Low-Dose SARS-CoV-2 S-Trimer with an Emulsion Adjuvant Induced Th1-Biased Protective Immunity
Abstract
:1. Introduction
2. Results
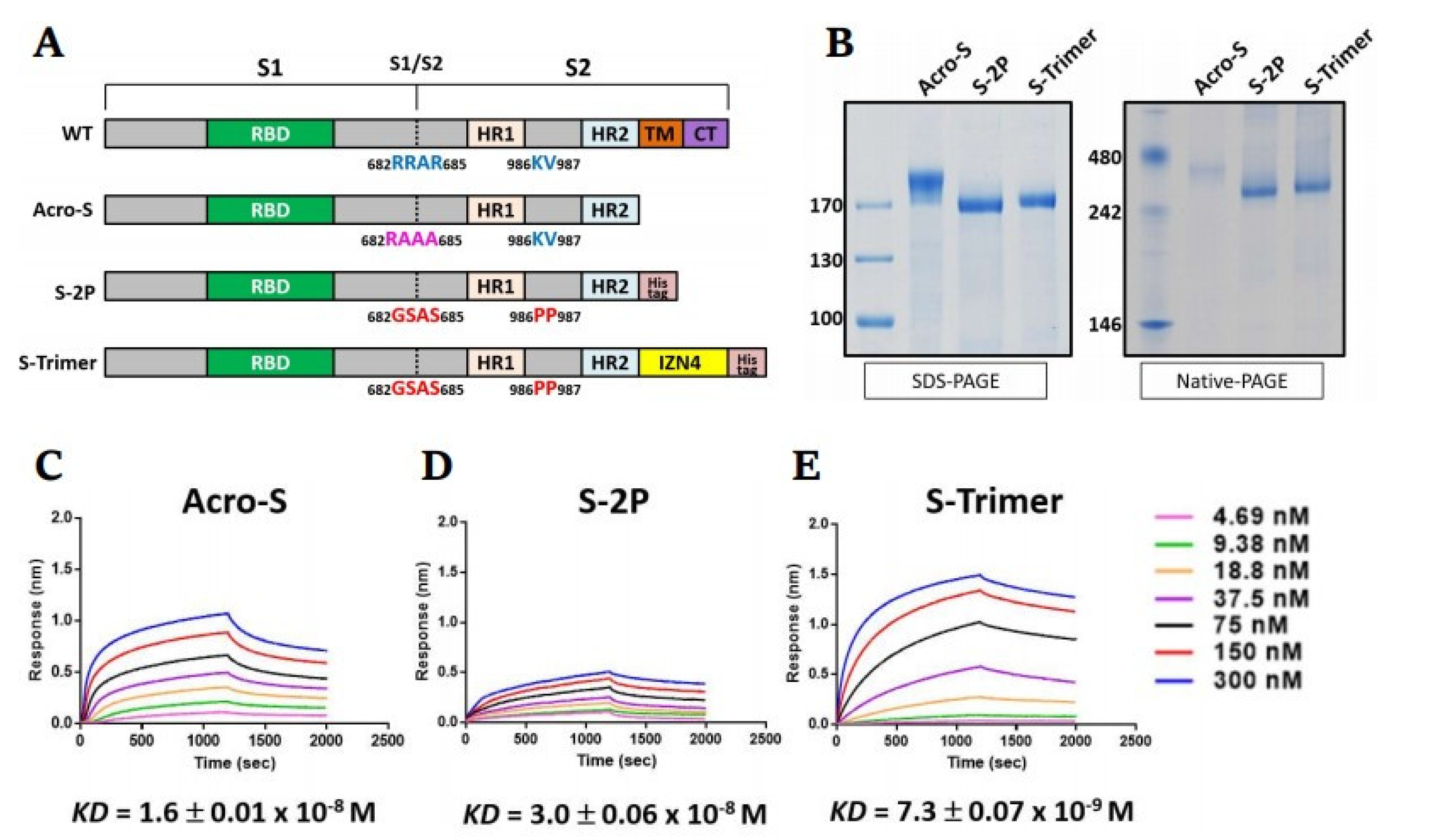
2.1. Design and Preparation of a Trimeric SARS-CoV-2 Spike Antigen
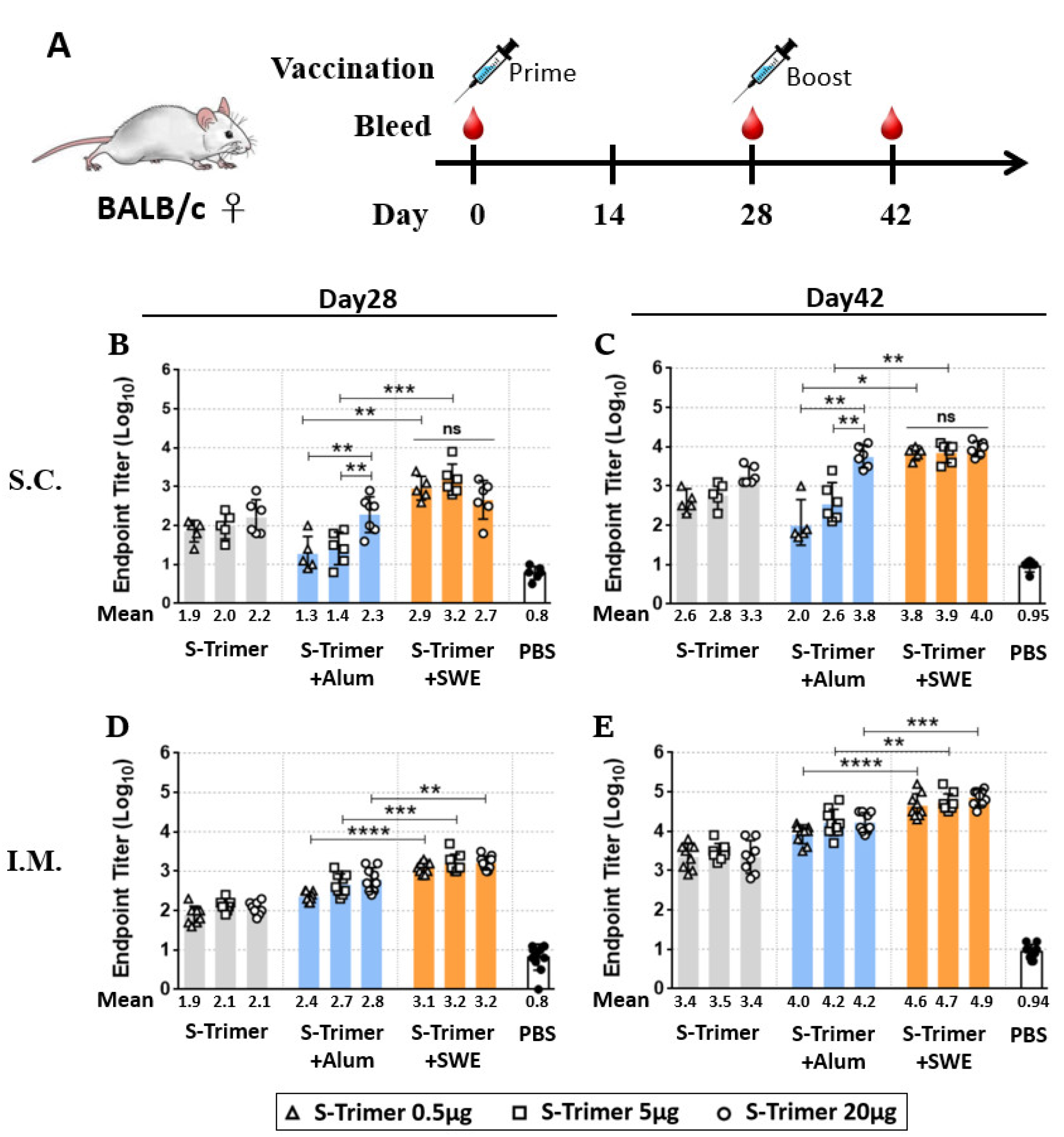
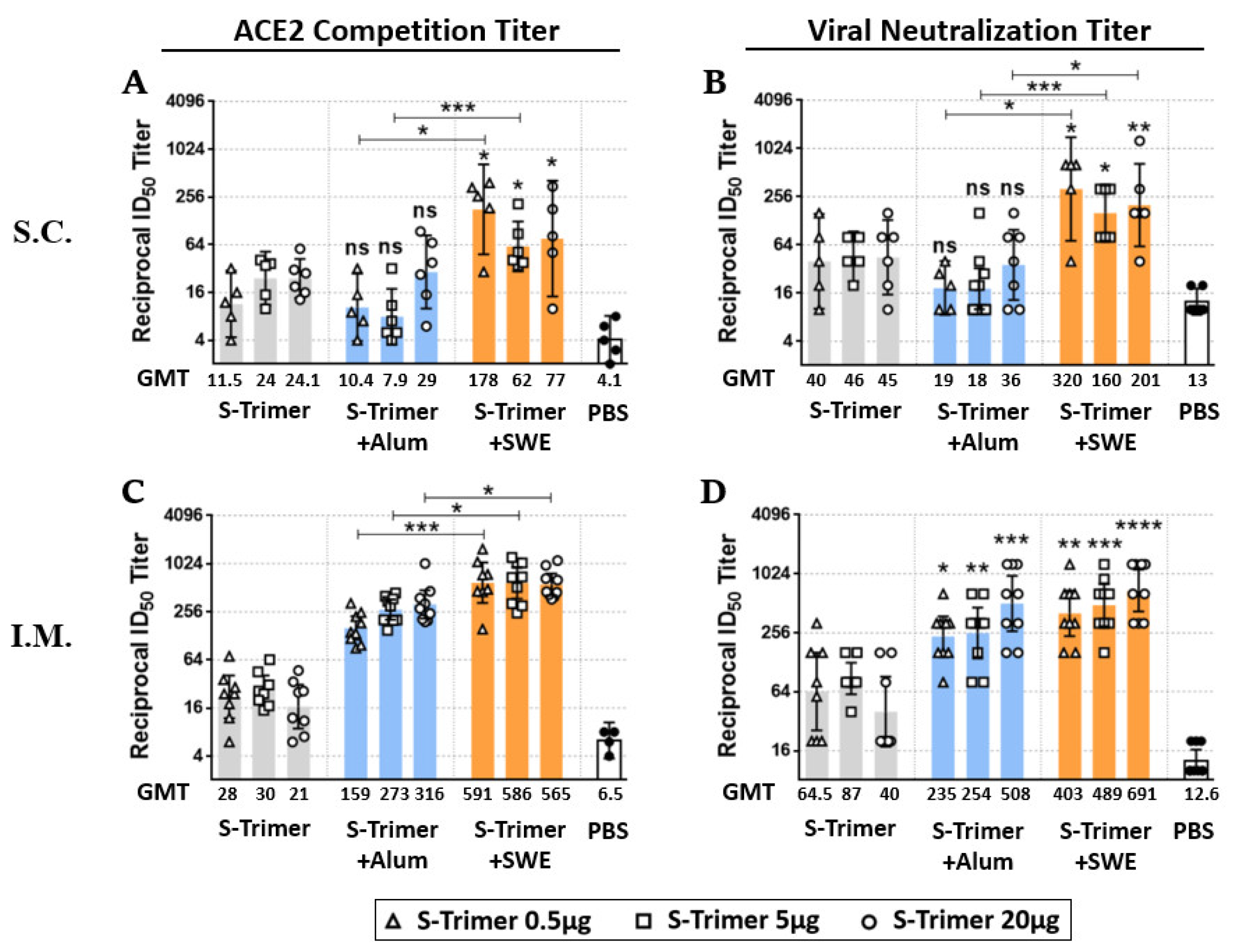
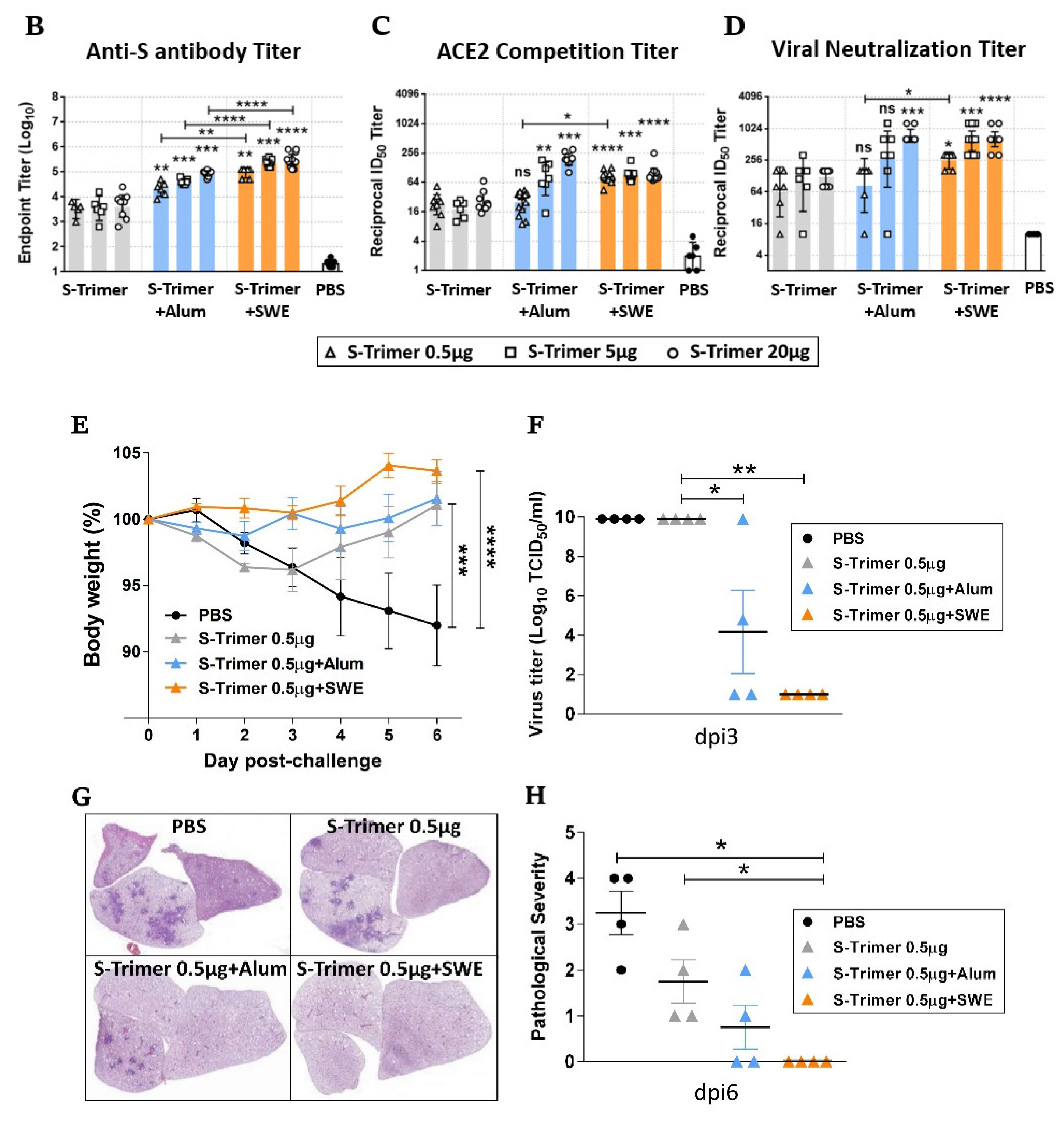
2.2. Vaccination of S-Trimer with SWE Elicits Robust Humoral Immunity in Mice
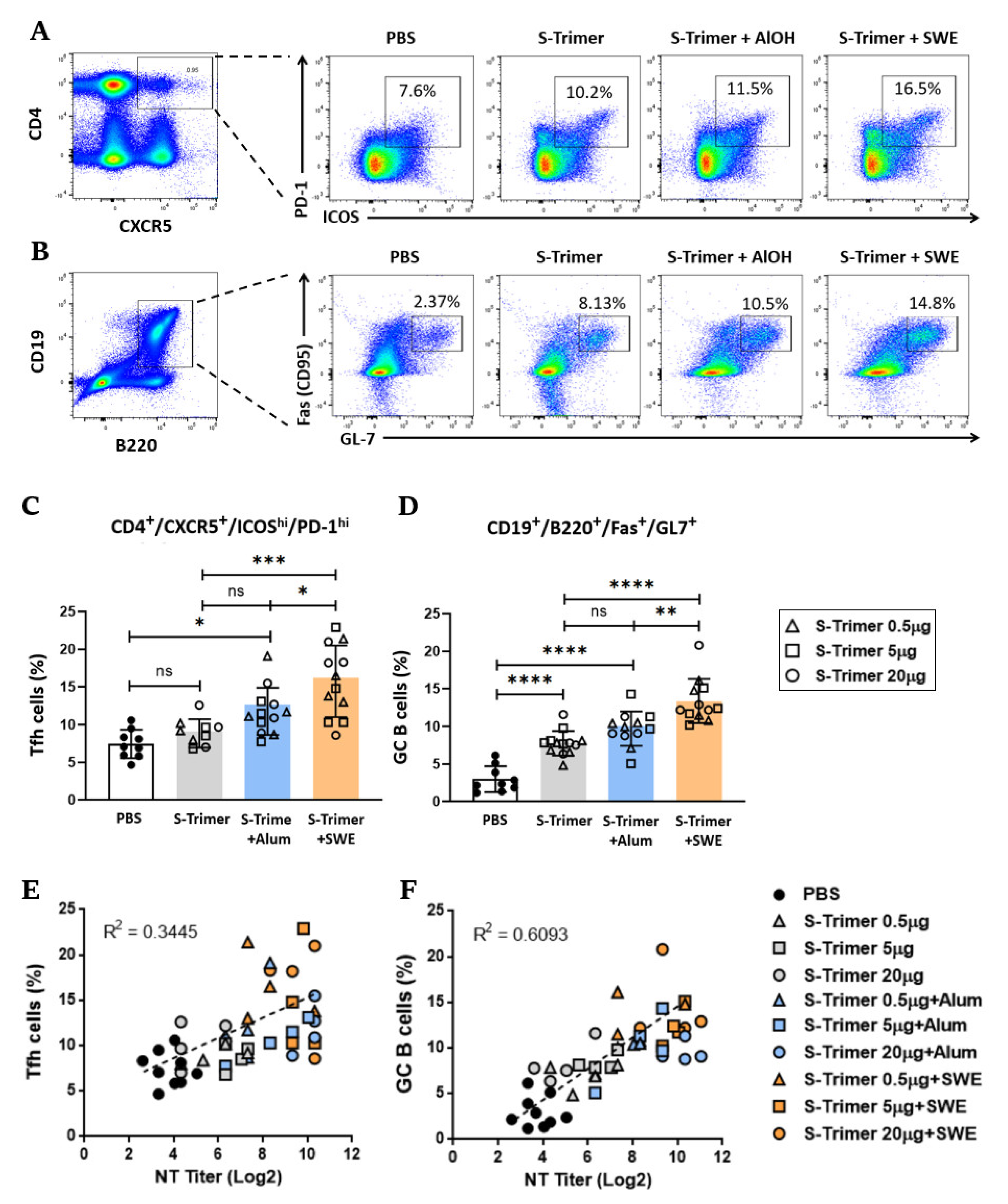
2.3. SWE Adjuvanticity Is Associated with the Induction of Tfh Cells and GC B Cells
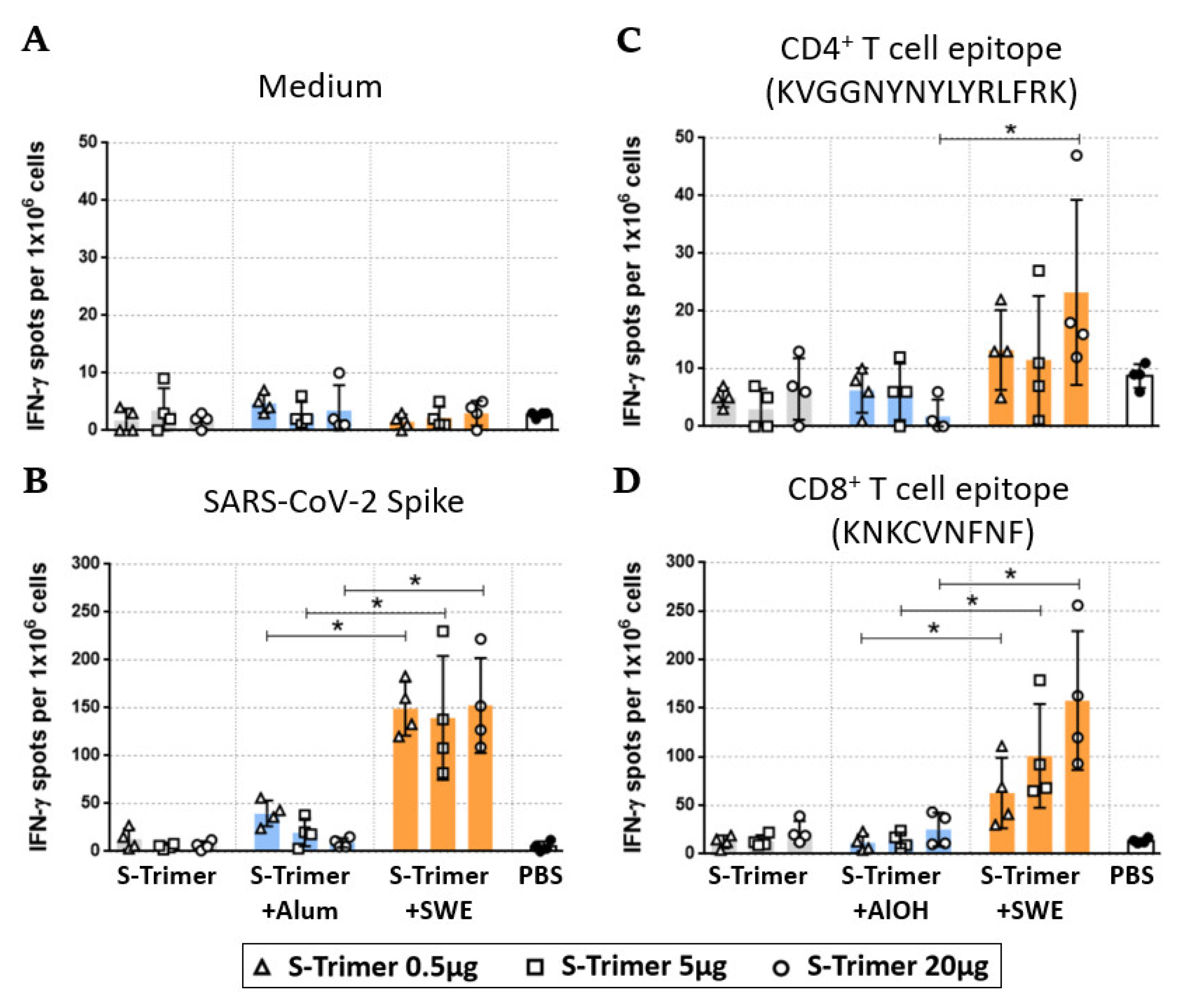
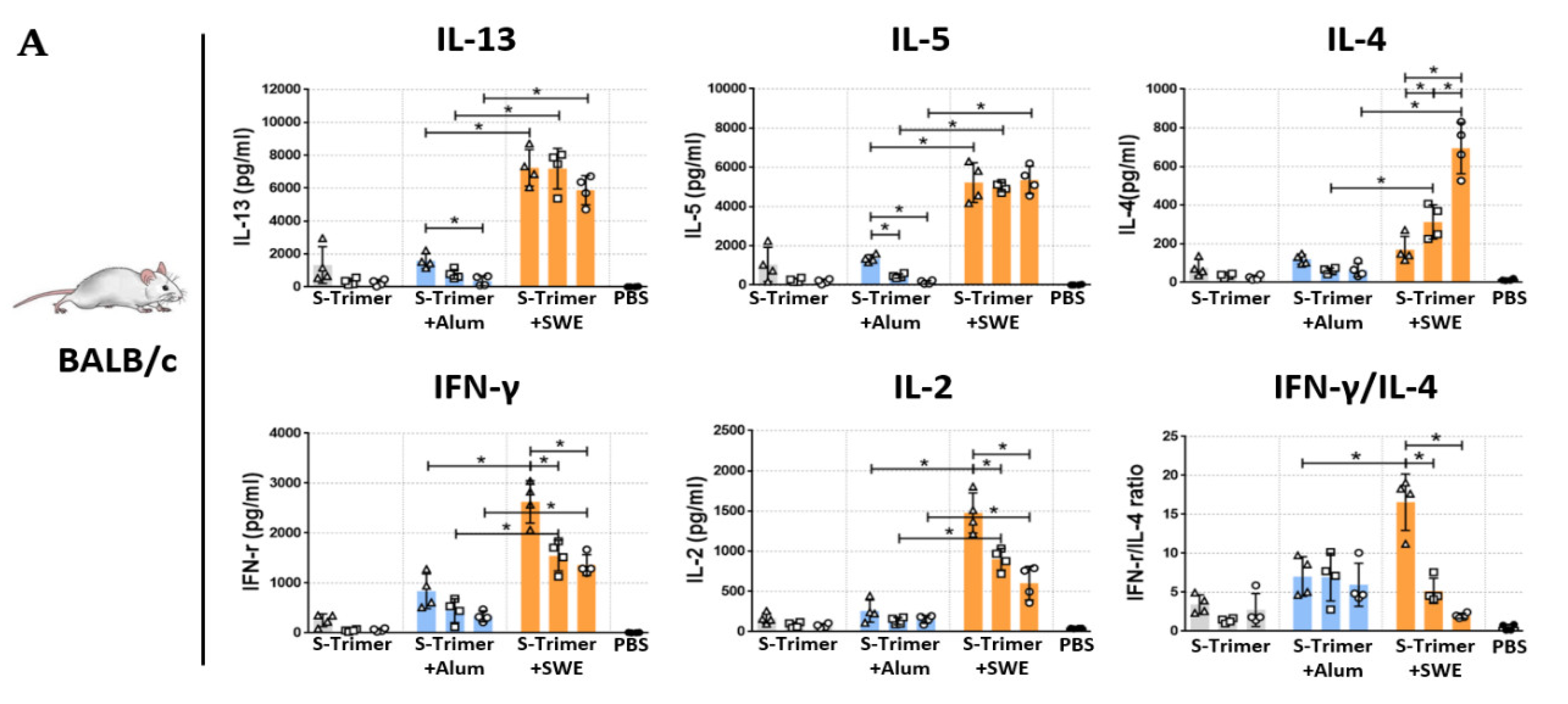
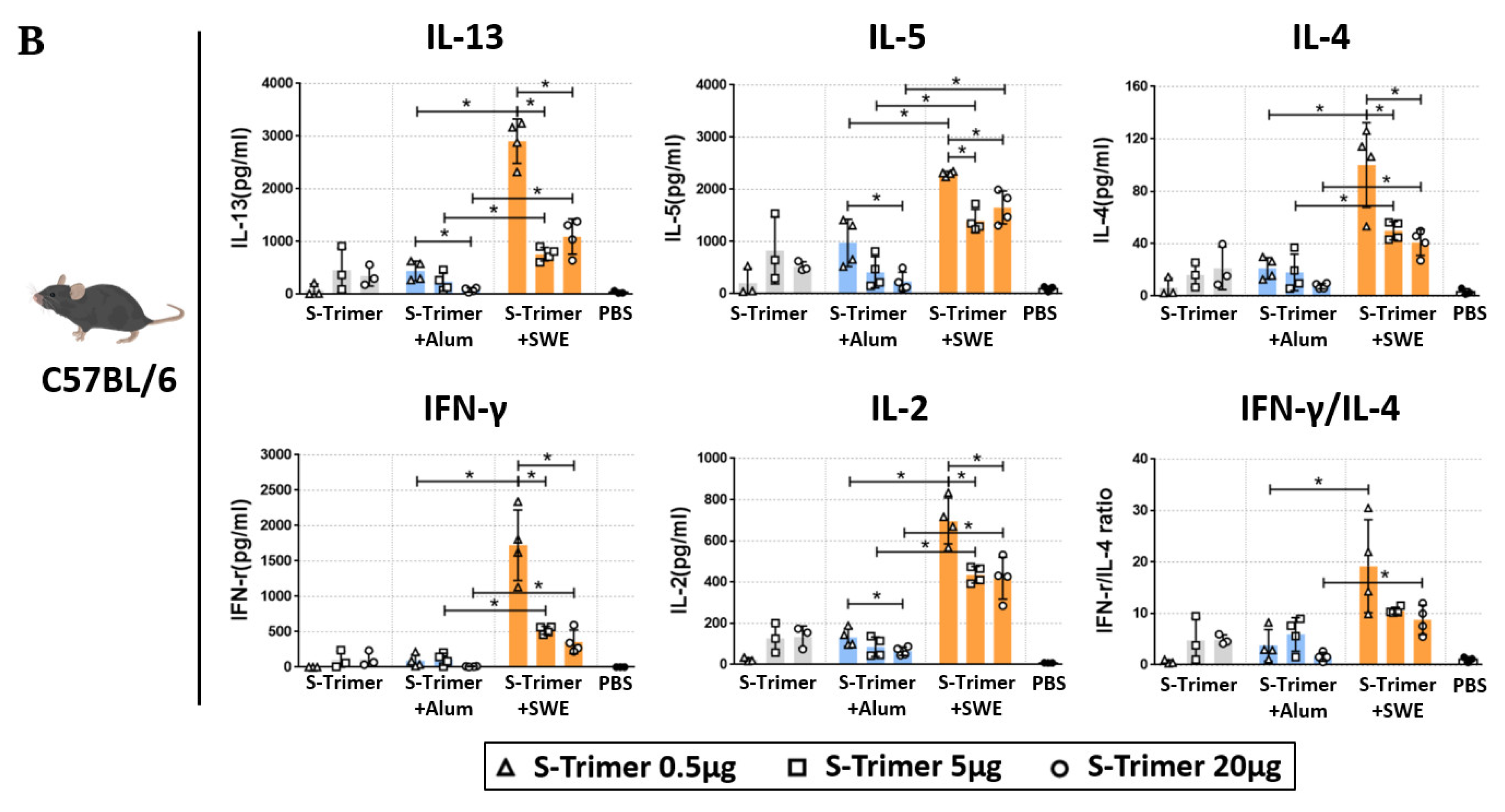
2.4. SWE-Adjuvated S-Trimer Enhances the T-Cell Response
2.5. Humoral Immune Responses and Protective Efficacy in Vaccinated Syrian Hamsters
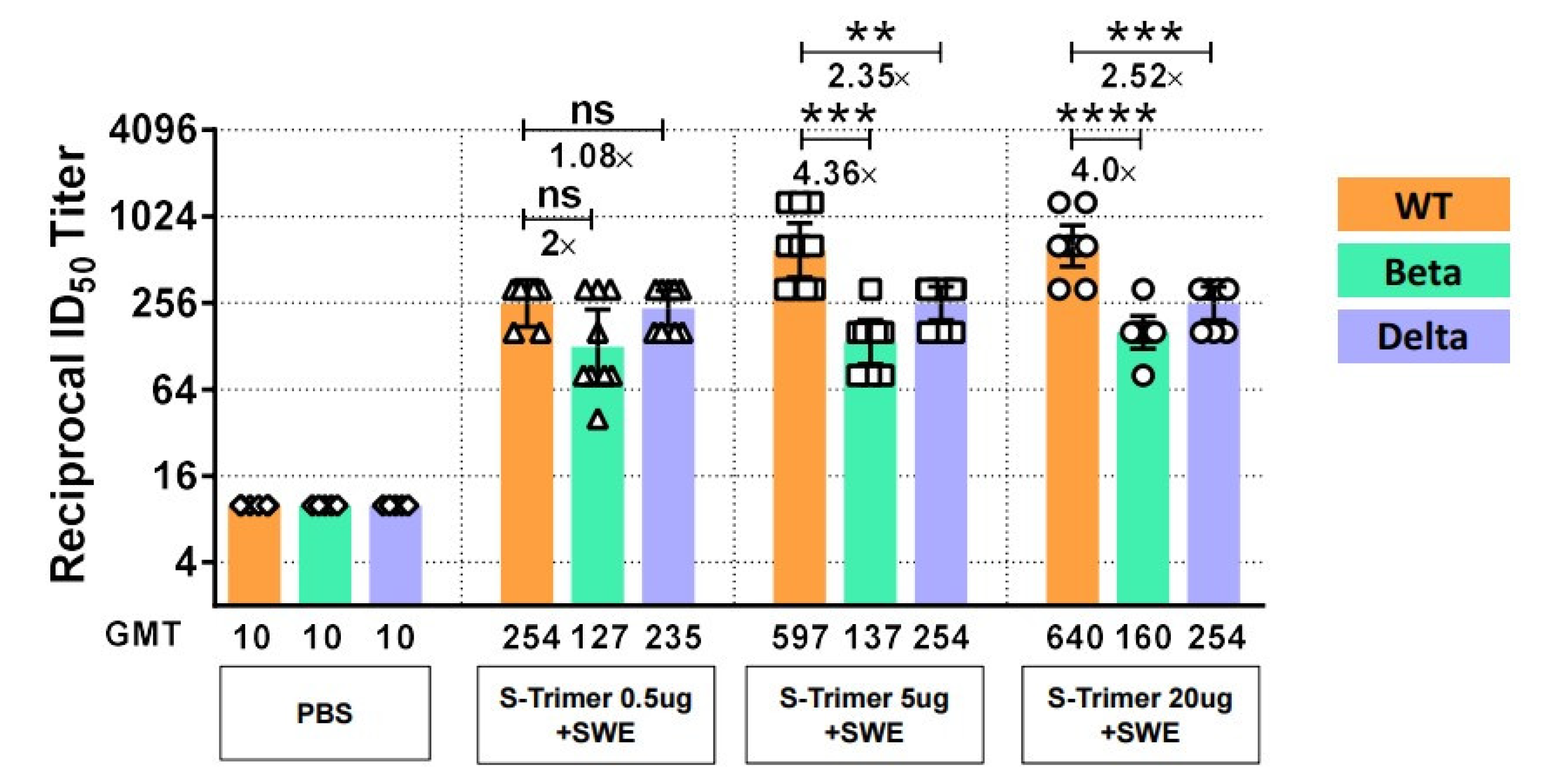
2.6. SWE-Adjuvanted S-Trimer Induces Cross-Neutralizing Activity against VOCs
3. Discussion
4. Materials and Methods
4.1. Production of SARS-CoV-2 S-Trimer
4.2. Animal Immunization
4.3. Immunoassay
4.4. ACE2 Competition ELISA
4.5. Neutralizing Antibody Analysis
4.6. Flow Cytometric Analysis
4.7. Cytokine Assay
4.8. ELISPOT Assay
4.9. Animal Challenge
4.10. H&E Staining
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/COVID-vaccinations (accessed on 20 February 2022).
- Cai, Y.; Zhang, J.; Xiao, T.; Peng, H.; Sterling, S.M.; Walsh, R.M., Jr.; Rawson, S.; Rits-Volloch, S.; Chen, B. Distinct conformational states of SARS-CoV-2 spike protein. Science 2020, 369, 1586–1592. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Juraszek, J.; Rutten, L.; Blokland, S.; Bouchier, P.; Voorzaat, R.; Ritschel, T.; Bakkers, M.J.G.; Renault, L.L.R.; Langedijk, J.P.M. Stabilizing the closed SARS-CoV-2 spike trimer. Nat. Commun. 2021, 12, 244. [Google Scholar] [CrossRef]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Watterson, D.; Wijesundara, D.K.; Modhiran, N.; Mordant, F.L.; Li, Z.; Avumegah, M.S.; McMillan, C.L.; Lackenby, J.; Guilfoyle, K.; van Amerongen, G.; et al. Preclinical development of a molecular clamp-stabilised subunit vaccine for severe acute respiratory syndrome coronavirus 2. Clin. Transl. Immunol. 2021, 10, e1269. [Google Scholar] [CrossRef]
- Liang, J.G.; Su, D.; Song, T.Z.; Zeng, Y.; Huang, W.; Wu, J.; Xu, R.; Luo, P.; Yang, X.; Zhang, X.; et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat. Commun. 2021, 12, 1346. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Guler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schafer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.E.; Lin, Y.J.; Chen, C.; Lian, W.C.; Kuo, T.Y.; Campbell, J.D.; Traquina, P.; Lin, M.Y.; Liu, L.T.; Chuang, Y.S.; et al. CpG-adjuvanted stable prefusion SARS-CoV-2 spike protein protected hamsters from SARS-CoV-2 challenge. Sci. Rep. 2021, 11, 8761. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.M.; Liu, M.C.; Chen, Y.H.; Lee, W.S.; Hwang, S.J.; Cheng, S.H.; Ko, W.C.; Hwang, K.P.; Wang, N.C.; Lee, Y.L.; et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: Interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J.; et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef]
- Chappell, K.J.; Mordant, F.L.; Li, Z.; Wijesundara, D.K.; Ellenberg, P.; Lackenby, J.A.; Cheung, S.T.M.; Modhiran, N.; Avumegah, M.S.; Henderson, C.L.; et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1383–1394. [Google Scholar] [CrossRef]
- Collin, N.; Dubois, P.M. The Vaccine Formulation Laboratory: A platform for access to adjuvants. Vaccine 2011, 29 (Suppl. S1), A37–A39. [Google Scholar] [CrossRef] [Green Version]
- Institute, V.F. SWE, an Squalene-in-Water Emulsion Adjuvant in Open-Access, for the Benefit of the Entire Vaccine Community. Available online: https://www.vaccineformulationinstitute.org/activities/swe-adjuvant/ (accessed on 20 February 2022).
- Westdijk, J.; Koedam, P.; Barro, M.; Steil, B.P.; Collin, N.; Vedvick, T.S.; Bakker, W.A.; van der Ley, P.; Kersten, G. Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains. Vaccine 2013, 31, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Vreman, S.; Stockhofe-Zurwieden, N.; Popma-de Graaf, D.J.; Savelkoul, H.F.J.; Barnier-Quer, C.; Collin, N.; Collins, D.; McDaid, D.; Moore, A.C.; Rebel, J.M.J. Immune responses induced by inactivated porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in neonatal pigs using different adjuvants. Vet. Immunol. Immunopathol. 2021, 232, 110170. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, J.; van Dijken, H.; de Heij, F.; Spijkers, S.; Mouthaan, J.; de Jong, R.; Roholl, P.; Adami, E.A.; Akamatsu, M.A.; Ho, P.L.; et al. H7N9 influenza split vaccine with SWE oil-in-water adjuvant greatly enhances cross-reactive humoral immunity and protection against severe pneumonia in ferrets. NPJ Vaccines 2020, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, K.; van Montfort, T.; Melchers, M.; Isik, G.; Sanders, R.W. Immunosilencing a highly immunogenic protein trimerization domain. J. Biol. Chem. 2015, 290, 7436–7442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintz, M.A.; Cyster, J.G. T follicular helper cells in germinal center B cell selection and lymphomagenesis. Immunol. Rev. 2020, 296, 48–61. [Google Scholar] [CrossRef]
- Verstegen, N.J.M.; Ubels, V.; Westerhoff, H.V.; van Ham, S.M.; Barberis, M. System-level scenarios for the elucidation of t cell-mediated germinal center b cell differentiation. Front. Immunol. 2021, 12, 734282. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lai, X.; Sun, J.; Chen, Z.; Zhang, Z.; Dai, J.; Liu, D.; Li, Y.; Li, F.; Wang, Y.; et al. Mapping and role of T cell response in SARS-CoV-2-infected mice. J. Exp. Med. 2021, 218, e20202187. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, G.; Wang, Y.; Ren, W.; Zhao, X.; Ji, F.; Zhu, Y.; Feng, F.; Gong, M.; Ju, X.; et al. Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2025373118. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Craven, J. COVID-19 Vaccine Tracker. Available online: https://www.raps.org/news-and-articles/news-articles/2020/3/COVID-19-vaccine-tracker (accessed on 26 February 2022).
- Ott, G.; Barchfeld, G.L.; Chernoff, D.; Radhakrishnan, R.; van Hoogevest, P.; Van Nest, G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm. Biotechnol. 1995, 6, 277–296. [Google Scholar]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Ma, J.; Su, D.; Sun, Y.; Huang, X.; Liang, Y.; Fang, L.; Ma, Y.; Li, W.; Liang, P.; Zheng, S. Cryo-EM structure of S-Trimer, a subunit vaccine candidate for COVID-19. J. Virol. 2021, 95, e00194-21. [Google Scholar] [CrossRef] [PubMed]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Israelow, B.; Mao, T.; Klein, J.; Song, E.; Menasche, B.; Omer, S.B.; Iwasaki, A. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci. Immunol. 2021, 6, eabl4509. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2523. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Edara, V.V.; Norwood, C.; Floyd, K.; Lai, L.; Davis-Gardner, M.E.; Hudson, W.H.; Mantus, G.; Nyhoff, L.E.; Adelman, M.W.; Fineman, R.; et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 2021, 29, 516–521.e3. [Google Scholar] [CrossRef]
- Planas, D.; Bruel, T.; Grzelak, L.; Guivel-Benhassine, F.; Staropoli, I.; Porrot, F.; Planchais, C.; Buchrieser, J.; Rajah, M.M.; Bishop, E.; et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021, 27, 917–924. [Google Scholar] [CrossRef]
- Wu, K.; Werner, A.P.; Moliva, J.I.; Koch, M.; Choi, A.; Stewart-Jones, G.B.E.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; Graham, B.S.; et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv 2021. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Dixon, G.; Oestreicher, J.; Legault, H.; Stewart-Jones, G.B.E.; Colpitts, T.; Pajon, R.; Bennett, H.; et al. Serum Neutralizing Activity of mRNA-1273 against SARS-CoV-2 Variants. J. Virol. 2021, 95, e0131321. [Google Scholar] [CrossRef]
- Valleriani, F.; Mancuso, E.; Vincifori, G.; Teodori, L.; Di Marcantonio, L.; Spedicato, M.; Leone, A.; Savini, G.; Morelli, D.; Bonfini, B.; et al. Neutralization of SARS-CoV-2 Variants by Serum from BNT162b2 Vaccine Recipients. Viruses 2021, 13, 2011. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S. The Novavax vaccine had 90% efficacy against COVID-19 >/=7 d after the second dose. Ann. Intern. Med. 2021, 174, JC124. [Google Scholar] [CrossRef] [PubMed]
- Chai, K.M.; Tzeng, T.T.; Shen, K.Y.; Liao, H.C.; Lin, J.J.; Chen, M.Y.; Yu, G.Y.; Dou, H.Y.; Liao, C.L.; Chen, H.W.; et al. DNA vaccination induced protective immunity against SARS CoV-2 infection in hamsterss. PLoS Negl. Trop. Dis. 2021, 15, e0009374. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.-C.; Wu, W.-L.; Chiang, C.-Y.; Huang, M.-S.; Shen, K.-Y.; Huang, Y.-L.; Wu, S.-C.; Liao, C.-L.; Chen, H.-W.; Liu, S.-J. Low-Dose SARS-CoV-2 S-Trimer with an Emulsion Adjuvant Induced Th1-Biased Protective Immunity. Int. J. Mol. Sci. 2022, 23, 4902. https://doi.org/10.3390/ijms23094902
Liao H-C, Wu W-L, Chiang C-Y, Huang M-S, Shen K-Y, Huang Y-L, Wu S-C, Liao C-L, Chen H-W, Liu S-J. Low-Dose SARS-CoV-2 S-Trimer with an Emulsion Adjuvant Induced Th1-Biased Protective Immunity. International Journal of Molecular Sciences. 2022; 23(9):4902. https://doi.org/10.3390/ijms23094902
Chicago/Turabian StyleLiao, Hung-Chun, Wan-Ling Wu, Chen-Yi Chiang, Min-Syuan Huang, Kuan-Yin Shen, Yu-Ling Huang, Suh-Chin Wu, Ching-Len Liao, Hsin-Wei Chen, and Shih-Jen Liu. 2022. "Low-Dose SARS-CoV-2 S-Trimer with an Emulsion Adjuvant Induced Th1-Biased Protective Immunity" International Journal of Molecular Sciences 23, no. 9: 4902. https://doi.org/10.3390/ijms23094902
APA StyleLiao, H.-C., Wu, W.-L., Chiang, C.-Y., Huang, M.-S., Shen, K.-Y., Huang, Y.-L., Wu, S.-C., Liao, C.-L., Chen, H.-W., & Liu, S.-J. (2022). Low-Dose SARS-CoV-2 S-Trimer with an Emulsion Adjuvant Induced Th1-Biased Protective Immunity. International Journal of Molecular Sciences, 23(9), 4902. https://doi.org/10.3390/ijms23094902






