Supplementation with SCFAs Re-Establishes Microbiota Composition and Attenuates Hyperalgesia and Pain in a Mouse Model of NTG-Induced Migraine
Abstract
:1. Introduction
2. Results
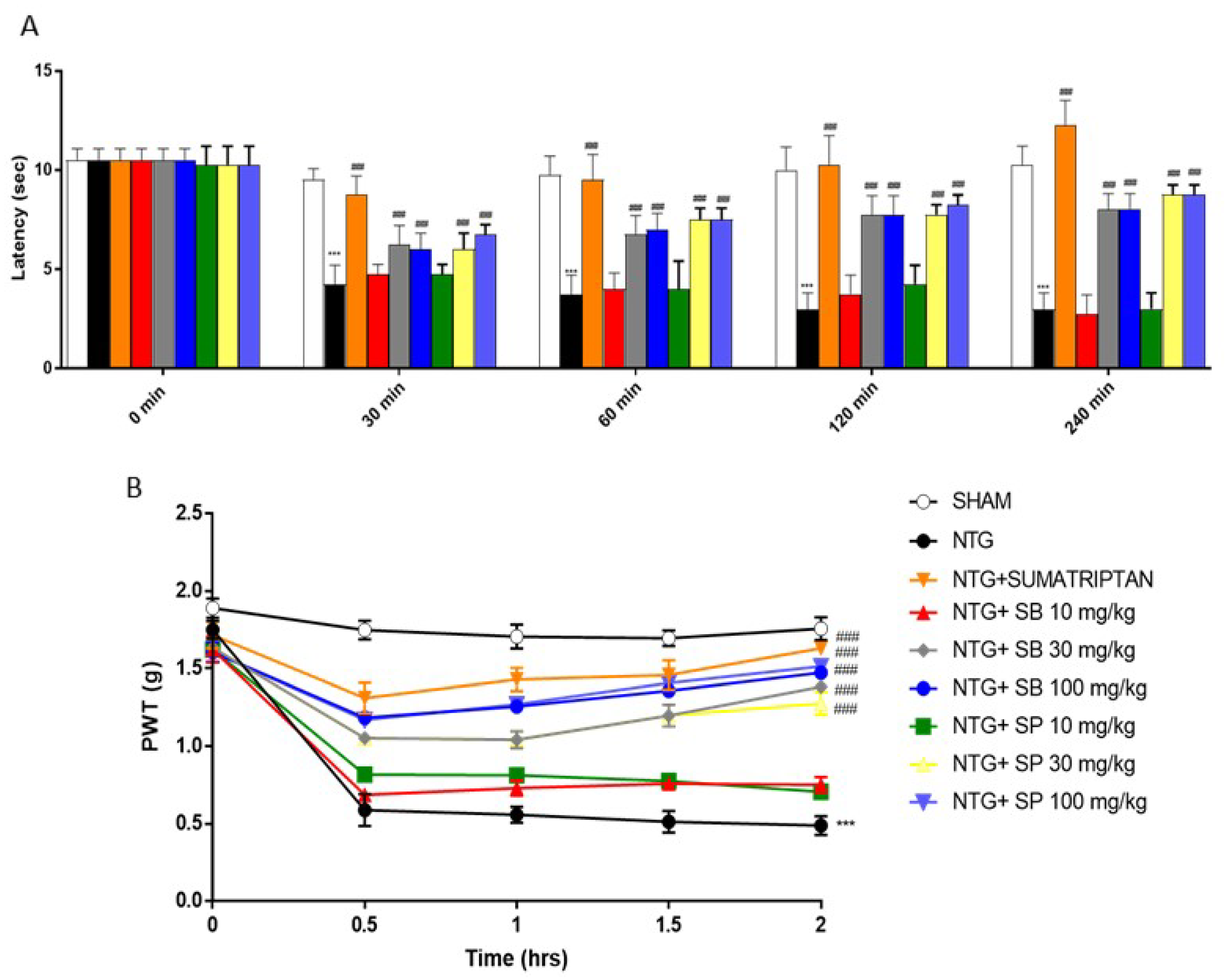
2.1. SCFAs Treatments Attenuates Hyperalgesia and Pain NTG-Induced
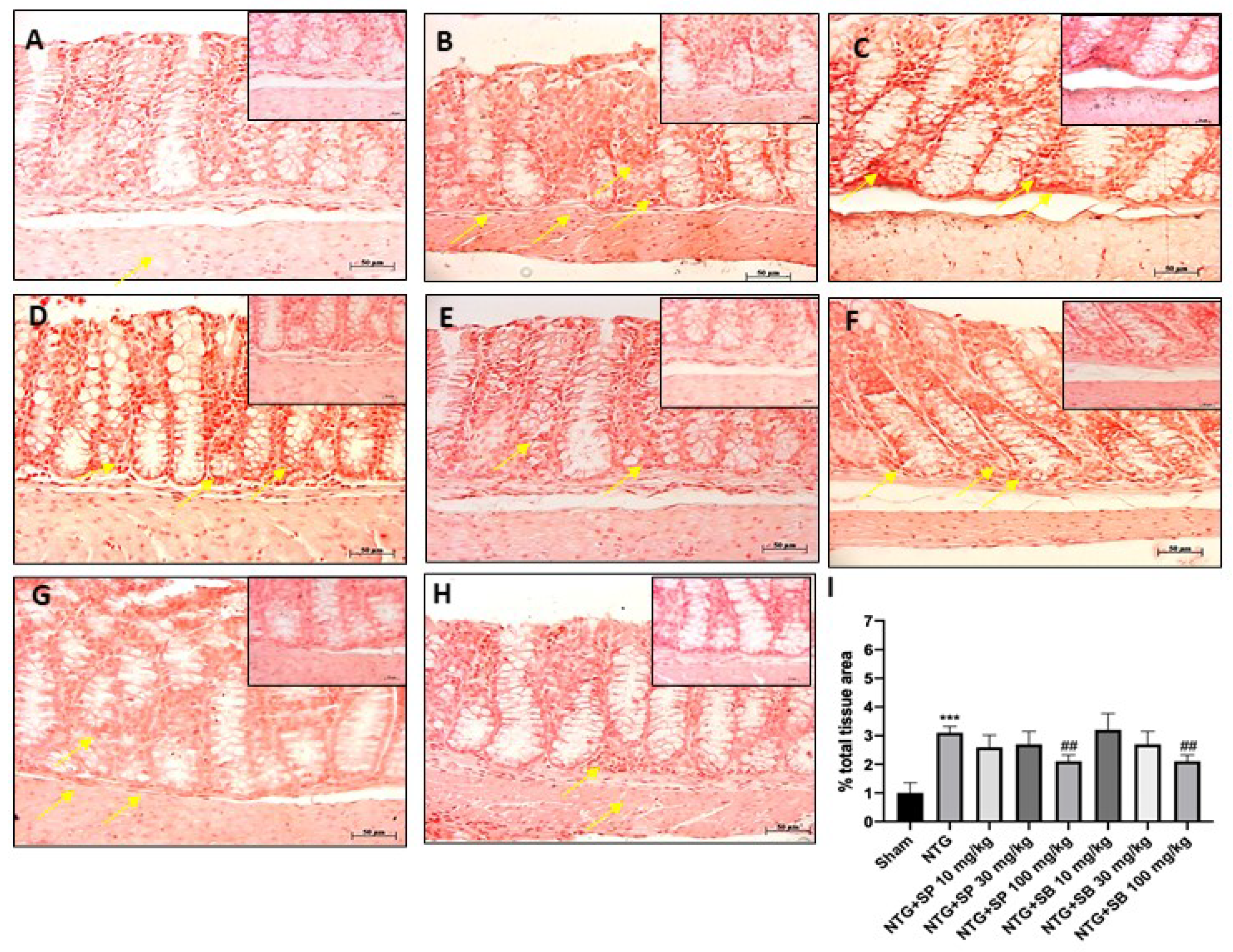
2.2. SCFAs Modulates Mast Cells Degranulation after NTG-Injection
2.3. Protective Effect of SCFAs on ICAM and P-Selectin Expression
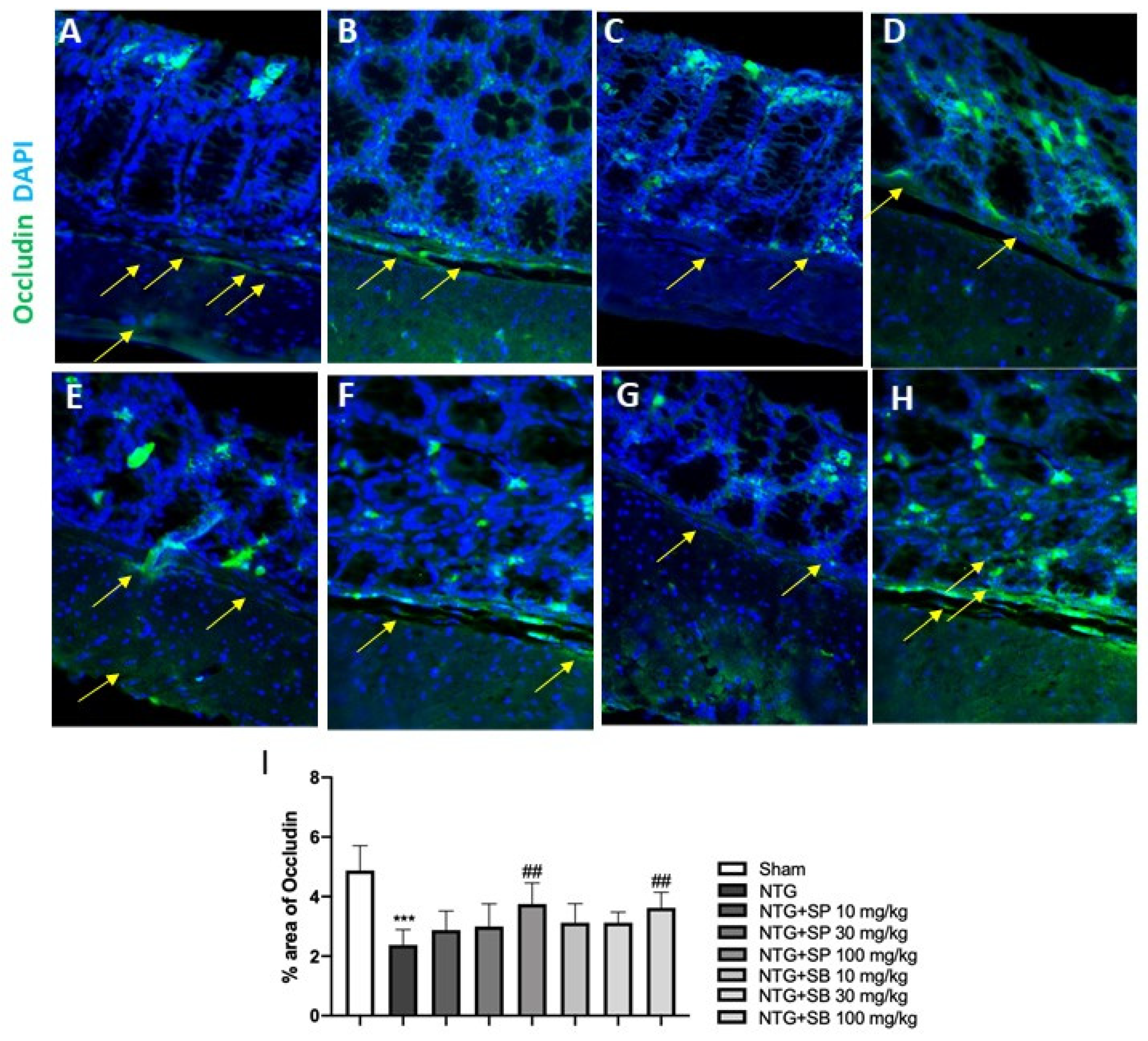
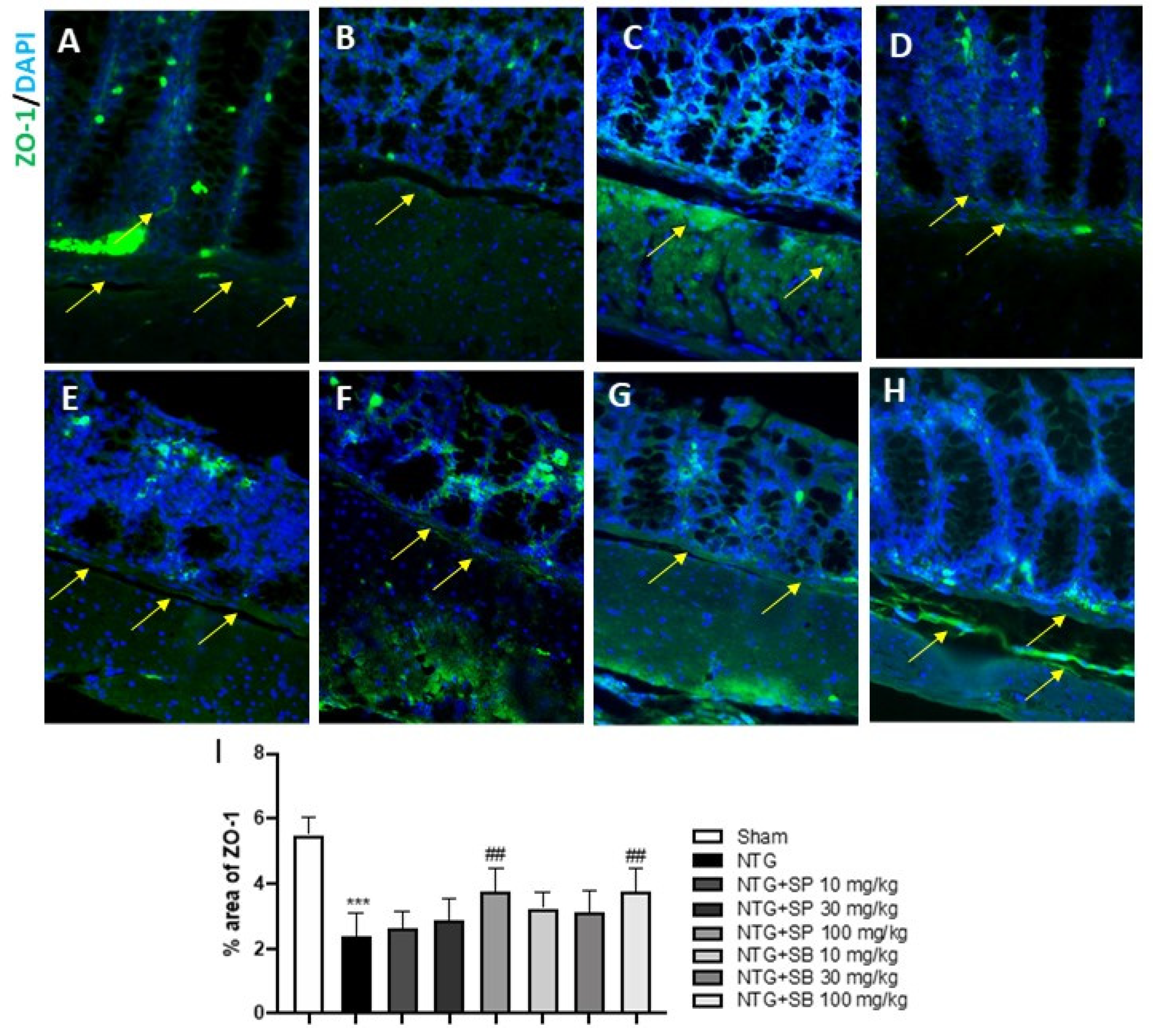
2.4. Effect of SCFAs on Tight Junctions (TJs) Expression
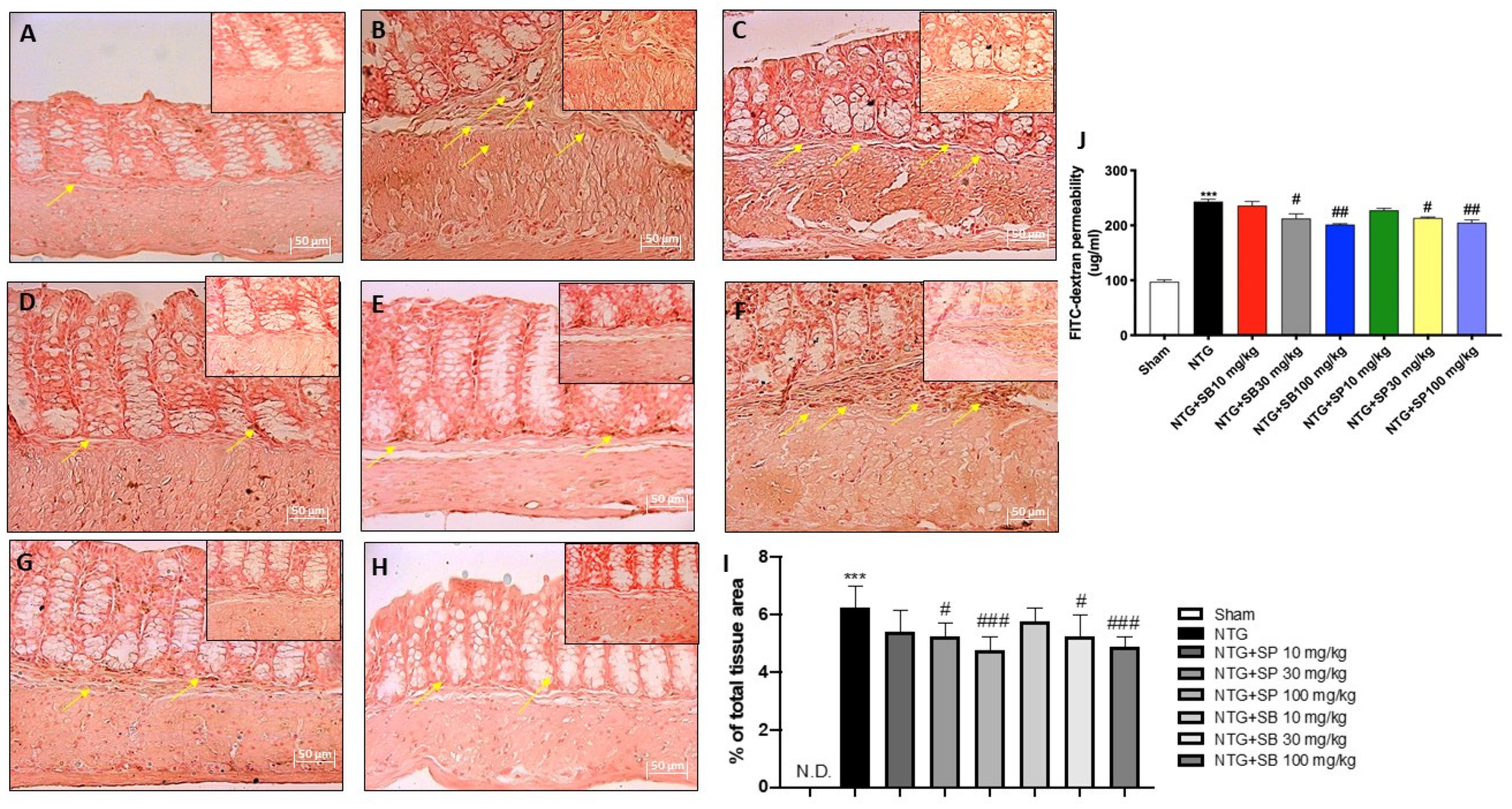
2.5. Effect of SCFAs on Intestinal Permeability
2.6. Effect of SCFAs on Microbiota Composition
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. NTG-Migraine Model
Experimental Groups
- −
- Group sham + vehicle (veh): mice received saline;
- −
- Group NTG: mice received NTG (10 mg/kg) intraperitoneally (i.p.);
- −
- Group NTG+ sumatriptan: mice received sumatriptan orally (600 μg/kg) 5 min after NTG (10 mg/kg) (i.p.);
- −
- Group NTG+ SP 10 mg/kg: mice received SP orally at a dose of 10 mg/kg 5 min after NTG injection (i.p.);
- −
- Group NTG+ SP 30 mg/kg: mice received SP orally at a dose of 30 mg/kg 5 min after NTG injection (i.p.);
- −
- Group NTG+ SP 100 mg/kg: mice received SP orally at a dose of 100 mg/kg 5 min after NTG injection (i.p.);
- −
- Group NTG+ SB 10 mg/kg: mice received SB orally at a dose of 10 mg/kg 5 min after NTG injection (i.p.);
- −
- Group NTG+ SB 30 mg/kg: mice received SB orally at a dose of 30 mg/kg 5 min after NTG injection (i.p.);
- −
- Group NTG+ SB 100 mg/kg: mice received SB orally at a dose of 100 mg/kg 5 min after NTG injection (i.p.).
4.3. Behavioral Tests
4.3.1. Hargreaves Test
4.3.2. Von Frey Test
4.4. Blue Toluidine Staining
4.5. Immunohistochemical Localization of ICAM, P-Selectin and E-Cadherin
4.6. Immunofluorescence Analysis of Occludin and ZO-1
4.7. Intestinal Permeability Measurement
4.8. DNA Extraction from Tools
4.9. Bioinformatics Pipeline
4.10. Materials
4.11. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache, F. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef] [Green Version]
- De Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Sun, P.; Su, L.; Zhu, H.; Li, X.; Guo, Y.; Du, X.; Zhang, L.; Qin, C. Gut Microbiota Regulation and Their Implication in the Development of Neurodegenerative Disease. Microorganisms 2021, 9, 2281. [Google Scholar] [CrossRef]
- Hindiyeh, N.; Aurora, S.K. What the Gut Can Teach Us About Migraine. Curr. Pain Headache Rep. 2015, 19, 33. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F.; Group, A.A. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Yu, Y.J.; Adeli, K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorganisms 2020, 8, 527. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine--current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Lanza, M.; Campolo, M.; Casili, G.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Sodium Butyrate Exerts Neuroprotective Effects in Spinal Cord Injury. Mol. Neurobiol. 2019, 56, 3937–3947. [Google Scholar] [CrossRef]
- Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells 2021, 10, 2756. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Protective effect of sodium propionate in Abeta1-42 -induced neurotoxicity and spinal cord trauma. Neuropharmacology 2020, 166, 107977. [Google Scholar] [CrossRef]
- Schilderink, R.; Verseijden, C.; de Jonge, W.J. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front. Immunol. 2013, 4, 226. [Google Scholar] [CrossRef] [Green Version]
- Casili, G.; Lanza, M.; Filippone, A.; Campolo, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl fumarate alleviates the nitroglycerin (NTG)-induced migraine in mice. J. Neuroinflamm. 2020, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Yeh, S.L.; Neuman, M.G. Gastrointestinal Inflammation and Repair: Role of Microbiome, Infection, and Nutrition. Gastroenterol. Res. Pract. 2016, 2016, 6516708. [Google Scholar] [CrossRef] [PubMed]
- de Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.R.; Dahlhoff, M.; Horst, D.; Hirschi, B.; Trulzsch, K.; Muller-Hocker, J.; Vogelmann, R.; Allgauer, M.; Gerhard, M.; Steininger, S.; et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS ONE 2010, 5, e14325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hemert, S.; Breedveld, A.C.; Rovers, J.M.; Vermeiden, J.P.; Witteman, B.J.; Smits, M.G.; de Roos, N.M. Migraine associated with gastrointestinal disorders: Review of the literature and clinical implications. Front. Neurol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Schoultz, I.; Keita, A.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Wlodarczyk, M.; Zielinska, A.; Poleszak, E.; Fichna, J.; Wlaz, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Balan, Y.; Gaur, A.; Sakthivadivel, V.; Kamble, B.; Sundaramurthy, R. Is the Gut Microbiota a Neglected Aspect of Gut and Brain Disorders? Cureus 2021, 13, e19740. [Google Scholar] [CrossRef]
- Sumagin, R.; Parkos, C.A. Epithelial adhesion molecules and the regulation of intestinal homeostasis during neutrophil transepithelial migration. Tissue Barriers 2015, 3, e969100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traina, G. Mast Cells in Gut and Brain and Their Potential Role as an Emerging Therapeutic Target for Neural Diseases. Front. Cell Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Wang, A.; Lin, Z. Structural and Functional Characterization of the Gut Microbiota in Elderly Women With Migraine. Front. Cell Infect. Microbiol. 2019, 9, 470. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Pukall, R.; Abt, B.; Foesel, B.U.; Meier-Kolthoff, J.P.; Kumar, N.; Bresciani, A.; Martinez, I.; Just, S.; Ziegler, C.; et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat. Microbiol. 2016, 1, 16131. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.E.; Sugiarto, E.; Taylor, A.M.W.; Pradhan, A.A.; Al-Hasani, R. Pain, Motivation, Migraine, and the Microbiome: New Frontiers for Opioid Systems and Disease. Mol. Pharmacol. 2020, 98, 433–444. [Google Scholar] [CrossRef]
- Dobranowski, P.A.; Tang, C.; Sauve, J.P.; Menzies, S.C.; Sly, L.M. Compositional changes to the ileal microbiome precede the onset of spontaneous ileitis in SHIP deficient mice. Gut Microbes 2019, 10, 578–598. [Google Scholar] [CrossRef] [Green Version]
- McNamara, M.P.; Singleton, J.M.; Cadney, M.D.; Ruegger, P.M.; Borneman, J.; Garland, T. Early-life effects of juvenile Western diet and exercise on adult gut microbiome composition in mice. J. Exp. Biol. 2021, 224, jeb239699. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- de Roos, N.M.; Giezenaar, C.G.; Rovers, J.M.; Witteman, B.J.; Smits, M.G.; van Hemert, S. The effects of the multispecies probiotic mixture Ecologic(R)Barrier on migraine: Results of an open-label pilot study. Benef. Microbes 2015, 6, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, J.; Johnson, M.; Staverosky, T. Treatment of migraine with targeted nutrition focused on improved assimilation and elimination. Altern. Med. Rev. 2001, 6, 488–494. [Google Scholar] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Bates, E.A.; Nikai, T.; Brennan, K.C.; Fu, Y.H.; Charles, A.C.; Basbaum, A.I.; Ptacek, L.J.; Ahn, A.H. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 2010, 30, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Cheah, M.; Fawcett, J.W.; Andrews, M.R. Assessment of Thermal Pain Sensation in Rats and Mice Using the Hargreaves Test. Bio Protoc. 2017, 7, e2506. [Google Scholar] [CrossRef] [Green Version]
- Edelmayer, R.M.; Ossipov, M.H.; Porreca, F. An experimental model of headache-related pain. Methods Mol. Biol. 2012, 851, 109–120. [Google Scholar] [CrossRef]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef]
- Woting, A.; Blaut, M. Small Intestinal Permeability and Gut-Transit Time Determined with Low and High Molecular Weight Fluorescein Isothiocyanate-Dextrans in C3H Mice. Nutrients 2018, 10, 685. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, A.S.M.; Erickson, L.C.; Mallikarjunn, A.; Thiessen, E.D.; Fennell, C.T. Dual language statistical word segmentation in infancy: Simulating a language-mixing bilingual environment. Dev. Sci. 2021, 24, e13050. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef] [PubMed]



| V3–V5 | |||||||
|---|---|---|---|---|---|---|---|
| Raw Reads | Merged | Clean Reads | Classified | Phylum | Genera | Species | |
| Sham | 111,002 | 97,172 | 96,915 | 90,264 | 8 | 51 | 70 |
| NTG+ SB 100 mg/kg | 108,808 | 98,049 | 97,731 | 92,342 | 6 | 56 | 82 |
| NTG+ SB 10 mg/kg | 132,836 | 119,373 | 118,943 | 114,293 | 8 | 55 | 81 |
| NTG+ SB 30 mg/kg | 83,828 | 75,125 | 74,849 | 71,517 | 8 | 58 | 76 |
| NTG+ SP 100 mg/kg | 122,776 | 109,779 | 109,443 | 104,130 | 8 | 52 | 78 |
| NTG+ SP 10 mg/kg | 106,006 | 93,551 | 93,251 | 89,616 | 8 | 56 | 77 |
| NTG+ SP 30 mg/kg | 110,765 | 97,279 | 96,941 | 92,845 | 9 | 54 | 73 |
| NTG | 119,745 | 107,072 | 106,740 | 101,596 | 8 | 58 | 80 |
| NTG+ Sumatriptan | 73,386 | 64,863 | 64,635 | 184,014 | 8 | 49 | 73 |
| Total | 969,152 | 862,263 | 859,448 | 940,617 | 11 * | 80 * | 126 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, M.; Filippone, A.; Casili, G.; Giuffrè, L.; Scuderi, S.A.; Paterniti, I.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Supplementation with SCFAs Re-Establishes Microbiota Composition and Attenuates Hyperalgesia and Pain in a Mouse Model of NTG-Induced Migraine. Int. J. Mol. Sci. 2022, 23, 4847. https://doi.org/10.3390/ijms23094847
Lanza M, Filippone A, Casili G, Giuffrè L, Scuderi SA, Paterniti I, Campolo M, Cuzzocrea S, Esposito E. Supplementation with SCFAs Re-Establishes Microbiota Composition and Attenuates Hyperalgesia and Pain in a Mouse Model of NTG-Induced Migraine. International Journal of Molecular Sciences. 2022; 23(9):4847. https://doi.org/10.3390/ijms23094847
Chicago/Turabian StyleLanza, Marika, Alessia Filippone, Giovanna Casili, Letterio Giuffrè, Sarah Adriana Scuderi, Irene Paterniti, Michela Campolo, Salvatore Cuzzocrea, and Emanuela Esposito. 2022. "Supplementation with SCFAs Re-Establishes Microbiota Composition and Attenuates Hyperalgesia and Pain in a Mouse Model of NTG-Induced Migraine" International Journal of Molecular Sciences 23, no. 9: 4847. https://doi.org/10.3390/ijms23094847
APA StyleLanza, M., Filippone, A., Casili, G., Giuffrè, L., Scuderi, S. A., Paterniti, I., Campolo, M., Cuzzocrea, S., & Esposito, E. (2022). Supplementation with SCFAs Re-Establishes Microbiota Composition and Attenuates Hyperalgesia and Pain in a Mouse Model of NTG-Induced Migraine. International Journal of Molecular Sciences, 23(9), 4847. https://doi.org/10.3390/ijms23094847













