An Overview of Cotton Gland Development and Its Transcriptional Regulation
Abstract
1. Introduction
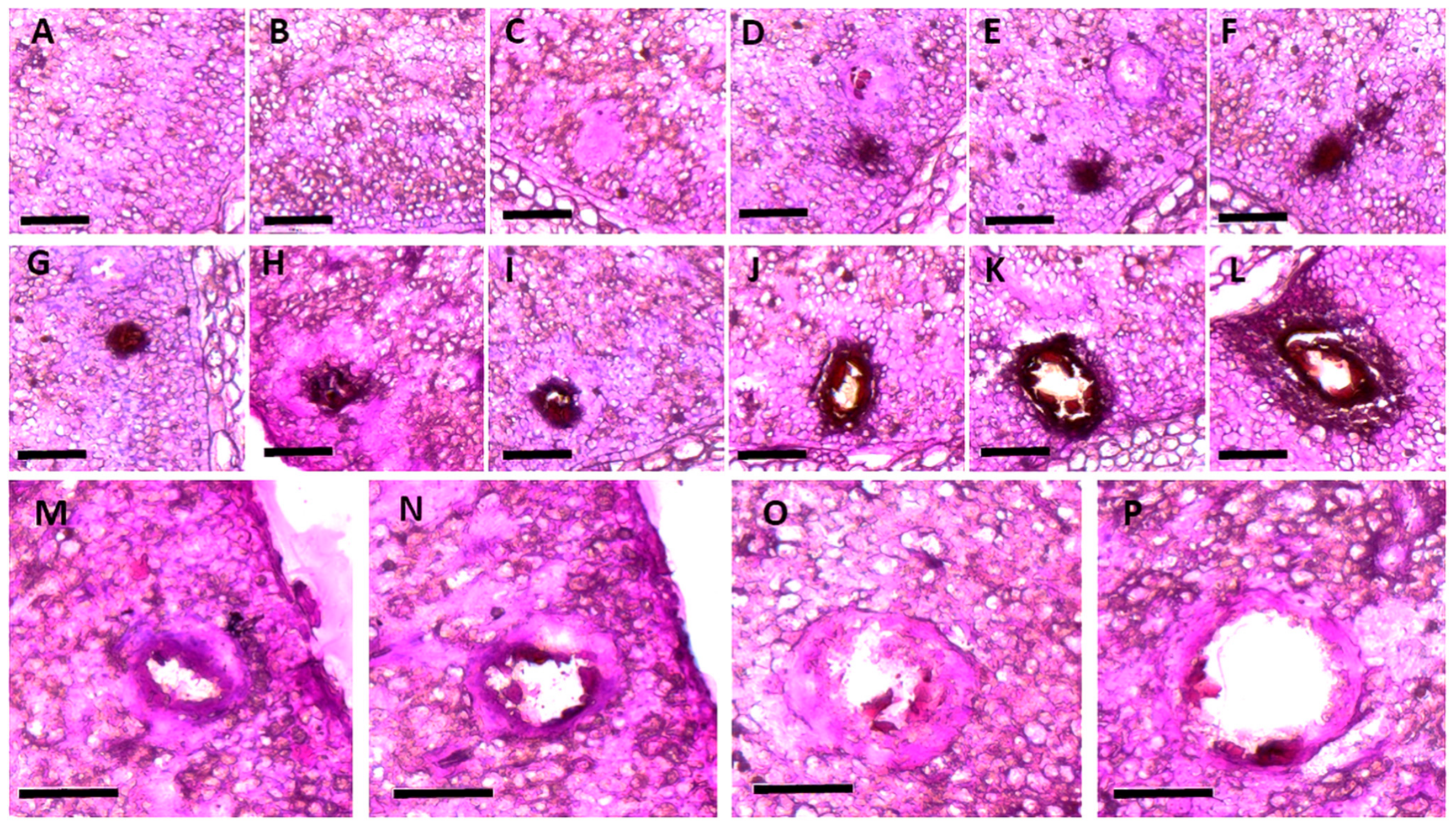
2. Developmental Changes in the Morphology of the Gossypol Gland in Cotton Plants
3. Functions of the Gossypol Gland in Cotton Plants
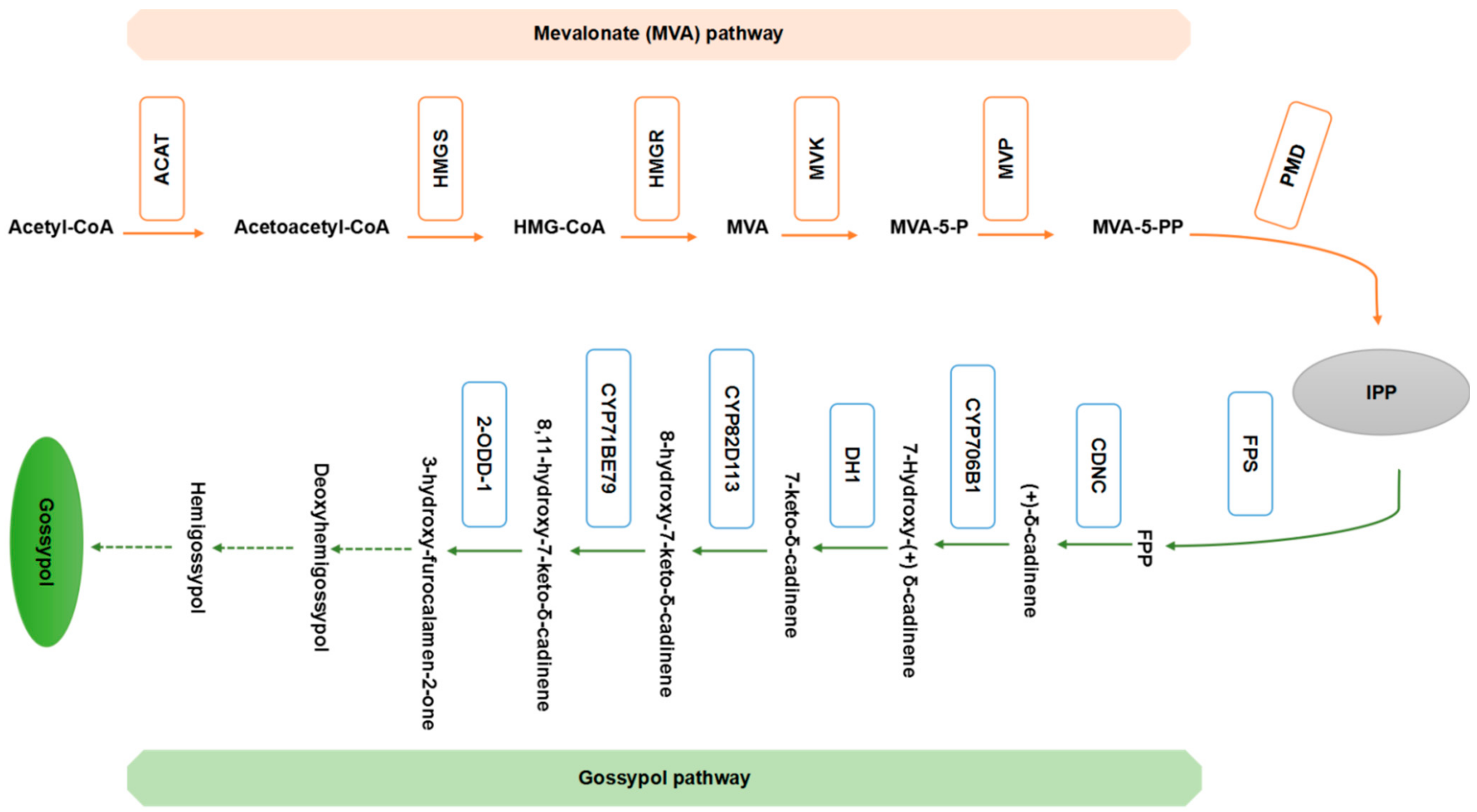
4. The Gossypol Biosynthetic Pathway
5. Molecular Cloning of Genes Associated with Gossypol Synthesis and Gossypol Glands
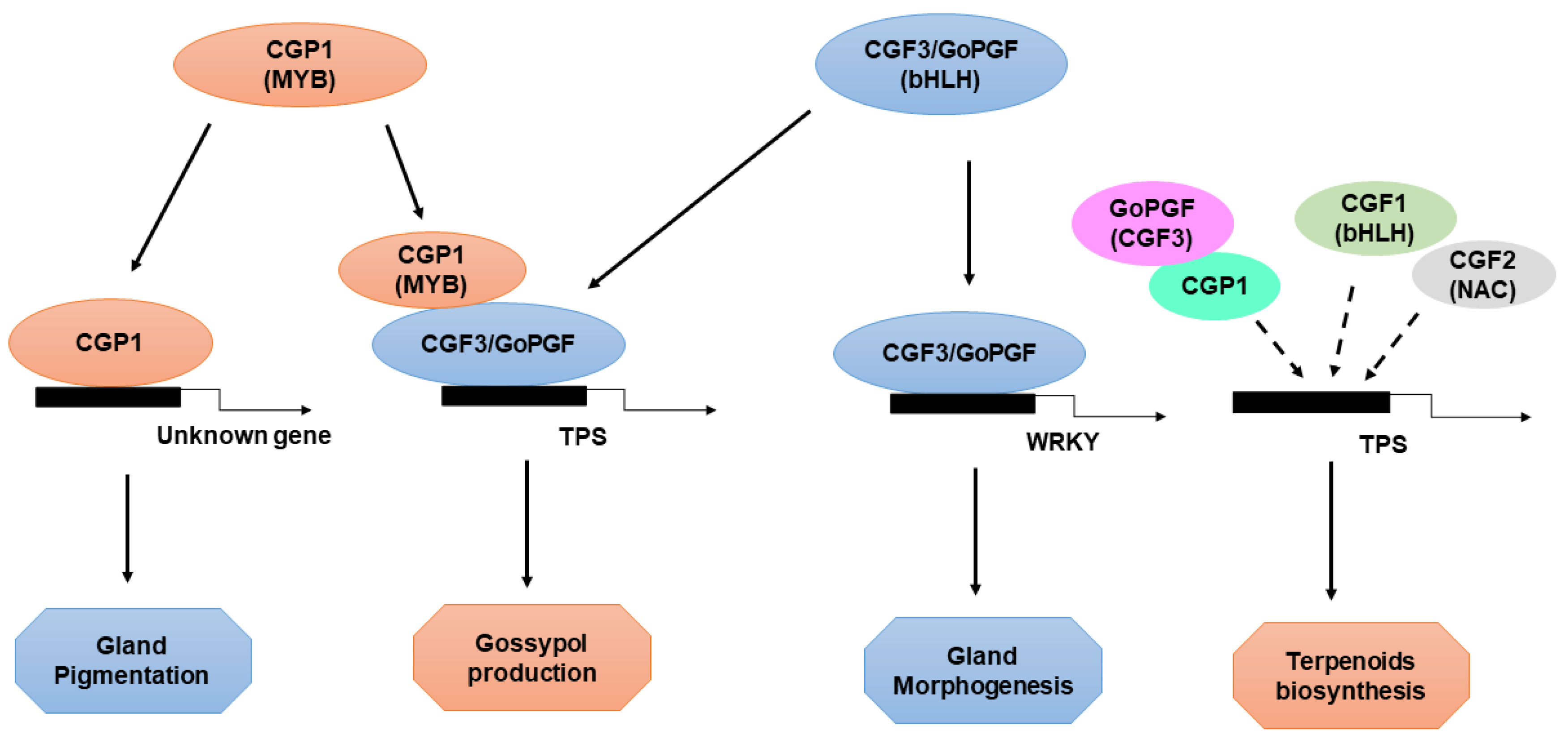
6. Transcriptional Regulation of Cotton Gland Morphogenesis and Pigmentation by CGP1, GoPGF, and CGFs
7. Challenges, Conclusions, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Zheng, S.; Tong, P.; Chen, Y.; Liu, W. Cytochemical localization of H2O2 in pigment glands of cotton (Gossypium hirsutum L.). J. Integr. Agric. 2016, 15, 1490–1498. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Xie, Y.; Li, H.; Liu, J.; Geng, R.; Wang, P.; Chu, Z.; Chang, Y.; Li, G.; Zhang, X.; et al. Cotton GhBRC1 regulates branching, flowering, and growth by integrating multiple hormone pathways. Crop J. 2022, 10, 75–87. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, Y.; Xie, Y.; Mo, J.; Yuan, Y.; Shi, Y.; Li, S.; Jiang, H.; Pan, Z.; Gao, Y.; et al. Gene expression profiling during gland morphogenesis of a mutant and a glandless upland cotton. Mol. Biol. Rep. 2010, 37, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Zhao, L.; Lv, Y.; Chen, J.; Hu, Y.; Zhang, T.; Zhou, B. Transcriptome sequencing and differential gene expression analysis of delayed gland morphogenesis in Gossypium australe during seed germination. PLoS ONE 2013, 8, e75323. [Google Scholar] [CrossRef]
- Zhang, J.; Wedegaertner, T. Genetics and Breeding for Glandless Upland Cotton with Improved Yield Potential and Disease Resistance: A Review. Front. Plant Sci. 2021, 12, 2227. [Google Scholar] [CrossRef]
- Liang, D.; Shuijin, Z.; Danyan, H.; Daofan, J. Observation on the Anatomical Structure of Pigment Glands and Analysis of the Gossypol Content in Gossypium stocksii. Acta Agron. Sin. 2004, 30, 100–103. [Google Scholar]
- Wang, Q.; Alariqi, M.; Wang, F.; Li, B.; Ding, X.; Rui, H.; Li, Y.; Xu, Z.; Qin, L.; Sun, L.; et al. The application of a heat-inducible CRISPR/Cas12b (C2c1) genome editing system in tetraploid cotton (G. hirsutum) plants. Plant Biotechnol. J. 2020, 18, 2436–2443. [Google Scholar] [CrossRef]
- Stanford, E.E.; Viehoever, A. Chemistry and Histology of the Glands of the Cotton Plant, with Notes on the Occurrence of Similar Glands in Related Plants; USDA: Washington, DC, USA, 1918; pp. 419–436.
- Hu, H.; He, X.; Tu, L.; Zhu, L.; Zhu, S.; Ge, Z.; Zhang, X. GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. Plant J. 2016, 88, 921–935. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, L.; Yang, Z.; Wu, Z.; Qin, W.; Yu, D.; Ren, Z.; Li, Y.; Wang, L.; Li, F.; et al. GhCaM7-like, a calcium sensor gene, influences cotton fiber elongation and biomass production. Plant Physiol. Biochem. PPB 2016, 109, 128–136. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Zhou, Y.-F.; Wang, X.; Jiao, Z.-J. Programmed cell death during pigment gland formation in Gossypium hirsutum leaves. Plant Biol. 2010, 12, 895–902. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Jiang, Z.; Zhao, Y.; Peng, J.; Jin, J.; Guo, H.; Luo, J. LSD: A leaf senescence database. Nucleic Acids Res. 2011, 39, D1103–D1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Luo, J.; Ji, J.; Gao, X.; Wu, C.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; et al. Transgenic insect-resistant Bt cotton expressing Cry1Ac/1Ab does not harm the insect predator Geocoris pallidipennis. Ecotoxicol. Environ. Saf. 2022, 230, 113129. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.T.; Rollins, M.L. New information on the morphology of the gossypol pigment gland of cottonseed. J. Am. Oil Chem. Soc. 1961, 38, 156–160. [Google Scholar] [CrossRef]
- Benedict, C.R.; Martin, G.S.; Liu, J.; Puckhaber, L.; Magill, C.W. Terpenoid aldehyde formation and lysigenous gland storage sites in cotton: Variant with mature glands but suppressed levels of terpenoid aldehydes. Phytochemistry 2004, 65, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.Y.; Xue, X.Y.; Huang, Y.P.; Chen, X.Y.; Mao, Y.B. Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Ecol. 2012, 21, 4371–4385. [Google Scholar] [CrossRef]
- Kline, M.P.; Rajkumar, S.V.; Timm, M.M.; Kimlinger, T.K.; Haug, J.L.; Lust, J.A.; Greipp, P.R.; Kumar, S. R-(-)−gossypol (AT-101) activates programmed cell death in multiple myeloma cells. Exp. Hematol. 2008, 36, 568–576. [Google Scholar] [CrossRef]
- Moon, D.-O.; Kim, M.-O.; Lee, J.-D.; Kim, G.-Y. Gossypol suppresses NF-κB activity and NF-κB-related gene expression in human leukemia U937 cells. Cancer Lett. 2008, 264, 192–200. [Google Scholar] [CrossRef]
- Bolek, Y.; Fidan, M.S.; Oglacki, M. Distribution of Gossypol Glands on Cotton (Gossypium hirsutum L.) Genotypes. Not. Bot. Horti Agrobot. 2010, 38, 81–87. [Google Scholar]
- Scheffler, J.A.; Romano, G.B.; Blanco, C.A. Evaluating host plant resistance in cotton (Gossypium hirsutum L.) with varying gland densities to tobacco budworm (Heliothis virescens F.) and bollworm (Helicoverpa zea Boddie) in the field and laboratory. Agric. Sci. 2012, 3, 14–23. [Google Scholar]
- Yang, Z.; Qanmber, G.; Wang, Z.; Yang, Z.; Li, F. Gossypium Genomics: Trends, Scope, and Utilization for Cotton Improvement. Trends Plant Sci. 2020, 25, 488–500. [Google Scholar] [CrossRef]
- Qu, Y.-K.; Cheng, S.; Duan, Y.; Lan, Y.; Liu, D.; Zhao, Y.; Zhang, L.; Huang, J. Correlation of Pigment Gland Formation and Gossypol Biosynthesis in Glanded and Glandless Trispecific Cotton Progenies. Int. J. Agric. Biol. 2019, 22, 553–560. [Google Scholar]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cheng, H.; Li, S.; Zuo, D.; Lin, Z.; Zhang, Y.; Lv, L.; Wang, Q.; Song, G. Molecular cloning and characterization of GhERF105, a gene contributing to the regulation of gland formation in upland cotton (Gossypium hirsutum L.). BMC Plant Biol. 2021, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; He, Z.; Ford, C.; Wyckoff, W.; Wu, Q. A Review of Cottonseed Protein Chemistry and Non-Food Applications. Sustain. Chem. 2020, 1, 256–274. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.L.; Zhao, J.H.; Wang, S.; Jin, Y.; Chen, Z.Q.; Fang, Y.Y.; Hua, C.L.; Ding, S.W.; Guo, H.S. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Ash, M.; Dohlman, E. Oil Crops Situation and Outlook Yearbook; Economic Research Service: Washington DC, USA, 2007. [Google Scholar]
- Gerasimidis, K.; Fillou, D.T.; Babatzimcpoulou, M.; Tassou, K.; Katsikas, H. Preparation of an edible cottonseed protein concentrate and evaluation of its functional properties. Int. J. Food Sci. Nutr. 2007, 58, 486–490. [Google Scholar] [CrossRef]
- Alchanati, I.; Patel, J.A.A.; Liu, J.; Benedict, C.R.; Stipanovic, R.D.; Bell, A.A.; Cui, Y.; Magill, C.W. The enzymatic cyclization of nerolidyl diphosphate by δ-cadinene synthase from cotton stele tissue infected with verticillium dahliae. Phytochemistry 1998, 47, 961–967. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Cai, J.; Li, D.; Song, F. NAC transcription factors in plant immunity. Phytopathol. Res. 2019, 1, 1–13. [Google Scholar] [CrossRef]
- Tian, X.; Ruan, J.-X.; Huang, J.-Q.; Yang, C.-Q.; Fang, X.; Chen, Z.-W.; Hong, H.; Wang, L.-J.; Mao, Y.-B.; Lu, S.; et al. Characterization of gossypol biosynthetic pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E5410–E5418. [Google Scholar] [CrossRef]
- Sun, Y.; Veerabomma, S.; Abdel-Mageed, H.A.; Fokar, M.; Asami, T.; Yoshida, S.; Allen, R.D. Brassinosteroid Regulates Fiber Development on Cultured Cotton Ovules. Plant Cell Physiol. 2005, 46, 1384–1391. [Google Scholar] [CrossRef]
- Oliver, C.L.; Miranda, M.B.; Shangary, S.; Land, S.; Wang, S.; Johnson, D.E. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol. Cancer Ther. 2005, 4, 23–31. [Google Scholar] [PubMed]
- Keller, P.A.; Birch, C.; Leach, S.P.; Tyssen, D.; Griffith, R. Novel pharmacophore-based methods reveal gossypol as a reverse transcriptase inhibitor. J. Mol. Graph. Model. 2003, 21, 365–373. [Google Scholar] [CrossRef][Green Version]
- Cherry, J.P. Cottonseed oil. J. Am. Oil Chem. Soc. 1983, 60, 360–367. [Google Scholar] [CrossRef]
- Brubaker, C.; Benson, C.; Miller, C.; Leach, D. Occurrence of terpenoid aldehydes and lysigenous cavities in the “Glandless” seeds of Australian Gossypium species. Aust. J. Bot. 1996, 44, 601–612. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, H.; Zeng, Y.; Mo, J.; Bao, J.; Miao, C.; Bai, J.; Yan, F.; Chen, F. An optimized gossypol high-performance liquid chromatography assay and its application in evaluation of different gland genotypes of cotton. J. Biosci. 2004, 29, 67–71. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, X.; Zuo, D.; Zhang, Y.; Wang, Q.; Lv, L.; Wu, C.; Li, S.; Dai, Y.; Qu, D.; et al. Gene expression correlation analysis reveals MYC-NAC regulatory network in cotton pigment gland development. Int. J. Mol. Sci. 2021, 22, 5007. [Google Scholar] [CrossRef]
- Martin, G.S.; Liu, J.; Benedict, C.R.; Stipanovic, R.D.; Magill, C.W. Reduced levels of cadinane sesquiterpenoids in cotton plants expressing antisense (+)-delta-cadinene synthase. Phytochemistry 2003, 62, 31–38. [Google Scholar] [CrossRef]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent advances and future perspectives in polymer-based nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, G.; Zhu, Y. Sustainable plant disease control: Biotic information flow and behavior manipulation. Sci. China Life Sci. 2019, 62, 1710–1713. [Google Scholar] [CrossRef]
- Zheng, S.; Luo, J.; Zhu, X.; Gao, X.; Hua, H.; Cui, J. Transcriptomic analysis of salivary gland and proteomic analysis of oral secretion in Helicoverpa armigera under cotton plant leaves, gossypol, and tannin stresses. Genomics 2022, 114, 110267. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Luo, J.; Ji, J.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Cui, J.; Niu, L. Transgenic Bt cotton expressing Cry1Ac/1Ab does not have detrimental effects on the predator Arma chinensis through its prey Helicoverpa armigera. J. Pest Sci. 2022, 230, 113129. [Google Scholar] [CrossRef]
- Maryam, H.; Ali, Z.; Saddique, M.A.B.; Nawaz, F. GhCDNC and GhCYP706B1 genes mediate gossypol biosynthesis in upland cotton. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Townsend, B.J.; Poole, A.; Blake, C.J.; Llewellyn, D.J. Antisense Suppression of a (+)-δ-Cadinene Synthase Gene in Cotton Prevents the Induction of This Defense Response Gene during Bacterial Blight Infection but Not Its Constitutive Expression. Plant Physiol. 2005, 138, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.; Zhang, X.; Ma, X.; Zhang, L.; Liao, Z.; Zhang, Q.; Wan, X.; Cheng, Y.; Zhang, J.; et al. The genome of kenaf (Hibiscus cannabinus L.) provides insights into bast fibre and leaf shape biogenesis. Plant Biotechnol. J. 2020, 18, 1796–1809. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, G.; Zhu, Y. Transposable elements play an important role during cotton genome evolution and fiber cell development. Sci. China. Life Sci. 2016, 59, 112–121. [Google Scholar] [CrossRef]
- Liu, J.; Benedict, C.R.; Stipanovic, R.D.; Bell, A.A. Purification and Characterization of S-Adenosyl-L-Methionine: Desoxyhemigossypol-6-O-Methyltransferase from Cotton Plants. An Enzyme Capable of Methylating the Defense Terpenoids of Cotton. Plant Physiol. 1999, 121, 1017–1024. [Google Scholar] [CrossRef]
- Li, B.; Liang, S.; Alariqi, M.; Wang, F.; Wang, G.; Wang, Q.; Xu, Z.; Yu, L.; Naeem Zafar, M.; Sun, L.; et al. The application of temperature sensitivity CRISPR/LbCpf1 (LbCas12a) mediated genome editing in allotetraploid cotton (G. hirsutum) and creation of nontransgenic, gossypol-free cotton. Plant Biotechnol. J. 2021, 19, 221–223. [Google Scholar] [CrossRef]
- Heinstein, P.F.; Herman, D.L.; Tove, S.B.; Smith, F.H. Biosynthesis of gossypol. Incorporation of mevalonate-2-14C and isoprenyl pyrophosphates. J. Biol. Chem. 1970, 245, 4658–4665. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Liu, D.; Wei, Y.-L.; Wang, Y.; Li, X.-B. Characterization of bHLH/HLH genes that are involved in brassinosteroid (BR) signaling in fiber development of cotton (Gossypium hirsutum). BMC Plant Biol. 2018, 18, 304. [Google Scholar] [CrossRef]
- Wang, K.; Wang, D.; Zheng, X.; Qin, A.; Zhou, J.; Guo, B.; Chen, Y.; Wen, X.; Ye, W.; Zhou, Y.; et al. Multi-strategic RNA-seq analysis reveals a high-resolution transcriptional landscape in cotton. Nat. Commun. 2019, 10, 4714. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Chen, Y.; Heinstein, P.; Davisson, V.J. Cloning, Expression, and Characterization of (+)-δ-Cadinene Synthase: A Catalyst for Cotton Phytoalexin Biosynthesis. Arch. Biochem. Biophys. 1995, 324, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Wang, J.; Gao, P.; Jiao, G.-L.; Zhao, P.-M.; Li, Y.; Wang, G.-L.; Xia, G.-X. Down-regulation of GhADF1 gene expression affects cotton fibre properties. Plant Biotechnol. J. 2008, 7, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; He, P.; Zhao, P.; Liu, H.; Zhang, L.; Pang, C.; Yu, J. Genome-wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J. Exp. Bot. 2018, 69, 4323–4337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ge, X.; Yang, Z.; Qin, W.; Sun, G.; Wang, Z.; Li, Z.; Liu, J.; Wu, J.; Wang, Y.; et al. Extensive intraspecific gene order and gene structural variations in upland cotton cultivars. Nat. Commun. 2019, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, Y.H.; Wang, G.D.; Essenberg, M.; Chen, X.Y. Molecular cloning and functional identification of (+)-delta-cadinene-8-hydroxylase, a cytochrome P450 mono-oxygenase (CYP706B1) of cotton sesquiterpene biosynthesis. Plant J. 2001, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Fang, X.; Tian, X.; Chen, P.; Lin, J.L.; Guo, X.X.; Li, J.X.; Fan, Z.; Song, W.M.; Chen, F.Y.; et al. Aromatization of natural products by a specialized detoxification enzyme. Nat. Chem. Biol. 2020, 16, 250–256. [Google Scholar] [CrossRef]
- Back, K.; Chappell, J. Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus muticus and its molecular comparison to related terpene cyclases. J. Biol. Chem. 1995, 270, 7375–7381. [Google Scholar] [CrossRef]
- Xie, Y.-F.; Wang, B.-C.; Li, B.; Cai, Y.-F.; Xie, L.; Xia, Y.-X.; Chang, P.-A.; Jiang, H.-Z. Construction of cDNA library of cotton mutant Xiangmian-18 library during gland forming stage. Colloids Surf. B Biointerfaces 2007, 60, 258–263. [Google Scholar] [CrossRef]
- Qin, L.; Li, J.; Wang, Q.; Xu, Z.; Sun, L.; Alariqi, M.; Manghwar, H.; Wang, G.; Li, B.; Ding, X.; et al. High-efficient and precise base editing of C•G to T•A in the allotetraploid cotton (Gossypium hirsutum) genome using a modified CRISPR/Cas9 system. Plant Biotechnol. J. 2020, 18, 45–56. [Google Scholar] [CrossRef]
- Huang, G.; Wu, Z.; Percy, R.G.; Bai, M.; Li, Y.; Frelichowski, J.E.; Hu, J.; Wang, K.; Yu, J.Z.; Zhu, Y. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat. Genet. 2020, 52, 516–524. [Google Scholar] [CrossRef]
- Janga, M.R.; Pandeya, D.; Campbell, L.M.; Konganti, K.; Villafuerte, S.T.; Puckhaber, L.; Pepper, A.; Stipanovic, R.D.; Scheffler, J.A.; Rathore, K.S. Genes regulating gland development in the cotton plant. Plant Biotechnol. J. 2019, 17, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ren, X.; Liu, Y. An Odorant Receptor from the Proboscis of the Cotton Narrowly Tuned to Indole. Insects 2022, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, T.; Sheng, K.; Sun, Y.; Han, Y.; Chen, Y.; Zhiying, E.; Zhu, S.; Chen, J. Root Illumination Promotes Seedling Growth and Inhibits Gossypol Biosynthesis in Upland Cotton. Plants 2022, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Hu, Y.; Yang, C.; Liu, B.; Fang, L.; Wan, Q.; Liang, W.; Mei, G.; Wang, L.; Wang, H.; et al. Genetic basis for glandular trichome formation in cotton. Nat. Commun. 2016, 7, 10456. [Google Scholar] [CrossRef] [PubMed]
- Caraban, C. Multidimensional Fluorescence Imaging of Embryonic and Postnatal Mammary Gland Development. Methods Mol. Biol. 2022, 2471, 19–48. [Google Scholar] [CrossRef]
- Gao, W.; Xu, F.-C.; Long, L.; Li, Y.; Zhang, J.-L.; Chong, L.; Botella, J.R.; Song, C.-P. The gland localized CGP1 controls gland pigmentation and gossypol accumulation in cotton. Plant Biotechnol. J. 2020, 18, 1573–1584. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Tang, K.; Zuo, K. Functional analysis of the seed coat-specific gene GbMYB2 from cotton. Plant Physiol. Biochem. PPB 2013, 73, 16–22. [Google Scholar] [CrossRef]
- Lu, S.X.; Knowles, S.M.; Andronis, C.; Ong, M.S.; Tobin, E.M. Circadian clock associated1 and late elongated hypocotyl function synergistically in the circadian clock of Arabidopsis. Plant Physiol. 2009, 150, 834–843. [Google Scholar] [CrossRef]
- Zang, Y.; Xu, C.; Xuan, L.; Ding, L.; Zhu, J.; Si, Z.; Zhang, T.; Hu, Y. Identification and characteristics of a novel gland-forming gene in cotton. Plant J. 2021, 108, 781–792. [Google Scholar] [CrossRef]
- Ioannidi, E.; Rigas, S.; Tsitsekian, D.; Daras, G.; Alatzas, A.; Makris, A.; Tanou, G.; Argiriou, A.; Alexandrou, D.; Poethig, S.; et al. Trichome patterning control involves TTG1 interaction with SPL transcription factors. Plant Mol. Biol. 2016, 92, 675–687. [Google Scholar] [CrossRef]
- Basch, J.; Chiang, S.-J. Cloning and expression of a cytochrome P450 hydroxylase gene from Amycolatopsis orientalis: Hydroxylation of epothilone B for the production of epothilone F. J. Ind. Microbiol. Biotechnol. 2007, 34, 171–176. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jan, M.; Liu, Z.; Guo, C.; Zhou, Y.; Sun, X. An Overview of Cotton Gland Development and Its Transcriptional Regulation. Int. J. Mol. Sci. 2022, 23, 4892. https://doi.org/10.3390/ijms23094892
Jan M, Liu Z, Guo C, Zhou Y, Sun X. An Overview of Cotton Gland Development and Its Transcriptional Regulation. International Journal of Molecular Sciences. 2022; 23(9):4892. https://doi.org/10.3390/ijms23094892
Chicago/Turabian StyleJan, Masood, Zhixin Liu, Chenxi Guo, Yaping Zhou, and Xuwu Sun. 2022. "An Overview of Cotton Gland Development and Its Transcriptional Regulation" International Journal of Molecular Sciences 23, no. 9: 4892. https://doi.org/10.3390/ijms23094892
APA StyleJan, M., Liu, Z., Guo, C., Zhou, Y., & Sun, X. (2022). An Overview of Cotton Gland Development and Its Transcriptional Regulation. International Journal of Molecular Sciences, 23(9), 4892. https://doi.org/10.3390/ijms23094892





