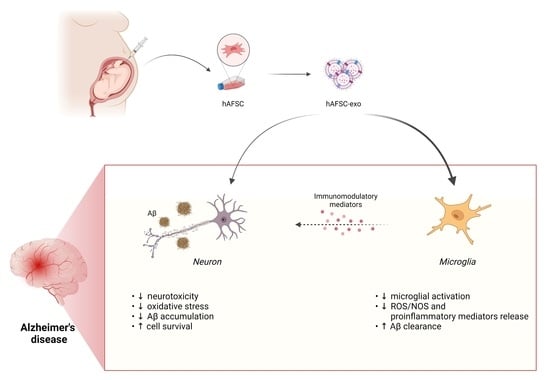
Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Aβ
Abstract
:1. Introduction
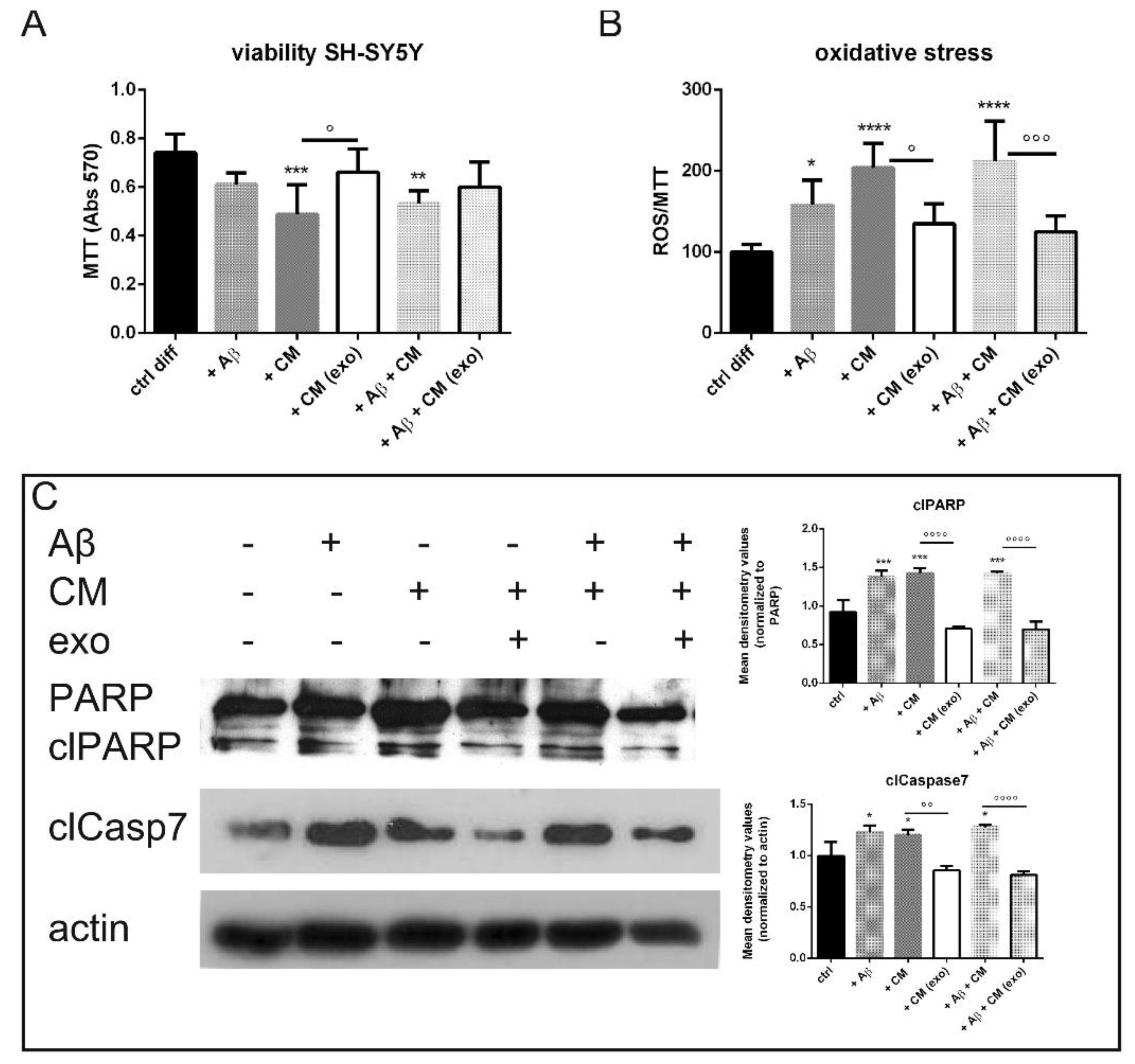
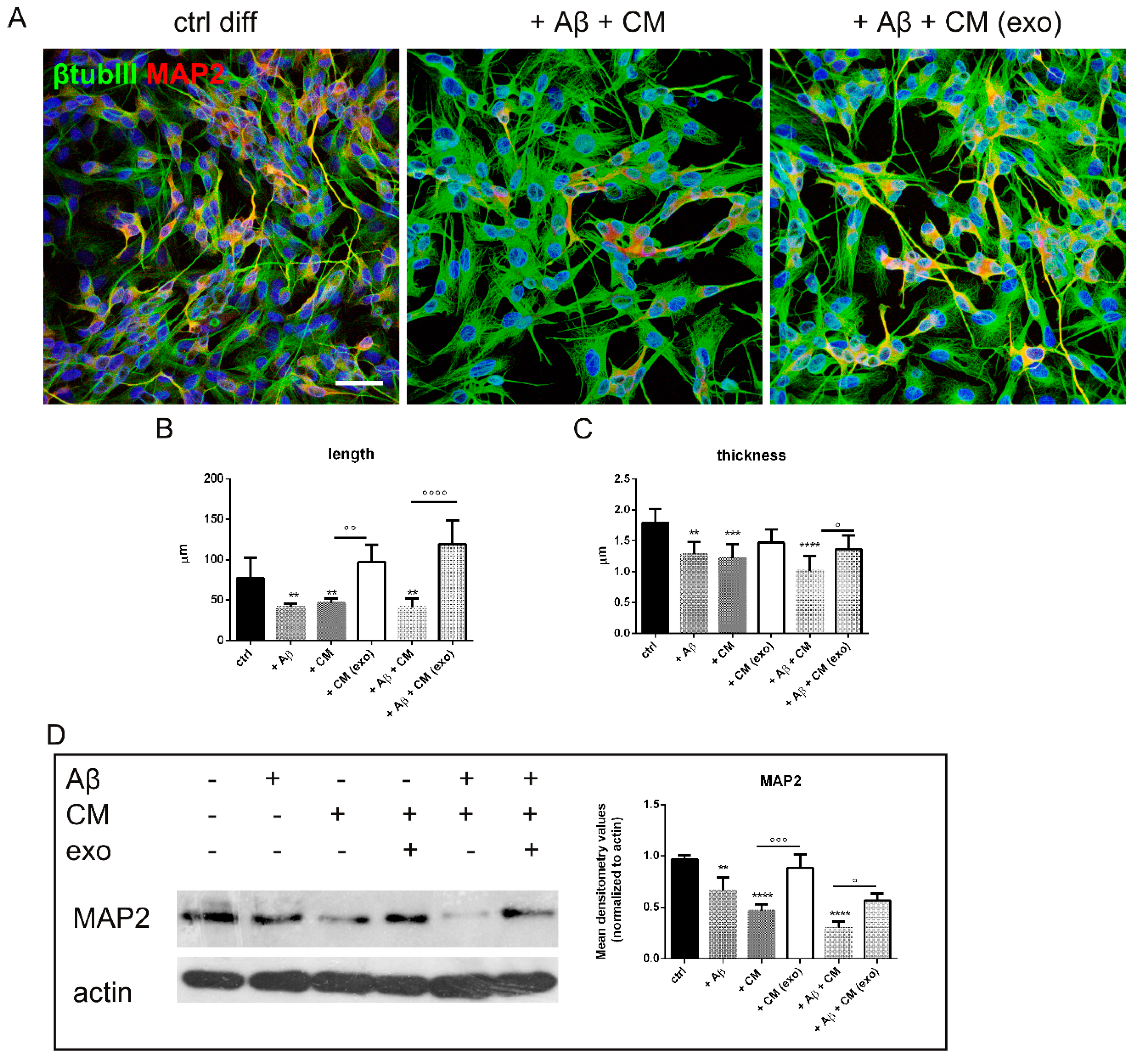
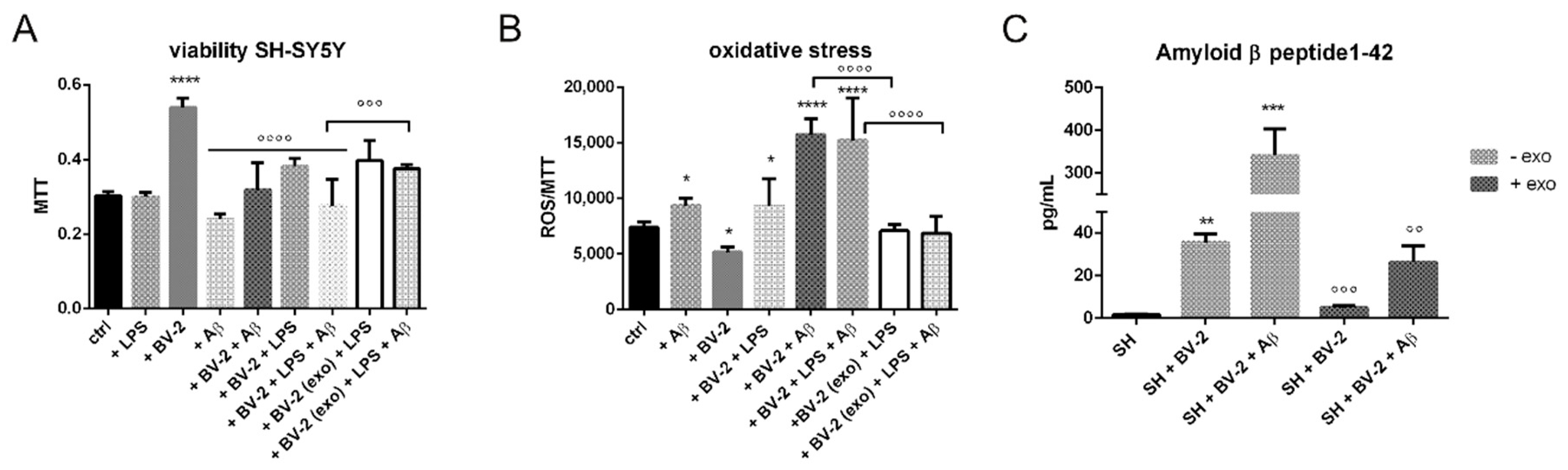
2. Results
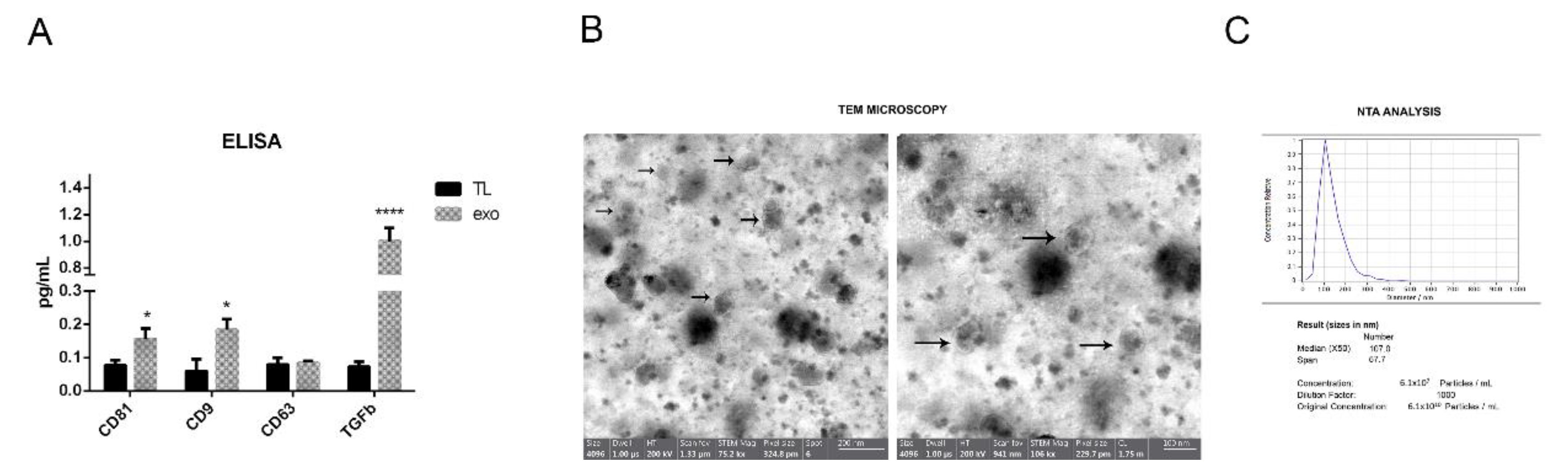
2.1. hAFSC Exosome Characterization
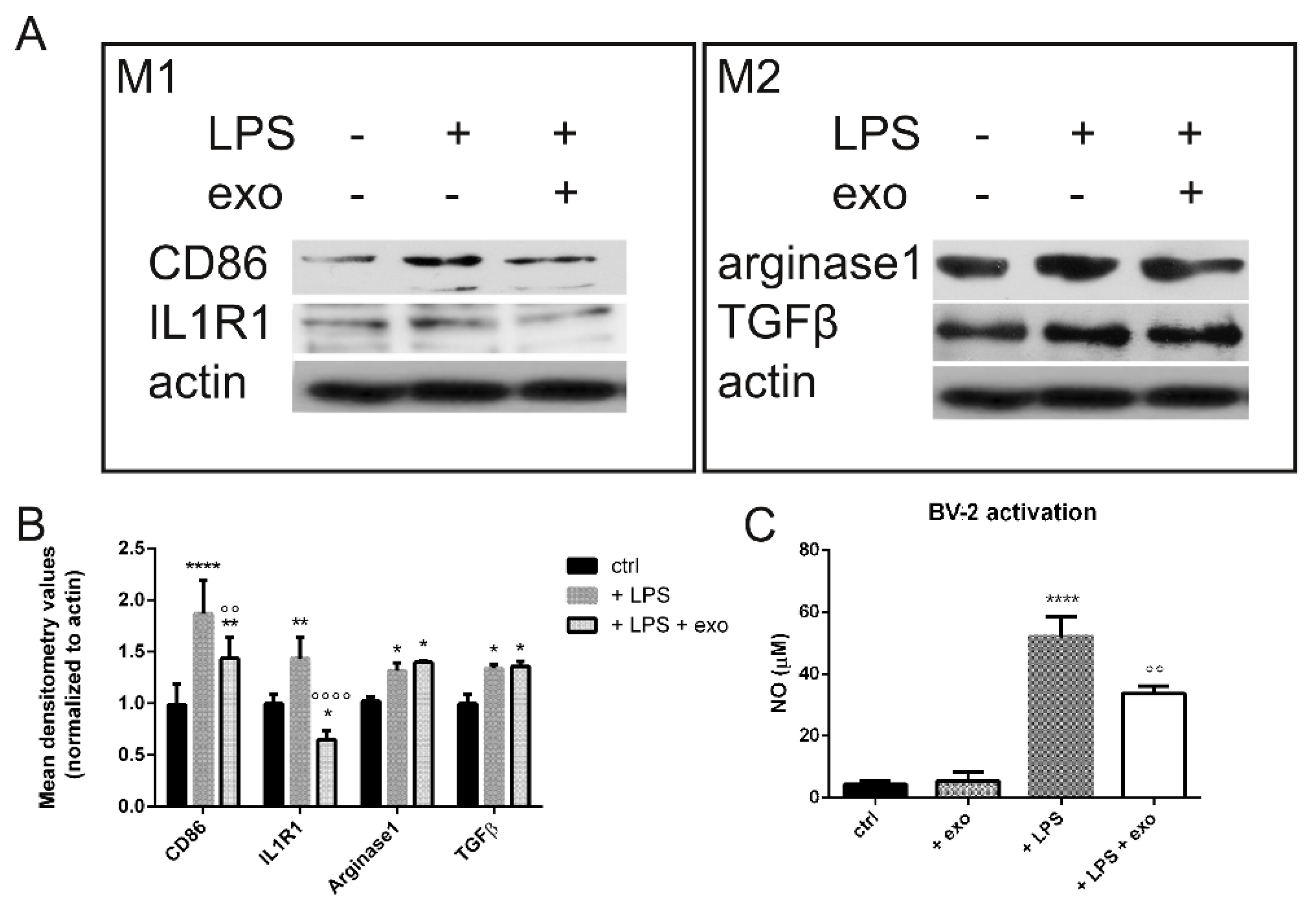
2.2. Anti-Inflammatory Activity
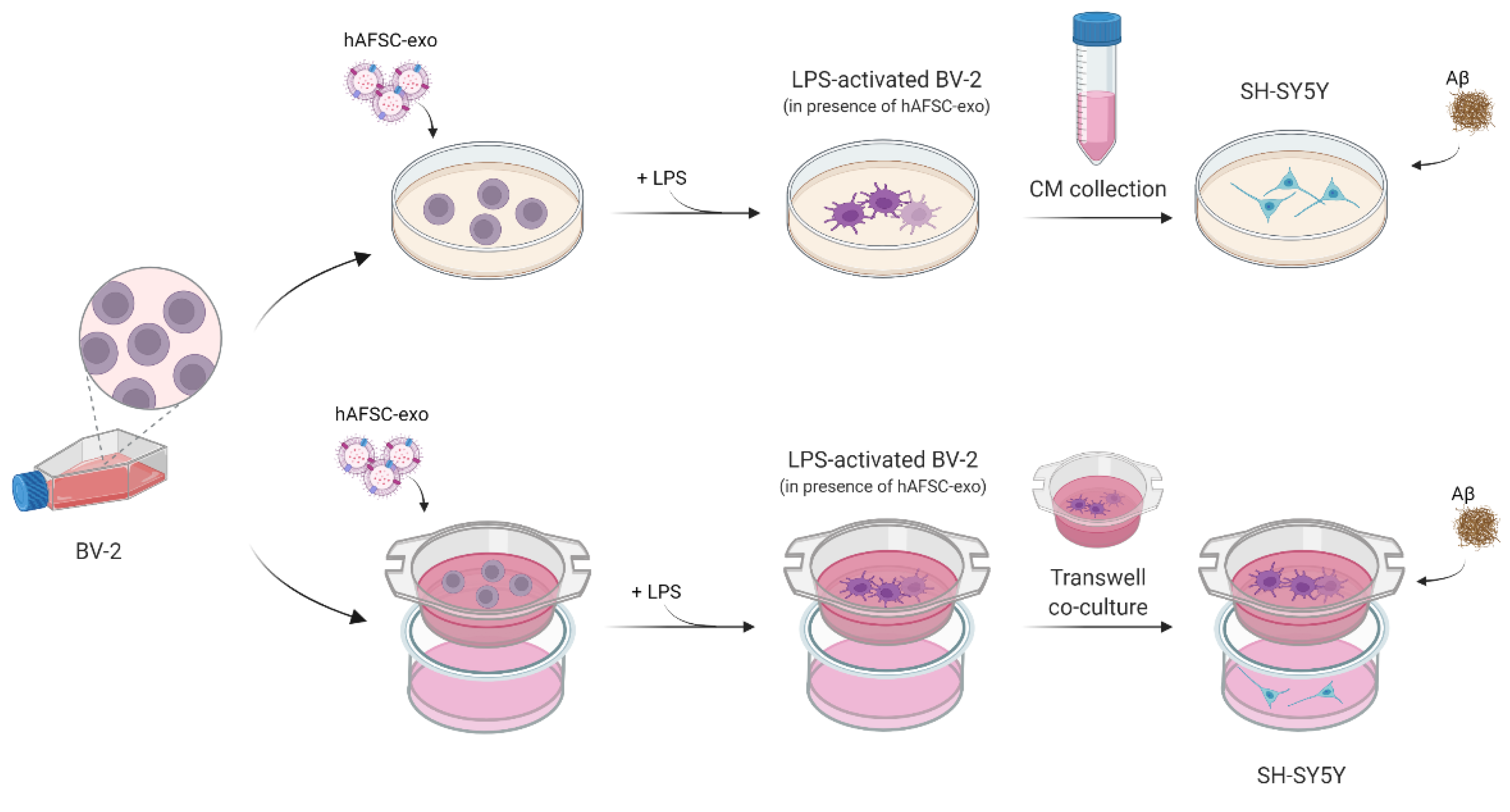
2.3. Does Exo Have a Protective Effect on the Co-Culture Systems?
3. Discussion
4. Materials and Methods
4.1. Amniotic Fluid Stem Cell Isolation
4.2. Exosome Isolation
4.3. Electron Microscopy
4.4. Cell Culture and Treatments
4.5. MTT Assay
4.6. ROS and NO Detection
4.7. Cellular Extract Preparation
4.8. SDS PAGE and Western Blot
4.9. ELISA Assays
4.10. Immunofluorescence and Confocal Microscopy
4.11. Cellular Morphology
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| CM | Conditioned medium |
| DCFH-DA | Dichlorodihydrofluorescein diacetate |
| DAPI | 4’,6-diamidino-2-phenylindole |
| EDTA | Ethylenediaminetetraacetic acid |
| HGF | Hepatocyte growth factor |
| LPS | Lipopolysaccharide |
| NO | Nitrogen species |
| NTA | Nanoparticle tracking analysis |
| PBS | Phosphate buffered saline |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulphate |
| TEM | Transmission electron microscopy |
| TL | Total lysate |
| WB | Western blot |
References
- Zhou, X.; Ashford, J.W. Advances in screening instruments for Alzheimer’s disease. AGING Med. 2019, 2, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalingam, K.B.; Radhakrishnan, A.; Ping, N.S.; Haleagrahara, N. Current Concepts of Neurodegenerative Mechanisms in Alzheimer’s Disease. Biomed. Res. Int. 2018, 2018, 3740461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitto, A.; Sell, C.; Crowe, E.; Lorenzini, A.; Malaguti, M.; Hrelia, S.; Torres, C. Stress-induced senescence in human and rodent astrocytes. Exp. Cell Res. 2010, 316, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef]
- Lee, C.Y.D.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef]
- Yang, H.; Yue, C.; Yang, H.; Xie, Z.; Hu, H.; Wei, L.; Wang, P.; Zhao, C.; Bi, J. Intravenous administration of human umbilical cord mesenchymal stem cells improves cognitive impairments and reduces amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Neurochem. Res. 2013, 38, 2474–2482. [Google Scholar] [CrossRef]
- Yokokawa, K.; Iwahara, N.; Hisahara, S.; Emoto, M.C.; Saito, T.; Suzuki, H.; Manabe, T.; Matsumura, A.; Matsushita, T.; Suzuki, S.; et al. Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress. J. Alzheimer’s Dis. 2019, 72, 867–884. [Google Scholar] [CrossRef] [Green Version]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Bodart-Santos, V.; de Carvalho, L.R.P.; de Godoy, M.A.; Batista, A.F.; Saraiva, L.M.; Lima, L.G.; Abreu, C.A.; De Felice, F.G.; Galina, A.; Mendez-Otero, R.; et al. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. Stem Cell Res. Ther. 2019, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Zavatti, M.; Beretti, F.; Giuliani, D.; Vandini, E.; Ottani, A.; Bertucci, E.; Maraldi, T. Oxidative Stress in Alzheimer’s Disease: In Vitro Therapeutic Effect of Amniotic Fluid Stem Cells Extracellular Vesicles. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef] [Green Version]
- Beretti, F.; Zavatti, M.; Casciaro, F.; Comitini, G.; Franchi, F.; Barbieri, V.; La Sala, G.B.; Maraldi, T. Amniotic fluid stem cell exosomes: Therapeutic perspective. BioFactors 2018, 44, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, H.; Gao, J. Natural Terpenoids as Neuroinflammatory Inhibitors in LPS-stimulated BV-2 Microglia. Mini Rev. Med. Chem. 2021, 21, 520–534. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Thakor, A.S. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discov. 2021, 7, 98. [Google Scholar] [CrossRef]
- Lee, D.Y.; Jin, M.S.; Manavalan, B.; Kim, H.K.; Song, J.H.; Shin, T.H.; Lee, G. Bidirectional Transcriptome Analysis of Rat Bone Marrow-Derived Mesenchymal Stem Cells and Activated Microglia in an In Vitro Coculture System. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Cargnoni, A.; Papait, A.; Masserdotti, A.; Pasotti, A.; Stefani, F.R.; Silini, A.R.; Parolini, O. Extracellular Vesicles From Perinatal Cells for Anti-inflammatory Therapy. Front. Bioeng. Biotechnol. 2021, 9, 637737. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.Y.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, P.; Bieler, L.; Scharler, C.; Pachler, K.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Mrowetz, H.; Benedetti, B.; Rivera, F.J.; et al. Extracellular Vesicles Can Deliver Anti-inflammatory and Anti-scarring Activities of Mesenchymal Stromal Cells After Spinal Cord Injury. Front. Neurol. 2019, 10, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Kim, D.H.; Lim, H.; Lee, D.; Choi, S.J.; Oh, W.; Yang, Y.S.; Oh, J.S.; Hwang, H.H.; Jeon, H.B. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Sci. Rep. 2018, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Cho, K.R.; Jang, H.; Lee, N.K.; Jung, Y.H.; Kim, J.P.; Lee, J.I.; Chang, J.W.; Park, S.; Kim, S.T.; et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase I clinical trial. Alzheimer’s Res. Ther. 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Spittau, B.; Dokalis, N.; Prinz, M. The Role of TGFβ Signaling in Microglia Maturation and Activation. Trends Immunol. 2020, 41, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Teng, Z.; Liu, C.; Li, Q.; Yin, Y.; Tang, Y. TREM2, microglia, and Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111438. [Google Scholar] [CrossRef]
- Yeh, F.L.; Wang, Y.; Tom, I.; Gonzalez, L.C.; Sheng, M. TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron 2016, 91, 328–340. [Google Scholar] [CrossRef]
- Andreone, B.J.; Przybyla, L.; Llapashtica, C.; Rana, A.; Davis, S.S.; van Lengerich, B.; Lin, K.; Shi, J.; Mei, Y.; Astarita, G.; et al. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat. Neurosci. 2020, 23, 927–938. [Google Scholar] [CrossRef]
- Guida, M.; Maraldi, T.; Resca, E.; Beretti, F.; Zavatti, M.; Bertoni, L.; La Sala, G.B.; De Pol, A. Inhibition of Nuclear Nox4 Activity by Plumbagin: Effect on Proliferative Capacity in Human Amniotic Stem Cells. Oxid. Med. Cell. Longev. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors 2019, 46, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Zambonin, L.; Rizzo, B.; Maraldi, T.; Angeloni, C.; Dalla Sega, F.V.; Fiorentini, D.; Hrelia, S. Glycosides from Stevia rebaudiana Bertoni Possess Insulin-Mimetic and Antioxidant Activities in Rat Cardiac Fibroblasts. Oxid. Med. Cell. Longev. 2017, 2017, 3724545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, C.; Maraldi, T.; Zambonin, L.; Fiorentini, D.; Hakim, G.; Landi, L. ROS production and Glut1 activity in two human megakaryocytic cell lines. Biofactors 2004, 20, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Maraldi, T.; Beretti, F.; Follo, M.Y.; Manzoli, L.; De Pol, A. Nuclear Nox4-derived reactive oxygen species in myelodysplastic syndromes. BioMed Res. Int. 2014, 2014, 456937. [Google Scholar] [CrossRef]
- Maraldi, T.; Guida, M.; Zavatti, M.; Resca, E.; Bertoni, L.; La Sala, G.B.; De Pol, A. Nuclear Nox4 Role in Stemness Power of Human Amniotic Fluid Stem Cells. Oxid. Med. Cell. Longev. 2015, 2015, 101304. [Google Scholar] [CrossRef] [Green Version]
- Zavatti, M.; Bertoni, L.; Maraldi, T.; Resca, E.; Beretti, F.; Guida, M.; La Sala, G.B.; De Pol, A. Critical-size bone defect repair using amniotic fluid stem cell/collagen constructs: Effect of oral ferutinin treatment in rats. Life Sci. 2015, 121, 174–183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavatti, M.; Gatti, M.; Beretti, F.; Palumbo, C.; Maraldi, T. Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Aβ. Int. J. Mol. Sci. 2022, 23, 4967. https://doi.org/10.3390/ijms23094967
Zavatti M, Gatti M, Beretti F, Palumbo C, Maraldi T. Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Aβ. International Journal of Molecular Sciences. 2022; 23(9):4967. https://doi.org/10.3390/ijms23094967
Chicago/Turabian StyleZavatti, Manuela, Martina Gatti, Francesca Beretti, Carla Palumbo, and Tullia Maraldi. 2022. "Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Aβ" International Journal of Molecular Sciences 23, no. 9: 4967. https://doi.org/10.3390/ijms23094967
APA StyleZavatti, M., Gatti, M., Beretti, F., Palumbo, C., & Maraldi, T. (2022). Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Aβ. International Journal of Molecular Sciences, 23(9), 4967. https://doi.org/10.3390/ijms23094967