Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA
Abstract
1. Introduction
2. Results
2.1. Characterization of Isolated DNA Aptamers to Human Urokinase Revealed Binding Sequences by EMSA and FLAA
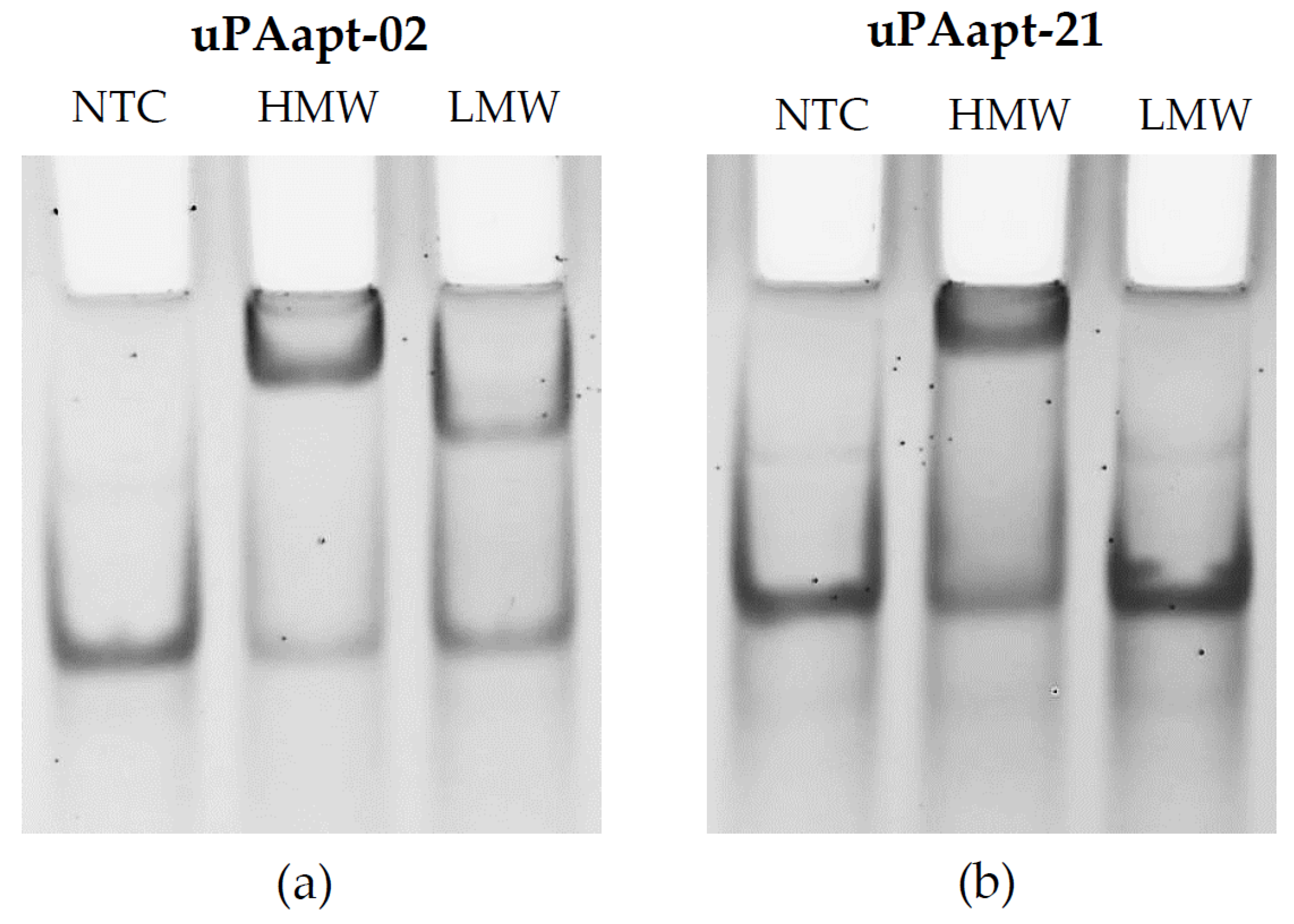
2.1.1. Gel Shift Experiments (EMSA)
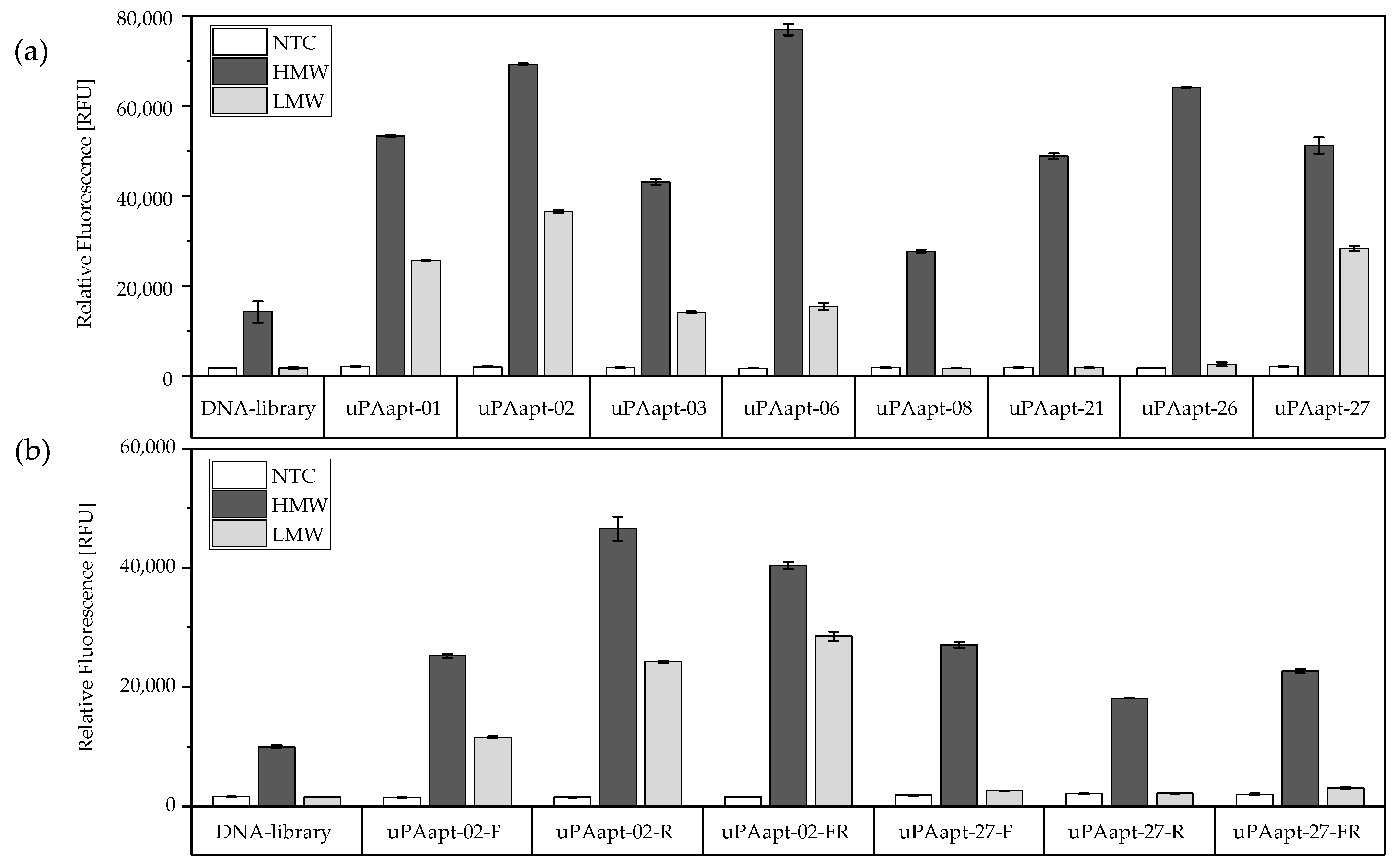
2.1.2. Fluorescent-Dye Linked Aptamer Assay (FLAA)
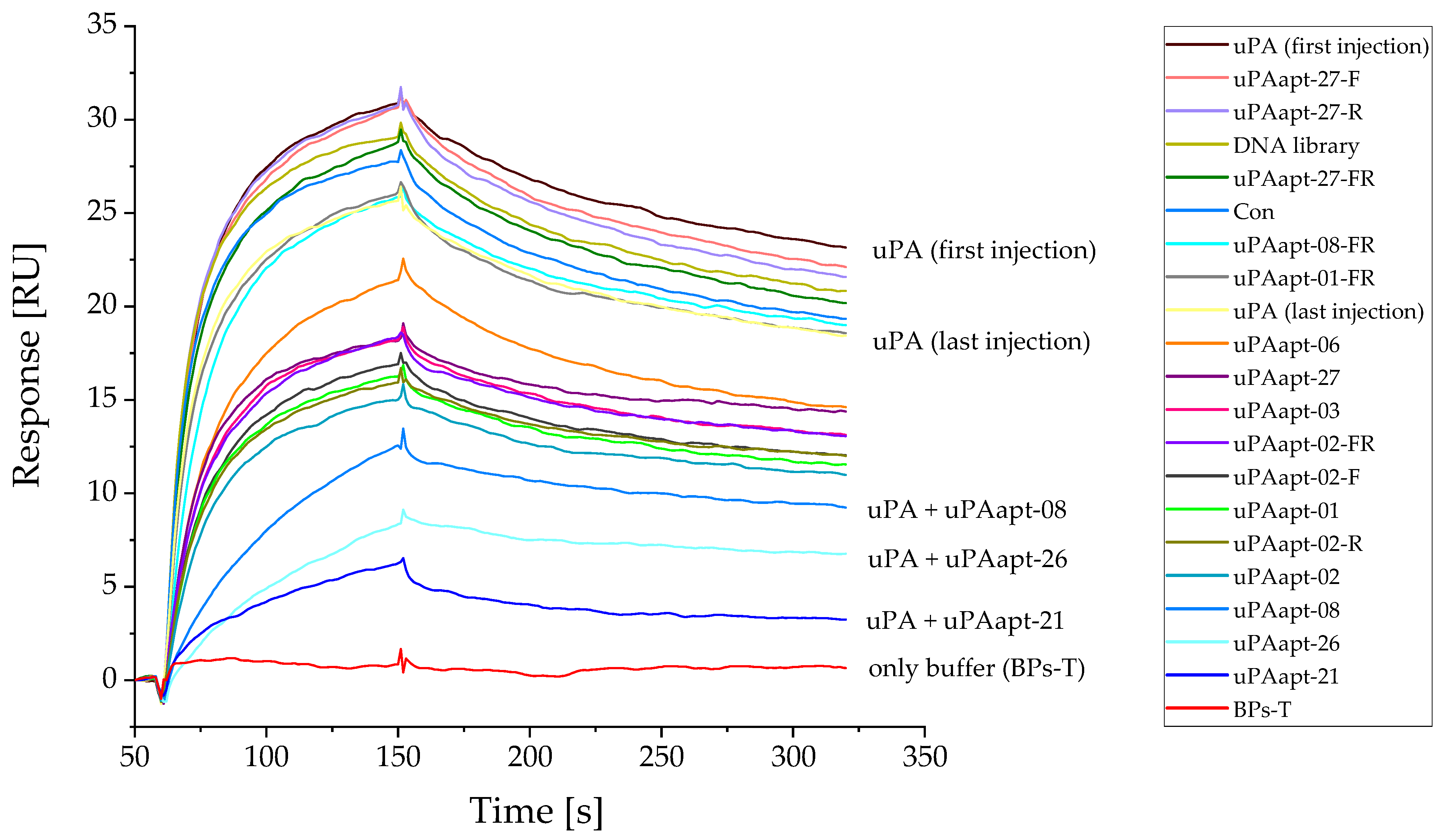
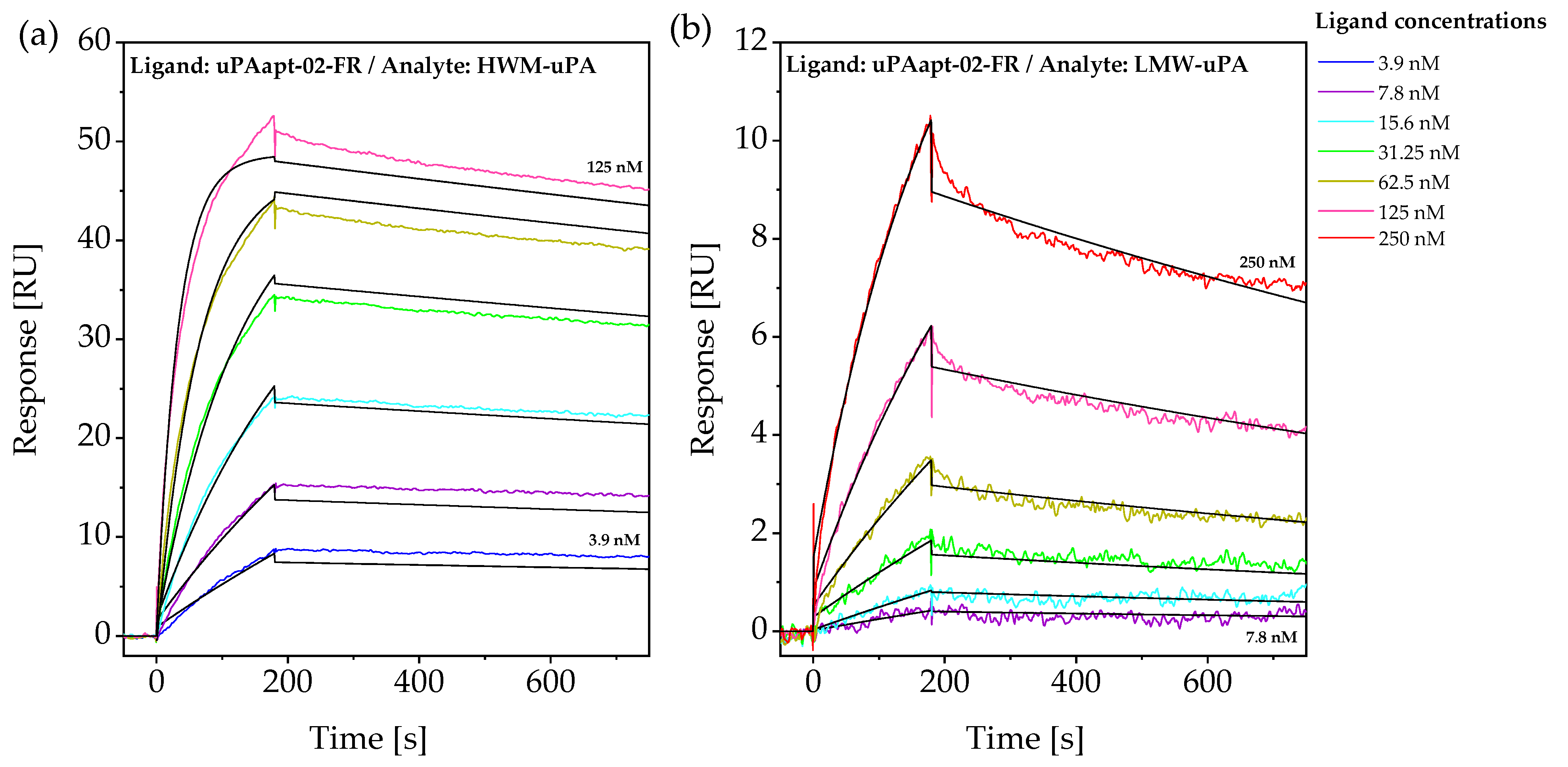
2.2. Aptamers Showed High Affinity to uPA with up to pM KD Values
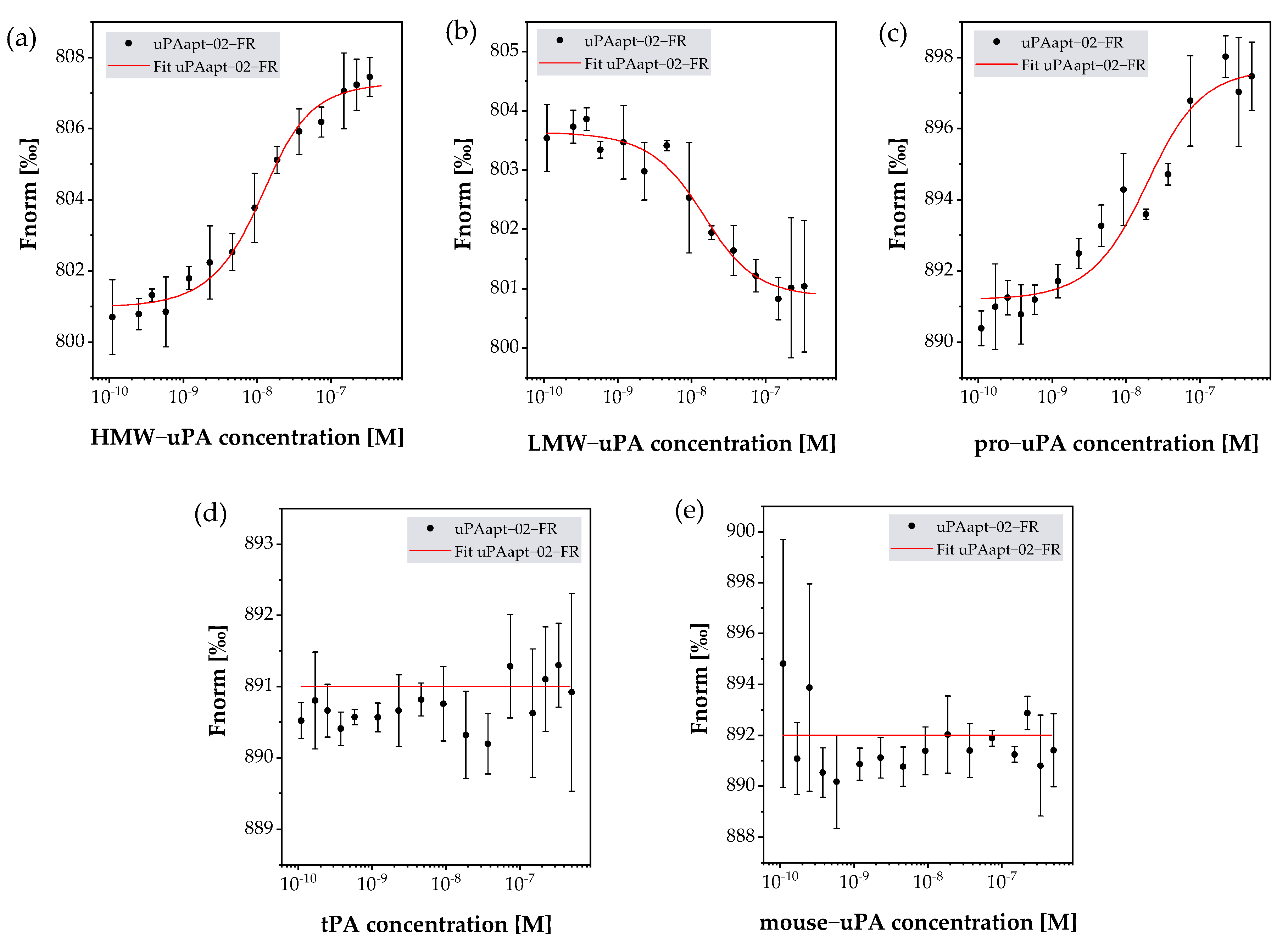
2.3. uPA Aptamer uPAapt-02-FR Demonstrated High Specificity for Human uPA
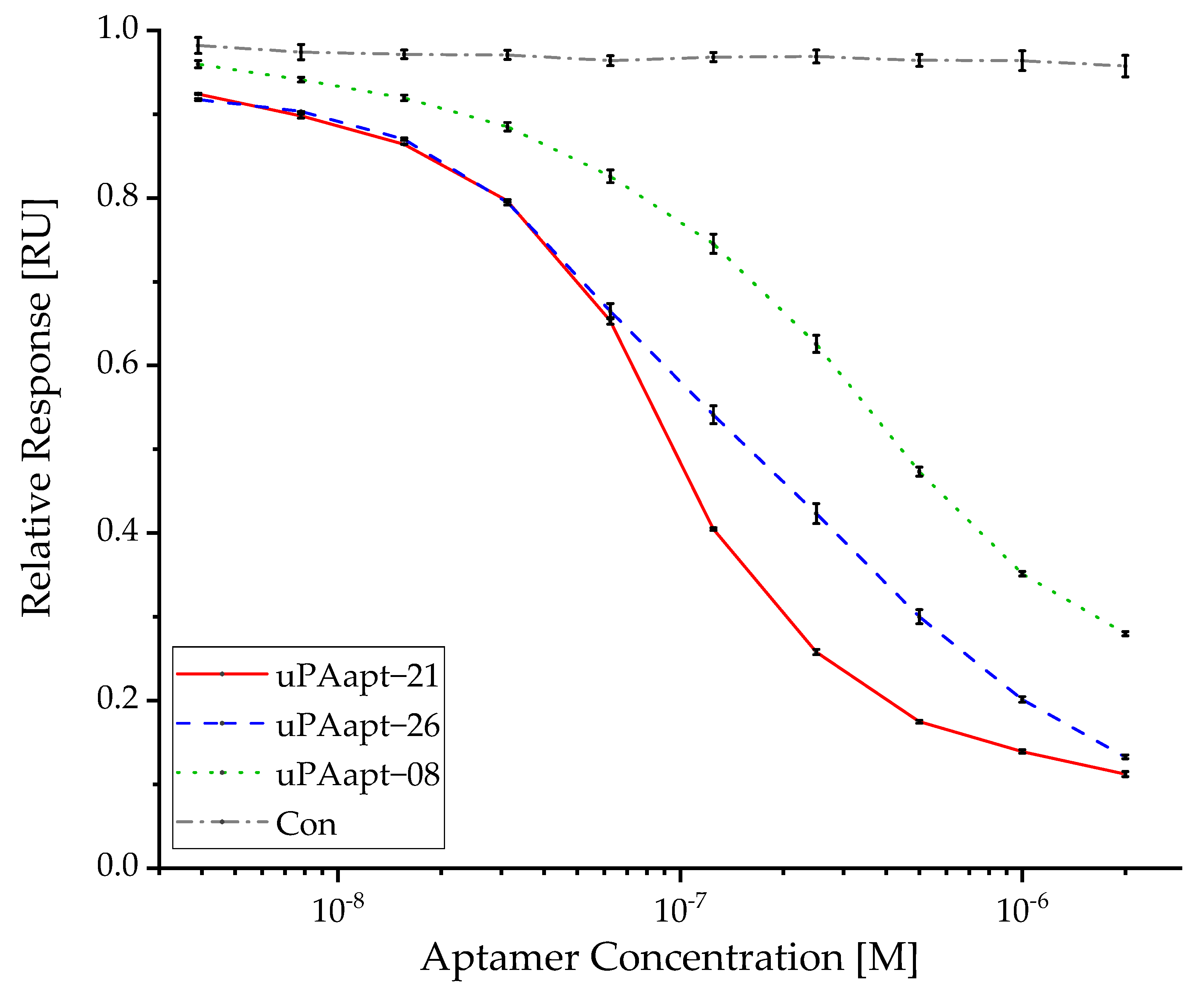
2.4. Various Aptamers Reveal an Inhibitory Effect toward Binding of uPA to uPAR with up to nM IC50 Values
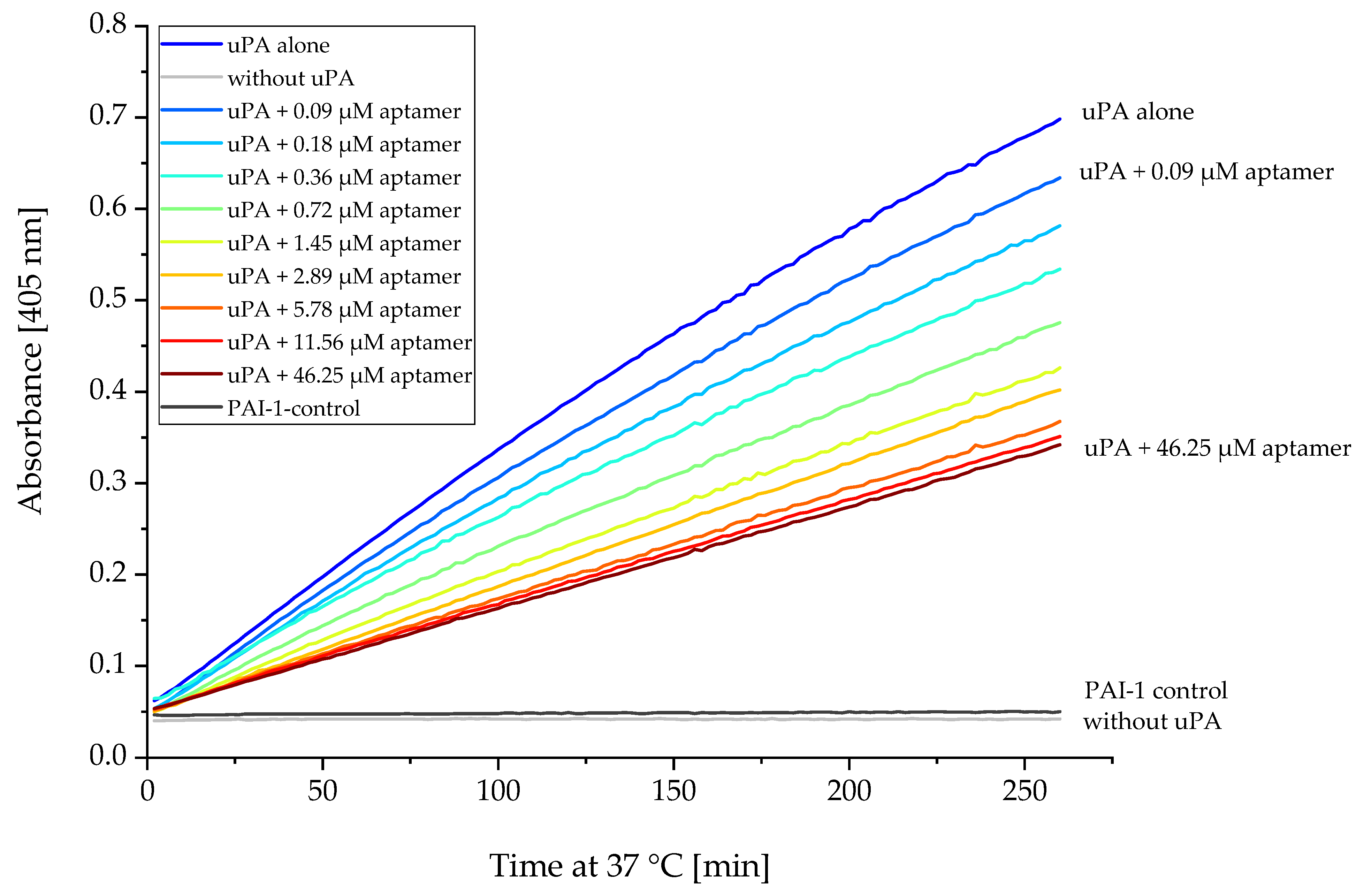
2.5. Proteolytic Activity of uPA Can Be Inhibited by Several Aptamers
3. Discussion
4. Materials and Methods
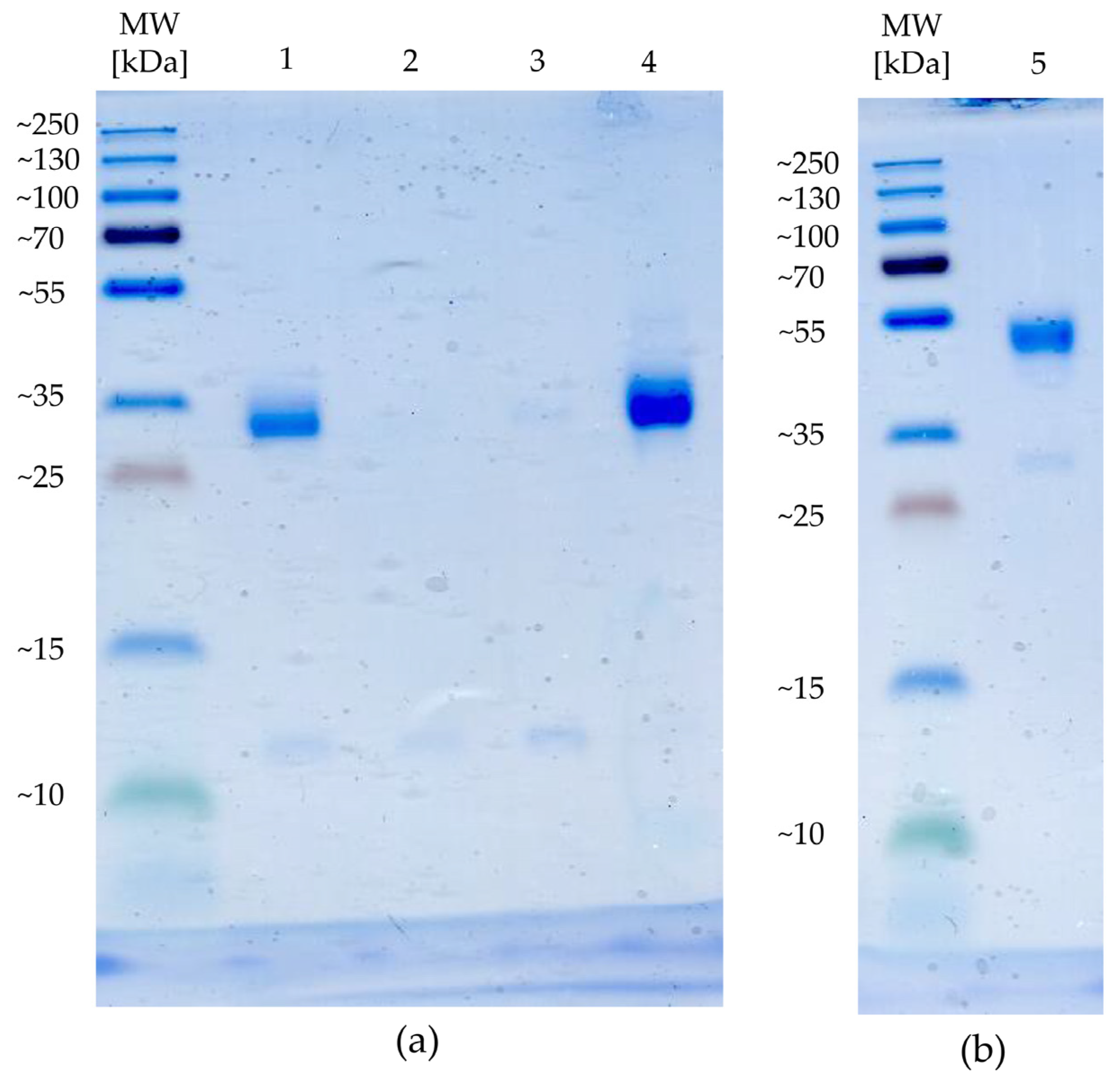
4.1. Target Preparation
4.2. Oligonucleotides and Semiautomatic In Vitro Selection
4.3. Electrophoretic Mobility Shift Assay (EMSA)
4.4. Fluorescent Dye-Linked Aptamer Assay (FLAA)
4.5. Surface Plasmon Resonance Spectroscopy
4.6. Microscale Thermophoresis
4.7. uPA Inhibition Assay
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Aptamer | EMSA | |
|---|---|---|
| HMW−uPA | LMW−uPA | |
| uPAapt−01 | + | + |
| uPAapt−02 | + | + |
| uPAapt−03 | + | (+) |
| uPAapt−06 | + | - |
| uPAapt-08 | + | - |
| uPAapt−21 | + | - |
| uPAapt−26 | + | - |
| uPAapt−27 | + | - |
| Aptamer | Target | ka [M−1 s−1] | Kd [s−1] | KD [M] | Chi2 |
|---|---|---|---|---|---|
| uPAapt−01 | HMW−uPA | 6.83 × 105 | 5.15 × 10−4 | 7.54 × 10−10 | 1.48 |
| LMW−uPA | 7.24 × 104 | 1.57 × 10−3 | 2.17 × 10−8 | 0.02 | |
| uPAapt−01−FR | HMW−uPA | 9.64 × 103 | 3.98 × 10−3 | 4.13 × 10−7 | 6.33 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−02 | HMW−uPA | 1.28 × 106 | 2.77 × 10−4 | 2.17 × 10−10 | 1.49 |
| LMW−uPA | 4.85 × 104 | 1.43 × 10−4 | 2.95 × 10−9 | 0.03 | |
| uPAapt−02−R | HMW−uPA | 8.98 × 105 | 2.93 × 10−4 | 3.26 × 10−10 | 6.98 |
| LMW−uPA | 6.55 × 104 | 3.75 × 10−4 | 5.73 × 10−9 | 0.02 | |
| uPAapt−02−FR | HMW−uPA | 2.42 × 105 | 1.72 × 10−4 | 7.08 × 10−10 | 1.80 |
| LMW−uPA | 2.47 × 104 | 5.30 × 10−4 | 2.14 × 10−8 | 0.02 | |
| uPAapt−03 | HMW−uPA | 1.94 × 105 | 6.50 × 10−4 | 3.34 × 10−9 | 0.02 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−06 | HMW−uPA | 9.39 × 104 | 9.99 × 10−4 | 1.06 × 10−8 | 3.55 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−08 | HMW−uPA | 2.14 × 104 | 8.53 × 10−4 | 4.00 × 10−8 | 0.20 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−08−FR | HMW−uPA | 7.77 × 102 | 1.57 × 10−4 | 2.02 × 10−7 | 0.47 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−21 | HMW−uPA | 1.58 × 105 | 5.93 × 10−4 | 3.77 × 10−9 | 0.51 |
| LMW−uPA | 4.74 × 104 | 2.78 × 10−3 | 5.86 × 10−8 | 0.05 | |
| uPAapt−26 | HMW−uPA | 1.90 × 105 | 2.46 × 10−4 | 1.30 × 10−9 | 0.64 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−27 | HMW−uPA | 2.84 × 105 | 1.31 × 10−3 | 4.59 × 10−9 | 0.11 |
| LMW−uPA | -* | -* | -* | -* | |
| uPAapt−27−FR | HMW−uPA | 1.93 × 105 | 1.07 × 10−3 | 5.54 × 10−9 | 0.23 |
| LMW−uPA | -* | -* | -* | -* | |
| Con | HMW−uPA | -* | -* | -* | -* |
| LMW−uPA | -* | -* | -* | -* |
References
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Higazi, A.; Cohen, R.L.; Henkin, J.; Kniss, D.; Schwartz, B.S.; Cines, D.B. Enhancement of the enzymatic activity of single-chain urokinase plasminogen activator by soluble urokinase receptor. J. Biol. Chem. 1995, 270, 17375–17380. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.C.; Lund, L.R.; Nielsen, L.S.; Danø, K.; Skriver, L. One-chain urokinase-type plasminogen activator from human sarcoma cells is a proenzyme with little or no intrinsic activity. J. Biol. Chem. 1988, 263, 11189–11195. [Google Scholar] [CrossRef]
- Mekkawy, A.H.; Pourgholami, M.H.; Morris, D.L. Involvement of urokinase-type plasminogen activator system in cancer: An overview. Med. Res. Rev. 2014, 34, 918–956. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Di Carluccio, G.; Motti, M.L.; Carriero, M.V. Therapeutic Strategies Targeting Urokinase and Its Receptor in Cancer. Cancers 2022, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.M.; Madsen, J.B.; Kristensen, T.; Bodker, J.S.; Blouse, G.E.; Wind, T.; Andreasen, P.A. Biochemical properties of plasminogen activator inhibitor-1. Front. Biosci. 2009, 14, 1337–1361. [Google Scholar] [CrossRef]
- Li Santi, A.; Napolitano, F.; Montuori, N.; Ragno, P. The Urokinase Receptor: A Multifunctional Receptor in Cancer Cell Biology. Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 4111. [Google Scholar] [CrossRef]
- Su, S.-C.; Lin, C.-W.; Yang, W.-E.; Fan, W.-L.; Yang, S.-F. The urokinase-type plasminogen activator (uPA) system as a biomarker and therapeutic target in human malignancies. Expert Opin. Ther. Targets 2016, 20, 551–566. [Google Scholar] [CrossRef]
- Dass, K.; Ahmad, A.; Azmi, A.S.; Sarkar, S.H.; Sarkar, F.H. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 2008, 34, 122–136. [Google Scholar] [CrossRef]
- Setyono-Han, B.; Stürzebecher, J.; Schmalix, W.A.; Muehlenweg, B.; Sieuwerts, A.M.; Timmermans, M.; Magdolen, V.; Schmitt, M.; Klijn, J.G.M.; Foekens, J.A. Suppression of rat breast cancer metastasis and reduction of primary tumour growth by the small synthetic urokinase inhibitor WX-UK1. Thromb. Haemost. 2005, 93, 779–786. [Google Scholar] [CrossRef]
- Ossowski, L.; Russo-Payne, H.; Wilson, E.L. Inhibition of urokinase-type plasminogen activator by antibodies: The effect on dissemination of a human tumor in the nude mouse. Cancer Res. 1991, 51, 274–281. [Google Scholar] [PubMed]
- Ma, D.; Gerard, R.D.; Li, X.Y.; Alizadeh, H.; Niederkorn, J.Y. Inhibition of metastasis of intraocular melanomas by adenovirus-mediated gene transfer of plasminogen activator inhibitor type 1 (PAI-1) in an athymic mouse model. Blood 1997, 90, 2738–2746. [Google Scholar] [CrossRef] [PubMed]
- Muehlenweg, B.; Sperl, S.; Magdolen, V.; Schmitt, M.; Harbeck, N. Interference with the urokinase plasminogen activator system: A promising therapy concept for solid tumours. Expert Opin. Biol. Ther. 2001, 1, 683–691. [Google Scholar] [CrossRef]
- Schmitt, M.; Harbeck, N.; Brünner, N.; Jänicke, F.; Meisner, C.; Mühlenweg, B.; Jansen, H.; Dorn, J.; Nitz, U.; Kantelhardt, E.J.; et al. Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev. Mol. Diagn. 2011, 11, 617–634. [Google Scholar] [CrossRef]
- Zhu, F.; Jia, S.; Xing, G.; Gao, L.; Zhang, L.; He, F. cDNA transfection of amino-terminal fragment of urokinase efficiently inhibits cancer cell invasion and metastasis. DNA Cell Biol. 2001, 20, 297–305. [Google Scholar] [CrossRef]
- Sato, S.; Kopitz, C.; Schmalix, W.A.; Muehlenweg, B.; Kessler, H.; Schmitt, M.; Krüger, A.; Magdolen, V. High-affinity urokinase-derived cyclic peptides inhibiting urokinase/urokinase receptor-interaction: Effects on tumor growth and spread. FEBS Lett. 2002, 528, 212–216. [Google Scholar] [CrossRef]
- Dupont, D.M.; Andersen, L.M.; Botkjaer, K.A.; Andreasen, P.A. Nucleic acid aptamers against proteases. Curr. Med. Chem. 2011, 18, 4139–4151. [Google Scholar] [CrossRef]
- Liu, M.; Zaman, K.; Fortenberry, Y.M. Overview of the Therapeutic Potential of Aptamers Targeting Coagulation Factors. Int. J. Mol. Sci. 2021, 22, 3897. [Google Scholar] [CrossRef]
- Dupont, D.M.; Madsen, J.B.; Hartmann, R.K.; Tavitian, B.; Ducongé, F.; Kjems, J.; Andreasen, P.A. Serum-stable RNA aptamers to urokinase-type plasminogen activator blocking receptor binding. RNA 2010, 16, 2360–2369. [Google Scholar] [CrossRef]
- Botkjaer, K.A.; Deryugina, E.I.; Dupont, D.M.; Gårdsvoll, H.; Bekes, E.M.; Thuesen, C.K.; Chen, Z.; Ploug, M.; Quigley, J.P.; Andreasen, P.A. Targeting Tumor Cell Invasion and Dissemination In Vivo by an Aptamer That Inhibits Urokinase-type Plasminogen Activator through a Novel Multifunctional Mechanism. Mol. Cancer Res. 2012, 10, 1532–1543. [Google Scholar] [CrossRef]
- Skrypina, N.A.; Savochkina, L.P.; Beabealashvilli, R.S. In Vitro Selection of Single-Stranded DNA Aptamers that Bind Human Pro-Urokinase. Nucleosides Nucleotides Nucleic Acids 2004, 23, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Thevendran, R.; Citartan, M. Assays to Estimate the Binding Affinity of Aptamers. Talanta 2022, 238, 122971. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.M.; Thuesen, C.K.; Bøtkjær, K.A.; Behrens, M.A.; Dam, K.; Sørensen, H.P.; Pedersen, J.S.; Ploug, M.; Jensen, J.K.; Andreasen, P.A. Protein-binding RNA aptamers affect molecular interactions distantly from their binding sites. PLoS ONE 2015, 10, e0119207. [Google Scholar] [CrossRef][Green Version]
- Dupont, D.M.; Madsen, J.B.; Hartmann, R.K.; Tavitian, B.; Ducongé, F.; Kjems, J.; Andreasen, P.A. Protein-binding RNA aptamers affect molecular interactions distantly from their binding sites. PLoS ONE 2015, 10, e0119207, Correction in PLoS ONE 2015, 10, e0126782. [Google Scholar] [CrossRef][Green Version]
- Schmidt, C.; Perbandt, M.; Klussmann, S.; Betzel, C. Molecular characterization of a ghrelin-l-aptamer complex. J. Mol. Struct. 2020, 1204, 127510. [Google Scholar] [CrossRef]
- Wang, R.E.; Wu, H.; Niu, Y.; Cai, J. Improving the stability of aptamers by chemical modification. Curr. Med. Chem. 2011, 18, 4126–4138. [Google Scholar] [CrossRef]
- Nobuhara, M.; Sakamaki, M.; Ohnishi, H.; Suzuki, Y. A comparative study of high molecular weight urokinase and low molecular weight urokinase. J. Biochem. 1981, 90, 225–232. [Google Scholar] [CrossRef]
- Gallagher, S.R. One-dimensional SDS gel electrophoresis of proteins. Curr. Protoc. Mol. Biol. 2006, 10. [Google Scholar] [CrossRef]
- Wochner, A.; Cech, B.; Menger, M.; Erdmann, V.A.; Glökler, J. Semi-automated selection of DNA aptamers using magnetic particle handling. Biotechniques 2007, 43, 346–348. [Google Scholar] [CrossRef]
- Garner, M.M.; Revzin, A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: Application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981, 9, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Wochner, A.; Glökler, J. Nonradioactive fluorescence microtiter plate assay monitoring aptamer selections. Biotechniques 2007, 42, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Sass, S.; Stöcklein, W.F.M.; Klevesath, A.; Hurpin, J.; Menger, M.; Hille, C. Binding affinity data of DNA aptamers for therapeutic anthracyclines from microscale thermophoresis and surface plasmon resonance spectroscopy. Analyst 2019, 144, 6064–6073. [Google Scholar] [CrossRef] [PubMed]
- Breitsprecher, D.; Schlinck, N.; Witte, D.; Duhr, S.; Baaske, P.; Schubert, T. Aptamer Binding Studies Using MicroScale Thermophoresis. Methods Mol. Biol. 2016, 1380, 99–111. [Google Scholar] [CrossRef] [PubMed]



| Name | Variable Region (5′−3′) |
|---|---|
| uPAapt−01 | ATGGTAACATGCACTAGGTCGCGATGGTTCCGTCTGATCGCT |
| uPAapt−02 | CAAGCGGGGGTGAGAGATCTGTCAGTACGAGCTGGGTTTGCG |
| uPAapt−03 | ACGGAGGCAGAGGGTGAGAGATCTGTTAGTACTAGCCCATGT |
| uPAapt−06 | GGGTGGGTGGGTGGGTGATGGCTCGACTCTACCCTGGCGCTG |
| uPAapt−08 | CAGCGGTAGGGGTTATATAGCTGCGCCATAGGGTACTCGTG |
| uPAapt−21 | GGAGGTACTCACCGACGCTGAACTCCATAGAATGTGGTGATG |
| uPAapt−26 | ATCCGTGGCGTGGTTGGTGGGGGAGGTGGGCGGGTAGACGGT |
| uPAapt−27 | ATGGGGTGTTATGGGGGGGGCTTATTGCGGTCAGGGGACGAT |
| Aptamer | Target | KD (M) | KD Confidence (M) |
|---|---|---|---|
| 5′−Cy5−uPAapt−02−FR | HMW−uPA | 6.69 × 10−9 | ±1.55 × 10−9 |
| LMW−uPA | 9.40 × 10−9 | ±3.10 × 10−9 | |
| pro−uPA | 1.40 × 10−8 | ±0.65 × 10−8 | |
| mouse uPA | -* | -* | |
| tPA | -* | -* | |
| 5′−Cy5−uPAapt−21 | HMW−uPA | 2.08 × 10−7 | ±0.63 × 10−7 |
| LMW−uPA | 1.41 × 10−6 | ±0.45 × 10−6 |
| Aptamer | IC50 (±Standard Error) (M) |
|---|---|
| uPAapt−21 | 8.70 × 10−8 (±0.40 × 10−8) |
| uPAapt−26 | 1.65 × 10−7 (±0.16 × 10−7) |
| uPAapt−08 | 2.98 × 10−7 (±0.17 × 10−7) |
| Con | -* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreymann, N.; Wuensche, J.; Sabrowski, W.; Moeller, A.; Czepluch, D.; Vu Van, D.; Fuessel, S.; Menger, M.M. Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA. Int. J. Mol. Sci. 2022, 23, 4890. https://doi.org/10.3390/ijms23094890
Dreymann N, Wuensche J, Sabrowski W, Moeller A, Czepluch D, Vu Van D, Fuessel S, Menger MM. Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA. International Journal of Molecular Sciences. 2022; 23(9):4890. https://doi.org/10.3390/ijms23094890
Chicago/Turabian StyleDreymann, Nico, Julia Wuensche, Wiebke Sabrowski, Anja Moeller, Denise Czepluch, Dana Vu Van, Susanne Fuessel, and Marcus M. Menger. 2022. "Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA" International Journal of Molecular Sciences 23, no. 9: 4890. https://doi.org/10.3390/ijms23094890
APA StyleDreymann, N., Wuensche, J., Sabrowski, W., Moeller, A., Czepluch, D., Vu Van, D., Fuessel, S., & Menger, M. M. (2022). Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA. International Journal of Molecular Sciences, 23(9), 4890. https://doi.org/10.3390/ijms23094890






