Quantification and Improvement of the Dynamics of Human Serum Albumin and Glycated Human Serum Albumin with Astaxanthin/Astaxanthin-Metal Ion Complexes: Physico-Chemical and Computational Approaches
Abstract
:1. Introduction
2. Results and Discussion
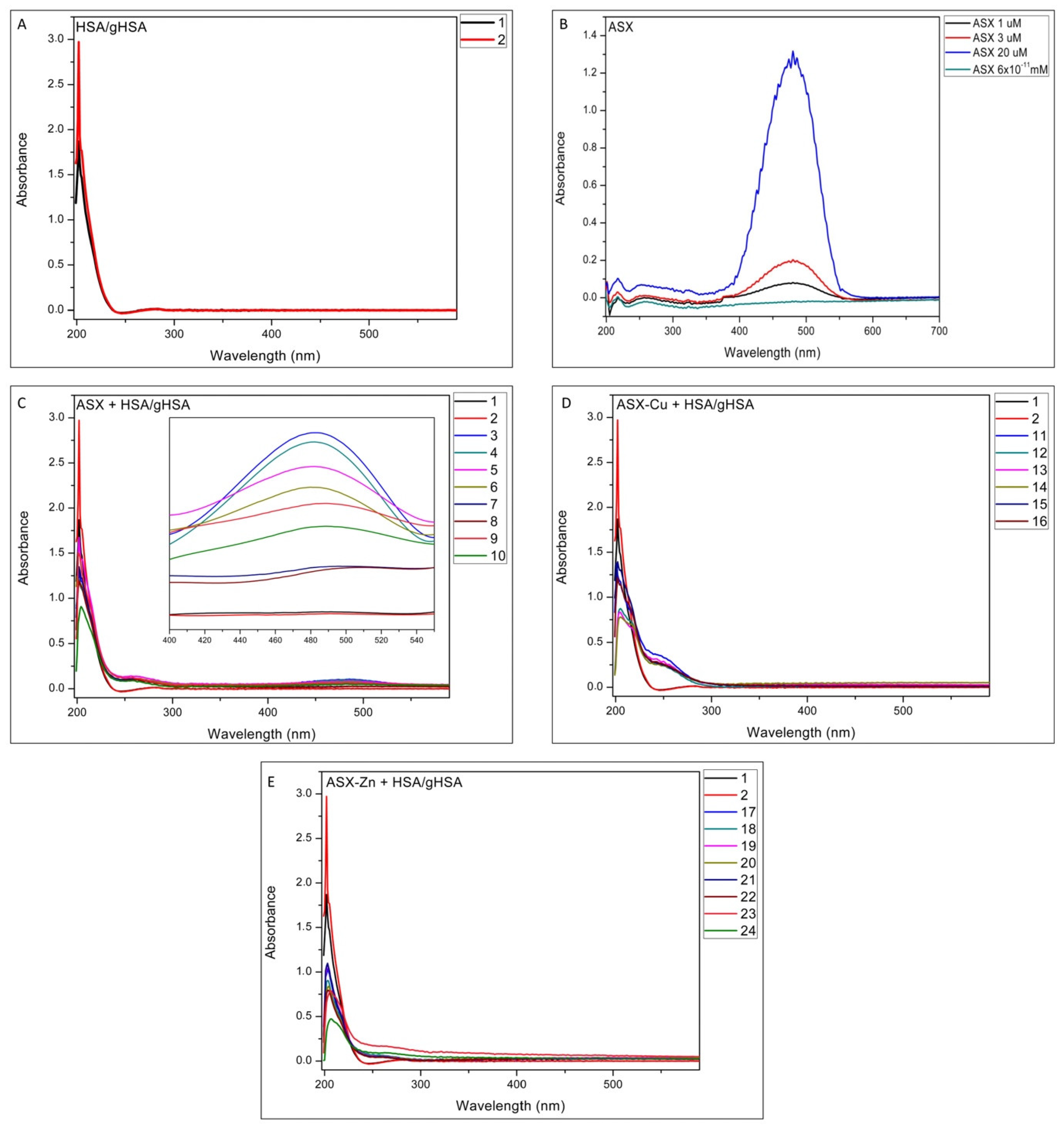
2.1. UV-Vis Analysis
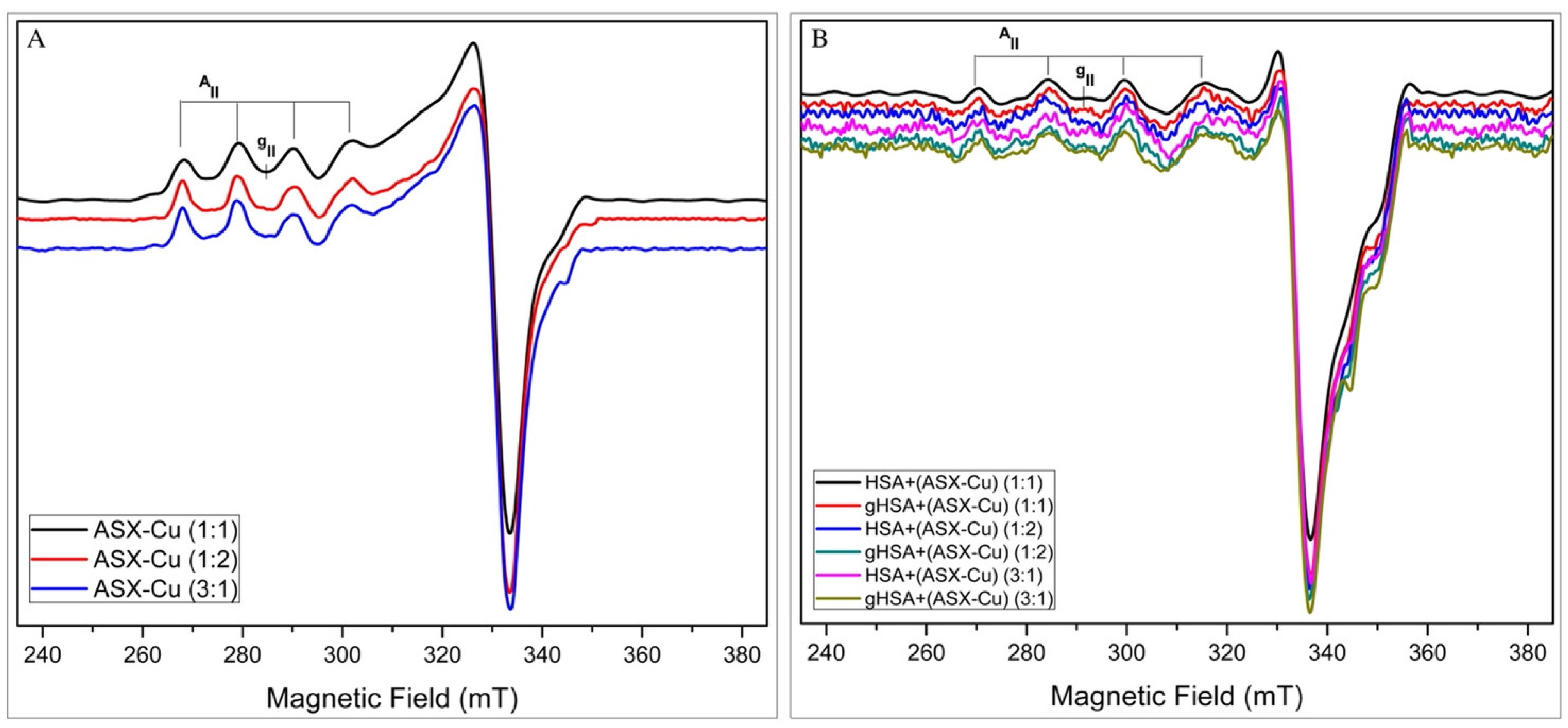
2.2. EPR Analysis
2.2.1. Complexation Ability
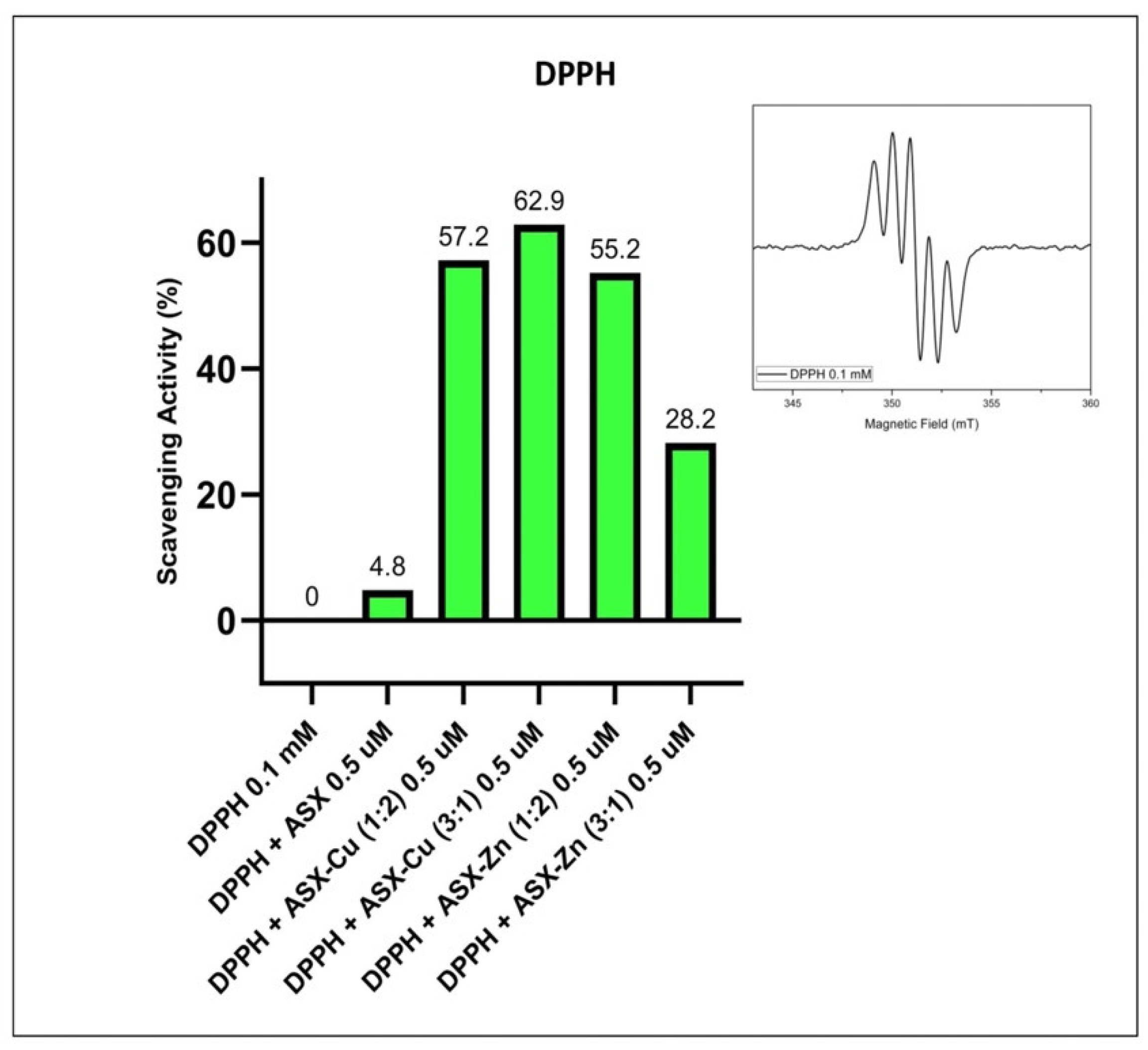
2.2.2. Antioxidant Activity
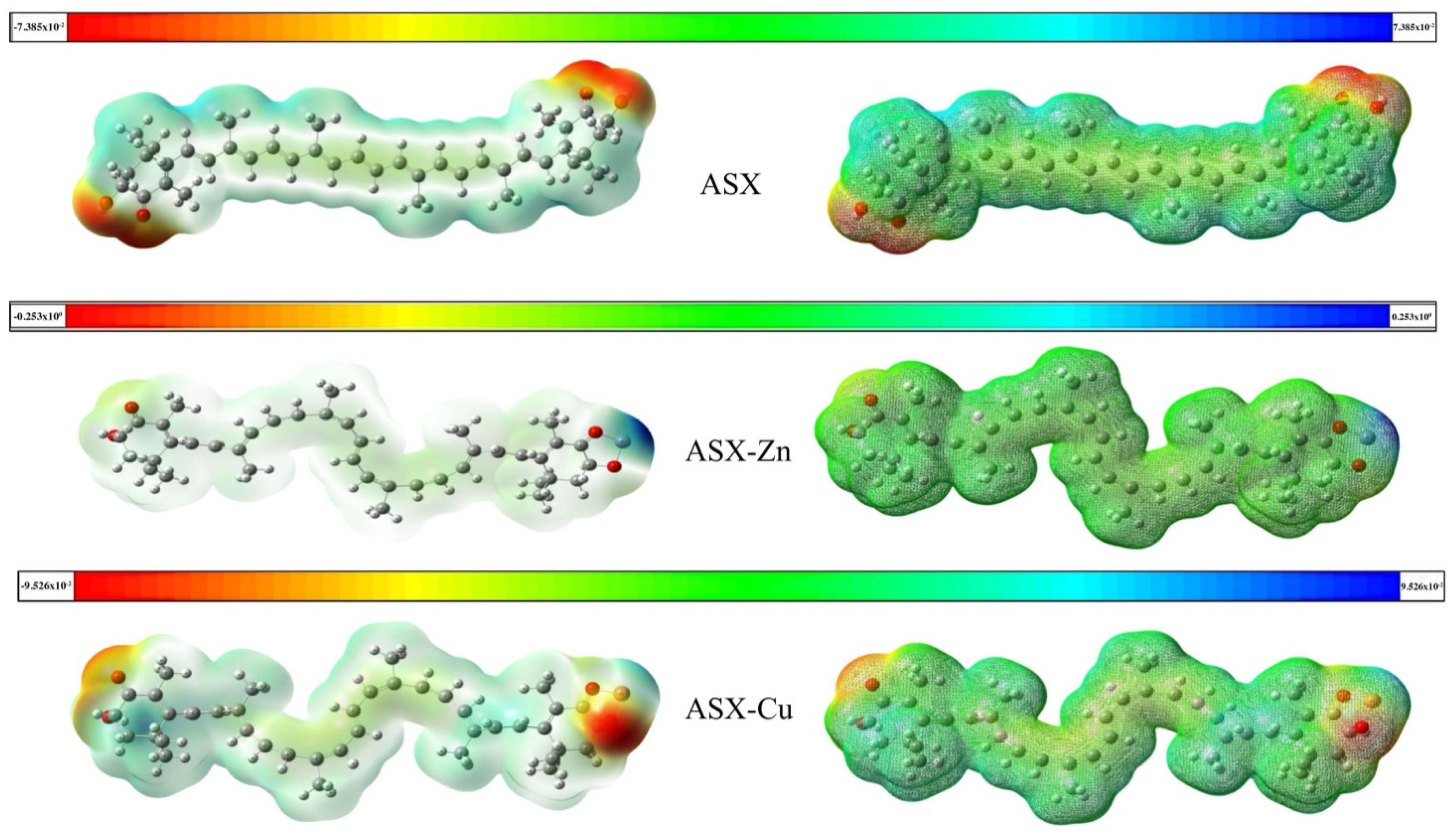
2.3. Molecular Electrostatic Potentials (MEP)
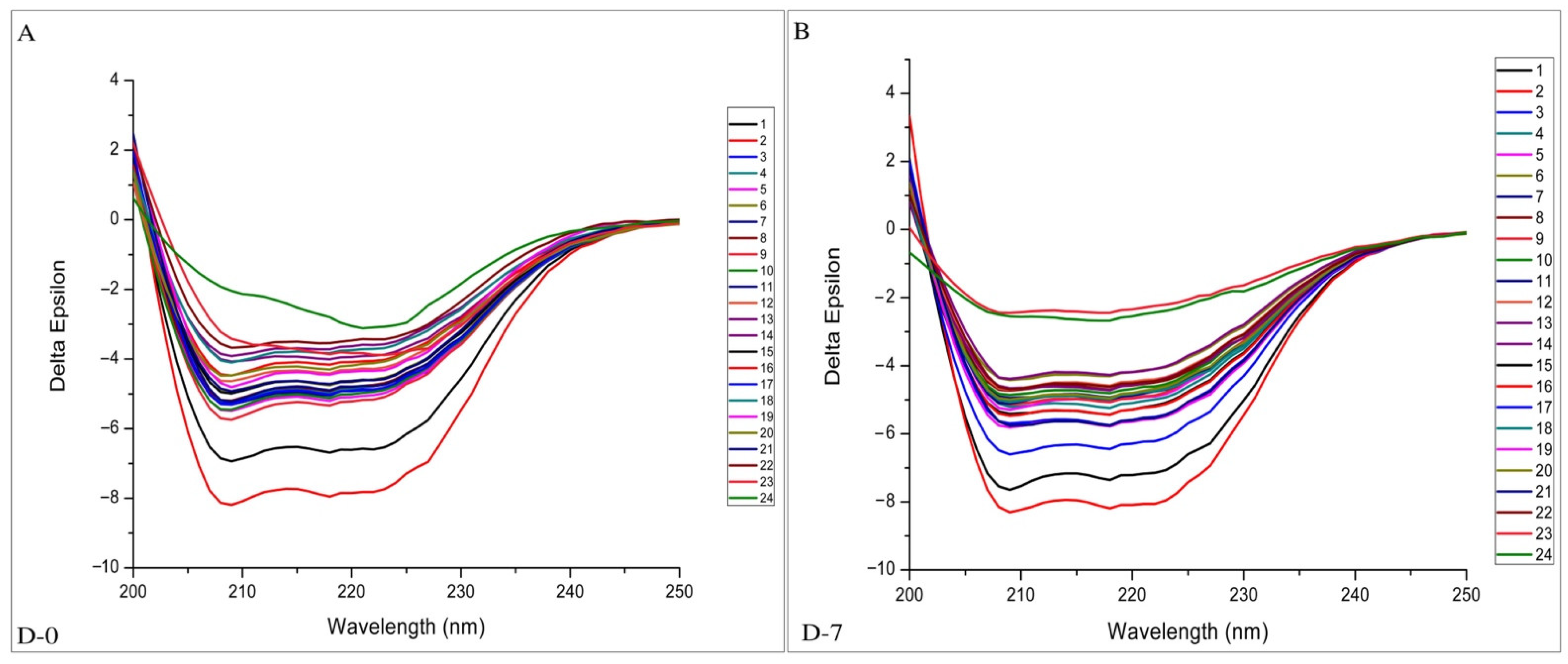
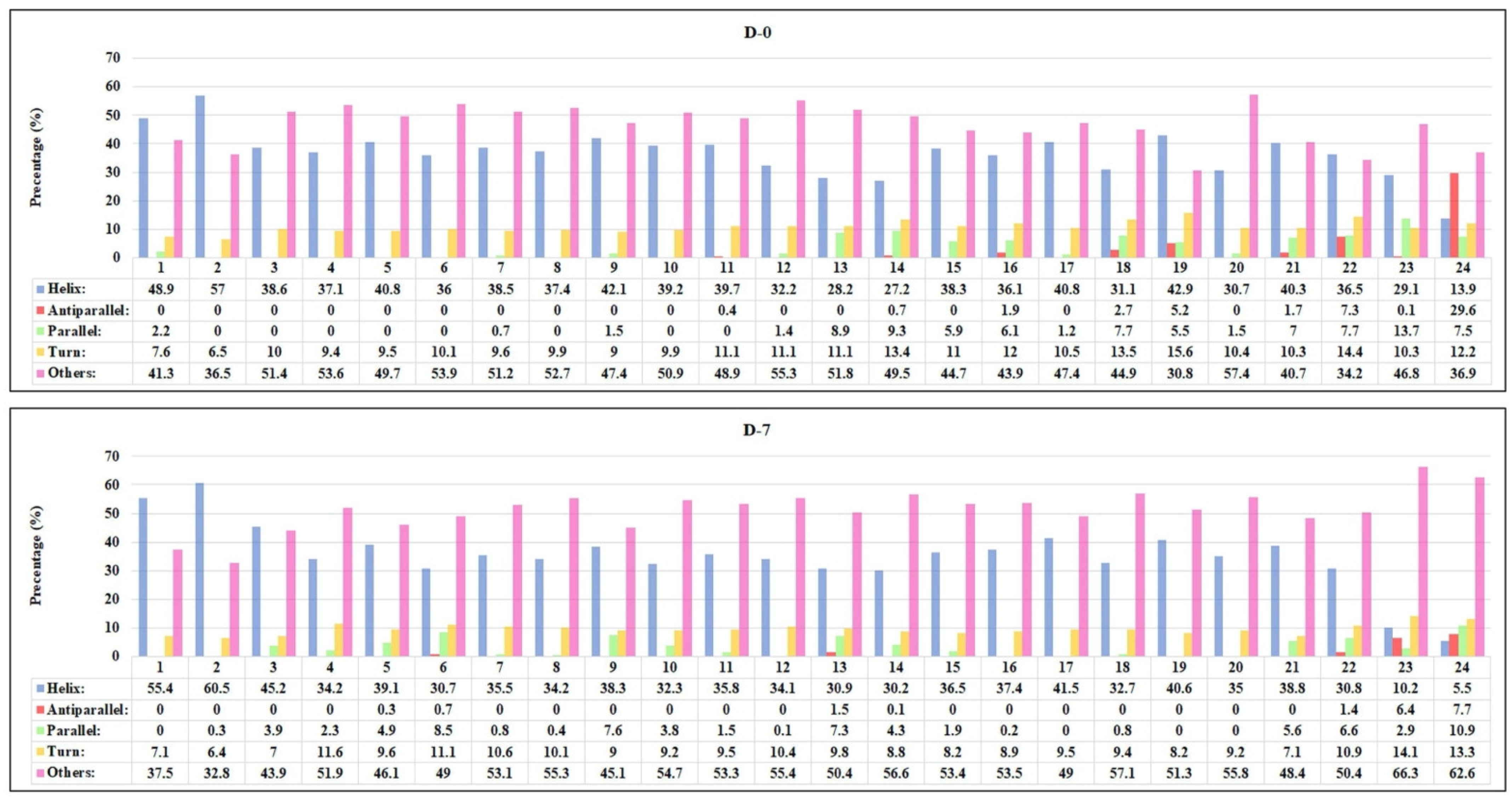
2.4. CD-Spectroscopy
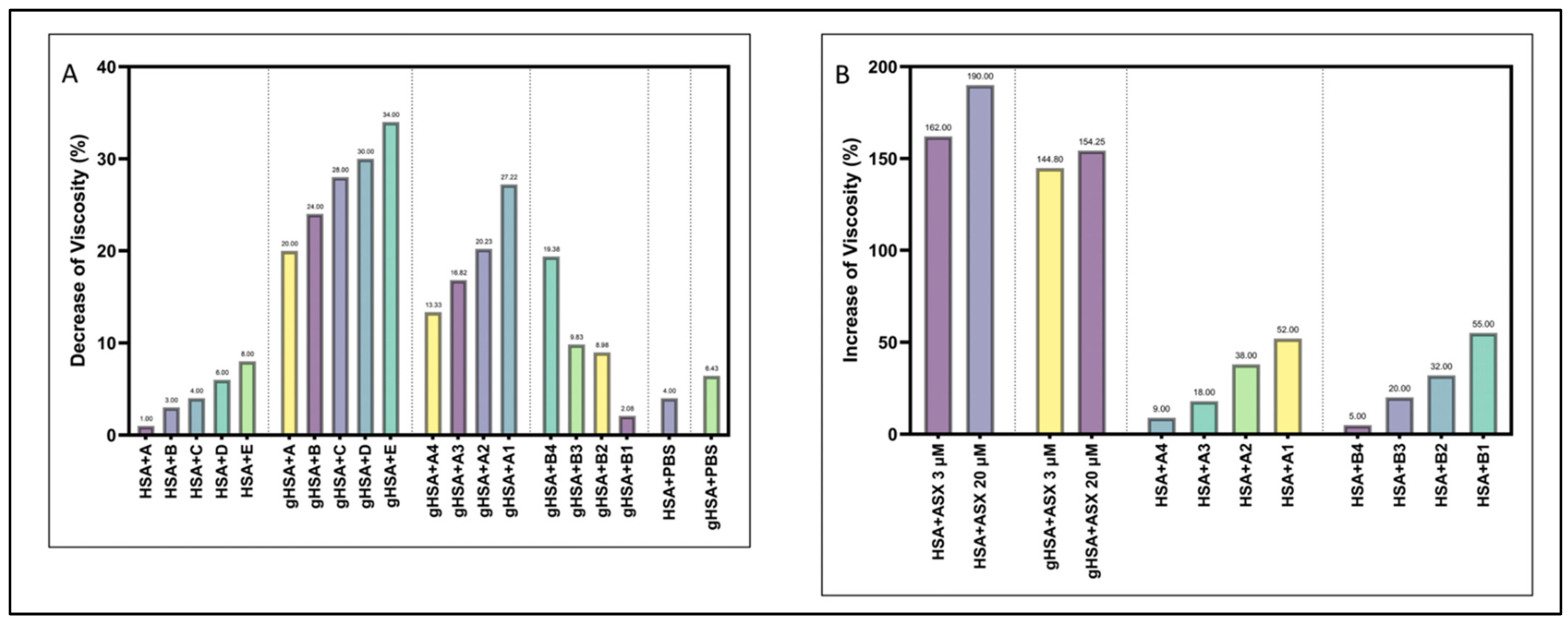
2.5. Viscosity, Particle Size and Zeta Potential Analysis
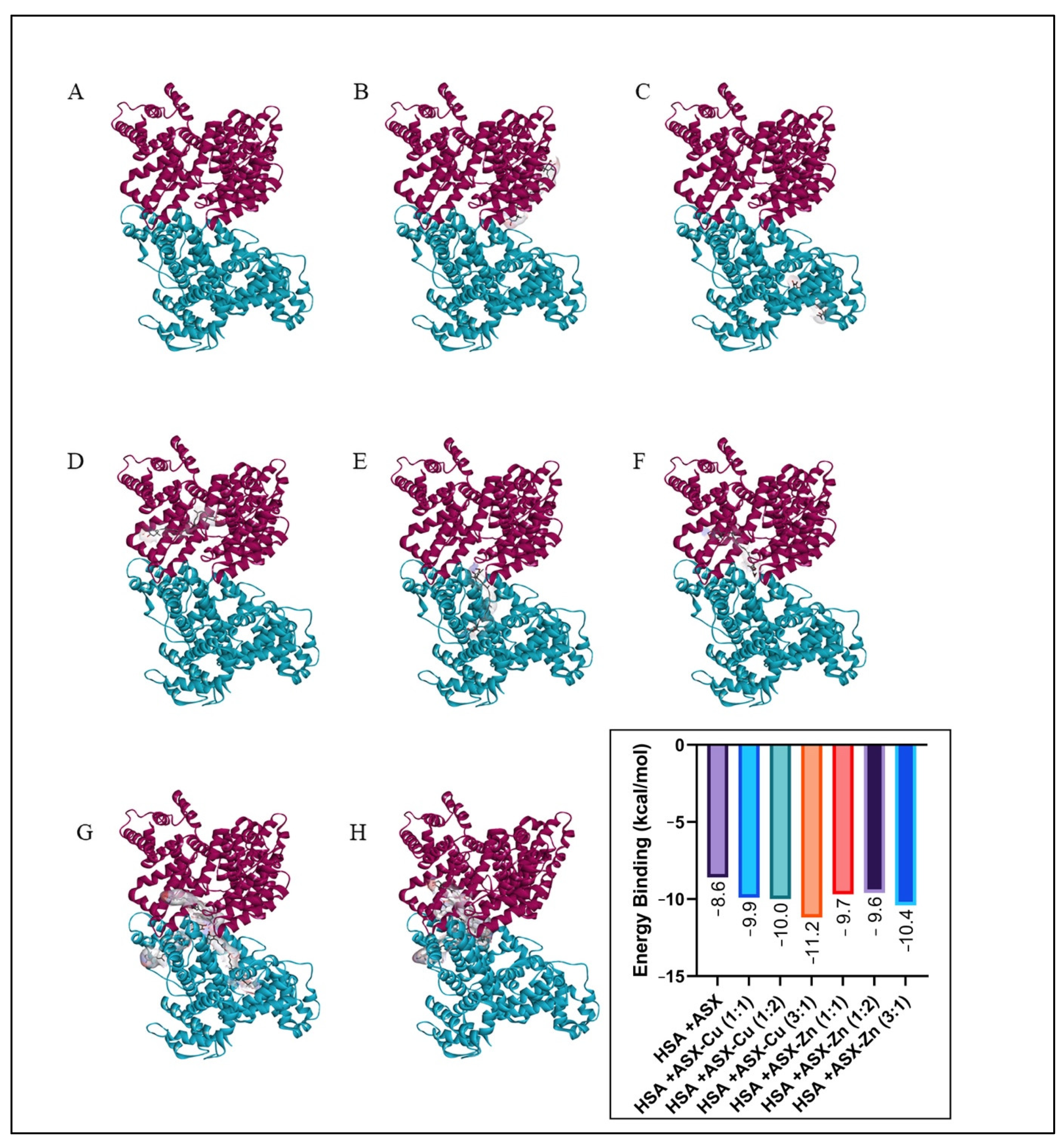
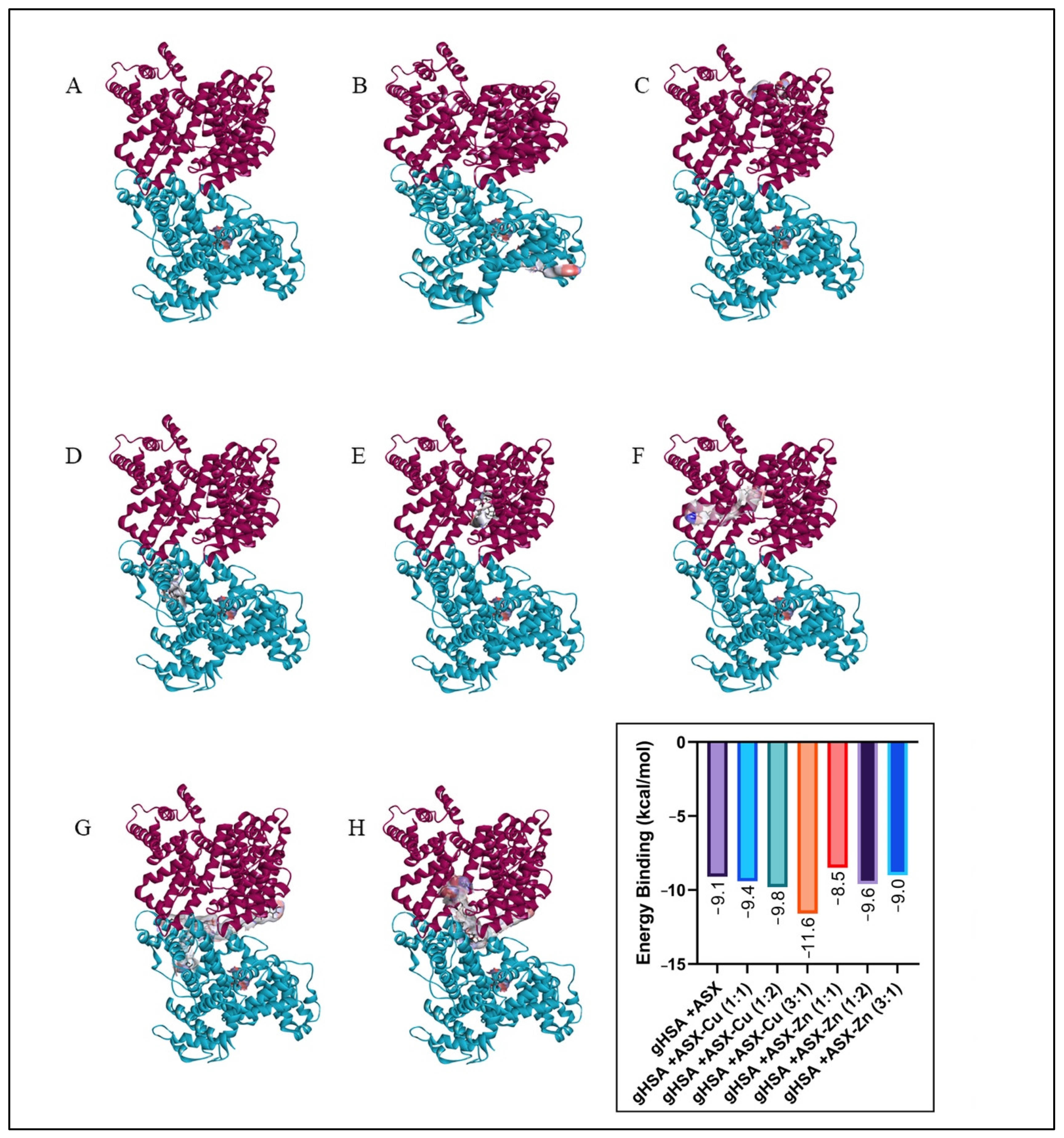
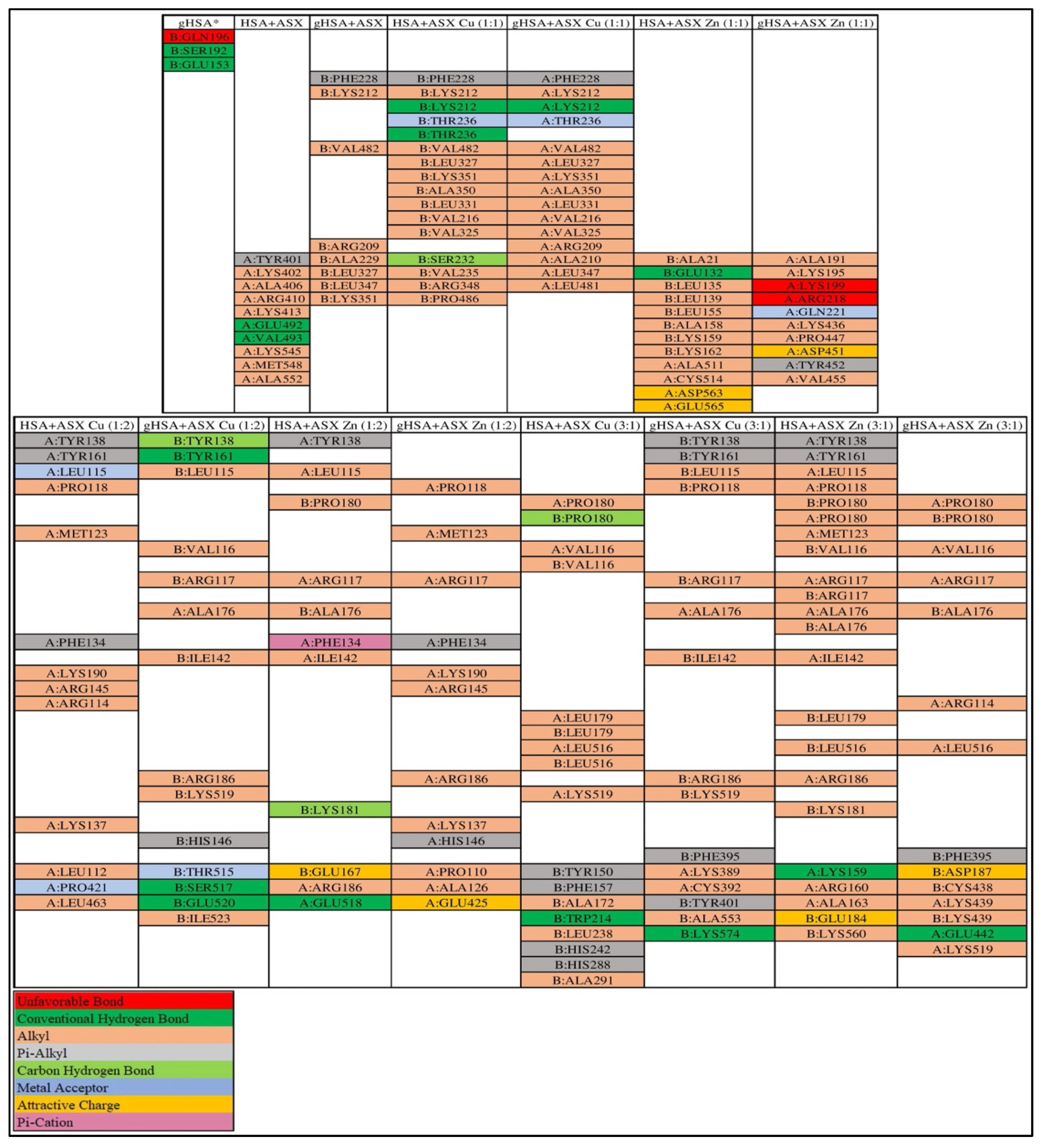
2.6. Molecular Docking
3. Materials and Methods
3.1. ASX-Transition Metal Ion Complexes
3.2. ASX-HSA and ASX-gHSA Complexes
3.3. UV-Vis Spectrophotometric Characterization
3.4. Viscosity Measurement
3.5. CD-Spectroscopy Analysis
3.6. Particle Size and Zeta Potential Analysis
3.7. Electron Paramagnetic Resonance Spectroscopy (EPR)
3.8. Molecular Electrostatic Potential (MEP) Analysis
3.9. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.; Focsan, A.; Bowman, M.; Kispert, L. Free Radical Formation in Novel Carotenoid Metal Ion Complexes of Astaxanthin. J. Phys. Chem. B 2010, 114, 16968–16977. [Google Scholar] [CrossRef] [Green Version]
- Murray, R.; Granner, D.; Mayes, P.; Rodwell, V. Harper’s Illustrated Biochemistry, 26th ed.; Mcgraw Hills Companies: Boston, MA, USA, 2003. [Google Scholar]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.; Martin, G.; Evans, T. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Sumitro, S.; Widyarti, S. Computational study of Cu2+, Fe2+, Fe3+, Mn2+ and Mn3+ binding sites identification on HSA 4K2C. IOP Conf. Ser.-Mat. Sci. 2020, 833, 012052. [Google Scholar] [CrossRef]
- Nagumo, K.; Tanaka, M.; Chuang, V.; Setoyama, H.; Watanabe, H.; Yamada, N.; Kubota, K.; Tanaka, M.; Matsushita, K.; Yoshida, A.; et al. Cys34-Cysteinylated Human Serum Albumin Is a Sensitive Plasma Marker in Oxidative Stress-Related Chronic Diseases. PLoS ONE 2014, 9, e85216. [Google Scholar] [CrossRef]
- Anraku, M.; Chuang, V.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. BBA-Gen. Subj. 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Wibowo, S.; Widyarti, S.; Sabarudin, A.; Soeatmadji, D.; Sumitro, S. The Role Of Astaxanthin Compared With Metformin In Preventing Glycated Human Serum Albumin From Possible Unfolding: A Molecular Dynamic Study. Asian J. Pharm. Clin. Res. 2019, 12, 276–282. [Google Scholar] [CrossRef]
- Cho, Y.; Mooney, M.; Cho, D. Hemorheological Disorders in Diabetes Mellitus. J. Diabetes Sci. Technol. 2008, 2, 1130–1138. [Google Scholar] [CrossRef] [Green Version]
- Levy, B.I.; Schiffrin, E.L.; Mourad, J.J.; Agostini, D.; Vicaut, E.; Safar, M.E.; Struijker-Boudier, H.A. Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008, 118, 968–976. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2014, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Grimmig, B.; Kim, S.; Nash, K.; Bickford, P.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 2017, 39, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant effects of astaxanthin in various diseases: A review. J. Mol. Pathophysiol. 2018, 7, 1. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Widyarti, S.; Sabarudin, A.; Soeatmadji, D.; Sumitro, S. DFT and molecular dynamics studies of astaxanthin-metal ions (Cu2+ and Zn2+) complex to prevent glycated human serum albumin from possible unfolding. Heliyon 2021, 7, e06548. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Prakoso, B.D.; Najihah, S.; Fajar, Z.; Widyarti, S.; Sabarudin, A.; Soeatmadji, D.; Sumitro, S.B. Synthesis and characterization of astaxanthin-metal ions (Cu2+ and Zn2+) complex. Rasayan J. Chem. 2021, 14, 877–886. [Google Scholar] [CrossRef]
- Bohlooli, M.; Ghaffari-Moghaddam, M.; Khajeh, M.; Sheibani, N. Determination of Amadori Product in Glycated Human Serum Albumin by Spectroscopy Methods. ChemistrySelect 2018, 3, 7018–7022. [Google Scholar] [CrossRef]
- Tokarz, D.; Cisek, R.; El-Ansari, O.; Espie, G.; Fekl, U.; Barzda, V. Organization of Astaxanthin within Oil Bodies of Haematococcus pluvialis Studied with Polarization-Dependent Harmonic Generation Microscopy. PLoS ONE 2014, 9, e107804. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhou, K.; Pan, D.; Shen, J.; Shi, J. Spectroscopic and molecular docking approaches for investigating conformation and binding characteristics of clonazepam with bovine serum albumin (BSA). J. Photochem. Photobiol. 2017, 167, 158–167. [Google Scholar] [CrossRef]
- Liu, J.; He, Y.; Liu, D.; He, Y.; Tang, Z.; Lou, H.; Huo, Y.; Cao, X. Characterizing the binding interaction of astilbin with bovine serum albumin: A spectroscopic study in combination with molecular docking technology. RSC Adv. 2018, 8, 7280–7286. [Google Scholar] [CrossRef] [Green Version]
- Peisach, J.; Blumberg, W. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef]
- Sciortino, G.; Lubinu, G.; Maréchal, J.; Garribba, E. DFT Protocol for EPR Prediction of Paramagnetic Cu(II) Complexes and Application to Protein Binding Sites. Magnetochemistry 2018, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Walter, E.; Chattopadhyay, M.; Millhauser, G. The Affinity of Copper Binding to the Prion Protein Octarepeat Domain: Evidence for Negative Cooperativity. Biochemistry 2006, 45, 13083–13092. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.; Walter, E.; Rodríguez, A.; Draper, D.; Davies, P.; Brown, D.; Millhauser, G. Early Onset Prion Disease from Octarepeat Expansion Correlates with Copper Binding Properties. PLoS Pathog. 2009, 5, e1000390. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Hernández-Marin, E.; Barbosa, A.; Martínez, A. The Metal Cation Chelating Capacity of Astaxanthin. Does This Have Any Influence on Antiradical Activity? Molecules 2012, 17, 1039–1054. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Peng, J.; Yin, K.; Wang, J. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2010, 55, 150–165. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient Radical Trapping at The Surface and Inside the phospholipid Membrane Is Responsible For Highly Potent Antiperoxidative Activity of The Carotenoid Astaxanthin. Biochim Biophys Acta Biomembr. 2001, 2, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, L.; Zhang, H.; Sun, Q.; Liu, R.; Li, J.; Wu, L.; Tsao, R. Rapid and Efficient Conversion of All-E-astaxanthin to 9Z- and 13Z-Isomers and Assessment of Their Stability and Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Martinez, H. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry 1972, 11, 4120–4131. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Ahmad, E.; Zaidi, N.; Fatima, S.; Khan, R. pH-Induced Molten Globule State of Rhizopus niveus Lipase is More Resistant Against Thermal and Chemical Denaturation Than Its Native State. Cell Biochem. Biophys. 2012, 62, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Moosavimovahedi, A.; Ahmad, F.; Hakimelahi, G.; Habibirezaei, M.; Saboury, A.; Sheibani, N. Formation of the molten globule-like state during prolonged glycation of human serum albumin. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 933–942. [Google Scholar] [CrossRef]
- Matei, I.; Hillebrand, M. Interaction of kaempferol with human serum albumin: A fluorescence and circular dichroism study. J. Pharm. Biomed. 2010, 51, 768–773. [Google Scholar] [CrossRef]
- Raghav, A.; Ahmad, J.; Alam, K. Impact of glycation on structural and antioxidant function of human serum albumin: Relevance in diabetic complications. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 96–101. [Google Scholar] [CrossRef]
- Yang, X.; Bolsa-Ferruz, M.; Marichal, L.; Porcel, E.; Salado-Leza, D.; Lux, F.; Tillement, O.; Renault, J.; Pin, S.; Wien, F.; et al. Human Serum Albumin in the Presence of AGuIX Nanoagents: Structure Stabilisation without Direct Interaction. Int. J. Mol. Sci. 2020, 21, 4673. [Google Scholar] [CrossRef]
- Çinar, Y.; Senyol, A.M.; Duman, K. Blood viscosity and blood pressure: Role of temperature and hyperglycemia. Am. J. Hypertens. 2001, 14, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Juárez, J.; Taboada, P.; Mosquera, V. Existence of Different Structural Intermediates on the Fibrillation Pathway of Human Serum Albumin. Biophys. J. 2009, 96, 2353–2370. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Liu, W.; Frost, L.; Wang, L.; Kong, L.; Dain, J.; Seeram, N. The hydrolyzable gallotannin, penta-O-galloyl-β-d-glucopyranoside, inhibits the formation of advanced glycation endproducts by protecting protein structure. Mol. Biosyst. 2015, 11, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.; Goto, Y.; Réfrégiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.; Parr, R. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Hay, P.; Wadt, W. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.; Wadt, W. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Wadt, W.; Hay, P. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry, 3rd ed.; Plenum: New York, NY, USA, 1977. [Google Scholar]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Zhanpeisov, N.; Ju, W.; Anpo, M. Local structure of highly dispersed lead containing zeolite. An ab initio and density functional theory study. J. Mol. Struct. Theochem. 2002, 592, 155–160. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Inagaki, S. Theoretical design of singlet localized σ-diradicals: C(MH2)3C (M=Si, Ge, Sn, Pb). Tetrahedron Lett. 2005, 46, 5567–5571. [Google Scholar] [CrossRef]
- Kaenkaew, S.; Sae-Khow, O.; Ruangpornvisuti, V. Cation recognition of thiacalix [2]thianthrene and p-tert-butylthiacalix [2]thianthrene and their conformers and complexes with Zn(II), Cd(II) and Hg(II): A theoretical investigation. J. Mol. Model. 2009, 16, 243–253. [Google Scholar] [CrossRef]
- Kandemirli, F.; Köksoy, B.; Arslan, T.; Sagdınc, S.; Berber, H. Synthesis and theoretical study of bis(fluoroisatinato) mercury(II). J. Mol. Struct. 2009, 921, 172–177. [Google Scholar] [CrossRef]
- Nguyen, N.; Nguyen, T.; Pham, N.; Huy, N.; Bay, M.; Pham, M.; Nam, P.; Vu, V.; Ngo, S. Autodock Vina Adopts More Accurate Binding Poses but Autodock4 From Better Finding Affinity. J. Chem. Inf. Model. 2020, 60, 204–211. [Google Scholar] [CrossRef]
| Samples | Z-Average (d.nm) | Polydispersity Index | Zeta Potential (mV) | Conductivity (mS/cm) |
|---|---|---|---|---|
| HSA (1 μM) | 199.4 | 0.248 | −10.0 | 15.9 |
| gHSA (1 μM) | 191.1 | 0.674 | −14.6 | 16.0 |
| ASX (6 × 10−11 mM) | 390.5 | 0.686 | −0.404 | 0.002 |
| HAS + ASX | 9331 | 0.354 | −2.88 | 4.37 |
| gHSA + ASX | 3969 | 0.377 | −4.82 | 5.58 |
| ASX-Cu2+ | 939.1 | 0.586 | 2.32 | 0.134 |
| ASX-Zn2+ | 889.8 | 0.762 | −2.70 | 0.953 |
| ASX 3 μM | 815.8 | 0.463 | −3.30 | 0.0184 |
| ASX 20 μM | 971.1 | 0.539 | −1.22 | 0.00683 |
| (ASX-Cu2+) + HSA | 6458 | 0.638 | −6.93 | 6.19 |
| (ASX-Cu2+) + gHSA | 6418 | 0.350 | −8.58 | 6.58 |
| (ASX-Zn2+) + HSA | 937.0 | 0.640 | −9.23 | 8.09 |
| (ASX-Zn2+) + gHSA | 990.2 | 0.661 | −10.1 | 7.61 |
| (ASX 3 μM) + HSA | 8995 | 0.492 | −5.09 | 5.18 |
| (ASX 3 μM) + gHSA | 7209 | 0.601 | −4.25 | 5.11 |
| (ASX 20 μM) + HSA | 7136 | 0.590 | −2.45 | 4.48 |
| (ASX 20 μM) + gHSA | 2673 | 0.449 | −2.36 | 3.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibowo, S.; Costa, J.; Baratto, M.C.; Pogni, R.; Widyarti, S.; Sabarudin, A.; Matsuo, K.; Sumitro, S.B. Quantification and Improvement of the Dynamics of Human Serum Albumin and Glycated Human Serum Albumin with Astaxanthin/Astaxanthin-Metal Ion Complexes: Physico-Chemical and Computational Approaches. Int. J. Mol. Sci. 2022, 23, 4771. https://doi.org/10.3390/ijms23094771
Wibowo S, Costa J, Baratto MC, Pogni R, Widyarti S, Sabarudin A, Matsuo K, Sumitro SB. Quantification and Improvement of the Dynamics of Human Serum Albumin and Glycated Human Serum Albumin with Astaxanthin/Astaxanthin-Metal Ion Complexes: Physico-Chemical and Computational Approaches. International Journal of Molecular Sciences. 2022; 23(9):4771. https://doi.org/10.3390/ijms23094771
Chicago/Turabian StyleWibowo, Syahputra, Jessica Costa, Maria Camilla Baratto, Rebecca Pogni, Sri Widyarti, Akhmad Sabarudin, Koichi Matsuo, and Sutiman Bambang Sumitro. 2022. "Quantification and Improvement of the Dynamics of Human Serum Albumin and Glycated Human Serum Albumin with Astaxanthin/Astaxanthin-Metal Ion Complexes: Physico-Chemical and Computational Approaches" International Journal of Molecular Sciences 23, no. 9: 4771. https://doi.org/10.3390/ijms23094771
APA StyleWibowo, S., Costa, J., Baratto, M. C., Pogni, R., Widyarti, S., Sabarudin, A., Matsuo, K., & Sumitro, S. B. (2022). Quantification and Improvement of the Dynamics of Human Serum Albumin and Glycated Human Serum Albumin with Astaxanthin/Astaxanthin-Metal Ion Complexes: Physico-Chemical and Computational Approaches. International Journal of Molecular Sciences, 23(9), 4771. https://doi.org/10.3390/ijms23094771










