Abstract
Chemoradiation-induced mucositis is a debilitating condition of the gastrointestinal tract eventuating from antineoplastic treatment. It is believed to occur primarily due to oxidative stress mechanisms, which generate Reactive Oxygen Species (ROS). The aim of this scoping review was to assess the role of oxidative stress in the development of Oral Mucositis (OM). Studies from the literature, published in MEDLINE and SCOPUS, that evaluated the oxidative stress pathways or antioxidant interventions for OM, were retrieved to elucidate the current understanding of their relationship. Studies failing inclusion criteria were excluded, and those suitable underwent data extraction, using a predefined data extraction table. Eighty-nine articles fulfilled criteria, and these were sub-stratified into models of study (in vitro, in vivo, or clinical) for evaluation. Thirty-five clinical studies evaluated antioxidant interventions on OM’s severity, duration, and pain, amongst other attributes. A number of clinical studies sought to elucidate the protective or therapeutic effects of compounds that had been pre-determined to have antioxidant properties, without directly assessing oxidative stress parameters (these were deemed “indirect evidence”). Forty-seven in vivo studies assessed the capacity of various compounds to prevent OM. Findings were mostly consistent, reporting reduced OM severity associated with a reduction in ROS, malondialdehyde (MDA), myeloperoxidase (MPO), but higher glutathione (GSH) and superoxide dismutase (SOD) activity or expression. Twenty-one in vitro studies assessed potential OM therapeutic interventions. The majority demonstrated successful a reduction in ROS, and in select studies, secondary molecules were assessed to identify the mechanism. In summary, this review highlighted numerous oxidative stress pathways involved in OM pathogenesis, which may inform the development of novel therapeutic targets.
1. Introduction
Oral mucositis (OM) is an acute inflammatory, ulcerative condition of the oral mucosa that commonly arises as a consequence of chemo- and/or radiotherapy. Treatments to manage cancer, such as radiotherapy, cisplatin or 5-fluorouracil (5-FU), generate ROS that target neoplastic cell DNA resulting in cell damage and death. ROS from these therapies also indiscriminately target healthy non-neoplastic DNA [1].
Production of ROS in excess leads to oxidative stress. Maintenance and regulation of ROS involves multiple enzymes with antioxidant properties including superoxide dismutase, catalase and glutathione peroxidase. The offset of this cell redox balance can be a result of oxidants that are formed following free-radical generation, or from a dysfunction in the antioxidant protective mechanism. The increase in oxidative stress is often associated with greater malondialdehyde and 4-hydroxynonenal, greater DNA damage, and protein structural impairment [2]. Biologically, oxidative stress can increase the risk of cancer and predisposition to inflammatory disease and conditions, among other things.
The resultant activation of the nuclear factor kappa B (NFκB) during chemoradiation causes an increase in pro-inflammatory cytokine production. This cascade of inflammatory pathways culminates in mucosal ulceration resulting in the clinical presentation of treatment-induced OM [3,4]. Patients developing OM are often debilitated by the inflammation and ulceration, affecting diet, appetite, and ability to conduct normal oral hygiene [5]. Furthermore, ulceration into the submucosa predisposes treated patients to secondary infections, causing a potential for further sequelae.
Chemo-/radiotherapy-induced ROS are implicated in OM, and therefore the oxidative stress pathway has been evaluated for therapeutic effect, including OM prophylaxis. However, it is yet to be elucidated if there is a specific mechanism in the oxidative stress pathway that can be modulated to prevent OM.
Glutathione (GSH), superoxide dismutase (SOD), catalase, myeloperoxidase (MPO), and hydrogen peroxide are some enzymes and compounds that are involved in the complex oxidative stress pathway [2]. This pathway also signals for the regulation of pro-apoptotic pathways such as NFκB, adding to further complication, but also potential therapeutic targets for OM prophylaxis.
Studies investigating oxidative stress and the role of antioxidants, in the context of chemo-/radiotherapy-induced OM, have assessed the effects of the improvement of OM in various settings. Various agents in these studies have been evaluated by assessing different enzymes of the oxidative stress pathway. This scoping review aims to collect the data generated from these studies to better understand the mechanisms by which the antioxidants can provide OM relief and improvement.
2. Results
There were 717 records identified in SCOPUS and PubMed, respectively. Out of these studies, 89 met the inclusion criteria and were included in the qualitative synthesis (Figure 1). A detailed description of each individual study is reported in Supplementary Materials. The main characteristics of the studies included are described in the manuscript.
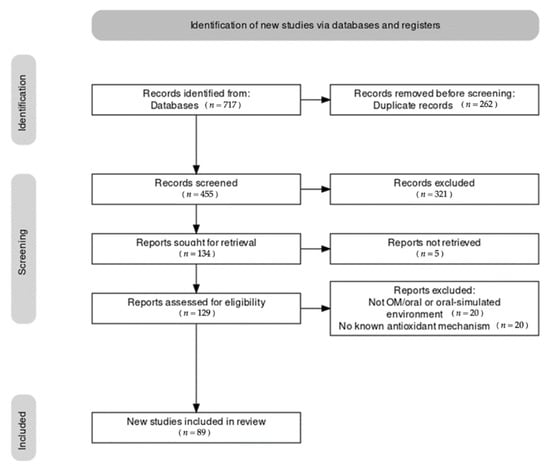
Figure 1.
Results of the literature search. Note that during the identification stage, an indeterminate number of non-English articles were excluded prior to screening via automatic tool.
2.1. In Vitro Studies
There were 21 in vitro studies that were suitable for inclusion. Cell lines cultured in the studies were sheep red blood cells [6], marrow cultures from mice [7], human dermal fibroblast cells [8], keratinocytes [9,10,11,12,13,14,15,16,17,18,19,20], human lung [21] and periodontal ligament fibroblasts [14,22], gingival cells [23], epithelial cells [24] and pharyngeal cells [25,26] (Table 1).

Table 1.
Summary of in vitro study designs and results.
Studies included potential novel therapeutic agents for OM. ROS scavenging activity was demonstrated by 5 studies, which investigated GS nitroxide JP4039, astaxanthin, green tea (polyphenol), amifostine and cyclooxygenase-1 inhibitor [7,8,9,14]. Decreased ROS production was demonstrated by 11 studies, which investigated rapamycin, rebamipide, novel compound 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione (KR22332), Korean ginseng, Salvia miltiorrhiza Bunge (SM), Onchumg eun (OCE), epicatechin, γ-tocotrienol, Daiokanzoto (TJ84), N-acetyl cysteine (NAC) and photomodulation [10,11,12,13,15,16,17,18,20,25,26]. Protection against generated ROS was demonstrated by 5 studies: NAC and Qingre Liyan, azelastine, mucosamin (hyaluronic acid-based compound), phenylbutyrate, GS nitroxide JP4-039 [6,13,19,21,24].
2.2. In Vivo Studies
There were 47 in vivo studies investigating the capacity for various compounds for prophylaxis and reductive effects on OM severity and onset (Table 2). The studies retrieved for analysis have been categorized by intervention types, as reported below.

Table 2.
Summary of in vivo studies and main results.
2.2.1. Chemical Compounds
Sixteen studies assessed the use of different chemicals in the prevention of OM. These drugs provided prophylactic therapy for OM by preventing onset [10,17] or reducing severity [27,28,29,30,31,32,33]. Histologically, limited or absence of oral epithelial destruction, reduced cell apoptosis, vasodilation, and inflammatory cell infiltrates were reported [10,29,30,31,32,33]. Ulcerated epithelium had improved recovery and thickness when administered the drugs [31]. Protection of or reduction in OM were associated with reduced ROS [15,17,34]. Compared to negative control groups, greater OM severity was associated with reduced γH2AX [9,31,34], MDA [30,35,36], and MPO [29,30] expression; additionally, there was increased GSH [37,38] and SOD expression [10].
2.2.2. Rebamipide
Three studies reported rebamipide exerting therapeutic effect by reducing ulcer-like lesions. The healing effect is suggested to be temporal-, frequency- and dose-dependent [39,40,41]. Rebamipide was associated with reduced pro-inflammatory protein expression [40,41].
2.2.3. Botanical Extracts
Thirteen studies demonstrated the protective effects of botanical extracts against OM. The studies assessed histological and oxidative stress biomarkers such as ROS production, MDA, MPO and SOD [26,42,43,44,45,46]. Treatments with plant extracts led to a significant reduction in severity of OM [47], and accelerated recovery of the epithelial layers [25,26,48,49,50,51].
2.2.4. MnBuOE
Two studies reported the protective effects of MnBuOE, a redox-active manganese porphyrin, against OM. MnBuOE treatment increased the GSH/GSSG ratio compared to the irradiated control group [52] and led to significant reduction in severity of radiation-induced OM [53].
2.2.5. Laser Therapy
Laser therapy, in two studies, reduced OM severity, most significantly in extra-oral laser irradiation. Furthermore, laser therapy improved the rate of healing of OM and OM score; these were associated with reduced ROS generation, and increased glutathione peroxidase (Glu.Px) and SOD activity [54,55].
2.2.6. Transplantation
A single study investigated the transplantation of CXCR2-overexpressing mesenchymal stem cells in mice following irradiation, reported improved OM healing. The macroscopic effect was associated with a reduction in ROS production [56].
2.2.7. Antioxidants
Six studies reported the protective effects of antioxidants against OM. The antioxidant effects were evaluated by assessing various oxidative stress biomarkers and histological changes. Antioxidant treatment resulted in reduced severity and area of OM [7,24,57,58,59,60], and greater epithelial thickness [58,59], as compared to the control. Moreover, it led to later onset of OM compared to the control group [60]. In treatment groups with antioxidants, higher levels of SOD and catalase and lower levels of MDA were described, which illustrated decreased oxidative stress [60].
2.2.8. Genes
Gene therapy. Three studies investigated gene therapy in the context of OM. They reported reduced ulcer area, cell apoptosis, and associated reduced expression of DNA damage markers (pH2AX and 8-OhdG) in Tat-Smad7 treated mice following radiotherapy [61]. Two papers examined therapeutic potential of SOD plasmids in OM therapy following radiation insult. Both reported greater SOD expression, by SOD plasmid treatment, resulted in improvement in OM [62,63].
MicroRNA. A single paper explored the modulatory effect of miR-200c in the pathogenesis of radiotherapy-induced oral mucositis (RIOM) in irradiated mice. The study demonstrated an increase in miR-141, miR-200a, miR-200b, and miR-200c expressions in irradiated mice that had developed OM [64].
2.3. Clinical Studies
Thirty-five clinical studies were included, some of which evaluated various interventions with antioxidant capabilities, whilst a small number of publications explored inherent protective factors in clinical subjects, such as genetic polymorphisms, hematological variations, and natively found constituents with antioxidant capabilities (Table 3).

Table 3.
Summary table of results of clinical studies.
2.3.1. Vitamin E
There were eight studies that evaluated vitamin E’s (VE) capacity as a treatment or prophylactic measure for OM. Four studies identified the effect of topical VE independently, in which three showed a significant effect of VE on OM improvement, whilst one did not [65,66,67,68].
Three studies evaluated VE in conjunction with other interventions [6,69,70]. Two of those reported less severe grades of OM by VE [6,70]. The other study concurred with these findings, after adjusting for age [69]. One study compared Vitamin E’s efficacy to Pycnogenol (Pine Bark Extract) [71]. This reported that both interventions were equally effective in reducing the severity of OM and its associated pain.
2.3.2. Genetic Influences & Inherent Antioxidants
Two studies evaluated the genetic link to OM, in particular genetic polymorphisms and their association with OM development. Both studies reported that particular polymorphisms (e.g., in XRCC1 or variants of NBN) are associated with a higher OM risk [72,73].
Two studies sought to correlate OM levels and plasma and buccal mucosa antioxidants levels. These studies found no correlation between antioxidant levels and OM severity [74,75].
One study investigated the effect of salivary antioxidants against OM [76]. They reported an increase in SOD levels coinciding with the development of OM, and a decrease in uric acid (UA) levels reflecting the progression of tissue damage. An association between severe acute OM and specific leukocyte lymphocyte, and plasma antioxidative capacity concentrations was also revealed [77].
2.3.3. Rebamipide
Two clinical studies that investigated Rebamipide as a treatment for OM, and both reported that rebamipide results in decreased severity of OM [78,79].
2.3.4. Zinc/Polaprezinc
Two clinical studies that investigated the effects of zinc supplementation on OM were included. Both demonstrated a decrease in the incidence of OM. One revealed that zinc promoted OM recovery [80,81].
2.3.5. Selenium
There were two studies that evaluated selenium. One determined that supplementation with selenium did not affect OM, and the efficacy of radiotherapy [82]. The other determined that selenium resulted in reduction in severe OM incidence, but not in the cumulative incidence [83].
2.3.6. Photobiomodulation
One study explored the modulatory effect of photobiomodulation on oxidative stress [20], specifically ROS reduction and antioxidant activity at different wavelengths. 800 nm laser light or a combination of 660, 800 and 970 nm light was discovered to result in the largest ROS reduction.
2.3.7. Hyaluronic Acid-Based Compounds
Two clinical studies assessed the efficacy of hyaluronic acid-based compounds. Patients treated with hyaluronic acid-based compounds have been reported to show reduced incidence and pain [84]. Used as OM prophylaxis, the compounds have demonstrated minimal intensity or no recurrence when applied three times daily via oral spray [19].
2.3.8. Single Study Per Intervention
For the following, only one study per intervention met inclusion criteria: NAC, propolis, genistein, glutamine, MF 5232, melatonin, actovegin, date palm pollen, β-carotene, GC4419, Calendula, allopurinol and erythropoietin (Table 4).

Table 4.
Interventions with statistically significant results for various outcomes.
3. Discussion
The aim of this scoping review was to systematically appraise the relevance of oxidative stress pathways in the pathogenesis of radio-/chemotherapy-induced OM. We used a comprehensive approach by including studies undertaken in all experimental settings (in vitro, in vivo, and clinical studies) and providing direct or indirect evidence for a role of oxidative stress or antioxidants in the development, prevention or treatment of OM. In total, 89 papers were included, and these were sub-stratified into models of study (in vitro, in vivo, or clinical) for evaluation. There were 22 in vitro studies, 47 in vivo studies, and 35 clinical studies. Discrepancies in the study count are owed to the fact that some studies had numerous intervention arms in different populations, allowing them to be evaluated in triplicate.
Whilst all papers evaluated OM and involved some element of antioxidant mechanisms, only some could provide direct evidence for the role of this pathway.
3.1. Direct Evidence
3.1.1. In Vitro
Most in vitro studies assessed the levels of ROS production by cells, providing direct evidence for the oxidative impact of each intervention at a cellular level. However, their connection to OM may yet remain unclear until in vivo or clinical trials are evaluated.
While demonstrating ROS reduction, the oxidative pathway, resulting in decreased oxidative stress, was further examined in some studies. Astaxanthin inhibited the cisplatin-induced release of intracellular ROS and inhibited human dermal fibroblast proliferation via peroxidation of the cytoplasmic lipids [8]. Rapamycin also demonstrated protection against senescence from DNA damage following H2O2 treatment, indicating it inhibits mTOR to suppress oxidative stress and reduce ROS accumulation [10]. KR22332 inhibited apoptosis-related genes such as p53, and TNF-α, suggesting its ROS protective capacity is related to TNF-α inhibition [11]. Korean red ginseng reduced ROS by stabilizing the change in MMP [12]. SM and OCE reduced intracellular ROS production and radical DPPH, demonstrating its antioxidant activity [17]. γ-tocotrienol and NAC advanced the accumulation of Nrf2, a transcriptional factor for cytoprotective gene, suggesting its protective effect during oxidative stress [18].
3.1.2. In Vivo
Botanical extracts, MnBuOE, chemical compounds, plasmid treatment, CXCR2 overexpression, and vitamin E and L-carnitine interventions were reported to act on the oxidative stress pathway similarly in vivo. These studies demonstrated that higher SOD [10,26,42,43,44,45,60,62], and GSH [52,53] levels and lower MPO and MDA levels were associated with prevention or reduced severity of OM [10,26,29,30,35,36,42,43,44,45,49,51,52,53,60]. Interestingly, in one study, catalase, an enzyme of the oxidative stress pathway that exerts antioxidant function, was reduced following cannabidiol treatment [49]. As non-treated irradiated mice had higher catalase levels, it is possible that ROS production was mitigated by cannabidiol treatment, and therefore the regulation of oxidative stress was not required. However, the nature of catalase in the study is unclear due to a lack of direct ROS assessment.
Further limitations of the in vivo studies are the inconsistency of verifying the SOD expression in the plasmid therapy [62,63] and limited evidence correlating signs of OM improvement with ROS [17,26,34,36,56], a more direct method of measuring oxidative stress.
3.1.3. Clinical Studies
The majority of clinical studies did not provide direct evidence as to how the interventions influenced the oxidative stress pathway. A small number of studies examined their antioxidant capacity, or inherent antioxidant pathways. We interpreted these studies as providing direct evidence.
Both photobiomodulation and Calendula provided direct evidence supporting their role in the oxidative stress pathway [20,97]. Photobiomodulation was shown to be associated with ROS generation due to its ability to excite cytochrome C oxidase at high wavelengths. However, the research in this field is limited, and a better understanding of how light interacts with biological tissues is necessary before adopting this intervention. Antioxidant activities were present in the Calendula intervention, including free radical scavenging and termination [97]. However, the literature claimed that a better understanding of optimal doses and administration frequency would better improve OM outcomes.
The study evaluating NAC provided some limited direct evidence for the role of the oxidative stress pathways and the development of OM. NAC stimulates the synthesis of GSH, which can scavenge free radicals. This study demonstrated that supplementation with parenteral NAC reduced the severity and duration of OM, and that serum levels of Glu.Px were higher in the intervention group—this implies that the antioxidant capacity of the compound is responsible for the changes in disease character [91].
Two studies evaluated OM’s association with particular genetic polymorphisms, providing direct evidence. Both studies assessed genes related to DNA damage and repair, particularly genes involved in protection against oxidative stress. Both studies concluded that variations in these genes are associated with a higher risk of developing OM [72,73]. This lends credibility to the assertion that the pathogenesis of OM is intrinsically linked to the oxidative stress pathway.
Three additional studies investigated the correlation between endogenous levels of antioxidants and OM. One study demonstrated that the natural salivary antioxidants, SOD, and uric acid levels were altered during OM progression [76]. Surprisingly, two studies reported no correlation between measures of plasma antioxidants and severity of OM, and that there was no particular antioxidant that had a predictive effect for severity or incidence of OM [74,75]. While this may seem to contradict the crux of the body of research, a more reasonable interpretation may be that there may be additional factors at play that affect the relationship between the oxidative stress pathway and OM. One finding from these studies that seems to support the rest of the available evidence is that there was a tendency for patients with sub-normal antioxidant concentrations to require a longer duration of parenteral nutrition, implying that their OM was symptomatic for longer. An interesting postulate that can also arise from this data is that perhaps the presence of antioxidant mechanisms is not protective, but the absence of sufficient antioxidant capacity is deleterious and could be the reason for the lack of protection.
A study on melatonin, a potent antioxidant, demonstrated its association with increased total antioxidant capacity (TAC) in patients with OM [94]. It further demonstrated that supplemented patients had reduced discomfort and pain. These results imply that the increased TAC is associated with improved OM outcomes.
Two studies about selenium provided direct evidence of its antioxidant effects. Increases in selenium levels were associated with an increase in GSH peroxidase levels which are important for endogenous detoxification of free radicals [82,83]. This was the assumed method by which selenium decreased OM severity.
3.2. Indirect Evidence
A number of clinical studies sought to elucidate the protective or therapeutic effects of compounds that have been pre-determined to have antioxidant properties. These include studies of propolis, genistein, glutamine, MF 5232, actovegin, vitamin E, date palm pollen, mucosamin (hyaluronic acid-based compound), rebamipide, mucosyte®, β-carotene, zinc, GC-4419, allopurinol and erythropoietin [6,19,65,66,68,69,70,71,78,79,80,81,84,85,86,87,88,89,90,92,93,95,96,97]. Whilst these studies provided evidence for the efficacy of these compounds, they did not explore the mechanisms behind how they produced an improvement in OM outcomes. Therefore, they only provided indirect evidence towards our research outcomes.
In vivo studies that demonstrated a reduction in ROS levels without assessing the oxidative stress mechanisms involved GS nitroxide JP05429, green tea (polyphenol), dexpanthenol, amifostine and cyclooxygenase-1-inhibitor (allopurinol), Daikanzoto, Hangeshashinto, rebamipide, L. reuteri, Z. jujuba, propolis, curcumin, velafermin, DMSO, GGsTop, antimicrobial laser or radiation therapy, Tat-Smad7 treatment, and tempol [7,9,14,22,23,27,31,33,39,40,41,42,47,48,50,54,55,57,58,59,61].
In vitro, phenylbutyrate [21], NAC and qingre liyan [13], and mucosamin [19] a direct or an indirect increase in ROS as indicated via changes in expression of particular proteins, such as heme-oxygenase-1 and mTOR [13,16]; or fibroblast senescence [19], suggested to be a result of reduced oxidative stress by free radical sequestration [19,20].
3.3. Limitations
There were a number of limitations within this scoping review. Whilst there has been a significant return of results, due to the immense heterogeneity, it is difficult to evaluate the quality of evidence. We have attempted to do so where we have found disagreement amongst the literature, but this is a primitive attempt. Furthermore, large differences in study populations and outcomes measured have prevented us from coming to a representative conclusion regarding our objective of determining the function of oxidative stress pathways in the development of OM. Due to constraints arising from the sheer amount of data retrieved, our scoping review made no attempt to evaluate the bias of the studies. However, since its inception, this study was planned to examine the breadth of the evidence rather than the depth, and these limitations are typical of scoping reviews. Future systematic reviews will build on the trends highlighted by our work and will focus on specific aspects on the oxidative stress pathway in OM.
4. Materials and Methods
4.1. Search Strategy
The scoping review was conducted in accordance with PRISMA-ScR guidelines [98].
The following databases were searched:
- PubMed (MEDLINE)
- SCOPUS
Inclusion criteria were studies on OM in patients receiving chemo-/radiotherapy, in vivo or in vitro models of OM, assessment of oxidative stress or related pathways and original articles (including case-reports). Gray literature was not considered in this review.
No restrictions were placed on the date of publication when searching databases. Publications released prior to the month of June 2021 were considered in this review.
The aim of this scoping review was to understand the mechanism of the oxidative stress pathway in chemo-/radiotherapy-induced OM. To initially screen a broad range of studies, synonyms for OM were considered in the search strategy. Furthermore, oxidative stress has recently been of interest in the prevention of OM. Therefore, the oxidative stress pathway was considered in the search strategy. Moreover, enzymes commonly studied in the oxidative stress pathway and terms associated with oxidative stress were included in the search strategy.
From this, the search strategy included three concepts:
- The condition: OM;
- Aetiology of the condition: chemotherapy and radiotherapy;
- Pathogenesis/pathway of interest: oxidative stress pathway.
The following keyword combinations were used to find relevant publications: (chemotherapy * OR “radiation-induced” OR radiotherapy *) AND (mucositis OR “Oral mucositis” OR “mucosal atrophy” OR “alimentary tract mucositis” OR “gastrointestinal tract mucositis” OR stomatitis) AND (“Reactive Oxygen Species” OR “oxidat *” OR “free radicals” OR ROS OR “Superoxide Dismutase” OR Catalase OR “Glutathione peroxidase” OR antiox *).
The search strategy was developed in tandem with the project supervisor. We ran a sensitivity analysis using additional keywords such as “superoxide”,”H2O2”, “hydrogenperoxide” or “redox stress” but these did not change the search results significantly, therefore the authors were satisfied that the search string used captured most variations in terminology for both the disease in question (OM), as well as including any nomenclature that would be associated with the oxidative stress pathway.
Studies that were not published in English were excluded via an automatic tool, and any studies that were: reviews/systematic reviews/meta-analyses, book chapters and non-peer-reviewed literature were manually excluded by reviewers.
4.2. Data Extraction
Two sets of two reviewers independently evaluated titles and abstracts of articles, to screen for exclusion criteria. A kappa score of agreement was calculated for each pair, and disagreements were resolved by a third-party judge. The kappa scores for each round and anonymous pair are presented below (Table 5).

Table 5.
Kappa scores for inclusion and exclusion.
A predefined data extraction sheet was used to distill information from the full texts of eligible studies.
4.3. Data Synthesis
Once suitable studies for inclusion were determined, the evidence from the studies were presented in two ways. Data from the final search was presented as extraction tables, as seen in the Supplementary Materials. This included the study type, population, intervention and comparator, outcome measurement, observed effect, and mechanism of action. Additionally, a narrative component was included in the results section. This detailed some of the interventions that were mentioned in multiple studies and compared the results between each of these studies.
5. Conclusions
This scoping review has revealed that there is a wide range of publications that have explored OM in the context of oxidative stress to varying extents, dating back to 1992, indicating that there has been interest in this field for some time. There has also been a revelation about the breadth of data that can actually directly support our area of interest—the majority of clinical studies do not delve into the antioxidant mechanisms behind their action, so perhaps this could be a future direction for research. This is likely the case due to clinical studies relying on prior in vitro and in vivo studies to define the mechanisms of action of these pathways.
Regarding the in vivo studies, it is clear that reduction in ROS, MPO, and MDA and increase in SOD and GSH are associated with prevention or improvement in OM severity. However, the exact mechanism by which oxidative stress contributes to chemo- and radiotherapy-induced OM is still largely unclear.
In regard to in vitro studies, further exploration on mechanisms of ROS reduction may be considered in future studies, in addition to ROS level detection.
Our data support a clear scope for further investigations into the oxidative stress pathways in OM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23094863/s1.
Author Contributions
Conceptualization: methodology, supervision and project administration: N.C.; Validation: D.H.S. and H.P.; Formal analysis: investigation, data curation and writing—original draft preparation: H.N., S.S., M.P., D.H.S. and H.P.; Writing—review and editing: H.N., S.S., M.P., D.H.S., H.P., A.I.M. and N.C.; Visualization: H.N. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S. The Biologic Role for Nuclear Factor-KappaB in Disease and its Potential Involvement in Mucosal Injury Associated with Anti-neoplastic Therapy. Crit Rev. Oral Biol Med. 2002, 13, 380–389. [Google Scholar] [CrossRef]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-induced oral mucositis. Front. Oncol. 2017, 7, 89–111. [Google Scholar] [CrossRef]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Osaki, T.; Ueta, E.; Yoneda, K.; Hirota, J.; Yamamoto, T. Prophylaxis of oral mucositis associated with chemoradiotherapy for oral carcinoma by Azelastine hydrochloride (Azelastine) with other antioxidants. Head Neck 1994, 16, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, J.S.; Berhane, H.; Shinde, A.; Rhieu, B.H.; Bernard, M.; Wipf, P.; Skoda, E.M.; Epperly, M.W. Can Radiosensitivity Associated with Defects in DNA Repair be Overcome by Mitochondrial-Targeted Antioxidant Radioprotectors. Front. Oncol. 2014, 4, 24. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tomihara, K.; Heshiki, W.; Sakurai, K.; Sekido, K.; Tachinami, H.; Moniruzzaman, R.; Inoue, S.; Fujiwara, K.; Noguchi, M. Astaxanthin ameliorates cisplatin-induced damage in normal human fibroblasts. Oral Sci. Int. 2019, 16, 171–177. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Ben Lagha, A.; Grenier, D. A green tea extract and epigallocatechin-3-gallate attenuate the deleterious effects of irinotecan in an oral epithelial cell model. Arch. Oral Biol. 2021, 126, 105135. [Google Scholar] [CrossRef]
- Iglesias-Bartolome, R.; Patel, V.; Cotrim, A.; Leelahavanichkul, K.; Molinolo, A.A.; Mitchell, J.B.; Gutkind, J.S. MTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 2012, 11, 401–414. [Google Scholar] [CrossRef]
- Baek, S.J.; Chang, J.W.; Park, K.H.; Yang, G.Y.; Hwang, H.S.; Koh, Y.W.; Jung, Y.S.; Kim, C.H. A novel synthetic compound 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione (KR22332) exerts a radioprotective effect via the inhibition of mitochondrial dysfunction and generation of reactive oxygen species. Yonsei Med. J. 2014, 55, 886–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, J.W.; Park, K.H.; Hwang, H.S.; Shin, Y.S.; Oh, Y.T.; Kim, C.H. Protective effects of Korean red ginseng against radiation-induced apoptosis in human HaCaT keratinocytes. J. Radiat Res. 2014, 55, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.P.; Kondapalli, L.; Parsa, C.; Mulamalla, H.C.; Orlando, R.; Pon, D.; Huang, Y.; Chow, M.S. Molecular signatures in the prevention of radiation damage by the synergistic effect of N-acetyl cysteine and qingre liyan decoction, a traditional chinese medicine, using a 3-dimensional cell culture model of oral mucositis. Evid.-Based Complement. Altern. Med. 2015, 2015, 425760. [Google Scholar] [CrossRef] [PubMed]
- Maiguma, T.; Kaji, H.; Makino, K.; Teshima, D. Protective effects of amifostine and cyclooxygenase-1 inhibitor against normal human epidermal keratinocyte toxicity induced by methotrexate and 5-fluorouracil. Basic Clin. Pharmacol. Toxicol. 2009, 105, 1–9. [Google Scholar] [CrossRef]
- Shin, Y.S.; Shin, H.A.; Kang, S.U.; Kim, J.H.; Oh, Y.T.; Park, K.H.; Kim, C.H. Effect of epicatechin against radiation-induced oral mucositis: In Vitro and In Vivo study. PLoS ONE 2013, 8, e69151. [Google Scholar] [CrossRef]
- Tsubaki, M.; Takeda, T.; Asano, R.T.; Matsuda, T.; Fujimoto, S.I.; Itoh, T.; Motohiro, I.; Satou, T.; Nishida, S. Rebamipide suppresses 5-fluorouracil-induced cell death via the activation of Akt/mTOR pathway and regulates the expression of Bcl-2 family proteins. Toxicol. In Vitro 2018, 46, 284–293. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, S.U.; Lee, Y.S.; Jang, J.Y.; Kang, H.; Kim, C.H. Protective effects of N-acetylcysteine against radiation-induced oral mucositis In Vitro and In Vivo. Cancer Res. Treat. 2020, 52, 1019–1030. [Google Scholar] [CrossRef]
- Takano, H.; Momota, Y.; Kani, K.; Aota, K.; Yamamura, Y.; Yamanoi, T.; Azuma, M. gamma-Tocotrienol prevents 5-FU-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-FU-induced activation of Nrf2. Int. J. Oncol. 2015, 46, 1453–1460. [Google Scholar] [CrossRef]
- Cirillo, N.; Vicidomini, A.; McCullough, M.; Gambardella, A.; Hassona, Y.; Prime, S.S.; Colella, G. A hyaluronic acid-based compound inhibits fibroblast senescence induced by oxidative stress In Vitro and prevents oral mucositis In Vivo. J. Cell Physiol. 2015, 230, 1421–1429. [Google Scholar] [CrossRef]
- Rupel, K.; Zupin, L.; Colliva, A.; Kamada, A.; Poropat, A.; Ottaviani, G.; Gobbo, M.; Fanfoni, L.; Gratton, R.; Santoro, M.; et al. Photobiomodulation at Multiple Wavelengths Differentially Modulates Oxidative Stress In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2018, 2018, 6510159. [Google Scholar] [CrossRef]
- Chung, Y.L.; Lee, M.Y.; Pui, N.N. Epigenetic therapy using the histone deacetylase inhibitor for increasing therapeutic gain in oral cancer: Prevention of radiation-induced oral mucositis and inhibition of chemical-induced oral carcinogenesis. Carcinogenesis 2009, 30, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.; Marquardt, Y.; Huth, L.; Schmitt, L.; Prescher, K.; Winterhalder, P.; Steiner, T.; Hölzle, F.; Eble, M.; Baron, J.M. Molecular effects of photon irradiation and subsequent aftercare treatment with dexpanthenol-containing ointment or liquid in 3D models of human skin and non-keratinized oral mucosa. Exp. Dermatol. 2021, 30, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yoshioka, M.; Okamura, H.; Moriyama, S.; Kawazoe, K.; Grenier, D.; Hinode, D. Preventive effect of Daiokanzoto (TJ-84) on 5-fluorouracil-induced human gingival cell death through the inhibition of reactive oxygen species production. PLoS ONE 2014, 9, e112689. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Berhane, H.; Rhieu, B.H.; Kalash, R.; Xu, K.; Goff, J.; Epperly, M.W.; Franicola, D.; Zhang, X.; Dixon, T.; et al. Intraoral Mitochondrial-Targeted GS-Nitroxide, JP4-039, Radioprotects Normal Tissue in Tumor-Bearing Radiosensitive Fancd2(-/-) (C57BL/6) Mice. Radiat. Res. 2016, 185, 134–150. [Google Scholar] [CrossRef]
- Kim, D.R.; Kim, J.; Oh, J.Y.; Kim, H.Y.; Kim, Y.J.; Chang, M.S. Protective effect of Salvia miltiorrhiza Bunge on 5-fluorouracil-induced oral mucositis. Int. J. Mol. Med. 2017, 40, 39–46. [Google Scholar] [CrossRef][Green Version]
- Park, J.W.; Oh, J.; Ko, S.J.; Chang, M.S.; Kim, J. Effects of Onchung-eum, an Herbal Prescription, on 5-Fluorouracil-Induced Oral Mucositis. Integr. Cancer Ther. 2018, 17, 1285–1296. [Google Scholar] [CrossRef]
- Ara, G.; Watkins, B.A.; Zhong, H.; Hawthorne, T.R.; Karkaria, C.E.; Sonis, S.T.; Larochelle, W.J. Velafermin (rhFGF-20) reduces the severity and duration of hamster cheek pouch mucositis induced by fractionated radiation. Int. J. Radiat. Biol. 2008, 84, 401–412. [Google Scholar] [CrossRef]
- Clémenson, C.; Liu, W.; Bricout, D.; Soyez-Herkert, L.; Chargari, C.; Mondini, M.; Haddad, R.; Wang-Zhang, X.; Benel, L.; Bloy, C.; et al. Preventing radiation-induced injury by topical application of an amifostine metabolite-loaded thermogel. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1141–1152. [Google Scholar] [CrossRef]
- Nakajima, N.; Watanabe, S.; Kiyoi, T.; Tanaka, A.; Suemaru, K.; Araki, H. Evaluation of edaravone against radiation-induced oral mucositis in mice. J. Pharm. Sci. 2015, 127, 339–343. [Google Scholar] [CrossRef]
- Mafra, C.A.C.C.; Vasconcelos, R.C.; de Medeiros, C.A.C.X.; Leitão, R.F.C.; Brito, G.A.C.; Costa, D.V.S.; Guerra, G.C.B.; de Araújo, R.F., Jr.; Medeiros, A.C.; de Araújo, A.A. Gliclazide prevents 5-FU-induced oral mucositis by reducing oxidative stress, inflammation, and P-selectin adhesion molecules. Front. Physiol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, L.; Peng, R.; Bai, F.; Shan, Y.; Yu, Z.; Zhou, P.; Cong, Y. Dimethyl sulfoxide prevents radiation-induced oral mucositis through facilitating DNA double-strand break repair in epithelial stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Acuña-Castroviejo, D.; Doerrier, C.; Dayoub, J.C.; López, L.C.; Venegas, C.; García, J.A.; López, A.; Volt, H.; Luna-Sánchez, M.; et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J. Pineal Res. 2015, 58, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Takeuchi, I.; Terada, H.; Makino, K. Therapeutic effect of GGsTop, selective gamma-glutamyl transpeptidase inhibitor, on a mouse model of 5-fluorouracil-induced oral mucositis. Anticancer Res. 2019, 39, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Im, K.I.; Nam, Y.S.; Kim, N.; Song, Y.; Lee, E.S.; Lim, J.Y.; Jeon, Y.W.; Cho, S.G. Regulation of HMGB1 release protects chemoradiotherapy-associated mucositis. Mucosal Immunol. 2019, 12, 1070–1081. [Google Scholar] [CrossRef]
- Ala, M.; Mohammad Jafari, R.; Ala, M.; Agbele, A.T.; Hejazi, S.M.; Tavangar, S.M.; Mahdavi, S.R.M.; Dehpour, A.R. Sumatriptan alleviates radiation-induced oral mucositis in rats by inhibition of NF-kB and ERK activation, prevention of TNF-α and ROS release. Arch. Oral Biol. 2020, 119, 104919. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A.; Nakajima, A.; Wada-Takahashi, S.; Takahashi, S.S.; Lee, M.C.I. Alteration of the redox state with reactive oxygen species for 5-fluorouracil-induced oral mucositis in hamsters. PLoS ONE 2013, 8, e82834. [Google Scholar]
- Vilar, C.J.F.; Ribeiro, S.B.; de Araújo, A.A.; Guerra, G.C.B.; de Araújo, R.F., Jr.; Brito, G.A.C.; Leitão, R.F.C.; Pontes, D.L.; Gasparotto, L.H.D.S.; Oliveira, M.M.B.; et al. Effect of gold nanoparticle on 5-fluorouracil-induced experimental oral mucositis in hamsters. Pharmaceutics 2020, 12, 304. [Google Scholar] [CrossRef]
- Gümüş, S.; Yarıktaş, M.; Nazıroğlu, M.; Uğuz, A.C.; Aynali, G.; Başpınar, Ş. Effect of a corticosteroid (triamcinolone) and chlorhexidine on chemotherapy-induced oxidative stress in the buccal mucosa of rats. Ear Nose Throat J. 2016, 95, E36–E43. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kamiki, Y.; Makino, K. Therapeutic efficacy of rebamipide-loaded PLGA nanoparticles coated with chitosan in a mouse model for oral mucositis induced by cancer chemotherapy. Colloids Surf. B Biointerfaces 2018, 167, 468–473. [Google Scholar] [CrossRef]
- Nakashima, T.; Sako, N.; Matsuda, T.; Uematsu, N.; Sakurai, K.; Ishida, T. Novel submicronized rebamipide liquid with moderate viscosity: Significant effects on oral mucositis in animal models. Biol. Pharma Bull. 2014, 37, 671–678. [Google Scholar] [CrossRef]
- Nakashima, T.; Uematsu, N.; Sakurai, K. Intra-oral administration of rebamipide liquid prevents tongue injuries induced by X-ray irradiation in rats. Support. Care Cancer 2017, 25, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Koohi-Hosseinabadi, O.; Andisheh-Tadbir, A.; Bahadori, P.; Sepehrimanesh, M.; Mardani, M.; Tanideh, N. Comparison of the therapeutic effects of the dietary and topical forms of Zizyphus jujuba extract on oral mucositis induced by 5-fluorouracil: A golden hamster model. J. Clin. Exp. Dent. 2015, 7, e304. [Google Scholar] [CrossRef] [PubMed]
- Koohi-Hosseinabadi, O.; Ranjbar, Z.; Sepehrimanesh, M.; AndisheTadbir, A.; Poorbaghi, S.L.; Bahranifard, H.; Tanideh, N.; Koohi-Hosseinabadi, M.; Iraji, A. Biochemical, hematological, and pathological related healing effects of Elaeagnus angustifolia hydroalcoholic extract in 5-fluorouracil-induced oral mucositis in male golden hamster. Environ. Sci. Pollut. Res. Int. 2017, 24, 24447–24453. [Google Scholar] [CrossRef] [PubMed]
- Tanideh, N.; Zareh, A.A.; Fani, M.M.; Mardani, M.; Farrokhi, F.; Talati, A.; Koohi Hosseinabadi, O.; Kamali, M. Evaluation of the Effect of a Topical Gel Form of Pistacia Atlantica and Trachyspermum Ammi on Induced Oral Mucositis in Male Golden Hamsters by Bio-Marker Indices and Stereological Assessment. J. Dent. 2019, 20, 240. [Google Scholar]
- Watanabe, S.; Suemaru, K.; Takechi, K.; Kaji, H.; Imai, K.; Araki, H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-fluorouracil–induced oral mucositis. J. Pharm. Sci. 2013, 121, 110–118. [Google Scholar] [CrossRef]
- Takuma, D.; Guangchen, S.; Yokota, J.; Hamada, A.; Onogawa, M.; Yoshioka, S.; Kusunose, M.; Miyamura, M.; Kyotani, S.; Nishioka, Y. Effect of Eriobotrya japonica seed extract on 5-fluorouracil-induced mucositis in hamsters. Biol. Pharm. Bull. 2008, 31, 250–254. [Google Scholar] [CrossRef]
- Rezvani, M.; Ross, G.A. Modification of radiation-induced acute oral mucositis in the rat. Int. J. Radiat. Biol. 2004, 80, 177–182. [Google Scholar] [CrossRef]
- Gupta, N.; Ferreira, J.; Hong, C.H.; Tan, K.S. Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 ameliorates chemotherapy-induced oral mucositis. Sci. Rep. 2020, 10, 16189. [Google Scholar] [CrossRef]
- Cuba, L.F.; Salum, F.G.; Guimaraes, F.S.; Cherubini, K.; Borghetti, R.L.; de Figueiredo, M.A.Z. Cannabidiol on 5-FU-induced oral mucositis in mice. Oral Dis. 2020, 26, 1483–1493. [Google Scholar] [CrossRef]
- Aghel, S.; Pouramir, M.; Moghadamnia, A.A.; Moslemi, D.; Molania, T.; Ghassemi, L.; Motallebnejad, M. Effect of Iranian propolis on salivary total antioxidant capacity in gamma-irradiated rats. J. Dent. Res. Clin. Dent. Prospect. 2014, 8, 235–239. [Google Scholar]
- Motallebnejad, M.; Zahedpasha, S.; Moghadamnia, A.A.; Kazemi, S.; Moslemi, D.; Pouramir, M.; Asgharpour, F. Protective effect of lycopene on oral mucositis and antioxidant capacity of blood plasma in the rat exposed to gamma radiation. Casp. J. Intern. Med. 2020, 11, 419–425. [Google Scholar]
- Birer, S.R.; Lee, C.T.; Choudhury, K.R.; Young, K.H.; Spasojevic, I.; Batinic-Haberle, I.; Crapo, J.D.; Dewhirst, M.W.; Ashcraft, K.A. Inhibition of the continuum of radiation-induced normal tissue injury by a redox-active Mn porphyrin. Radiat. Res. 2017, 188, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, K.A.; Boss, M.K.; Tovmasyan, A.; Roy Choudhury, K.; Fontanella, A.N.; Young, K.H.; Palmer, G.M.; Birer, S.R.; Landon, C.D.; Park, W.; et al. Novel Manganese-Porphyrin Superoxide Dismutase-Mimetic Widens the Therapeutic Margin in a Preclinical Head and Neck Cancer Model. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Cruz, É.D.; Campos, L.; da Silva Pereira, F.; Magliano, G.C.; Benites, B.M.; Arana-Chavez, V.E.; Ballester, R.Y.; Simões, A. Clinical, biochemical and histological study of the effect of antimicrobial photodynamic therapy on oral mucositis induced by 5-fluorouracil in hamsters. Photodiagnosis Photodyn. Ther. 2015, 12, 298–309. [Google Scholar] [CrossRef]
- Thieme, S.; Ribeiro, J.T.; Dos Santos, B.G.; de Almeida Zieger, R.; Severo, M.L.; Martins, M.A.; Matté, C.; Martins, M.D. Comparison of photobiomodulation using either an intraoral or an extraoral laser on oral mucositis induced by chemotherapy in rats. Supportive Care Cancer 2020, 28, 867–876. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, J.; Huang, Q.; Shi, Y.; Wei, Z.; Zheng, X.; Qiu, Y.; Zhang, M.; Wang, Y.; Qin, W.; et al. Genetic modification to induce CXCR2 overexpression in mesenchymal stem cells enhances treatment benefits in radiation-induced oral mucositis. Cell Death Dis. 2018, 9, 229. [Google Scholar] [CrossRef]
- Willis, J.; Epperly, M.W.; Fisher, R.; Zhang, X.; Shields, D.; Hou, W.; Wang, H.; Li, S.; Wipf, P.; Parmar, K.; et al. Amelioration of head and neck radiation-induced mucositis and distant marrow suppression in Fanca−/− and Fancg−/− mice by intraoral administration of GS-nitroxide (JP4-039). Radiat Res. 2018, 189, 560–578. [Google Scholar] [CrossRef]
- Cotrim, A.P.; Yoshikawa, M.; Sunshine, A.N.; Zheng, C.; Sowers, A.L.; Thetford, A.D.; Cook, J.A.; Mitchell, J.B.; Baum, B.J. Pharmacological protection from radiation ± cisplatin-induced oral mucositis. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1284–1290. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Y.; Cotrim, A.P.; Zhu, Z.; Gao, R.; Zheng, C.; Goldsmith, C.M.; Jin, L.; Zhang, C.; Mitchell, J.B.; et al. Effect of Tempol on the prevention of irradiation-induced mucositis in miniature pigs. Oral Dis. 2017, 23, 801–808. [Google Scholar] [CrossRef]
- Uçüncü, H.; Ertekin, M.V.; Yörük, O.; Sezen, O.; Ozkan, A.; Erdoğan, F.; Kiziltunç, A.; Gündoğdu, C. Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced oral mucositis and myelosuppression: A controlled study in a rat model. J. Radiat. Res. 2006, 47, 91–102. [Google Scholar] [CrossRef]
- Luo, J.; Bian, L.; Blevins, M.A.; Wang, D.; Liang, C.; Du, D.; Wu, F.; Holwerda, B.; Zhao, R.; Raben, D.; et al. Smad7 promotes healing of radiotherapy-induced oral mucositis without compromising oral cancer therapy in a xenograft mouse model. Clin. Cancer Res. 2019, 25, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Seixas-Silva, J.A., Jr.; Epperly, M.W.; Gretton, J.E.; Shin, D.M.; Bar-Sagi, D.; Archer, H.; Greenberger, J.S. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat. Res. 2003, 159, 361–370. [Google Scholar] [CrossRef]
- Epperly, M.W.; Carpenter, M.; Agarwal, A.; Mitra, P.; Nie, S.; Greenberger, J.S. Intraoral manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo 2004, 18, 401–410. [Google Scholar] [PubMed]
- Tao, J.; Fan, M.; Zhou, D.; Hong, Y.; Zhang, J.; Liu, H.; Sharma, S.; Wang, G.; Dong, Q. MiR-200c modulates the pathogenesis of radiation-induced oral mucositis. Oxid. Med. Cell. Longev. 2019, 2019, 2352079. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.; Tomlinson, G.A.; Greenberg, M.L.; Koren, G.; Judd, P.; Ota, S.; Feldman, B.M. Serial controlled N-of-1 trials of topical vitamin E as prophylaxis for chemotherapy-induced oral mucositis in paediatric patients. Eur. J. Cancer 2007, 43, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.R.; Fleck, J.F.; Diehl, A.; Barletta, D.; Braga-Filho, A.; Barletta, A.; Ilha, L. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: A double-blind randomized trial. Head Neck 2004, 26, 313–321. [Google Scholar] [CrossRef]
- El-Housseiny, A.A.; Saleh, S.M.; El-Masry, A.A.; Allam, A.A. The effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapy. J. Clin. Pediatr. Dent. 2007, 31, 167–170. [Google Scholar] [CrossRef]
- Wadleigh, R.G.; Redman, R.S.; Graham, M.L.; Krasnow, S.H.; Anderson, A.; Cohen, M.H. Vitamin E in the treatment of chemotherapy-induced mucositis. Am. J. Med. 1992, 92, 481–484. [Google Scholar] [CrossRef]
- Sayed, R.; El Wakeel, L.; Saad, A.S.; Kelany, M.; El-Hamamsy, M. Pentoxifylline and vitamin E reduce the severity of radiotherapy-induced oral mucositis and dysphagia in head and neck cancer patients: A randomized, controlled study. Med. Oncol. 2019, 37, 8. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Pourpasha, M.; Amanlou, M.; Moosavi, M.S. Mouthwash containing vitamin E, triamcinolon, and hyaluronic acid compared to triamcinolone mouthwash alone in patients with radiotherapy-induced oral mucositis: Randomized clinical trial. Front. Oncol. 2021, 11, 614877. [Google Scholar] [CrossRef]
- Khurana, H.; Pandey, R.K.; Saksena, A.K.; Kumar, A. An evaluation of Vitamin E and Pycnogenol in children suffering from oral mucositis during cancer chemotherapy. Oral Dis. 2013, 19, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.H.; Manjunath, V.B.; Mumbrekar, K.D.; Negi, H.; Fernandes, D.J.; Sharan, K.; Banerjee, S.; Bola Sadashiva, S.R. Polymorphisms in radio-responsive genes and its association with acute toxicity among head and neck cancer patients. PLoS ONE 2014, 9, e89079. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, N.; Mangoni, M.; Mancini, I.; Paiar, F.; Simi, L.; Livi, L.; Cassani, S.; Buglione, M.; Grisanti, S.; Almici, C.; et al. Association between single nucleotide polymorphisms in the XRCC1 and RAD51 genes and clinical radiosensitivity in head and neck cancer. Radiother. Oncol. 2011, 99, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Raynor, A.; Bertz, H.; Lambert, C.; Biesalski, H.K. Role of antioxidants in buccal mucosa cells and plasma on the incidence and severity of oral mucositis after allogeneic haematopoietic cell transplantation. Support. Care Cancer 2012, 20, 1831–1838. [Google Scholar] [CrossRef]
- Wardman, P.; Folkes, L.K.; Bentzen, S.M.; Stratford, M.R.; Hoskin, P.J.; Phillips, H.; Jackson, S. Influence of plasma glutathione levels on radiation mucositis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 460–464. [Google Scholar] [CrossRef]
- Bachmeier, E.; Mazzeo, M.A.; Lopez, M.M.; Linares, J.A.; Jarchum, G.; Wietz, F.M.; Finkelberg, A.B. Mucositis and salivary antioxidants in patients undergoing bone marrow transplantation (BMT). Med. Oral Patol Oral Cir. Bucal. 2014, 19, e444-50. [Google Scholar] [CrossRef] [PubMed]
- Severin, E.; Greve, B.; Pascher, E.; Wedemeyer, N.; Hacker-Klom, U.; Silling, G.; Kienast, J.; Willich, N.; Göhde, W. Evidence for predictive validity of blood assays to evaluate individual radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 242–250. [Google Scholar] [CrossRef]
- Chaitanya, B.; Pai, K.M.; Yathiraj, P.H.; Fernandes, D.; Chhaparwal, Y. Rebamipide gargle in preventive management of chemo-radiotherapy induced oral mucositis. Oral Oncol. 2017, 72, 179–182. [Google Scholar] [CrossRef]
- Ishii, N.; Kawano, Y.; Sakai, H.; Hayashi, S.; Akizuki, N.; Komoda, M.; Hanawa, T. Effects of a rebamipide mouthwash on stomatitis caused by cancer chemotherapy-evaluation of the efficacy by patients themselves. Yakugaku Zasshi 2017, 137, 1027–1034. [Google Scholar] [CrossRef][Green Version]
- Gholizadeh, N.; Mehdipour, M.; Chavoshi, S.H.; Kahani, S.; Sadrzadeh-Afshar, M.S. The effect of orally-administered zinc in the prevention of chemotherapy-induced oral mucositis in patients with acute myeloid leukemia. Int. J. Cancer Manag. 2017, 10, e9252. [Google Scholar] [CrossRef]
- Doi, H.; Fujiwara, M.; Suzuki, H.; Niwa, Y.; Nakayama, M.; Shikata, T.; Odawara, S.; Takada, Y.; Kimura, T.; Kamikonya, N.; et al. Polaprezinc reduces the severity of radiation-induced mucositis in head and neck cancer patients. Mol. Clin. Oncol. 2015, 3, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Büntzel, J.; Micke, O.; Kisters, K.; Bruns, F.; Glatzel, M.; Schonekaes, K.; Kundt, G.; Schäfer, U.; Mücke, R. Selenium substitution during radiotherapy of solid tumours-laboratory data from two observation studies in gynaecological and head and neck cancer patients. Anticancer Res. 2010, 30, 1783–1786. [Google Scholar] [PubMed]
- Jahangard-Rafsanjani, Z.; Gholami, K.; Hadjibabaie, M.; Shamshiri, A.R.; Alimoghadam, K.; Sarayani, A.; Mojtahedzadeh, M.; Ostadali-Dehaghi, M.; Ghavamzadeh, A. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: A randomized clinical trial. Bone Marrow Transplant. 2013, 48, 832–836. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Schumacher, R.F.; D’Ippolito, C.; Porta, F.; Majorana, A. Efficacy of a Solution Composed by Verbascoside, Polyvinylpyrrolidone (PVP) and Sodium Hyaluronate in the Treatment of Chemotherapy-induced Oral Mucositis in Children With Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2016, 38, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Tacyildiz, N.; Ozyoruk, D.; Yavuz, G.; Unal, E.; Dincaslan, H.; Dogu, F.; Sahin, K.; Kucuk, O. Soy isoflavones ameliorate the adverse effects of chemotherapy in children. Nutr. Cancer 2010, 62, 1001–1005. [Google Scholar] [CrossRef]
- Wu, S.X.; Cui, T.T.; Zhao, C.; Pan, J.J.; Xu, B.Y.; Tian, Y.; Cui, N.J. A prospective, randomized, multi-center trial to investigate Actovegin in prevention and treatment of acute oral mucositis caused by chemoradiotherapy for nasopharyngeal carcinoma. Radiother. Oncol. 2010, 97, 113–118. [Google Scholar] [CrossRef]
- Anderson, C.M.; Lee, C.M.; Saunders, D.P.; Curtis, A.; Dunlap, N.; Nangia, C.; Lee, A.S.; Gordon, S.M.; Kovoor, P.; Arevalo-Araujo, R.; et al. Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin for Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3256–3265. [Google Scholar] [CrossRef]
- Yokomizo, H.; Yoshimatsu, K.; Hashimoto, M.; Ishibashi, K.; Umehara, A.; Yoshida, K.; Fujimoto, T.; Watanabe, K.; Ogawa, K. Prophylactic efficacy of allopurinol ice ball for leucovorin/5-fluorouracil therapy-induced stomatitis. Anticancer Res. 2004, 24, 1131–1134. [Google Scholar]
- Hosseinjani, H.; Hadjibabaie, M.; Gholami, K.; Javadi, M.; Radfar, M.; Jahangard-Rafsanjani, Z.; Hosseinjani, E.; Shabani, N.; Vaezi, M.; Ghavamzadeh, A. The efficacy of erythropoietin mouthwash in prevention of oral mucositis in patients undergoing autologous hematopoietic SCT: A double-blind, randomized, placebo-controlled trial. Hematol. Oncol. 2017, 35, 106–112. [Google Scholar] [CrossRef]
- Vidal-Casariego, A.; Calleja-Fernandez, A.; Ballesteros-Pomar, M.D.; Cano-Rodriguez, I. Efficacy of glutamine in the prevention of oral mucositis and acute radiation-induced esophagitis: A retrospective study. Nutr. Cancer 2013, 65, 424–429. [Google Scholar] [CrossRef]
- Moslehi, A.; Taghizadeh-Ghehi, M.; Gholami, K.; Hadjibabaie, M.; Jahangard-Rafsanjani, Z.; Sarayani, A.; Javadi, M.; Esfandbod, M.; Ghavamzadeh, A. N-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: A double-blind, randomized, placebo-controlled trial. Bone Marrow Transplant. 2014, 49, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Saeedi, M.; Ghorbani, A.; Ghodrati, P.; Moosazadeh, M.; Rostamkalaei, S.; Hatkehlouei, M.B.; Molania, T. The effect of propolis tablet on oral mucositis caused by chemotherapy. Gazi Med. J. 2018, 29, 196–201. [Google Scholar]
- Naidu, M.U.; Ramana, G.V.; Ratnam, S.V.; Sudhavani, T.; Naidu, K.J.; Roy, P.; Suresh, P.; Rani, P.U.; Mohan, I.K. A randomised, double-blind, parallel, placebo-controlled study to evaluate the efficacy of MF 5232 (Mucotrol), a concentrated oral gel wafer, in the treatment of oral mucositis. Drugs R D 2005, 6, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Elsabagh, H.H.; Moussa, E.; Mahmoud, S.A.; Elsaka, R.O.; Abdelrahman, H. Efficacy of Melatonin in prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral Dis. 2020, 26, 566–572. [Google Scholar] [CrossRef]
- Elkerm, Y.; Tawashi, R. Date palm pollen as a preventative intervention in radiation- and chemotherapy-induced oral mucositis: A pilot study. Integr. Cancer Ther. 2014, 13, 468–472. [Google Scholar] [CrossRef]
- Mills, E.E. The modifying effect of beta-carotene on radiation and chemotherapy induced oral mucositis. Br. J. Cancer 1988, 57, 416–417. [Google Scholar] [CrossRef]
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: A randomized controlled clinical study. DARU J. Pharm. Sci. 2013, 21, 18. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).