The Impact of Mouse Preterm Birth Induction by RU-486 on Microglial Activation and Subsequent Hypomyelination
Abstract
:1. Introduction
2. Results
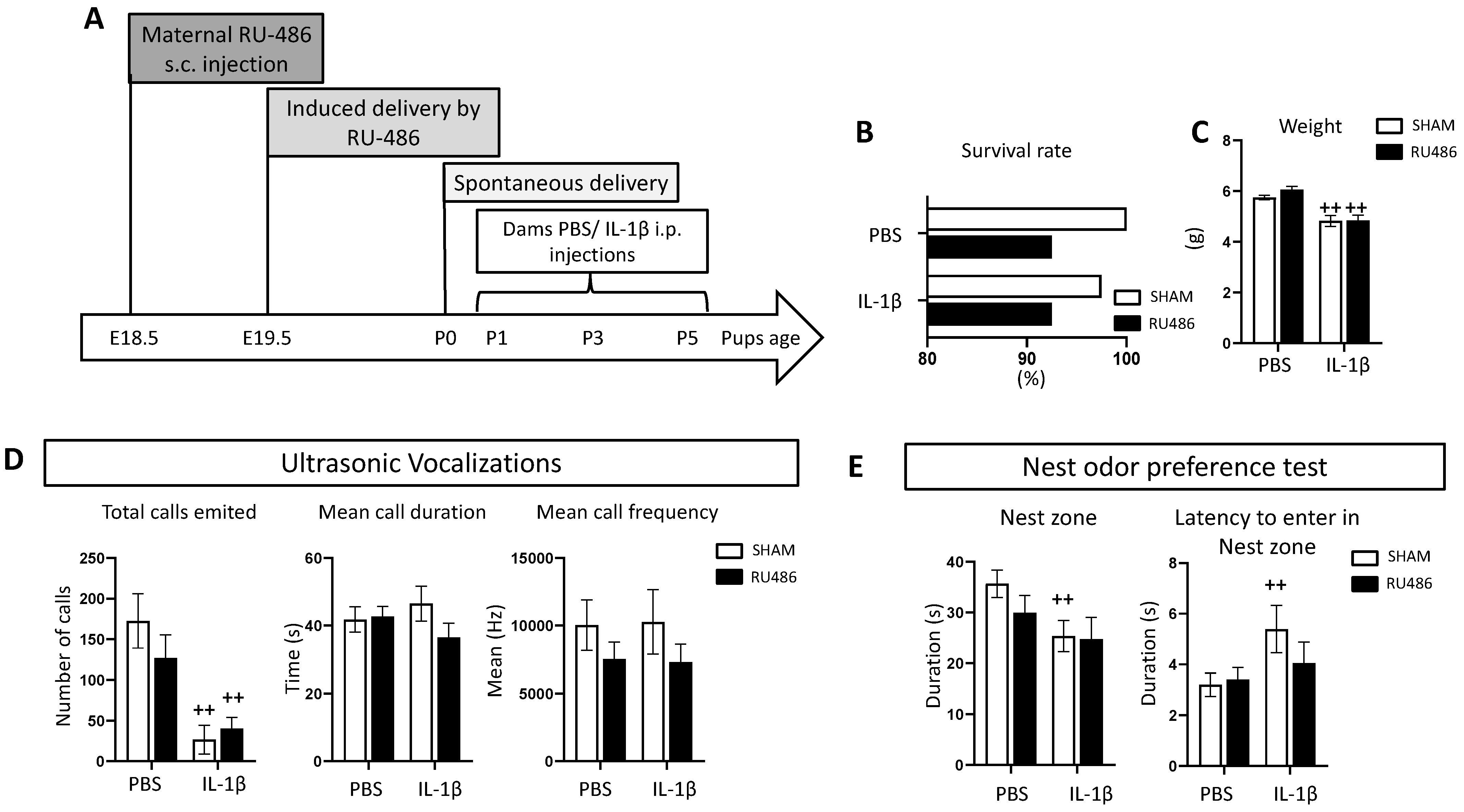
2.1. Effect of RU-486 In Vivo on Dam Survival, Weight, and Juvenile Behavior
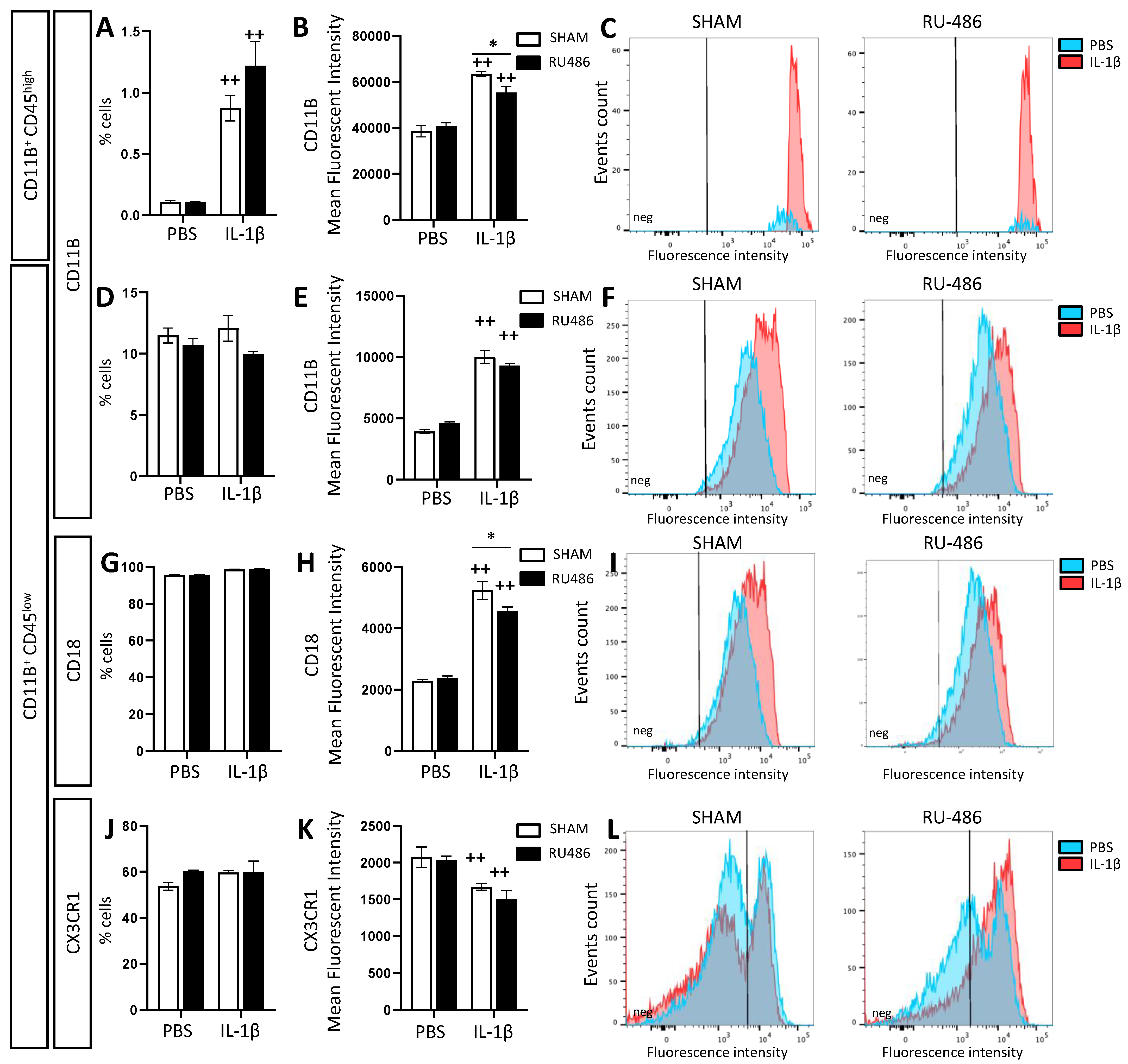
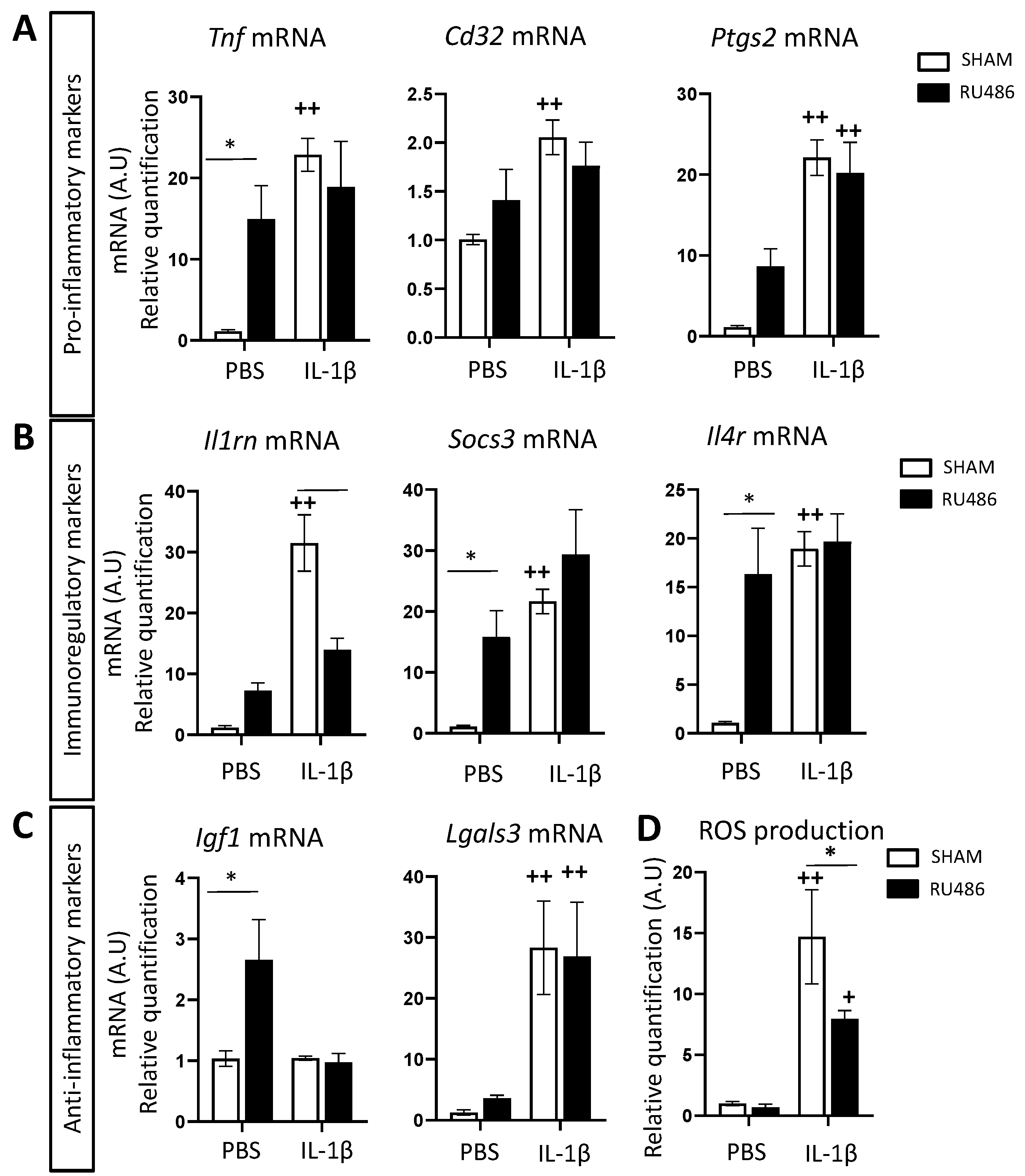
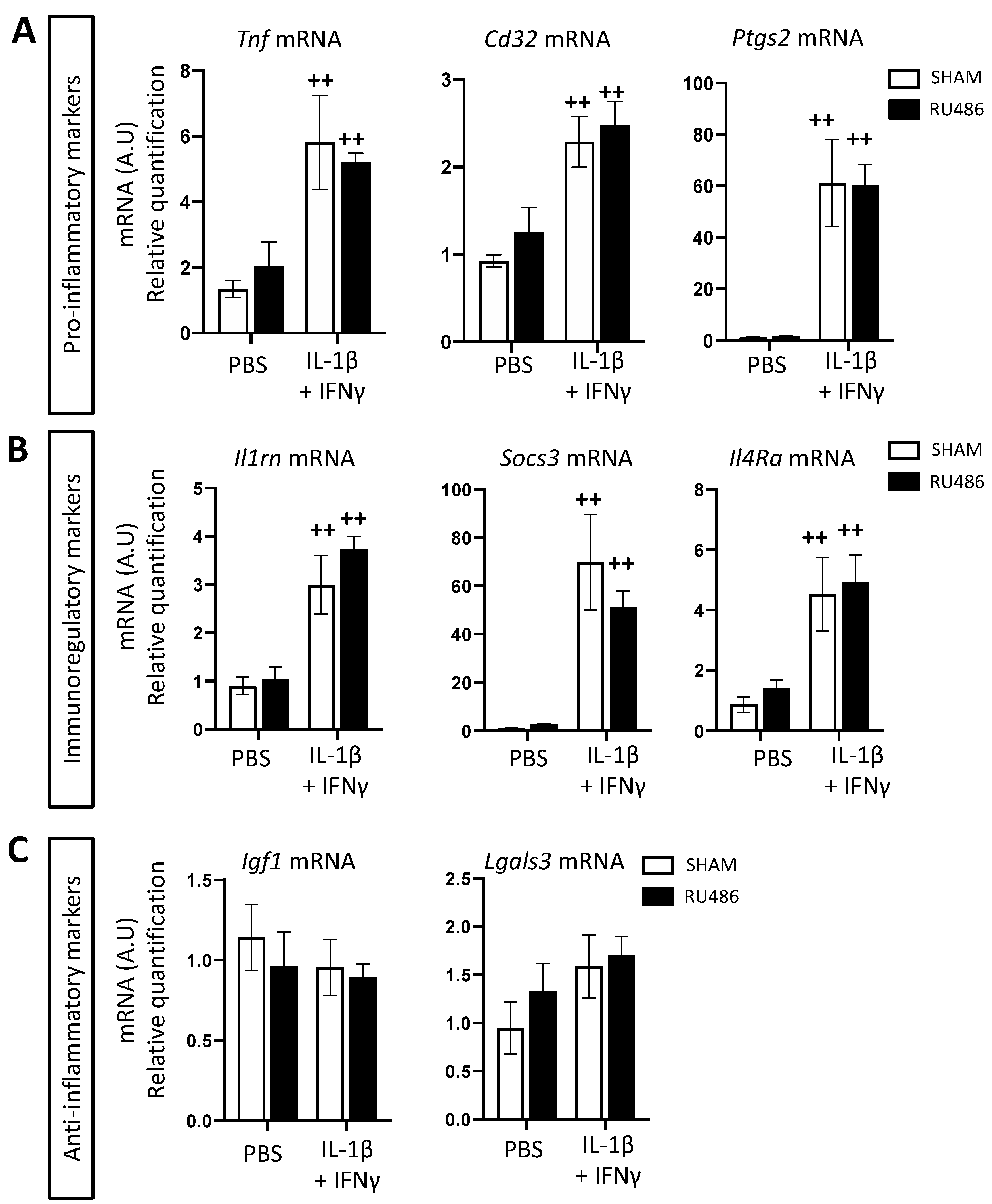
2.2. Effect of RU-486 on Microglial Reactivity In Vivo
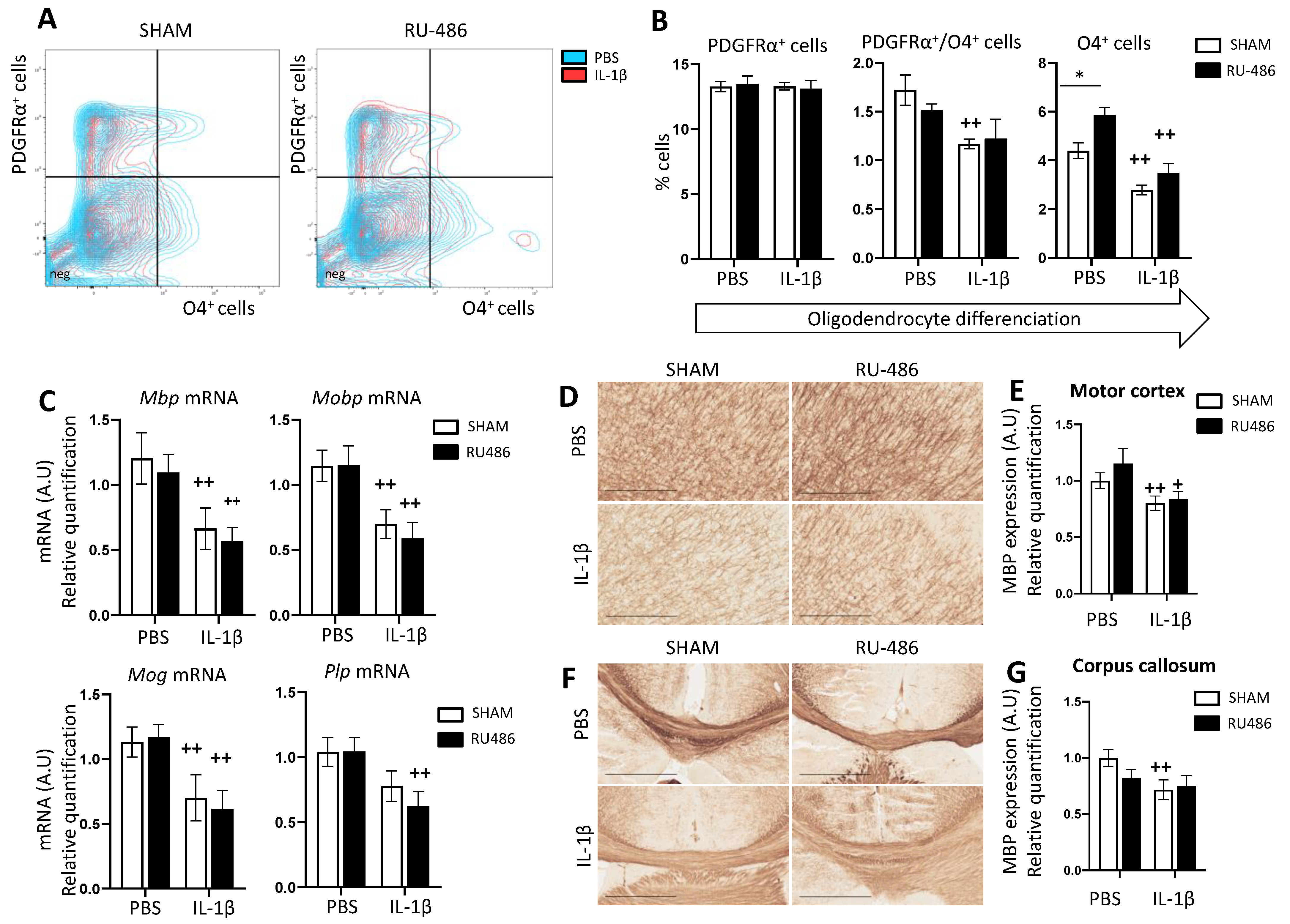
2.3. RU-486 Impacts on Oligodendrocyte Differentiation and Subsequent Myelination
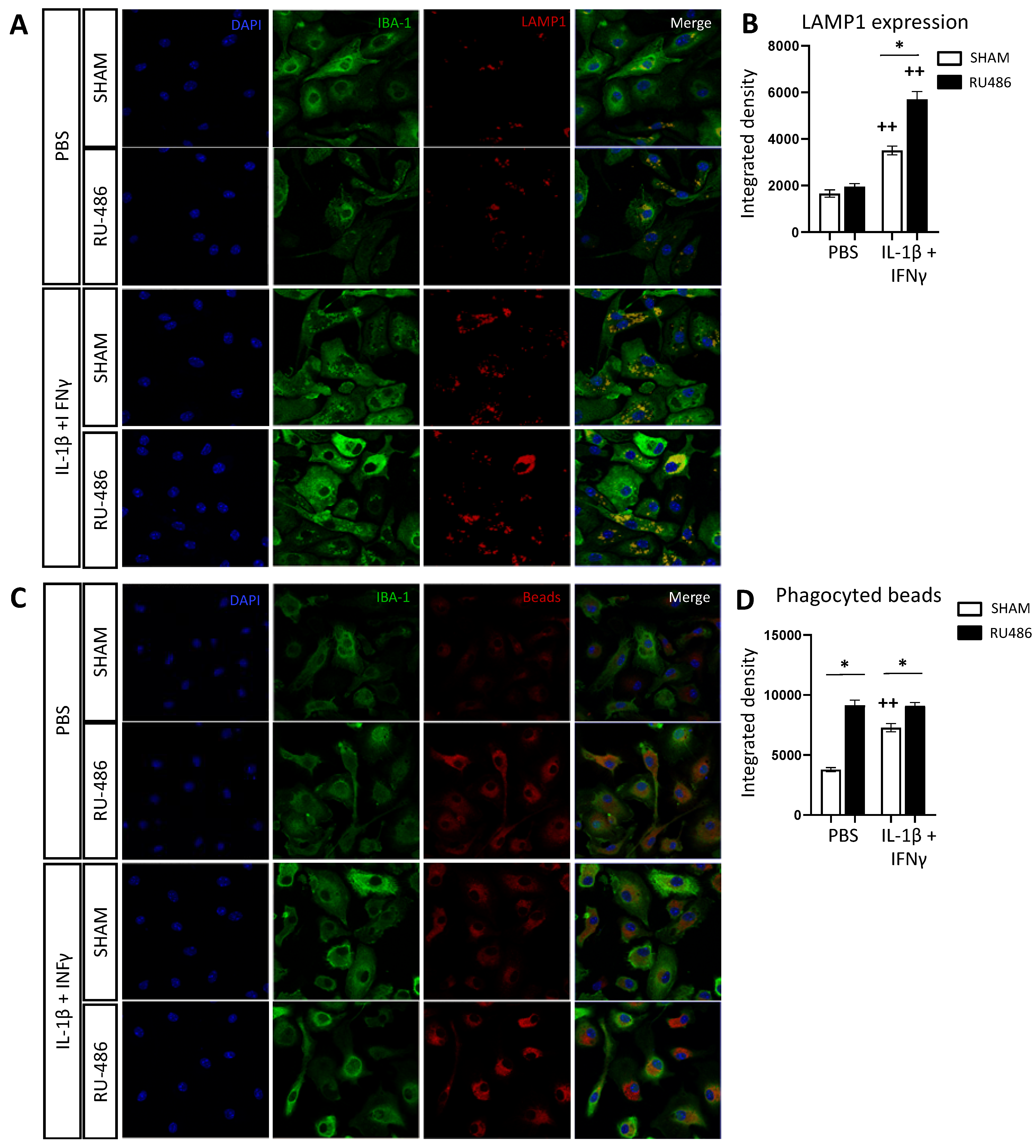
2.4. RU-486 Increased Microglia Phagocytosis In Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Handling, Labor Induction by RU-486, and EoP Model
4.2. Brain Collection, Dissociation, and Microglia Magnetic Cell Sorting
4.3. Ex Vivo Microglia Cell Culture
4.4. RNA Extraction and Real-Time qPCR
4.5. Measurements of Reactive Oxygen Species Production by Luminometry
4.6. Flow Cytometry Analyses
4.7. Immunofluorescence
4.8. Immunohistochemistry
4.9. Behavioral Tests
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierrat, V.; Marchand-Martin, L.; Marret, S.; Arnaud, C.; Benhammou, V.; Cambonie, G.; Debillon, T.; Dufourg, M.-N.; Gire, C.; Goffinet, F.; et al. Neurodevelopmental outcomes at age 5 among children born preterm: EPIPAGE-2 cohort study. BMJ 2021, 373, n741. [Google Scholar] [CrossRef] [PubMed]
- Penn, A.A.; Gressens, P.; Fleiss, B.; Back, S.A.; Gallo, V. Controversies in preterm brain injury. Neurobiol. Dis. 2015, 92, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moster, D.; Lie, R.T.; Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Bokobza, C.; Van Steenwinckel, J.; Mani, S.; Mezger, V.; Fleiss, B.; Gressens, P. Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2018, 85, 155–165. [Google Scholar] [CrossRef]
- Volpe, J.J. Neurobiology of Periventricular Leukomalacia in the Premature Infant. Pediatr. Res. 2001, 50, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favrais, G.; van de Looij, Y.; Fleiss, B.; Ramanantsoa, N.; Bonnin, P.; Stoltenburg-Didinger, G.; Lacaud, A.; Saliba, E.; Dammann, O.; Gallego, J.; et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011, 70, 550–565. [Google Scholar] [CrossRef]
- Bennet, L.; Dhillon, S.; Lear, C.A.; van den Heuij, L.; King, V.; Dean, J.M.; Wassink, G.; Davidson, J.O.; Gunn, A.J. Chronic inflammation and impaired development of the preterm brain. J. Reprod. Immunol. 2018, 125, 45–55. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Hagberg, H.; Gressens, P.; Mallard, C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2011, 71, 444–457. [Google Scholar] [CrossRef]
- Verney, C.; Pogledic, I.; Biran, V.; Adle-Biassette, H.; Fallet-Bianco, C.; Gressens, P. Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J. Neuropathol. Exp. Neurol. 2012, 71, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verney, C.; Monier, A.; Fallet-Bianco, C.; Gressens, P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J. Anat. 2010, 217, 436–448. [Google Scholar] [CrossRef]
- Michell-Robinson, M.; Touil, H.; Healy, L.; Owen, D.; Durafourt, B.; Bar-Or, A.; Antel, J.; Moore, C.S. Roles of microglia in brain development, tissue maintenance and repair. Brain 2015, 138, 1138–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, M.L.; Van Steenwinckel, J.; Schang, A.-L.; Yan, J.; Arnadottir, J.; Le Charpentier, T.; Csaba, Z.; Dournaud, P.; Cipriani, S.; Auvynet, C.; et al. Integrative genomics of microglia implicates DLG4 (PSD95) in the white matter development of preterm infants. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwinckel, J.; Schang, A.L.; Krishnan, M.L.; Degos, V.; Delahaye-Duriez, A.; Bokobza, C.; Csaba, Z.; Verdonk, F.; Montané, A.; Sigaut, S.; et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain J. Neurol. 2019, 142, 3806–3833. [Google Scholar] [CrossRef]
- Bokobza, C.; Joshi, P.; Schang, A.; Csaba, Z.; Faivre, V.; Montané, A.; Galland, A.; Benmamar-Badel, A.; Bs, E.B.; Lebon, S.; et al. miR -146b Protects the Perinatal Brain against Microglia-Induced Hypomyelination. Ann. Neurol. 2021, 91, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- McCarthy, R.; Martin-Fairey, C.; Sojka, D.K.; Herzog, E.D.; Jungheim, E.S.; Stout, M.J.; Fay, J.C.; Mahendroo, M.; Reese, J.; Herington, J.L.; et al. Mouse models of preterm birth: Suggested assessment and reporting guidelines†. Biol. Reprod. 2018, 99, 922–937. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, E.; Saotome, I.; Hirsch, D. A model of intrauterine infection and preterm delivery in mice. Am. J. Obstet. Gynecol. 1995, 172, 1598–1603. [Google Scholar] [CrossRef]
- Kaga, N.; Katsuki, Y.; Obata, M.; Shibutani, Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: A model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am. J. Obstet. Gynecol. 1996, 174, 754–759. [Google Scholar] [CrossRef]
- Dudley, D.J.; Branch, D.W.; Edwin, S.S.; Mitchell, M. Induction of preterm birth in mice by RU486. Biol. Reprod. 1996, 55, 992–995. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Mazor, M.; Tartakovsky, B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am. J. Obstet. Gynecol. 1991, 165, 969–971. [Google Scholar] [CrossRef]
- Hapangama, D.; Neilson, J.P. Mifepristone for induction of labour. Cochrane Database Syst. Rev. 2009, 2009, CD002865. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.C.W.; Selinger, M.; Ferguson, J.; MacKenzie, I.Z. The placental transfer of mifepristone (RU 486) during the second trimester and its influence upon maternal and fetal steroid concentrations. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.P.; Chillik, C.F.; Itskovitz, J.; Weyman, D.; Anderson, T.L.; Ulmann, A.; Baulieu, E.E.; Hodgen, G.D. Transplacental passage of a progesterone antagonist in monkeys. Am. J. Obstet. Gynecol. 1988, 159, 238–242. [Google Scholar] [CrossRef]
- Castillo-Ruiz, A.; Hite, T.A.; Yakout, D.W.; Rosen, T.J.; Forger, N.G. Does Birth Trigger Cell Death in the Developing Brain? eNeuro 2020, 7, NEURO.0517-19.2020. [Google Scholar] [CrossRef] [Green Version]
- Oligino, T.; Poliani, P.; Wang, Y.; Tsai, S.; O’Malley, B.; Fink, D.; Glorioso, J. Drug inducible transgene expression in brain using a herpes simplex virus vector. Gene Ther. 1998, 5, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Sierra, A.; Gottfried-Blackmore, A.; Milner, T.A.; McEwen, B.S.; Bulloch, K. Steroid hormone receptor expression and function in microglia. Glia 2008, 56, 659–674. [Google Scholar] [CrossRef]
- Carrillo-de Sauvage, M.Á.; Maatouk, L.; Arnoux, I.; Pasco, M.; Sanz Diez, A.; Delahaye, M.; Herrero, M.T.; Newman, T.A.; Calvo, C.F.; Audinat, E.; et al. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 2013, 20, 1546–1557. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Xu, H.; Chai, J.; Ofori, E.; Elovitz, M.A. Preterm and term cervical ripening in CD1 Mice (Mus musculus): Similar or divergent molecular mechanisms? Biol. Reprod. 2009, 81, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Burd, I.; Bentz, A.I.; Chai, J.; Gonzalez, J.; Monnerie, H.; Le Roux, P.D.; Cohen, A.S.; Yudkoff, M.; Elovitz, M. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J. Neurosci. Res. 2010, 88, 1872–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouda, J.; Wöhr, M.; Del Rey, A. Immunity and ultrasonic vocalization in rodents. Ann. N. Y. Acad. Sci. 2018, 1437, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Shiow, L.R.; Favrais, G.; Schirmer, L.; Schang, A.-L.; Cipriani, S.; Andres, C.; Wright, J.; Nobuta, H.; Fleiss, B.; Gressens, P.; et al. Reactive astrocyte COX2-PGE2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia 2017, 65, 2024–2037. [Google Scholar] [CrossRef]
- Calvo, B.; Rubio-Arranz, F.; Fernández, M.; Tranque, P. Dissociation of neonatal and adult mice brain for simultaneous analysis of microglia, astrocytes and infiltrating lymphocytes by flow cytometry. IBRO Rep. 2020, 8, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef] [Green Version]
- Kurpius, D.; Wilson, N.; Fuller, L.; Hoffman, A.; Dailey, M.E. Early activation, motility, and homing of neonatal microglia to injured neurons does not require protein synthesis. Glia 2006, 54, 58–70. [Google Scholar] [CrossRef]
- Walter, T.J.; Vetreno, R.P.; Crews, F.T. Alcohol and Stress Activation of Microglia and Neurons: Brain Regional Effects. Alcohol. Clin. Exp. Res. 2017, 41, 2066–2081. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Cornell, J.; Salinas, S.; Huang, H.-Y. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705. [Google Scholar] [CrossRef] [PubMed]
- Chhor, V.; Le Charpentier, T.; Lebon, S.; Oré, M.-V.; Celador, I.L.; Josserand, J.; Degos, V.; Jacotot, E.; Hagberg, H.; Sävman, K.; et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 2013, 32, 70–85. [Google Scholar] [CrossRef]
- Nayernia, Z.; Jaquet, V.; Krause, K.-H. New Insights on NOX Enzymes in the Central Nervous System. Antioxid. Redox Signal. 2014, 20, 2815–2837. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, F.; Pezzè, L.; Ciribilli, Y. LAMPs: Shedding light on cancer biology. Semin. Oncol. 2017, 44, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Von Zahn, J.; Möller, T.; Kettenmann, H.; Nolte, C. Microglial phagocytosis is modulated by pro- and anti-inflammatory cytokines. Neuroreport 1997, 8, 3851–3856. [Google Scholar] [CrossRef] [PubMed]
- Canini, M.; Cavoretto, P.; Scifo, P.; Pozzoni, M.; Petrini, A.; Iadanza, A.; Pontesilli, S.; Scotti, R.; Candiani, M.; Falini, A.; et al. Subcortico-Cortical Functional Connectivity in the Fetal Brain: A Cognitive Development Blueprint. Cereb. Cortex Commun. 2020, 1, tgaa008. [Google Scholar] [CrossRef] [Green Version]
- Della Rosa, P.A.; Miglioli, C.; Caglioni, M.; Tiberio, F.; Mosser, K.H.H.; Vignotto, E.; Canini, M.; Baldoli, C.; Falini, A.; Candiani, M.; et al. A hierarchical procedure to select intrauterine and extrauterine factors for methodological validation of preterm birth risk estimation. BMC Pregnancy Childbirth. 2021, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.H.; McAllister, A.; Flynn, A.N.; Martin, B.; Jiang, E.; Koelper, N.; Schreiber, C.A. The effect of mifepristone pretreatment on bleeding and pain during medical management of early pregnancy loss. Contraception 2021, 104, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Hjelm, B.; Grunseich, C.; Gowing, G.; Avalos, P.; Tian, J.; Shelley, B.C.; Mooney, M.; Narwani, K.; Shi, Y.; Svendsen, C.N.; et al. Mifepristone-inducible transgene expression in neural progenitor cells in vitro and in vivo. Gene Ther. 2016, 23, 424–437. [Google Scholar] [CrossRef] [Green Version]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA—Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2011, 26, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Glezer, I.; Rivest, S. Oncostatin M is a novel glucocorticoid-dependent neuroinflammatory factor that enhances oligodendrocyte precursor cell activity in demyelinated sites. Brain Behav. Immun. 2010, 24, 695–704. [Google Scholar] [CrossRef]
- Baud, O. Postnatal steroid treatment in preterm infants: Risk/benefit ratio. J. Gynecol. Obstet. Biol. Reprod. 2005, 34 (Suppl. 1), S118–S126. [Google Scholar] [CrossRef]
- Zia, M.T.; Vinukonda, G.; Vose, L.R.; Bhimavarapu, B.B.; Iacobas, S.; Pandey, N.K.; Beall, A.M.; Dohare, P.; LaGamma, E.F.; Iacobas, D.A.; et al. Postnatal glucocorticoid-induced hypomyelination, gliosis, and neurologic deficits are dose-dependent, preparation-specific, and reversible. Exp. Neurol. 2014, 263, 200–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vielkind, U.; Walencewicz, A.; Levine, J.M.; Bohn, M.C. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J. Neurosci. Res. 1990, 27, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 2: Mechanisms. Nat. Rev. Endocrinol. 2014, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zeger, M.; Popken, G.; Zhang, J.; Xuan, S.; Lu, Q.R.; Schwab, M.H.; Nave, K.A.; Rowitch, D.; D’Ercole, A.J.; Ye, P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia 2007, 55, 400–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, A.N.; Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 2020, 23, 1055–1066. [Google Scholar] [CrossRef]
- Audinat, E.; Arnoux, I. Microglia: Immune cells sculpting and controlling neuronal synapses. Med. Sci. M/S 2014, 30, 153–159. [Google Scholar]
- Horchar, M.J.; Wohleb, E.S. Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain Behav. Immun. 2019, 81, 329–340. [Google Scholar] [CrossRef]
- Baccan, G.; Oliveira, R.D.; Mantovani, B. Stress and immunological phagocytosis: Possible nongenomic action of corticosterone. Life Sci. 2004, 75, 1357–1368. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sood, A.; Shukla, R.; Hanif, K. AT2R Activation Prevents Microglia Pro-inflammatory Activation in a NOX-Dependent Manner: Inhibition of PKC Activation and p47phox Phosphorylation by PP2A. Mol. Neurobiol. 2018, 56, 3005–3023. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Chaumont, F.; Lemière, N.; Coqueran, S.; Bourgeron, T.; Ey, E. LMT USV Toolbox, a Novel Methodological Approach to Place Mouse Ultrasonic Vocalizations in Their Behavioral Contexts-A Study in Female and Male C57BL/6J Mice and in Shank3 Mutant Females. Front. Behav. Neurosci. 2021, 15, 735920. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Description | Forward | Reverse |
|---|---|---|---|
| Rpl13a | Ribosomal protein L13 a | ACA GCC ACT CTG GAG GAG AA | GAG TCC GTT GGT CTT GAG GA |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 (=Cox2) | TCA TTC ACC AGA CAG ATT GCT | AAG CGT TTG CGG TAC TCA TT |
| Tnf | Tumor necrosis factor | GCC TCT TCT CAT TCC TGC TT | AGG GTC TGG GCC ATA GAA CT |
| Il1rn | Interleukin 1 receptor antagonist | TTG TGC CAA GTC TGG AGA TG | TTC TCA GAG CGG ATG AAG GT |
| Il4ra | Interleukin 4 receptor alpha | GGA TAA GCA GAC CCG AAG C | ACT CTG GAG AGA CTT GGT TGG |
| Socs3 | Suppressor of cytokines 3 | CGT TGA CAG TCT TCC GAC AA | TAT TCT GGG GGC GAG AAG AT |
| Lgals3 | Lectin galactoside-binding soluble 3 | GAT CAC AAT CAT GGG CAC AG | ATT GAA GCG GGG GTT AAA GT |
| Igf1 | Insulin-like growth factor 1 | TGG ATG CTC TTC AGT TCG TG | GCA ACA CTC ATC CAC AAT GC |
| Mbp | Myelin basic protein | CCG GAC CCA AGA TGA AAA C | CTT GGG ATG GAG GTG GTG T |
| Mog | Myelin-oligodendrocyte glycoprotein | AAG AGG CAG CAA TGG AGT TG | GAC CTG CAG GAG GAT |
| Plp | Proteolipid protein | CCA AAT GAC CTT CCA CCT GT | CGA AGT TGT AAG TGG CAG CA |
| Mobp | Myelin-associated oligodendrocyte basic protein | TCCACAGGAAC CTTTCACAA | TCCT GGCCATTTTCTGACT |
| Cd32 | Cluster of differentiation 32 | CTG GAA GAA GCT GCC AAA AC | CCA ATG CCA AGG GAG ACT AA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, C.; Guenoun, D.; Sautet, I.; Faivre, V.; Csaba, Z.; Schwendimann, L.; Young-Ten, P.; Van Steenwinckel, J.; Gressens, P.; Bokobza, C. The Impact of Mouse Preterm Birth Induction by RU-486 on Microglial Activation and Subsequent Hypomyelination. Int. J. Mol. Sci. 2022, 23, 4867. https://doi.org/10.3390/ijms23094867
Morin C, Guenoun D, Sautet I, Faivre V, Csaba Z, Schwendimann L, Young-Ten P, Van Steenwinckel J, Gressens P, Bokobza C. The Impact of Mouse Preterm Birth Induction by RU-486 on Microglial Activation and Subsequent Hypomyelination. International Journal of Molecular Sciences. 2022; 23(9):4867. https://doi.org/10.3390/ijms23094867
Chicago/Turabian StyleMorin, Cécile, David Guenoun, Irvin Sautet, Valérie Faivre, Zsolt Csaba, Leslie Schwendimann, Pierrette Young-Ten, Juliette Van Steenwinckel, Pierre Gressens, and Cindy Bokobza. 2022. "The Impact of Mouse Preterm Birth Induction by RU-486 on Microglial Activation and Subsequent Hypomyelination" International Journal of Molecular Sciences 23, no. 9: 4867. https://doi.org/10.3390/ijms23094867
APA StyleMorin, C., Guenoun, D., Sautet, I., Faivre, V., Csaba, Z., Schwendimann, L., Young-Ten, P., Van Steenwinckel, J., Gressens, P., & Bokobza, C. (2022). The Impact of Mouse Preterm Birth Induction by RU-486 on Microglial Activation and Subsequent Hypomyelination. International Journal of Molecular Sciences, 23(9), 4867. https://doi.org/10.3390/ijms23094867






