whole exome and RNA sequencing. Oncotarget 2015, 6, 43452–43471. [Google Scholar] [CrossRef]
- Le Calvé, B.; Rynkowski, M.; Le Mercier, M.; Bruyère, C.; Lonez, C.; Gras, T.; Haibe-Kains, B.; Bontempi, G.; Decaestecker, C.; Ruysschaert, J.-M.; et al. Long-term In Vitro Treatment of Human Glioblastoma Cells with Temozolomide Increases Resistance In Vivo through Up-regulation of GLUT Transporter and Aldo-Keto Reductase Enzyme AKR1C Expression. Neoplasia 2010, 12, 727–739. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Zhang, S.; Dong, W.; Lou, X.; Liu, S. Quantitative Evaluation of Aldo–keto Reductase Expression in Hepatocellular Carcinoma (HCC) Cell Lines. Genomics. Proteom. Bioinform. 2013, 11, 230–240. [Google Scholar] [CrossRef]
- Sinreih, M.; Anko, M.; Zukunft, S.; Adamski, J.; Rižner, T.L. Important roles of the AKR1C2 and SRD5A1 enzymes in progesterone metabolism in endometrial cancer model cell lines. Chem. Biol. Interact. 2015, 234, 297–308. [Google Scholar] [CrossRef]
- Belelli, D.; Gee, K.W. 5α-pregnan-3α,20α-diol behaves like a partial agonist in the modulation of GABA-stimulated chlride ion uptake by synaptoneurosomes. Eur. J. Pharmacol. 1989, 167, 173–176. [Google Scholar] [CrossRef]
- Hosie, A.M.; Wilkins, M.E.; Da Silva, H.M.A.; Smart, T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 2006, 444, 486–489. [Google Scholar] [CrossRef]
- Magnaghi, V.; Ballabio, M.; Consoli, A.; Lambert, J.J.; Roglio, I.; Melcangi, R.C. GABA receptor-mediated effects in the peripheral nervous system. J. Mol. Neurosci. 2006, 28, 89–102. [Google Scholar] [CrossRef]
- Magnaghi, V.; Parducz, A.; Frasca, A.; Ballabio, M.; Procacci, P.; Racagni, G.; Bonanno, G.; Fumagalli, F. GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J. Neurochem. 2010, 112, 980–990. [Google Scholar] [CrossRef]
- Cucchiara, F.; Pasqualetti, F.; Giorgi, F.S.; Danesi, R.; Bocci, G. Epileptogenesis and oncogenesis: An antineoplastic role for antiepileptic drugs in brain tumours? Pharmacol. Res. 2020, 156, 104786. [Google Scholar] [CrossRef]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ε (mPRδ and mPRε) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Membrane progesterone receptors: Evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology 2012, 96, 162–171. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P. Membrane progesterone receptor α (mPRα/PAQR7) promotes migration, proliferation and BDNF release in human Schwann cell-like differentiated adipose stem cells. Mol. Cell. Endocrinol. 2021, 531, 111298. [Google Scholar] [CrossRef]
- Frye, C.A.; Koonce, C.J.; Walf, A.A. The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats. Front. Syst. Neurosci. 2014, 8, 60. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Sánchez, C.J.; Bello-Alvarez, C.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone Promotes Migration and Invasion of Human Glioblastoma Cells through the Protein Tyrosine Kinase c-Src Activation. Int. J. Mol. Sci. 2022, 23, 4996. https://doi.org/10.3390/ijms23094996
Zamora-Sánchez CJ, Bello-Alvarez C, Rodríguez-Dorantes M, Camacho-Arroyo I. Allopregnanolone Promotes Migration and Invasion of Human Glioblastoma Cells through the Protein Tyrosine Kinase c-Src Activation. International Journal of Molecular Sciences. 2022; 23(9):4996. https://doi.org/10.3390/ijms23094996
Chicago/Turabian StyleZamora-Sánchez, Carmen J., Claudia Bello-Alvarez, Mauricio Rodríguez-Dorantes, and Ignacio Camacho-Arroyo. 2022. "Allopregnanolone Promotes Migration and Invasion of Human Glioblastoma Cells through the Protein Tyrosine Kinase c-Src Activation" International Journal of Molecular Sciences 23, no. 9: 4996. https://doi.org/10.3390/ijms23094996
APA StyleZamora-Sánchez, C. J., Bello-Alvarez, C., Rodríguez-Dorantes, M., & Camacho-Arroyo, I. (2022). Allopregnanolone Promotes Migration and Invasion of Human Glioblastoma Cells through the Protein Tyrosine Kinase c-Src Activation. International Journal of Molecular Sciences, 23(9), 4996. https://doi.org/10.3390/ijms23094996





