Detection of Myosin 1g Overexpression in Pediatric Leukemia by Novel Monoclonal Antibodies
Abstract
:1. Introduction
2. Results
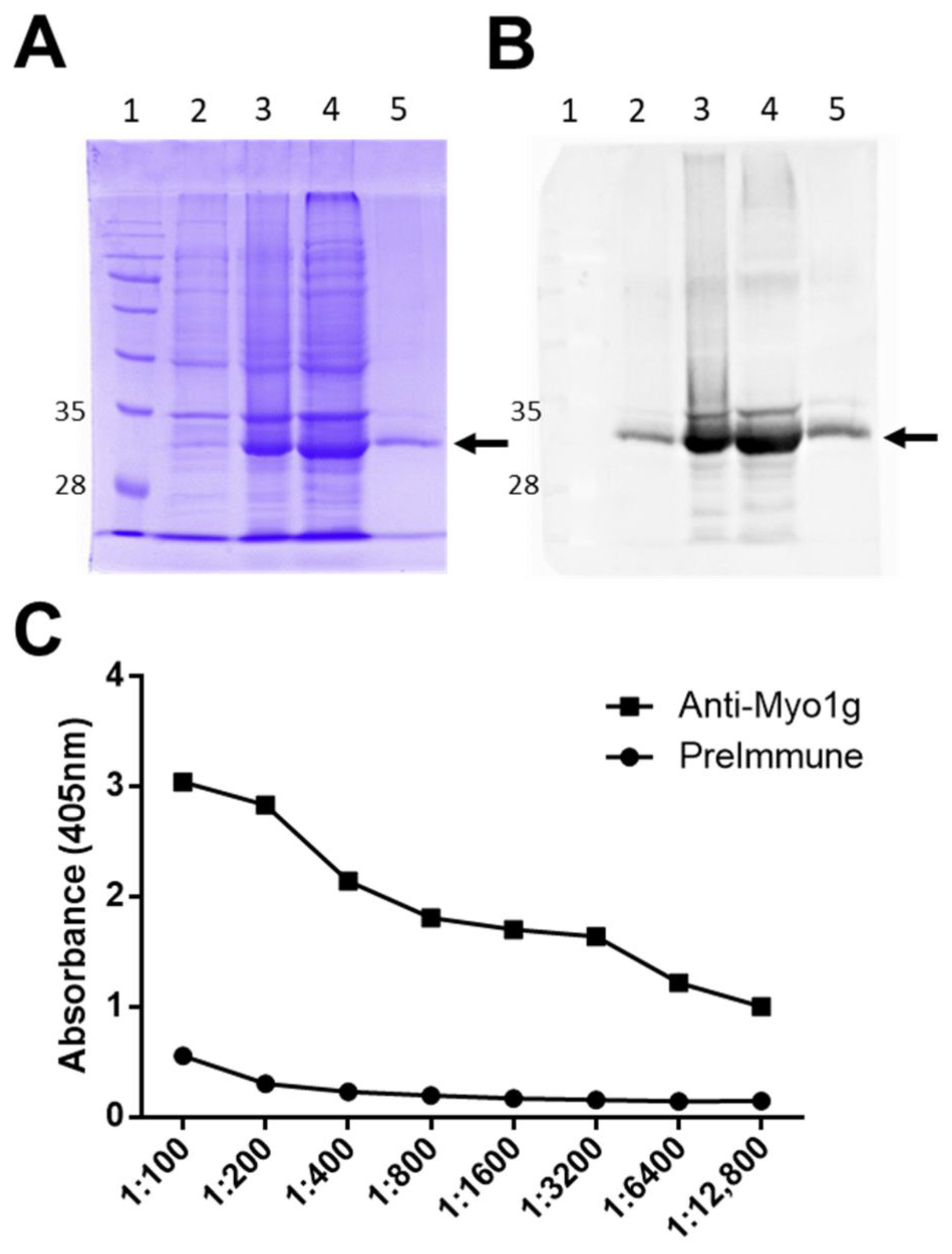
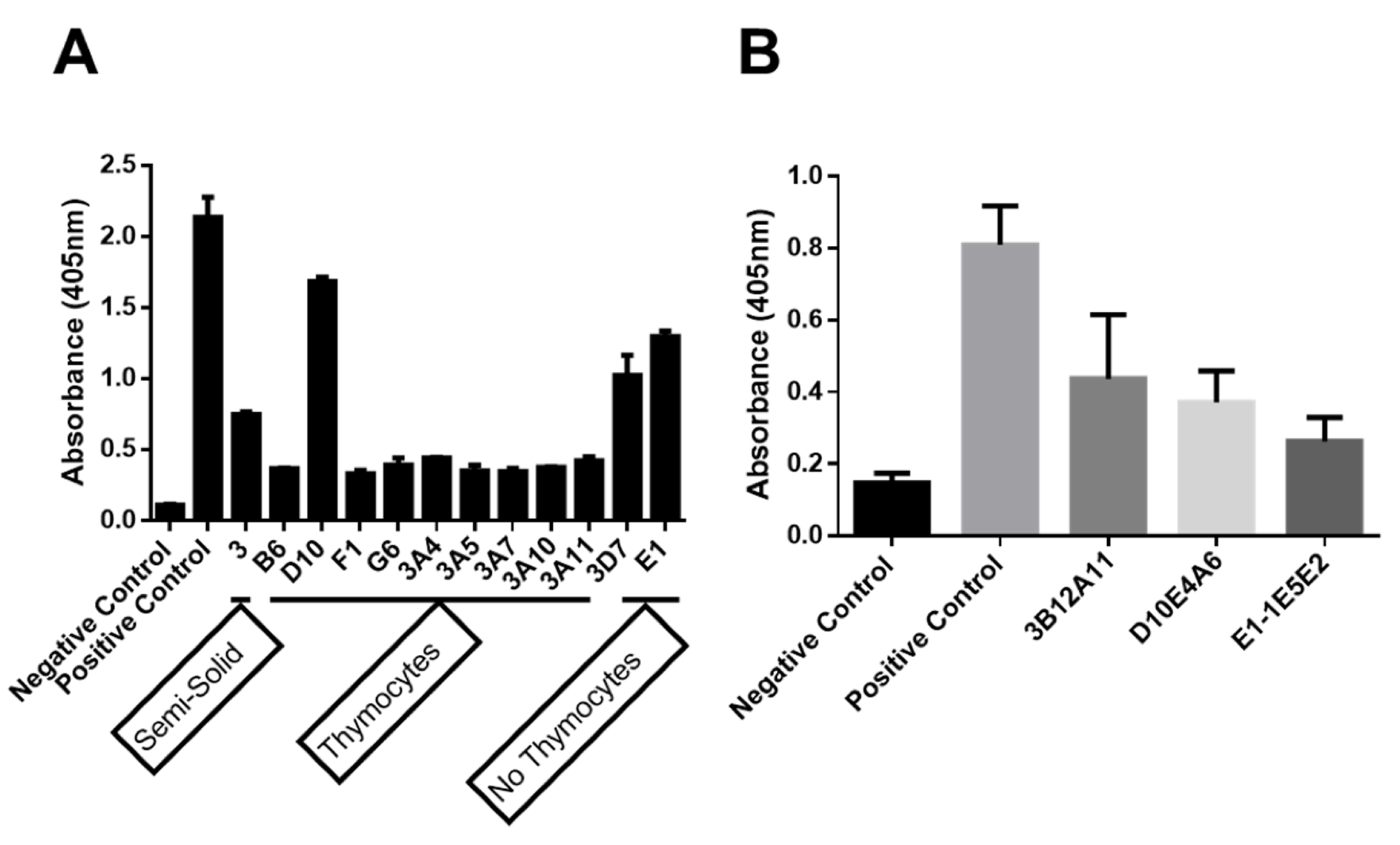
2.1. Production of Anti-Human Myo1g Monoclonal Antibodies
2.1.1. The New Monoclonal Antibodies Detect Endogenous Myo1g
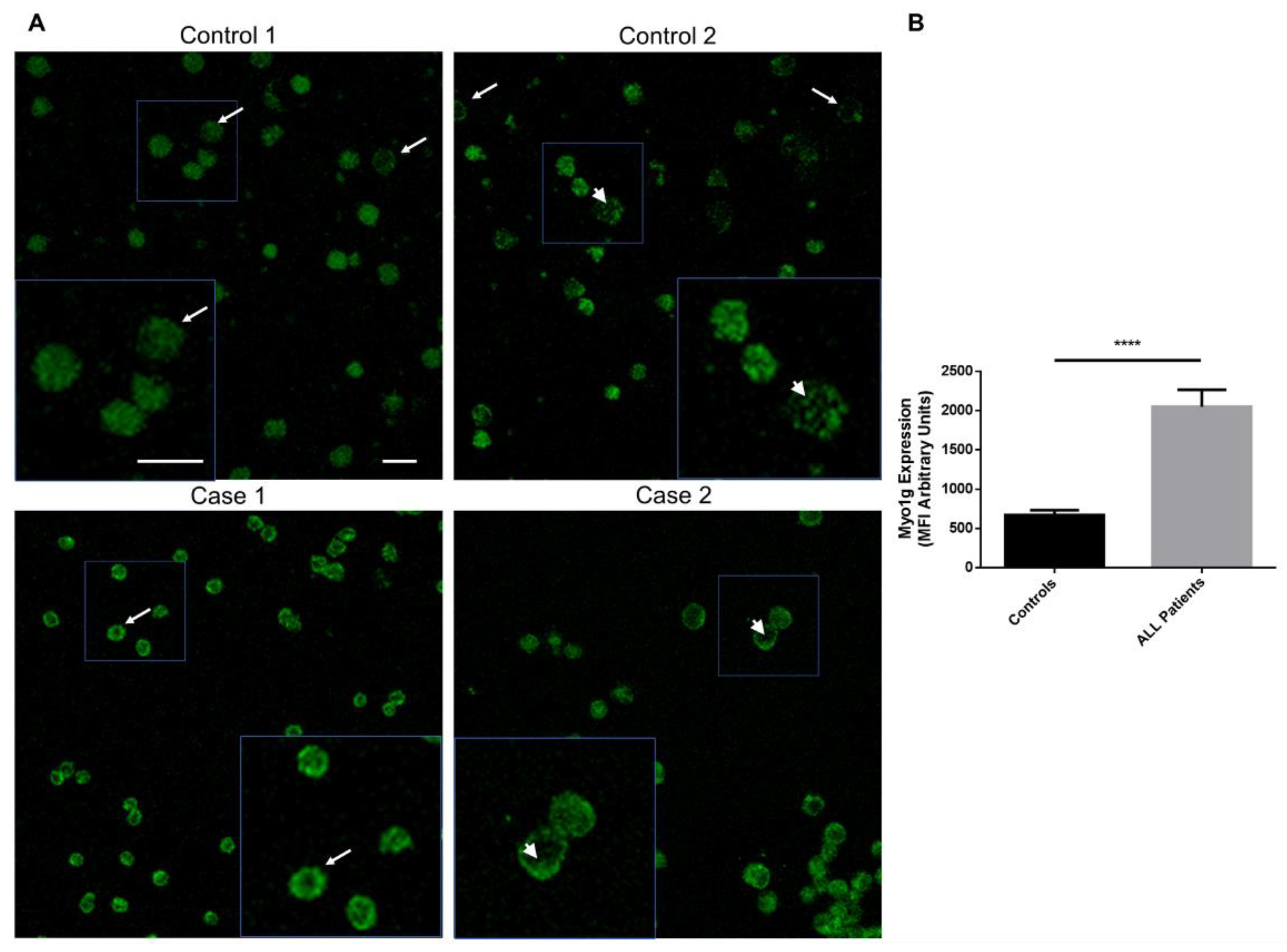
2.1.2. Myo1g Overexpression in Pediatric Patients with Acute Lymphoblastic Leukemia
3. Discussion
4. Materials and Methods
4.1. Purification of Recombinant Human Myo1g IQ-Tail Protein
4.2. Cell Lines
4.3. Mice Immunization
4.4. ELISA
4.5. Western Blot
4.6. Immunofluorescence Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheney, R.E.; Mooseker, M.S. Unconventional myosins. Curr. Opin. Cell Biol. 1992, 4, 27–35. [Google Scholar] [CrossRef]
- Olivert, T.N.; Berg, J.S.; Cheney, R.E. Tails of unconventional myosins. Cell. Mol. Life Sci. 1999, 56, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.V.; Flavell, R.A. Myosin I: From yeast to human. Cell. Mol. Life Sci. 2008, 65, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, R.; McConnell, R.E.; Tyska, M.J. Control of cell membrane tension by myosin-I. Proc. Natl. Acad. Sci. USA 2009, 106, 11972–11977. [Google Scholar] [CrossRef] [Green Version]
- Hammer, J.A. Novel myosins. Trends Cell Biol. 1991, 1, 50–56. [Google Scholar] [CrossRef]
- Adams, R.J.; Pollard, T.D. Propulsion of organelles isolated from Acanthamoeba along actin filaments by myosin-I. Nature 1986, 320, 264–265. [Google Scholar] [CrossRef]
- Hokanson, D.E.; Ostap, E.M. Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. USA 2006, 103, 3118–3123. [Google Scholar] [CrossRef] [Green Version]
- Patino-Lopez, G.; Aravind, L.; Dong, X.; Kruhlak, M.J.; Ostap, E.M.; Shaw, S. Myosin 1G is an abundant class I myosin in lymphocytes whose localization at the plasma membrane depends on its ancient divergent pleckstrin homology (PH) domain (Myo1PH). J. Biol. Chem. 2010, 285, 8675–8686. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Saldivar, M.L.; Fajardo-Gutiérrez, A.; Bernáldez-Ríos, R.; Martínez-Avalos, A.; Medina-Sanson, A.; Espinosa-Hernández, L.; Flores-Chapa, J.D.D.; Amador-Sánchez, R.; Peñaloza-González, J.G.; Álvarez-Rodríguez, F.J.; et al. Childhood acute leukemias are frequent in Mexico City: Descriptive epidemiology. BMC Cancer 2011, 11, 355. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, CA. Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, CA. Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Gerard, A.; Patino-Lopez, G.; Beemiller, P.; Nambiar, R.; Ben-Aissa, K.; Liu, Y.; Totah, F.J.; Tyska, M.J.; Shaw, S.; Krummel, M.F. Detection of rare antigen-presenting cells through T cell-intrinsic meandering motility, mediated by Myo1g. Cell 2014, 158, 492–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dart, A.E.; Tollis, S.; Bright, M.D.; Frankel, G.; Endres, R.G. The motor protein myosin 1G functions in FcγR-mediated phagocytosis. J. Cell Sci. 2012, 125, 6020–6029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Ortega, O.; Santos-Argumedo, L. Myosin 1g Contributes to CD44 Adhesion Protein and Lipid Rafts Recycling and Controls CD44 Capping and Cell Migration in B Lymphocytes. Front. Immunol. 2017, 8, e35218. [Google Scholar] [CrossRef] [Green Version]
- Maravillas-Montero, J.L.; López-Ortega, O.; Patiño-López, G.; Santos-Argumedo, L. Myosin 1g regulates cytoskeleton plasticity, cell migration, exocytosis, and endocytosis in B lymphocytes. Eur. J. Immunol. 2014, 44, 877–886. [Google Scholar] [CrossRef]
- Cruz-Zárate, D.; López-Ortega, O.; Girón-Pérez, D.A.; Gonzalez-Suarez, A.M.; García-Cordero, J.L.; Schnoor, M.; Santos-Argumedo, L. Myo1g is required for efficient adhesion and migration of activated B lymphocytes to inguinal lymph nodes. Sci. Rep. 2021, 11, 7197. [Google Scholar] [CrossRef]
- Estrada-Abreo, L.A.; Rodríguez-Cruz, L.; Garfias-Gómez, Y.; Araujo-Cardenas, J.E.; Antonio-Andrés, G.; Salgado-Aguayo, A.R.; Orozco-Ruiz, D.; Torres-Nava, J.R.; Díaz-Valencia, J.D.; Huerta-Yépez, S.; et al. High expression of Myosin 1g in pediatric acute lymphoblastic leukemia. Oncotarget 2021, 12, 1937–1945. [Google Scholar] [CrossRef]
- Ouderkirk, J.L.; Krendel, M. Non-muscle myosins in tumor progression, cancer cell invasion, and metastasis. Cytoskeleton 2014, 71, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Peckham, M. How myosin organization of the actin cytoskeleton contributes to the cancer phenotype. Biochem. Soc. Trans. 2016, 44, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-R.; Yang, W.-X. Myosins as fundamental components during tumorigenesis: Diverse and indispensable. Oncotarget 2016, 7, 46785–46812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olety, B.; Wälte, M.; Honnert, U.; Schillers, H.; Bähler, M. Myosin 1G (Myo1G) is a haematopoietic specific myosin that localises to the plasma membrane and regulates cell elasticity. FEBS Lett. 2010, 584, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Argumedo, L.; Maravillas-Montero, J.L.; López-Ortega, O. Class I myosins in B-cell physiology: Functions in spreading, immune synapses, motility, and vesicular traffic. Immunol. Rev. 2013, 256, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Krendel, M. Myosins: Tails (and Heads) of Functional Diversity. Physiology 2005, 20, 239–251. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, C.B.; Tyska, M.J.; Mooseker, M.S. Myosin at work: Motor adaptations for a variety of cellular functions. Biochim. Biophys. Acta-Mol. Cell Res. 2007, 1773, 615–630. [Google Scholar] [CrossRef] [Green Version]
- López-Ortega, O.; Ovalle-García, E.; Ortega-Blake, I.; Antillón, A.; Chávez-Munguía, B.; Patiño-López, G.; Fragoso-Soriano, R.; Santos-Argumedo, L. Myo1g is an active player in maintaining cell stiffness in B-lymphocytes. Cytoskeleton 2016, 73, 258–268. [Google Scholar] [CrossRef]
- Portell, C.A.; Advani, A.S. Novel targeted therapies in acute lymphoblastic leukemia. Leuk. Lymphoma 2014, 55, 737–748. [Google Scholar] [CrossRef]
- Meyer, L.H.; Eckhoff, S.M.; Queudeville, M.; Kraus, J.M.; Giordan, M.; Stursberg, J.; Zangrando, A.; Vendramini, E.; Möricke, A.; Zimmermann, M.; et al. Early Relapse in ALL Is Identified by Time to Leukemia in NOD/SCID Mice and Is Characterized by a Gene Signature Involving Survival Pathways. Cancer Cell 2011, 19, 206–217. [Google Scholar] [CrossRef]
- Shahid, S.; Shahid, W.; Shaheen, J.; Akhtar, M.W. Circulating miR---146a expression as a non---invasive predictive biomarker for acute lymphoblastic leukemia. Sci. Rep. 2021, 11, 22783. [Google Scholar] [CrossRef]
- Mazzolini, R.; Dopeso, H.; Mateo-Lozano, S.; Chang, W.; Rodrigues, P.; Bazzocco, S.; Alazzouzi, H.; Landolfi, S.; Hernandez-Losa, J.; Andretta, E.; et al. Brush border Myosin Ia has tumor suppressor activity in the intestine. Proc. Natl. Acad. Sci. USA 2012, 109, 1530–1535. [Google Scholar] [CrossRef] [Green Version]
- Ouderkirk-Pecone, J.L.; Goreczny, G.J.; Chase, S.E.; Tatum, A.H.; Turner, C.E.; Krendel, M. Myosin 1e promotes breast cancer malignancy by enhancing tumor cell proliferation and stimulating tumor cell de-differentiation. Oncotarget 2016, 7, 46419–46432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmura, G.; Tsujikawa, T.; Yaguchi, T.; Kawamura, N.; Mikami, S.; Sugiyama, J.; Nakamura, K.; Kobayashi, A.; Iwata, T.; Nakano, H.; et al. Aberrant Myosin 1b Expression Promotes Cell Migration and Lymph Node Metastasis of HNSCC. Mol. Cancer Res. 2015, 13, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Téllez, R.I.; Ribas-Aparicio, R.M.; Patiño-López, G. Detection of Myosin 1g Overexpression in Pediatric Leukemia by Novel Monoclonal Antibodies. Int. J. Mol. Sci. 2022, 23, 3912. https://doi.org/10.3390/ijms23073912
Rodríguez-Téllez RI, Ribas-Aparicio RM, Patiño-López G. Detection of Myosin 1g Overexpression in Pediatric Leukemia by Novel Monoclonal Antibodies. International Journal of Molecular Sciences. 2022; 23(7):3912. https://doi.org/10.3390/ijms23073912
Chicago/Turabian StyleRodríguez-Téllez, Rosa Isela, Rosa María Ribas-Aparicio, and Genaro Patiño-López. 2022. "Detection of Myosin 1g Overexpression in Pediatric Leukemia by Novel Monoclonal Antibodies" International Journal of Molecular Sciences 23, no. 7: 3912. https://doi.org/10.3390/ijms23073912
APA StyleRodríguez-Téllez, R. I., Ribas-Aparicio, R. M., & Patiño-López, G. (2022). Detection of Myosin 1g Overexpression in Pediatric Leukemia by Novel Monoclonal Antibodies. International Journal of Molecular Sciences, 23(7), 3912. https://doi.org/10.3390/ijms23073912






