Osteopontin on the Dental Implant Surface Promotes Direct Osteogenesis in Osseointegration
Abstract
:1. Introduction
2. Results
2.1. Histological Changes in the Opn-KO Mice
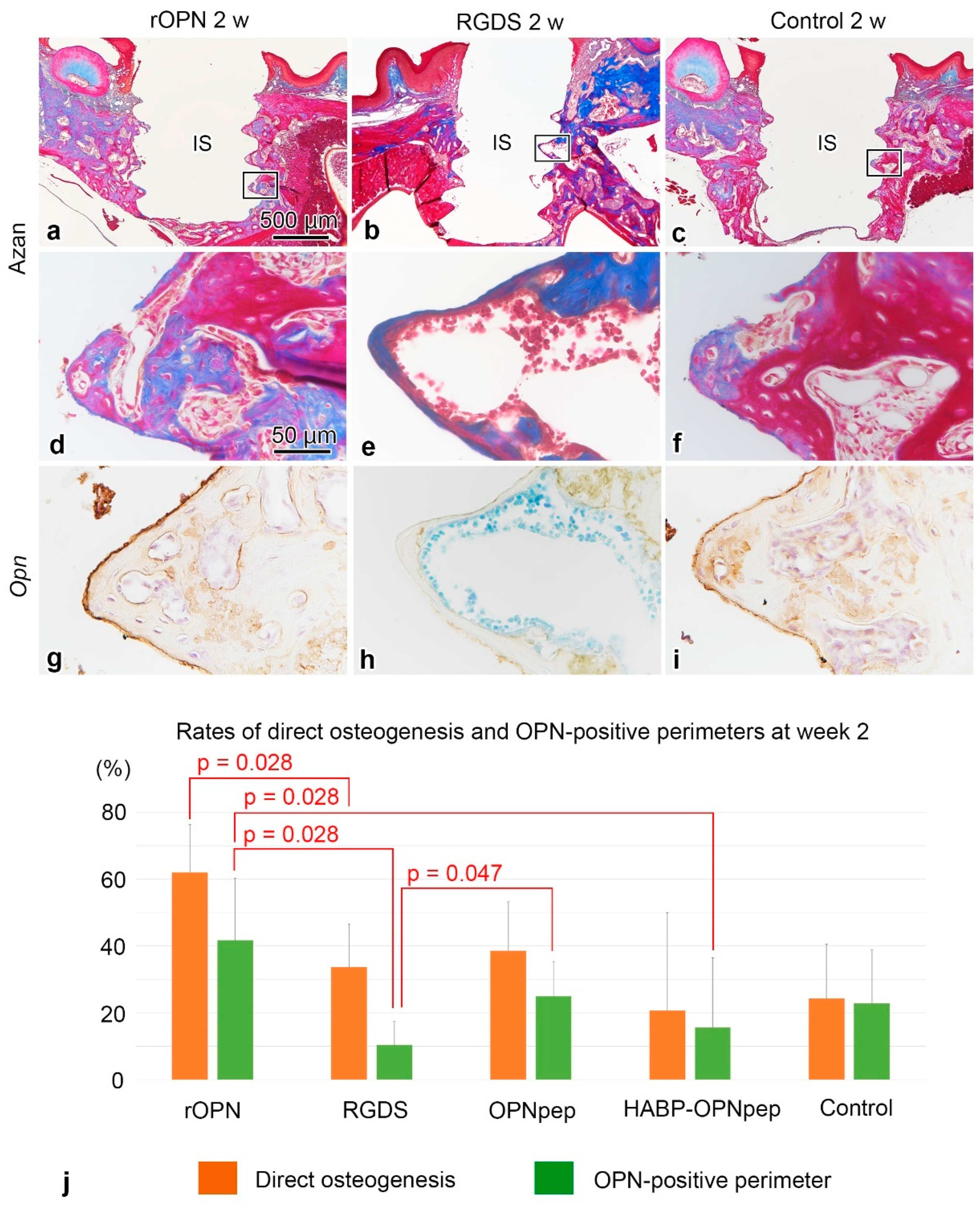
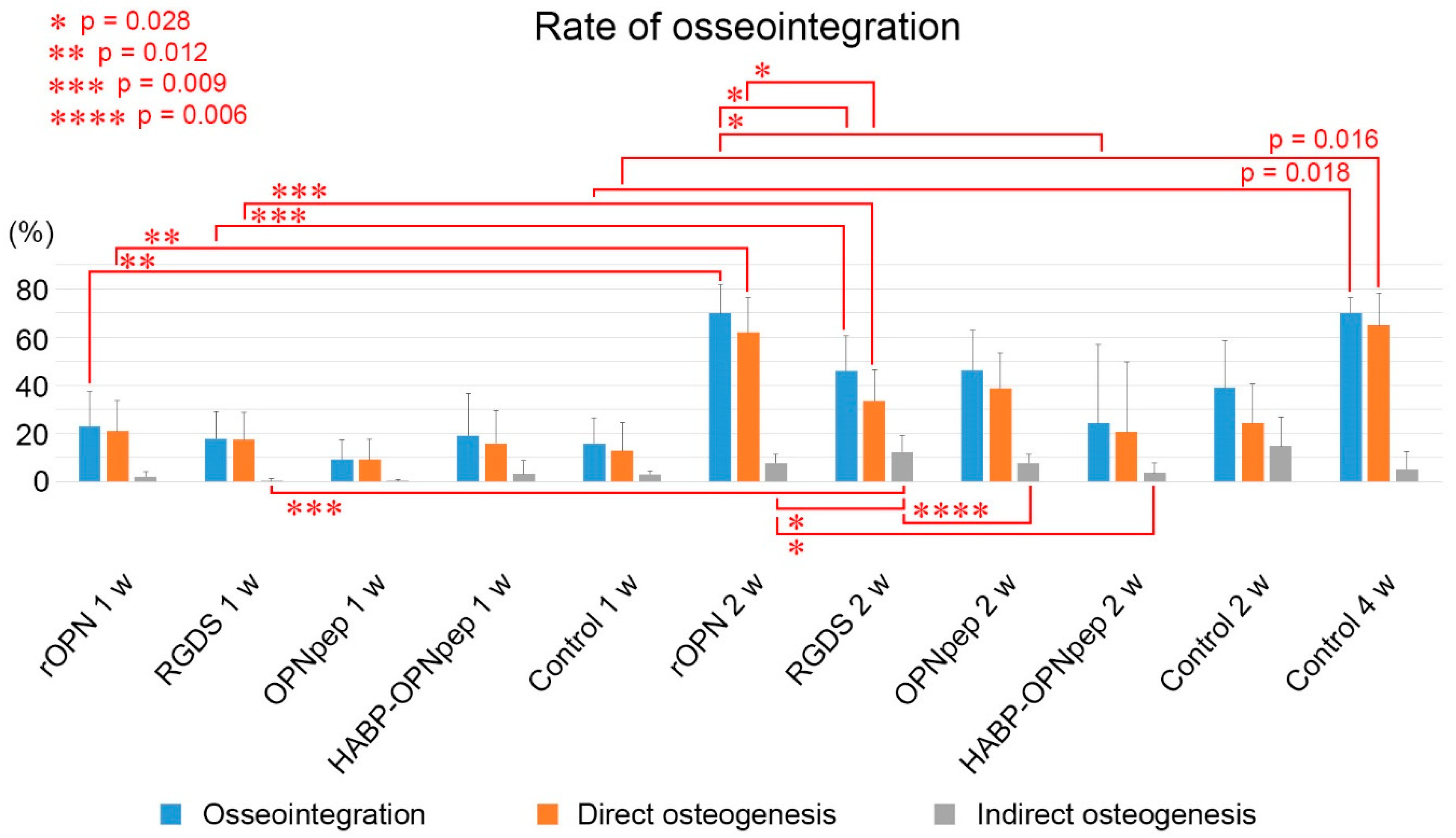
2.2. On Day 5 to Week 2 in the WT Mice
2.3. Cell Proliferation in the WT Mice
2.4. Tartrate-Resistant Acid Phosphatase (TRAP) Activity in the WT Mice
3. Discussion
4. Materials and Methods
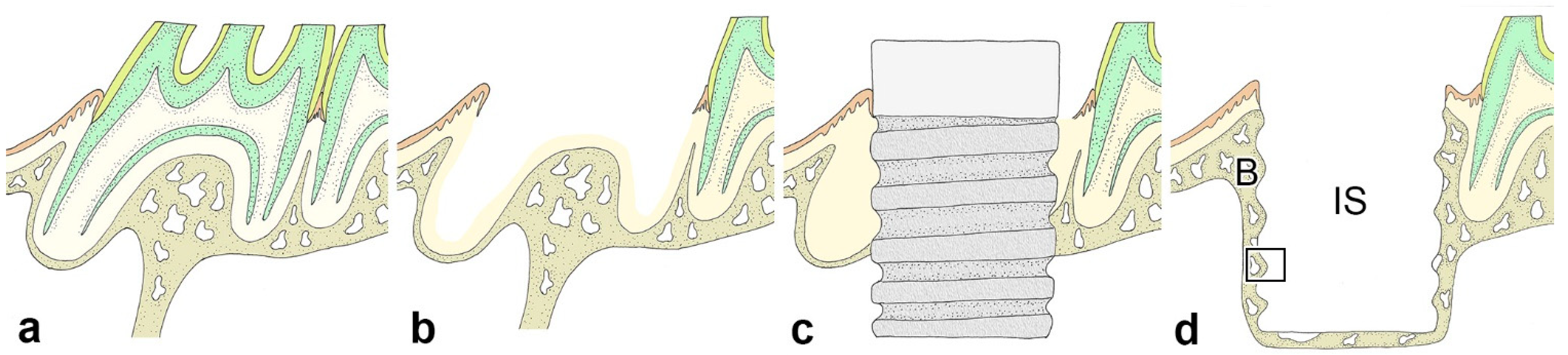
4.1. Animals and Experimental Procedure
4.2. Immediate Implant Placement
4.3. Histological Procedure
4.4. Immunohistochemical and Histochemical Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Young, M.F. Skeletal biology: Where matrix meets mineral. Matrix Biol. 2016, 52–54, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering; Eberli, D., Ed.; IntechOpen Limited: London, UK, 2011. [Google Scholar]
- Narducci, P.; Nicolin, V. Differentiation of activated monocytes into osteoclast-like cells on a hydroxyapatite substrate: An in vitro study. Ann. Anat. 2009, 191, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.M.; Brooks, R.A.; Spence, G.; McFarlane, I.; Lopes, M.A.; Best, S.M.; Santos, J.D.; Rushton, N.; Bonfield, W. Differentiation of mononuclear precursors into osteoclasts on the surface of Si-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2006, 78, 709–720. [Google Scholar] [CrossRef]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef]
- Lin, Y.; Gallucci, G.O.; Buser, D.; Bosshardt, D.; Belser, U.C.; Yelick, P.C. Bioengineered periodontal tissue formed on titanium dental implants. J. Dent. Res. 2011, 90, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adell, R.; Lekholm, U.; Rockler, B.; Branemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Makishi, S.; Saito, K.; Ohshima, H. Osteopontin-deficiency disturbs direct osteogenesis in the process of achieving osseointegration following immediate placement of endosseous implants. Clin. Implant. Dent. Relat. Res. 2016, 19, 496–504. [Google Scholar] [CrossRef]
- Bassir, S.H.; El Kholy, K.; Chen, C.Y.; Lee, K.H.; Intini, G. Outcome of early dental implant placement versus other dental implant placement protocols: A systematic review and meta-analysis. J. Periodontol. 2019, 90, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nakagawa, E.; Saito, K.; Ohshima, H. Differences in Healing Patterns of the Bone-Implant Interface between Immediately and Delayed-Placed Titanium Implants in Mouse Maxillae. Clin. Implant. Dent. Relat. Res. 2016, 18, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2005, 20, 425–431. [Google Scholar]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Wai, P.Y.; Kuo, P.C. The role of Osteopontin in tumor metastasis. J. Surg Res. 2004, 121, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Razzouk, S.; Brunn, J.C.; Qin, C.; Tye, C.E.; Goldberg, H.A.; Butler, W.T. Osteopontin posttranslational modifications, possibly phosphorylation, are required for in vitro bone resorption but not osteoclast adhesion. Bone 2002, 30, 40–47. [Google Scholar] [CrossRef]
- Nakamura, I.; Takahashi, N.; Sasaki, T.; Jimi, E.; Kurokawa, T.; Suda, T. Chemical and physical properties of the extracellular matrix are required for the actin ring formation in osteoclasts. J. Bone Miner. Res. 1996, 11, 1873–1879. [Google Scholar] [CrossRef]
- Nakayama, T.; Thirukonda, G.J.; Nagasawa, S.; Kawahara, I.; Udagawa, N.; Yagami, K.; Kawatani, M.; Hiroyuki Osada, H.; Doi, Y.; Yoshinari, N.; et al. Polarization of osteoclasts on dental implant materials is similar to that observed on bone. J. Oral Biosci. 2014, 56, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Decup, F.; Six, N.; Palmier, B.; Buch, D.; Lasfargues, J.J.; Salih, E.; Goldberg, M. Bone sialoprotein-induced reparative dentinogenesis in the pulp of rat’s molar. Clin. Oral Investig. 2000, 4, 110–119. [Google Scholar] [CrossRef]
- O’Toole, G.C.; Salih, E.; Gallagher, C.; FitzPatrick, D.; O’Higgins, N.; O’Rourke, S.K. Bone sialoprotein-coated femoral implants are osteoinductive but mechanically compromised. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2004, 22, 641–646. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A. Osteopontin and the bone remodeling sequence. Colloidal-gold immunocytochemistry of an interfacial extracellular matrix protein. Ann. N. Y. Acad. Sci. 1995, 760, 177–189. [Google Scholar] [CrossRef]
- Dodds, R.A.; Connor, J.R.; James, I.E.; Rykaczewski, E.L.; Appelbaum, E.; Dul, E.; Gowen, M. Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: An in vitro and ex vivo study of remodeling bone. J. Bone Miner. Res. 1995, 10, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Spence, G.; Patel, N.; Brooks, R.; Rushton, N. Carbonate substituted hydroxyapatite: Resorption by osteoclasts modifies the osteoblastic response. J. Biomed. Mater. Res. A 2009, 90, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Pampena, D.A.; Robertson, K.A.; Litvinova, O.; Lajoie, G.; Goldberg, H.A.; Hunter, G.K. Inhibition of hydroxyapatite formation by osteopontin phosphopeptides. Biochem. J. 2004, 378, 1083–1087. [Google Scholar] [CrossRef]
- Hunter, G.K.; Goldberg, H.A. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 8562–8565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzopardi, P.V.; O’Young, J.; Lajoie, G.; Karttunen, M.; Goldberg, H.A.; Hunter, G.K. Roles of electrostatics and conformation in protein-crystal interactions. PLoS ONE 2010, 5, e9330. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Carlsen, B.; Rudkin, G.; Berry, M.; Ishida, K.; Yamaguchi, D.T.; Miller, T.A. Osteopontin is a negative regulator of proliferation and differentiation in MC3T3-E1 pre-osteoblastic cells. Bone 2004, 34, 799–808. [Google Scholar] [CrossRef]
- Katayama, Y.; House, C.M.; Udagawa, N.; Kazama, J.J.; McFarland, R.J.; Martin, T.J.; Findlay, D.M. Casein kinase 2 phosphorylation of recombinant rat osteopontin enhances adhesion of osteoclasts but not osteoblasts. J. Cell. Physiol. 1998, 176, 179–187. [Google Scholar] [CrossRef]
- Ek-Rylander, B.; Flores, M.; Wendel, M.; Heinegard, D.; Andersson, G. Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase. Modulation of osteoclast adhesion in vitro. J. Biol. Chem. 1994, 269, 14853–14856. [Google Scholar] [CrossRef]
- Helfrich, M.H.; Nesbitt, S.A.; Horton, M.A. Integrins on rat osteoclasts: Characterization of two monoclonal antibodies (F4 and F11) to rat beta 3. J. Bone Miner. Res. 1992, 7, 345–351. [Google Scholar] [CrossRef]
- Flores, M.E.; Norgard, M.; Heinegard, D.; Reinholt, F.P.; Andersson, G. RGD-directed attachment of isolated rat osteoclasts to osteopontin, bone sialoprotein, and fibronectin. Exp. Cell Res. 1992, 201, 526–530. [Google Scholar] [CrossRef]
- Kazanecki, C.C.; Uzwiak, D.J.; Denhardt, D.T. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J. Cell. Biochem. 2007, 102, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Perruzzi, C.A.; Papadopoulos-Sergiou, A.; Van de Water, L. Adhesive properties of osteopontin: Regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell 1994, 5, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Yokosaki, Y.; Tanaka, K.; Higashikawa, F.; Yamashita, K.; Eboshida, A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005, 24, 418–427. [Google Scholar] [CrossRef]
- Patarca, R.; Freeman, G.J.; Singh, R.P.; Wei, F.Y.; Durfee, T.; Blattner, F.; Regnier, D.C.; Kozak, C.A.; Mock, B.A.; Morse, H.C., 3rd; et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J. Exp. Med. 1989, 170, 145–161. [Google Scholar] [CrossRef] [Green Version]
- Chabas, D.; Baranzini, S.E.; Mitchell, D.; Bernard, C.C.; Rittling, S.R.; Denhardt, D.T.; Sobel, R.A.; Lock, C.; Karpuj, M.; Pedotti, R.; et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001, 294, 1731–1735. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, S.; Huang, G.; Pan, J.; Li, J.; Zhang, X.; Fang, L.; Liu, B.; Meng, W.; Zhang, Y.; Liu, X. Involvement of osteopontin as a core protein in craniopharyngioma calcification formation. J. Neuro-Oncol. 2010, 98, 21–30. [Google Scholar] [CrossRef]
- O’Regan, A.; Berman, J.S. Osteopontin: A key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 2000, 81, 373–390. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.; da Silva, C.L.; Vashishth, D. Synergistic effect of extracellularly supplemented osteopontin and osteocalcin on stem cell proliferation, osteogenic differentiation, and angiogenic properties. J. Cell. Biochem. 2019, 120, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.; Birk, D.E.; Ballas, C.B.; Whitsitt, J.S.; Davidson, J.M.; Hogan, B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 1998, 101, 1468–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto-Tanaka, H.; Ikegame, M.; Takagi, R.; Harada, H.; Ohshima, H. Histochemical and immunocytochemical study of hard tissue formation in dental pulp during the healing process in rat molars after tooth replantation. Cell Tissue Res. 2006, 325, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Gong, K.; Zhang, X.; Wu, S.; Cui, Y.; Qian, B.Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 2019, 144, 235–244. [Google Scholar] [CrossRef] [PubMed]


| Strain | Method | Day 1 | Day 3 | Day 5 | Week 1 | Week 2 | Week 4 | Total |
|---|---|---|---|---|---|---|---|---|
| WT mice | Histological section | 3 | 20 {3 1} | 19 {3 1} | 21 | 23 | 4 {4 1} | 90 {10 1} |
| Ki67 | - | (20 {3 1}) | (19 {3 1}) | (21) | (23) | - | (86 {6 1}) | |
| OPN | (3) | (20 {3 1}) | (19 {3 1}) | (21) | (23) | - | (86 {6 1}) | |
| TRAP | - | (20 {3 1}) | (19 {3 1}) | (21) | - | - | (60 {6 1}) | |
| Opn-KO mice | Histological section | - | - | - | - | 6 | - | 6 |
| OPN | - | - | - | - | (6) | - | (6) | |
| Total | 3 | 20 {3 1} | 19 {3 1} | 21 | 29 | 4 {4 1} | 96 {10 1} | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makishi, S.; Yamazaki, T.; Ohshima, H. Osteopontin on the Dental Implant Surface Promotes Direct Osteogenesis in Osseointegration. Int. J. Mol. Sci. 2022, 23, 1039. https://doi.org/10.3390/ijms23031039
Makishi S, Yamazaki T, Ohshima H. Osteopontin on the Dental Implant Surface Promotes Direct Osteogenesis in Osseointegration. International Journal of Molecular Sciences. 2022; 23(3):1039. https://doi.org/10.3390/ijms23031039
Chicago/Turabian StyleMakishi, Sanako, Tomohiko Yamazaki, and Hayato Ohshima. 2022. "Osteopontin on the Dental Implant Surface Promotes Direct Osteogenesis in Osseointegration" International Journal of Molecular Sciences 23, no. 3: 1039. https://doi.org/10.3390/ijms23031039
APA StyleMakishi, S., Yamazaki, T., & Ohshima, H. (2022). Osteopontin on the Dental Implant Surface Promotes Direct Osteogenesis in Osseointegration. International Journal of Molecular Sciences, 23(3), 1039. https://doi.org/10.3390/ijms23031039








