Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review
Abstract
1. Introduction
2. Data Collection
3. Phenolic Compounds
4. The Linkage between Phenolics Structure and Health Benefits
4.1. Antioxidant Effects of Phenolics
4.2. Anti-Inflammatory Properties of Phenolics
5. Effects of Phenolic Compounds and Phenolic-Rich Sources on Exercise Performance and Recovery
5.1. Phenolic Compounds
5.1.1. Rodent Models
5.1.2. Clinical Studies
| Phenolic Compound | Study Design | Main Outcomes | Reference |
|---|---|---|---|
| Rodent Models | |||
| Catechin | |||
| Effects of 8-week supplementation (0.35% catechins/day) in mice subjected to downhill exercise | Attenuated the downhill running-induced decrease in muscle force ↓↓ pro-inflammatory markers in both plasma and gastrocnemius muscle ↓↓ oxidative stress, by ↑↑ glutathione reductase activity | [181] | |
| (−)-Epigallocatechin-3-O-gallate | |||
| Effects of 16-week supplementation (0.32%/day) in the skeletal muscle of high fat-fed mice | ↓↓ fasting blood glucose (−18.5%), plasma insulin (−25.3%), and insulin resistance (−33.9%), and markers of obesity-related fatty liver disease in high fat-fed mice ↑↑ mRNA levels of the nuclear respiratory factor, medium-chain acyl-CoA decarboxylase, uncoupling protein 3, and peroxisome proliferator responsive element | [198] | |
| Quercetin | |||
| Effects of 7-day supplementation (12.5 mg/kg/day) against influenza infection before an intensive run in rats-model | Quercetin administration effectively ↓↓ the susceptibility to influenza infection following stressful exercise | [199] | |
| Effects on brain and muscle mitochondrial biogenesis and exercise tolerance in rats at doses of 12.5 and 25 mg/kg | ↑↑ mRNA expression of PGC-1α, SIRT1, mtDNA and cytochrome C ↑↑ maximal endurance capacity and voluntary wheel-running activity | [139] | |
| Resveratrol | |||
| Effects of 4-week supplementation (10 mg/kg/day) in rats subjected to an acute swimming exercise bout for 30 min | ↓↓ lipid peroxidation and genotoxicity, by diminishing MDA and 8-OhdG levels ↑↑ total antioxidant levels | [143] | |
| Effects of 12-week supplementation (146 mg/kg/day) in rats subjected to a progressive treadmill running | ↑↑ exercise training-induced improvements (+21% than placebo) ↑↑ twitch and tetanic forces generated during isometric contraction (+18 and 58%, respectively) and cardiac fatty acid oxidation (+20% than placebo) Favourable changes in cardiac gene expression and structure, and signal transduction pathways | [200] | |
| Effects of 4-week supplementation (10 mg/kg/day) in rats subjected to an acute swimming exercise bout for 30 min | Prevented the decrease of glycogen in the liver after intense exercise in both exercised and non-exercised rats | [201] | |
| Effects of 12-week supplementation (10 mg/kg/day) in rats subjected to a treadmill endurance exercise program | Prevented exercise-induced oxidative stress by avoiding lipid peroxidation (lower levels of MDA and higher superoxide dismutase activity and total antioxidant capacity on plasma) | [202] | |
| Effects of 6-week supplementation (7.5 mg/kg/day) in male and female rats subjected to regular aerobic exercise | ↑↑ antioxidant capacity and the expression of non-selective PDE1, 2, 3 and cAMP selective PDE4 There were not observed effects on cGMP selective PDE5 expression in the aorta | [150] | |
| Effects of one-year supplementation (0.7 mg/kg/day) on skeletal muscle of active old mice | ↑↑ antioxidant defences, blocking SkM protein carbonylation increments ↓↓ IL-6 | [203] | |
| Effects of 6-week supplementation (10 mg/kg/day) in aged rats subjected to swimming high-intensity interval exercise | ↑↑ recognition memory and modulate anxiety-like behaviours | [145] | |
| Effects of 4-week supplementation (10 mg/kg/day) in rats subjected to acute swimming exercise | Protective effect on muscle glycogen in exercised rats | [144] | |
| Effects of 4-week supplementation (25 mg/kg) in skeletal muscle adaptation involved in exercise-induced weight loss in obese mice | ↑↑ whole-body glucose, lipid homeostasis and the expression levels of PGC-1α and its downstream transcription factors | [204] | |
| Effects of 2-week supplementation (12.5 mg/kg) in rats’ gastrocnemius muscles | Protective effects against oxidative stress and muscle force loss in hindlimb suspension ↓↓ hydrogen peroxide, lipid peroxidation, and caspase-9 levels in muscles of animals after hindlimb suspension subjected to hindlimb suspension | [205] | |
| Effects of 4-week supplementation (25, 50, and 100 mg/kg) in rats subjected to a strenuous exercise on the treadmill | ↓↓ lipid peroxidation by diminishing LDH, CK, MDA, 4-HNE, and 8-OHdG levels | [206] | |
| Effects of 8-week supplementation (20 mg/kg) in diabetic rats subjected to continuous exercise | ↓↓ hepatocyte apoptosis caused by diabetes | [29] | |
| Effects of 10-day supplementation in young adult and aged mice gastrocnemius muscles subjected to short-term isometric exercise | ↓↓ oxidative stress and oxidative damage in gastrocnemius muscles from young adult and aged mice subjected to short-term isometric exercise | [207] | |
| Effects of 8-week supplementation (20 mg/kg) in diabetic rats subjected to regular continuous exercise | ↓↓ BAX and caspase-3 ↑↑ BCL-2 concentration in hepatocyte tissue | [148] | |
| Effects of 6-month supplementation (16.5 mg/kg/day) in rats subject to running exercises | ↑↑ antioxidant defenses by increasing glutathione, glutathione peroxidase, glutathione transferase, and NAD(P)H: quinone acceptor oxidoreductase activities | [208] | |
| Effects of 9-week supplementation (50 mg/kg/day) in rats’ spermatogenic dysfunction caused by high-intensity exercise | ↑↑ significantly sperm density, testosterone and FSH levels, protamine, superoxide dismutase activity, and spermatogenic epithelial cells number ↓↓ IL-6, TNF-α and MDA content | [146] | |
| Effects of supplementation (50 mg/kg/day) in rats 6 h before intensive swimming | ↑↑ accelerated the recovery of LDH, ammonia, CPK, and glucose levels after exercise | [149] | |
| Effects of 5-month supplementation (4 g/kg/day) in induced-Alzheimer’s disease rats subjected to the treadmill belt | ↓↓ neuroinflammation, accumulation of Aβ oligomers and markers of apoptosis, autophagy, endolysosomal degradation, and ubiquitination ↑↑ increased levels of neurotrophins, synaptic markers, and silent information regulator | [142] | |
| Effects of 4-week supplementation (10 mg/kg/day) in rats subjected to an endurance exercise and acute exercise training | ↓↓ carbonyl and 8-OHdG levels | [209] | |
| Effects of 12-week supplementation (10 mg/kg/day) in rats subjected to endurance exercises | ↓↓ CPR and IL-6 levels No effects regarding TNF-α and IL-17 content were observed | [210] | |
| Effects of training and resveratrol 12-week supplementation (100 mg/kg) on the muscle of colon cancer mice | ↑↑ muscle regeneration by increasing Myosin heavy chain-embryonic protein (+34.7% than placebo) No effects regarding myoblast determination protein, body weight and tumour size were observed | [211] | |
| Effects of training and resveratrol 3-week supplementation (25, 50, and 125 mg/kg/day) in rats subjected to forelimb grip strength and exhaustive swimming | ↓↓ serum LDH, ammonia, and CK levels in a dose-dependent manner ↑↑ grip strength and glucose levels in a dose-dependent manner | [212] | |
| Effects of 4-week supplementation (25 mg/kg/day) in rats subjected to forelimb grip strength exercises | ↓↓ plasma LDH and ammonia levels ↑↑ muscle strength and endurance performance | [213] | |
| Effects of training and 6-week supplementation (40 mg/kg/day) in rats subjected to swimming | ↓↓ kainate-induced seizure activity and oxidative stress, by increasing superoxide dismutase activity | [214] | |
| Effects of training and 6-month supplementation (16.67 mg/kg/day) in rats | ↑↑ catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, glutathione S-transferase and NAD(P)H: quinone oxidoreductase 1 | [215] | |
| Effects of training and 4-month supplementation (4 g/kg/day) in Alzheimer’s disease model | ↑↑ improves fracture resistance and cross-sectional geometric indicators of bone strength | [216] | |
| Effects of 6-week supplementation (16–17 mg/kg/day) in old mice | ↓↓ frailty ↑↑ muscle performance, coordination and strength practising a moderate exercise | [217] | |
| Effects of training and 6-week supplementation (150 mg/kg/day) in rats subjected to running | Strong effects on weight loss, which could be beneficial for suppressing exercise loads | [218] | |
| Effects of training and 9-week supplementation (100 mg/kg/day) in rats subjected to running | ↑↑ PGC-1α and SirT1 to the nucleus, stimulating mitochondrial biogenesis | [138] | |
| Effects of training and 4-week supplementation (15 mg/kg/day) in rats subjected to swimming | ↑↑ PGC-1α, and hence, stimulating mitochondrial biogenesis, and also citrate synthase enzyme activity | [219] | |
| Effects of training and 13-week supplementation (0.2% w/w) in rats subjected to running | ↑↑ senescence-accelerated prone mice levels Improve energy metabolism in skeletal muscle | [137] | |
| Effects of resistance exercise and 4-week supplementation (25 mg/kg) in rats subjected to aerobic and anaerobic exercises | ↑↑ muscular hypertrophy, physiological adaption, and aerobic and anaerobic performances | [220] | |
| Effects of 12-week supplementation (100 mg/kg) in rats subjected to treadmill exercise training | ↑↑ aerobic performance, mitochondrial quality, control, and biogenesis, namely by activating the AMPK-SIRT1-PGC-1α pathway | [221] | |
| Effects of 8-week supplementation (10 mg/kg) in rats subjected to forced running | Better exhausted time and running distance | [222] | |
| Effects of training and 8-week supplementation (25 mg/kg/day) in the liver of elderly rats with nonalcoholic fatty liver | ↑↑ Sirt1, Lxr, and Fxr, ↓↓ AST, ALT, ALP enzymes, and apoptotic cells | [223] | |
| Resveratrol conjugated with nonsteroidal anti-inflammatory drugs | |||
| Effects of 7-day supplementation (25 mg/kg) in rats after contusion induced muscle injury | ↑↑ muscle recovery, by lowering uric acid, creatinine, LDH, and CK serum levels | [171] | |
| Caffeic acid phenethyl ester | |||
| Effects of 5-day supplementation (5 and 10 mg/kg/day) in rats subjected to eccentric exercises | ↓↓ COX2, iNOS, and IL-1β and MCP1 levels, by suppressing NF-κB activation | [197] | |
| Salidroside from Rhodiola Sachalinensis A. Bor | |||
| Effects of 2-week supplementation (50 mL/kg/day) in mice forced to swim for 120 min without loads on the last day of the assay | ↑↑ superoxide dismutase and glutathione peroxidase activities, and liver glycogen and muscle glycogen reserve and free fatty acid concentrations ↓↓ MDA levels Stabilize blood sugar and prevent blood sugar, liver glycogen, and muscle glycogen levels from reducing in long time exercise on mice Promote the use of fat | [151] | |
| Clinical Trials | |||
| Phenolics extracted from Camellia sinensis | |||
| Effects of 7-day supplementation (2000 mg/day) in untrained men subjected to resistance exercise | ↓↓ IL-8 levels No effects regarding granulocyte percentage and CD11b adhesion molecule expression levels were observed | [178] | |
| Tea catechins | |||
| Effects of 3-month supplementation (350 mL of a tea beverage fortified with 540 mg catechins) in elderly women with sarcopenia subjected to 60 min of a comprehensive training program twice a week | ↑↑ leg muscle mass and walking speed | [179] | |
| Catechins and theaflavins | |||
| Effects of 13-week supplementation (2000 mg/day) in active male subjected to eccentric exercise challenge | ↓↓ peak torque, whole body and hamstring soreness, serum cortisol, and ↑↑ ferric reducing ability of plasma | [182] | |
| (−)-Epigallocatechin-3-O-gallate | |||
| Effects of 7-day supplementation (135 mg/day) in healthy adults before specific exercise training tests | ↑↑ VO2max without affecting maximal cardiac output, and also arterial-venous oxygen difference | [185] | |
| Effects of 14-day supplementation (1800 mg/day) in skeletal muscle proteolytic gene expression of healthy males after eccentric exercise section | ↓↓ muscle ring-finger 1, ubiquitin-protein ligase 3B, and m-calpain expression after exercise | [224] | |
| Effects of 12-week supplementation (150 mg 2×day) combined with exercise 3 times/week in obese postmenopausal women | ↓↓ heart rate and plasma glucose levels | [184] | |
| Effects of 14-day supplementation (1800 mg/day) in healthy active males after being subjected to a single bout of eccentric muscle contractions daily | ↓↓ neutrophils count, CK, 8-isoprostane, cortisol, and TNF-α levels No effects regarding cytochrome C, caspase-3 content, caspase-3 enzyme activity, and total DNA were observed | [186] | |
| Anthocyanins juice extracted from apples, plums, blueberries, maqui berries, raspberries, and cranberries | |||
| Effects of 9-day supplementation (240 mL 2×day) in healthy young men subjected to downhill running | Faster recovery ↓↓ CK levels | [187] | |
| Epicatechin | |||
| Effects of 8-week supplementation (1 mg/kg/day) in sarcopenic older adults subjected to an intensive training program per week | ↑↑ muscle strength and circulatory levels of plasma follistatin and ↓↓ plasma myostatin levels | [183] | |
| Quercetin | |||
| Effects of 6-week supplementation (300 mg/day) in elite male cyclists before a competition | Better high-intensity cycling performance through the enhancement of power output No differences regarding carbohydrate and fat oxidation were obtained | [160] | |
| Effects of 2-week supplementation (1000 mg/day) in moderately active young men | Capacity to ↓↓ the severity of muscle weakness caused by eccentric-induced myofibrillar disruption and sarcolemmal action potential propagation impairment ↑↑ isometric strength (+4.7% when compared to baseline) | [161] | |
| Effects of 2-week supplementation (1000 mg/day) in untrained young male adults | ↑↑ mRNA expression of mtDNA and cytochrome c Significant improvement in a 12 min treadmill time trial performance (+15% when compared to placebo) | [225] | |
| Effects of 1-week supplementation (1000 mg/day) in untrained males | ↑↑ VO2max (+3.9%) and ride time to fatigue (+13.2%) | [163] | |
| Effects of 3-week supplementation (1000 mg/day) before, during, and 2-week after a 3-day of intense exercise (at approximately 57% Wmax) in trained male cyclists | No significant changes in NKCA, PHA-LP, POBA, and sIgA ↓↓ URTI incidence in cyclists after intensified exercise | [133] | |
| Effects of 8-week supplementation (500 mg/day) in athletic students | ↑↑ basal metabolic rate, lean body mass, total body water, and total energy expenditure | [226] | |
| Quercetin conjugated with vitamin C | |||
| Effects of 8-week daily supplementation with quercetin (500 mg)-vitamin C (250 mg) in men physical education students | Its conjugation with Vitamin C can reduce pro-inflammatory markers and oxidative stress (↓↓ CRP, IL-6, E-selectin, and F2-isoprostane levels) | [227] | |
| Effects of 8-week daily supplementation with quercetin (500 mg)-vitamin C (200 mg) in male athletes | ↓↓ plasma CK levels and body fat percentage | [228] | |
| Quercetin conjugated with epigallocatechin 3-gallate | |||
| Effects of 2-week daily supplementation with quercetin (1000 mg)-epigallocatechin 3-gallate (120 mg) in trained male and female cyclists | ↑↑ granulocyte oxidative burst activity ↓↓ CRP levels No effects regarding peroxisome proliferator-activated receptor γ coactivator α, citrate synthase, and cytochrome C were observed | [229] | |
| Resveratrol | |||
| Effects of 3-month supplementation (100 mg/day) in military firefighters | ↓↓ IL-6 and TNF-α | [165] | |
| Effects of 4-day supplementation (480 mg/day) in male athletes subjected to a high-intensity cycling challenge | ↓↓ IL-6 | [167] | |
| Effects of 12-week supplementation (500 mg/day) older men and women skeletal muscle | ↑↑ mitochondrial density, muscle fatigue resistance, knee extensor muscle peak torque (8%), average peak torque (14%), power (14%), and mean fiber (+45.3%) and total myonuclei (+20%) in muscle fibers | [146] | |
| Effects of 6-week supplementation (40 mg/day) in male professional basketball players | ↓↓ IL-6 and TNF-α | [168] | |
| Effects of 12-week supplementation (250 mg/day) on postexercise endothelial function in estrogen-deficient postmenopausal women | ↑↑ basal flow-mediated dilation | [166] | |
| Effects of supplementation (500 mg/day) on untrained healthy young individuals 3 days prior to isometric ankle dorsiflexion exercises | Attenuate pain perception following exercise-induced muscle damage No effects on force preservation were observed | [230] | |
| Effects of 8-week supplementation (250 mg/day) in aged man | ↑↑ muscle TIMP-1 protein levels | [231] | |
| Effects of 7-day supplementation (1000 mg/day) in non-athletic men subjected to plyometric exercise | ↓↓ muscle damage and inflammation levels, and soreness caused by plyometric-exercise-induced muscle damage | [232] | |
| Resveratrol conjugated with other polyphenolic-rich extracts | |||
| Effects of 30-day supplementation (60 mg/day) in healthy volunteers | ↓↓ IL-6 | [233] | |
| Resveratrol combined with piperine | |||
| Effects of 4-week supplementation (500 mg resveratrol plus 10 mg of piperine) in young adults subjected to forearm wrist flexor exercises | ↑↑ forearm skeletal muscle mitochondrial capacity | [172] | |
| Resveratrol combined with quercetin | |||
| Effects of supplementation with 120 mg resveratrol and 225 mg quercetin for 6 days and 240 mg resveratrol and 450 mg quercetin on day 7 in young adults subjected to forearm wrist flexor | ↓↓ F2-isoprostanes levels and exercise-induced lipid peroxidation | [173] | |
| Resveratrol conjugated with carotenoids astaxanthin and β-carotene | |||
| Effects of 10-week supplementation (100 mg/day) in healthy men | ↑↑ resistance training-induced strength, metabolic adaptations, and moderated fatigue and oxidative damage | [174] | |
| Ellagitannins from pomegranate extract | |||
| Effects of 9-day supplementation (500 mL/day) in recreationally active males after a damaging bout of eccentric exercise. | ↑↑ strength after 48 and 72 h of exercise No effects regarding serum markers of inflammation and muscle damage were observed | [176] | |
| Hesperetin | |||
| Effects of 4-week supplementation (500 mg/day) in trained male athletes subjected to cycling time-trial performance | ↑↑ absolute power (+5% than placebo) ↓↓ oxygen consumption/power ratio | [177] | |
| Caffeic acid phenethyl ester | |||
| Effects of 1, 2, and 4 µg/mL exposure in peripheral blood mononuclear cells of competitive cyclists against hyperthermal stress | ↓↓ hyperthermia-induced survival inhibition, necrosis, superoxide levels, glutathione depletion | [196] | |
| Curcumin | |||
| Effects of supplementation (5 g/day) in men 2 days before and to 3 days after eccentric single-leg press exercise | ↓↓ in pain during a single-leg squat, gluteal stretch, squat jump, IL-6 levels, and CK activity ↑↑ single-leg jump performance | [234] | |
| Effects of 8-week supplementation (200 mg/day) in physically active men and women after completion of a downhill running bout | ↓↓ peak extension torque values after 1 and 24 h of muscle-damaging exercise | [193] | |
| Effects of 7-day supplementation (180 mg/day) in healthy men subjected to eccentric exercise | ↓↓ IL-8, muscle soreness, and CK activity | [106] | |
| Effects of 400 mg/day supplementation in 2 days before and 4 days after exercise | ↓↓ exercise-induced muscle damage, by lowering CK, TNF-α, and IL-8 (-48, -25, and -21% than placebo) No significant differences regarding IL-6 and IL-10 levels and quadriceps muscle soreness were observed | [235] | |
| Effects of 3-day supplementation (500 mg/day) in non-heat acclimated male and female participants subjected to treadmill runs | ↓↓ indicators of cellular energy status SIRT1 and p-AMPK (-47.8 and -48.5% than placebo), and mediators of cellular heat shock response HSP70 protein (-11.0% than placebo) No effects regarding protein expression in peripheral blood mononuclear cells | [236] | |
| Effects of 3-day supplementation (500 mg/day) in male recreational athletes subjected to 2 h of endurance cycling | Ameliorates psychological stress ↓↓ IL-6 and CK levels | [194] | |
| Effects of 90 mg supplementation 2 h before and immediately after exercise | Improves antioxidant potential by ↓↓ derivatives of reactive oxygen metabolites | [237] | |
| Effects of 3-day supplementation (500 mg/day) in non-heat-acclimated humans subjected to treadmill exercises | Improves exertional heat stress responses by ↓↓ I-FABP and IL-1RA, TNF-α, and IL-10 levels | [238] | |
| Effects 4-day supplementation (180 mg/day) in healthy young men after eccentric exercise of the elbow flexors | Ameliorates muscle soreness by ↓↓ CK levels | [192] | |
| Effects in untrained young men of 150 mg before and 12 hafter being subjected to eccentric exercise | ↓↓ maximal voluntary contraction torque and CK levels Faster recovery No effects in IL-6 and TNF-α were observed | [239] | |
| Effects of 150 mg in untrained young men after being subjected to heavy eccentric exercise | ↓↓ muscle pain, CK, alanine aminotransferase, and aspartate aminotransferase ↑↑ antioxidant capacity | [191] | |
| Effects of 3-week supplementation (500 mg/day) in active healthy men subjected to aerobic exercises | ↓↓ CK levels and muscle soreness No effects regarding antioxidant capacity, and TNF-α and MDA levels were found | [195] | |
| Effects 400 mg/day supplementation in active healthy men 48 hbefore downhill running test and 24 h after the test | ↓↓ muscle pain in the lower limb and IL-8 levels | [240] | |
| Effects of 200 mg supplementation in moderately trained men before, immediately post, 1-h post, and 24, 48, and 72 h after a downhill running protocol | ↓↓ muscle sourness, CK levels Faster recovery | [241] | |
| Effects on 500 mg supplementation in male recreational athletes followed 2 h of endurance cycling | ↓↓ IL-6 and reduce psychological stress | [242] | |
| Curcumin combined with piperine | |||
| Effects of supplementation (2000 mg of curcumin and 20 mg of piperine×3 times a day) in 48 h before and 48 h of exercise | Improves recovery of the muscle function after the exercise, however, this effect is due to power output loss | [243] | |
| Curcumin combined with Boswellia serrata | |||
| Effects of 12-week supplementation (10 mg of curcumin and 140 mg of Boswellia) in master athletes | ↓↓ soluble receptor for advanced glycation end-products, advanced glycation end-products, and MDA levels | [244] | |
| Isoflavones | |||
| Effects of one-year supplementation (75 mg/day) combined with walking 3 times/week in postmenopausal women | ↓↓ trunk fat mass | [245] | |
| Effects of 6-month supplementation (70 mg/day) combined with exercise 3 times/week in obese postmenopausal women | Improvements in body composition parameters (body weight, total and abdominal fat mass, body mass index, appendicular fat-free mass, fat-free mass/fat mass ratio, and sex hormone-binding globulin | [188] | |
| Effects of 6-month supplementation (70 mg/day) combined with aerobic and resistance training per week in overweight and obese postmenopausal women | ↓↓ fat mass and CRP levels | [189] | |
| Effects of 6-month supplementation (70 mg/day) combined with aerobic and resistance training per week in postmenopausal women | ↓↓ fatty liver index and plasma γ-glutamyl-transferase | [190] | |
5.2. Phenolic-Rich Sources
5.2.1. Rodent Models
5.2.2. Clinical Trials
| Phenolic-Rich Source | Study Design | Main Outcomes | Reference |
|---|---|---|---|
| Rodent Models | |||
| Apple | |||
| Preventive effects of 3-week supplementation (0.05%) against lengthening contraction-induced muscle injuries in rats | ↓↓ torque deficits after the eccentric contractions, and TBARS protein carbonyl levels ↑↑ glutathione-S-transferase | [249] | |
| Green tea | |||
| Effects of 10-week supplementation (0.5% green tea) in mice subjected to pool exercises | ↑↑ endurance capacity and muscle lipid β-oxidation ↓↓ plasma LDH levels Together, both results suggest that lipids were used as an energy source | [247] | |
| Effects of 10-week supplementation (0.5% green tea) in rats subjected to running exercises | ↑↑ metabolic capacity and utilization of fatty acid as a source of energy in skeletal muscle during running exercise | [248] | |
| Honey | |||
| Effects of 8-week supplementation (1 g/kg body weight) in female rats subjected to 5-day high-intensity jumping exercise | Exert beneficial effects on bone mass and bone metabolism markers Protective effects on disturbance of reproductive hormone levels induced by high and low intensities of jumping exercise | [251] | |
| Pomegranate peel | |||
| Anti-fatigue effects of 3-week supplementation (25 mg/day) in rats subjected to swimming exercises | ↑↑ swimming time and glycogen content without change in liver fat content ↓↓ LDH, CPK, ATP, glycogen content, and MDA levels | [282] | |
| Red wine | |||
| Effect of 24-week supplementation (25 and 75 mg/kg/day) in young rats subjected to treadmill exercises | ↑↑ endothelial dysfunction, normalized oxidative stress and the expression of proteins involved in the formation of nitric oxide, and the angiotensin II pathway | [250] | |
| Clinical Trials | |||
| Blackcurrants fruits | |||
| Effects of 7-day supplementation (600 mg/day) in trained football players | Benefit repeated sprint performance, by reducing sprint slowing | [265] | |
| Effects of 7-day supplementation (600 mg/day) in endurance-trained females subjected to prolonged cycling | ↑↑ enhances fat oxidation (+27% than placebo) ↑↑ non-esterified fatty acids and glycerol concentrations | [266] | |
| Effects of 7-day supplementation (300 mg/day) in cyclists | ↑↑ fat oxidation (+65% than placebo) and VO2max (+27% than placebo) Improve 16.1 km time trial performance by 2.4% | [334] | |
| Effects of 7-day supplementation (300 mg/day) in male trained cyclists | ↑↑ performance (+0.82% than placebo) No effects regarding heart rate and LDH were observed | [267] | |
| Effects of 7-day supplementation (300 mg/day) in active male subjected to treadmill running protocol to exhaustion | ↑↑ total running distance (+10.6%), distance during sprints increased (+10.8%), and higher LDH values at exhaustion (+15%) No effects regarding heart rate, VO2max, and rating of perceived exertion were observed | [268] | |
| Effects of 7-day supplementation (300 mg/day) in active male with experience in high-intensity intermittent running | Bette and faster time sprint (+50%) No effects regarding LDH, heart rate, time to exhaustion, and vertical jump power were observed | [269] | |
| Blackcurrants juices | |||
| Effect of 100 mL/day in college students | ↓↓ postprandial glycemia, insulinemia and incretin secretion levels | [270] | |
| Effect of 1-week supplementation (500 mL 2×day) college students subjected to a bout of eccentric knee extensions | ↓↓ muscle damage and inflammation, by lowering CK, and IL-6 ↑↑antioxidant capacity | [271] | |
| Blueberries, and bananas fruits, and apple juice | |||
| Effects of 200 g blueberries, 50 g banana, and 200 mL apple juice supplementation in active females subjected to recreational level resistance and aerobic based exercises | ↓↓ isometric, concentric, and eccentric torque followed the exercise Faster rate of recovery, and for concentric and eccentric strength | [277] | |
| Mixed blueberry, bilberry, cranberry, elderberry, raspberry seeds and strawberry lemonade | |||
| Effects of 250 mL supplementation in sedentary college-aged males before eccentric bout exercises | Mitigate eccentric-induced decrements in muscle function Ameliorates muscle soreness | [276] | |
| Blueberry juice | |||
| Effects of 2 ×200 mL 5 days before the race, on race day, and 2 days after the race in trained runners | ↓↓ delayed onset muscle soreness and CRP | [273] | |
| Lychee fruits combined with vitamins C and E | |||
| Effect of 1-month supplementation (200 mg/day) in college students subjected to a bout of eccentric knee extensions | ↑↑ endurance capacity by increasing anaerobic threshold (+7.4%) ↓↓ oxidative stress | [304] | |
| Yerba Mate plant | |||
| Effect of supplementation (5 g/day) in well-trained male cyclists 5 days and 1 h before experimental trial | ↑↑ fat utilization during submaximal exercise and improved time-trial performance, by increasing power output and VO2max | [328] | |
| Carob | |||
| Effects of 6-week supplementation (40 g of carob powder diluted in 250 mL of water) in taekwondo athletes | ↑↑ aerobic performance score and rating of perceived exertion ↓↓ body weight No significant effects regarding body fat and muscular volume were found | [329] | |
| Banana | |||
| Effect of 2-week supplementation of bananas in cyclist men before their 75 Km trial | Improve metabolic recovery and diminish post-exercise inflammation, by ↓↓ COX-2 mRNA expression by THP-1 monocytes and the reliance on glycolysis for ATP production | [279] | |
| Bananas and pears | |||
| Effect of 2-week supplementation of bananas and pears in cyclist men before their 75 Km trial | Improves 75 Km cycling performance (+5.0% and 3.3% faster regarding banana and pear versus water), by ↑↑ the ferric reducing ability of plasma, blood glucose, and insulin levels ↓↓ fatty acid utilization and oxidation, IL-10, cortisol, and total leucocytes after exercise | [278] | |
| Red grape skin | |||
| Effects of 6-week supplementation (390 mg × 3 times a day) in healthy male subjects subjected to interval swimming tests | Improves antioxidant status by ↑↑ superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activities Faster swimming performance ↓↓ CK and uric acid levels | [281] | |
| Grapes and apples | |||
| Effect of supplementation (500 mg/day) in physically active men | ↑↑ maximal endurance and perceived exertion | [280] | |
| Grapes, pomegranates, and green tea | |||
| Effect of supplementation (1000 mg/day) in healthy recreationally-active men 1 h before exercise tests | ↑↑ total power output (+5%), maximal peak power output (+3.7%), and average power developed (+5%), without inducing more fatigue or greater heart rate Oxidative homeostasis stabilized | [262] | |
| Lemon | |||
| Effect of 10-day supplementation (400 mg/day) in physically active men and female | Faster and full recovery than placebo ↓↓ muscle damage, exercise-related loss of muscle strength, and movement induced pain ↑↑ glutathione peroxidase activity No effects regarding CK, and IL-6 were observed | [303] | |
| Citrus aurantium combined with caffeine | |||
| Effect of supplementation (100 mg Citrus aurantium + 100 mg caffeine) in physically active men | ↓↓ blood glucose levels ↑↑ plasma epinephrine and norepinephrine levels | [114] | |
| Dark chocolate | |||
| Effects of a 3-month supplementation (20 g/day) in healthy and sedentary individuals | ↓↓ triglycerides and protein carbonylation ↑↑ mitochondria efficiency and VO2max, high-density cholesterol levels, and LKB1, AMPK, PGC-1α, glutathione, and citrate synthase activities | [310] | |
| Effects of 100 g supplementation in men cyclists 2 h before prolonged exercise | ↑↑ pre-exercise antioxidant status and plasma insulin concentration ↓↓ F2-isoprostane levels Better maintenance of plasma glucose concentration No differences regarding stress hormones and IL-6 levels, leukocytosis magnitude and neutrophilia, and changes in neutrophil function were observed | [318] | |
| Effects of 2-week supplementation (40 g/day) in men cyclists | ↓↓ oxidized low-density lipoproteins ↑↑ free fatty acids during exercise No effects regarding circulating insulin, IL-6, IL-10, and IL-1ram glucose, glucagon and cortisol levels, and VO2max were observed | [317] | |
| Effects of 2-week supplementation (40 g/day) in moderately-trained male participants | ↑↑ gas exchange threshold, time trial performance, and VO2max | [311] | |
| Effects of 1-month supplementation (40 g/day) in elite football athletes | ↑↑ antioxidant power ↓↓ CK and LDH levels | [313] | |
| Effects of 25.1 g/day supplementation in soccer players | ↓↓ CK, LDH, carbonyl groups, thiol groups, and MDA levels ↑↑ total antioxidant capacity and glutathione peroxidase activity, and in physical performance | [312] | |
| Milk chocolate containing flavanols | |||
| Effects of 2-week supplementation (105 g/day) in young soccer players | ↓↓ diastolic blood pressure, mean blood pressure, plasma cholesterol, LDL, MDA, urate, and LDH ↑↑ vitamin E/cholesterol | [316] | |
| Effects of 500 mL supplementation in healthy trained cyclists and triathletes immediately before, postexercise, and 2 and 4 h after exercise | Faster time trial and muscle glycogen resynthesis ↓↓ rapamycin phosphorylation | [321] | |
| Effects of 510 mL supplementation between two training sessions in male and endurance-trained cyclists | ↑↑ recovery between both exercise bouts | [335] | |
| Effects of 240 mL supplementation between two training sessions in soccer players | ↑↑ time to fatigue | [315] | |
| Effects of ≈500 mL in trained male cyclists immediately before and 2 h after endurance exercises | ↑↑ recovery after performance | [320] | |
| Effects of 5-day supplementation (1000 mL/day) in trained male judo athletes subjected to intensive training | ↓↓ cortisol, saliva flow rate, and delayed onset muscle soreness ↑↑ salivary testosterone/cortisol ratio No effects regarding weight loss were observed | [322] | |
| Effects of 5-week supplementation (1000 mL/day) between different training programs in trained male cyclists | Faster recovery after glycogen-lowering exercise | [314] | |
| Effects of supplementation in male climbers following an exhaustive bout of high-intensity endurance climbing | ↓↓ muscle soreness | [319] | |
| Cacao mucilage juice | |||
| Effect of 10-day supplementation (330 mL/day) in young and healthy recreationally active male subjected to intensive knee extension exercise 7 days before and 2 days after exercise | Faster recovery No effects regarding knee extension maximum voluntary contraction and blood markers were found | [323] | |
| Cocoa | |||
| Effects of a 3-week supplementation (300 mL/day) in male cyclists | ↑↑ total antioxidant capacity in rest and during exercise, by reducing uric acid levels No effects regarding exercise-induced lipid peroxidation, inflammation, nitric oxide production, performance, and recovery were observed | [324] | |
| Effects of 7-day supplementation (400 mg/day) in sedentary middle-aged adults subjected to a series of ‘step’ moderate- and severe-intensity exercise teat day 7 | ↑↑ VO2max kinetics during moderate, but not severe-intensity exercise | [325] | |
| Flavanol-rich cocoa drink | |||
| Effects of a 100 mL supplementation in male volunteers 2 and 4 hafter strenuous physical exercise | ↓↓ F2-isoprostanes | [326] | |
| Effects of 7-day supplementation (1765 mg/day) in well-trained male cyclists | ↓↓ lipid peroxidation and oxidative stress during exhaustive exercise in hypoxia Beneficial effects on endothelial function and prefrontal oxygenation at rest and during moderate-intensity exercise | [327] | |
| Tart cherry fruit | |||
| Effects of a 3 or more days supplementation (500 mg/day) in men subjected to barbell back squat resistance exercises | ↓↓ oxidative stress, markers of muscle cardiac damage and central fatigue, by lowering CK and creatine kinase myocardial band content | [105] | |
| Effects of 10-day supplementation (480 mg/day) in resistance-trained males | ↓↓ muscle soreness perception in the vastus medialis and the vastus lateral, serum creatinine and total proteins, AST, bilirubin, and ALT No effects regarding inflammatory and oxidative stress markers were observed | [295] | |
| Effects of 10-day supplementation (480 mg/day) in male endurance-trained runners and triathletes | Better running performance times ↑↑ total antioxidant capacity ↓↓ deviations from predicted race pace, and creatinine, urea/blood urea nitrogen, total protein, cortisol, and inflammatory markers levels | [298] | |
| Tart cherry juice | |||
| Effects of 7-day supplementation (50 mL 2×day) in well-trained male cyclists | ↓↓ CRP, IL-6 and lipid hydroperoxides | [297] | |
| Effects of 4-day supplementation (60 mL/day) in male college students subjected to eccentric elbow flexion contractions | ↓↓ strength loss and pain | [301] | |
| Effects of 30 mL 2×day supplementation in well-trained male 7 days before and 48 h after intensive unilateral leg exercise | Improves isometric muscle strength recovery after intensive exercise, by lowering oxidative stress markers ↓↓ CK levels | [292] | |
| Effects of 8-day supplementation (30 mL 2×day) in trained male cyclists | Maintains muscle function after an exercise stress-induced exclusively through a metabolic challenge ↓↓ IL-6 and CRP | [293] | |
| Effects of 8-day supplementation (30 mL 2×day) in semi-professional male soccer player | Improves performance indices recovered faster, agility and muscle soreness ↓↓ IL-6 No effects regarding CK and oxidative stress markers were observed | [261] | |
| Effects of 8-day supplementation (30 mL 2×day) in females subjected to repeated-sprint at day 4 | ↑↑ recovery of countermovement jump height ↓↓ muscle soreness | [299] | |
| Effects of 8-day supplementation (30 mL 2×day) in marathon runners | Faster isometric strength recovered ↑↑ total antioxidant capacity ↓↓ IL-6, CRP, uric acid, and TBARS levels No effects regarding protein carbonyls and LDH levels were observed | [300] | |
| Effects of 8-day supplementation (30 mL 2×day) in male and female sports players | Faster recovery ↓↓ CK levels and muscle soreness | [296] | |
| Effects of 355 mL 2×day supplementation in healthy runners 7 days before and on the day of the race event | ↓↓ pain | [294] | |
| Effects of 7-day supplementation (240 mL/day supplementation in active individuals subjected to plyometric exercises | ↑↑ total antioxidant capacity ↓↓ soreness, CK, LDH, and myeloperoxidase species | [298] | |
| Camellia sinensis | |||
| Effects of 4-week supplementation (320 mg of polyphenols 2×day) in untrained healthy men subjected to strength training | ↓↓ plasma lipid hydroperoxides at rest and CK levels | [336] | |
| Green tea | |||
| Effects of the intake of 3 green tea capsules in healthy young men 24 h before undergoing cycling exercise | ↑↑ fat oxidation rates and consequently, ↑↑ fat oxidation to total energy expenditure ↑↑ insulin sensitivity and glucose tolerance | [337] | |
| Effects of 4-week supplementation (450 mg/day) in male sprinters | Prevents oxidative stress induced by high-intensity cycle sprint test, by decreasing MDA and superoxide radical levels, and probably by inhibiting xanthine oxidase | [254] | |
| Effects of 6-week supplementation (250 mg/day) in CrossFit individuals | ↑↑ ferric reducing ability of plasma ↓↓ TBARS | [253] | |
| Effects of 3-week consumption (500 mg/day) on whole-body metabolism during cycling exercise in endurance-trained men | ↑↑ high-density-lipoprotein cholesterol ↓↓ plasma CK levels No effects regarding fat and energy metabolism, IL-6, CRP, and oxidative stress markers (TBAR and, oxidized low-density-lipoprotein cholesterol) were observed | [338] | |
| Effect of 15-day supplementation (500 mg/day) in male cyclists | ↓↓ muscle damage and oxidative stress, ↓↓ CK and TBARS, and exerts positive effects regarding neuromuscular parameters related to muscle activation and muscle fatigue | [256] | |
| Effect of 7-day supplementation (2 g of leaves in 200 mL of water, three times per day) in weight-trained men | ↓↓ lipid hydroperoxide, CK, AST, reduced glutathione, xanthine oxidase, and uric acid levels before and after exercise ↑↑ the ferric reducing ability of plasma | [339] | |
| Effects of 4-week supplementation (250 mg/day) in male sprinters | ↑↑ total antioxidant capacity and erythrocyte superoxide dismutase activity ↓↓ MDA levels No effects regarding sprint performance were observed | [254] | |
| Effects of 4-week supplementation (2000 mg/day) in untrained men subjected to resistance training | ↑↑ Total antioxidant capacity No effects regarding hinder strength gains were observed | [340] | |
| Effects of 2-week supplementation (500 mg/day) in untrained men subjected to sessions of exercise to induce delayed onset muscle soreness in the triceps sural muscle group | ↓↓ markers of muscle damage after exercise, by lowering CK No effects regarding delayed onset muscle soreness were observed | [257] | |
| Effects of 4-week supplementation (250 mg/day) in sedentary men subjected to exhaustive run | ↑↑ Total antioxidant capacity ↓↓ MDA and CK levels | [255] | |
| Effects of 20 g of green tea leaves mixed with 600 mL supplementation in well-trained male cyclists during training | Maintenance post-exercise testosterone and lymphocyte concentration ↓↓ neutrophils count | [341] | |
| Effects of 780 mg/day supplementation in sportive male university gymnastics before training | ↓↓ LDH concentration ↑↑ fat oxidation | [180] | |
| Effects of 8-week supplementation (500 mg/day) in overweight middle-aged men subjected to endurance training | ↓↓ IL-6 and CRP levels, and body weight, body mass index, body fat percentage, and visceral fat | [258] | |
| Effects of 10-week supplementation (572.8 mg/day) in healthy males subjected to a 60 min/day, 3 days/week of ergometer exercises | ↓↓ respiratory exchange ratio by increasing the proportion of whole-body fat utilization during exercise | [342] | |
| Green tea combined with caffeine | |||
| Effects of 24-hcapsule supplementation 3 times/day (50 mg caffeine and 90 mg green tea) in healthy young men | Exhibits thermogenic properties, by increasing energy expenditure and promoting fat oxidation | [259] | |
| Black tea | |||
| Effects of 4-week supplementation (900 mg/day) in college-age males with weight training experience | ↑↑ Recovery ↓↓ oxidative stress levels, IL-6 and delayed onset muscle soreness responses to acute anaerobic intervals | [260] | |
| Propolis | |||
| Effects of 9-day supplementation (1760 mg/day) in healthy active individuals | ↑↑ total antioxidant capacity and glutathione levels ↓↓ IL-6, total oxidant status, MDA, and oxidative stress index No differences regarding IL-10, fat mass, fat-free mass, anaerobic powers, fatigue index, and VO2max were observed | [308] | |
| Honey | |||
| Effects of 70 g supplementation in healthy nonprofessional male road cyclists before each training session during 8 weeks | ↓↓ IL-1β, IL-6, IL-8, TNF-α, reactive oxygen species and MDA levels ↑↑ superoxide dismutase, catalase, and total antioxidant capacity | [306] | |
| Effects of 1 g/kg body weight supplementation in healthy volunteer men before exercise during 3 weeks | ↓↓ MDA levels | [305] | |
| Effects of 0.75 and 105 g/kg supplementation in female athletes | Protective effects against lipid peroxidation and oxidative stress by ↓↓ MDA levels | [307] | |
| Effects of 70 g supplementation 90 min before each training session in male road cyclists during 16 weeks | ↓↓ lymphocytes DNA damage, cytokines, peroxidative biomarkers ↑↑ antioxidative biomarkers | [264] | |
| Ginkgo biloba leaves | |||
| Effects of 6-week supplementation (80 mg/day) in healthy and physically active young men | ↑↑ endurance performance, VO2max and blood antioxidant capacity, and better neuroprotection through increased exercise-induced production of brain-derived neurotrophic factor, by ↓↓ TBARS and ↑↑ superoxide dismutase, the ferric reducing ability of plasma, and reduced glutathione | [330] | |
| Ecklonia cava | |||
| Effect of 100 mL supplementation in college students 30 min before treadmill tests | Better endurance performance, by ↓↓ LDH levels and ↑↑ glucose oxidation | [331] | |
| Mangosteen, pomegranate, and black elderberry | |||
| Effects of 5-week supplementation (500 mg/day) in recreationally active men and women | ↓↓ delayed onset muscle soreness, by reducing myoglobin, creatinine, and CK levels | [288] | |
| Blueberry and green tea polyphenol-rich soy protein-based product | |||
| Effects of 17-day supplementation (40 mg/day) in long-distance runners | ↑↑ gut-derived phenolic signature and ketogenesis during recovery from 3 days of heavy exertion | [275] | |
| Effect of 17-day supplementation (12.5 mg/kg/day) against virus infection before an intensive run in long-distance runners | ↓↓ the susceptibility to influenza infection following stressful exercise | [274] | |
| Jabuticaba peel beverage | |||
| Effect of supplementation (100 mL/day) in soccer athletes 1 h before intensive training | Positive effects in attenuating muscle damage and oxidative stress, by lowering urea, ALT, AST, and CK levels, and increasing GST amounts | [302] | |
| Pomegranate | |||
| Effect of 8-week supplementation (815–1350 mg/day) in male and female cyclists | ↑↑ VO2max required during submaximal exercise Ameliorate long time-trials exercises | [283] | |
| Effect of 3-day supplementation (1000 mg/day and 1300 mg/day) in active male and female | ↑↑ vessel diameter and blood flow Delay fatigue during treadmill runs | [284] | |
| Pomegranate juice | |||
| Effect of 2-day supplementation (250 mL/day) in elite weightlifters | Capacity to attenuate the acute plasma response after exercise, by ↓↓ cortisol and homocysteine levels, and ↑↑ testosterone/cortisol ratio | [103] | |
| Effect of 250 mL supplementation in elite weightlifters 48 h before training | ↑↑ antioxidant responses, by ↓↓ MDA levels (−12.5% than placebo) and ↑↑ catalase and glutathione peroxidase activities (+8.6 and 6.8%, respectively) | [287] | |
| Effect of 2-week supplementation (250 mL 2×day) in resistance-trained men | Attenuates weakness ↓↓ soreness of the elbow flexor No effects regarding isometric strength and muscle soreness were observed when compared to placebo | [263] | |
| Effects of 8-day supplementation (650 mg/day and 1300 mg/day) in resistance-trained men | ↑↑ strength recovery in leg and arm muscles following eccentric exercise No dose-response effect was observed | [286] | |
| Effects of 1-week supplementation (500 mL/day) in participants subjected to a 30 min moderate treadmill exercise 2 different occasions | ↓↓ MDA, urinary free cortisol, and systolic blood pressure pre-exercise and post- and diastolic blood pressure | [285] | |
| Effects of 2-month supplementation (50 mL/day) in well-trained rowers | ↑↑ plasma antioxidant potential No effects regarding inflammatory markers were observed | [343] | |
| Effects of 22-day supplementation (200 mL/day) in endurance athletes | Capacity to modulate fat and protein damage by ↑↑ LDH levels and ↓↓ MDA, and carbonyl levels | [344] | |
| Effects of 1-week supplementation (500 mL/day) in overweight and obese individuals subjected to 30 min of treadmill tests | ↓↓ MDA, cortisol, and systolic and diastolic blood pressure before and after exercise | [285] | |
| Beetroot juice | |||
| Effects 250 mL/day supplementation in healthy male participants subjected to muscle-damaging exercise at day 1 and on the following 3 mornings | ↓↓ muscle pain No effects regarding CK, and CPR were observed | [332] | |
| Effects of 150 mL 2×day 3 days before exercise, on the day trial, and 3 days after exercise in soccer players | ↓↓ muscle pain Better performance during the recovery period | [333] | |
| Effects of 250 mL/day supplementation on the day, 24 h, and 48 h after muscle-damaging exercises in male p | ↓↓ countermovement jumps reactive strength index following repeated sprint test, and muscle pain No effects regarding sprint performance or oxidative stress were observed | [345] | |
| Effects of 250 mL supplementation in soccer players 24, and 48 h after 100-drop jumps | ↓↓ muscle soreness No effects regarding maximal isometric voluntary contractions, CK, IL-6, TNF-α, and IL-8 were observed | [333] | |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Rodrigues, M.; Fortuna, A.; Falcão, A.; Alves, G. Flavonoid compounds as reversing agents of the P-glycoprotein-mediated multidrug resistance: An in vitro evaluation with focus on antiepileptic drugs. Food Res. Int. 2018, 103, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Neurol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement. Altern. Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.d.l.L.; Arráez-Román, D.; Segura-Carretero, A. Functional ingredients based on nutritional phenolics. A case study against inflammation: Lippia Genus. Nutrients 2019, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, Z.A.; Fulford, J.; Gates, P.E.; Jackman, X.S.R.; Jones, A.M.; Bond, B.; Bowtell, J.L.; Bond, B.; Bowtell, J.L. Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight, middle-aged men. J. Appl. Physiol. 2019, 126, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. Phytochemicals for improving aspects of cognitive function and psychological state potentially relevant to sports performance. Sport. Med. 2019, 49, 39–58. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Polyphenols and athletic performance: A review on human data. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2012; p. 13. [Google Scholar]
- Bowtell, J.; Kelly, V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sport. Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A.; Ugbolue, U.C.; He, Y.; Meng, Y.; Gu, Y. Effect of running exercise on oxidative stress biomarkers: A systematic review. Front. Physiol. 2021, 11, 610112. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological signaling functions of reactive oxygen species in stem cells: From flies to man. Front. Cell Dev. Biol. 2021, 9, 4370. [Google Scholar] [CrossRef]
- Dannecker, E.A.; Liu, Y.; Rector, R.S.; Thomas, T.R.; Fillingim, R.B.; Robinson, M.E. Sex differences in exercise-induced muscle pain and muscle damage. J. Pain 2012, 13, 1242–1249. [Google Scholar] [CrossRef]
- Bontemps, B.; Vercruyssen, F.; Gruet, M.; Louis, J. Downhill running: What are the effects and how can we adapt? A narrative review. Sport. Med. 2020, 50, 2083–2110. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Gatta, P.A.D.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Muñoz, D.; Olcina, G.; Timón, R.; Robles, M.C.; Caballero, M.J.; Maynar, M. Effect of different exercise intensities on oxidative stress markers and antioxidant response in trained cyclists. J. Sports Med. Phys. Fitness 2010, 50, 93–98. [Google Scholar]
- Clemente, F.M.; González-Fernández, F.T.; Ceylan, H.I.; Silva, R.; Younesi, S.; Chen, Y.S.; Badicu, G.; Wolański, P.; Murawska-Ciałowicz, E. Blood biomarkers variations across the pre-season and interactions with training load: A study in professional soccer players. J. Clin. Med. 2021, 10, 5576. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, F.J.; Villalón, J.M.; Zamorano-León, J.J.; Rosas, L.F.; Proietti, R.; Mateos-Caceres, P.J.; González-Armengol, J.J.; Villarroel, P.; Macaya, C.; López-Farré, A.J. Functional status and inflammation after preseason training program in professional and recreational soccer players: A proteomic approach. J. Sport. Sci. Med. 2011, 10, 45–51. [Google Scholar]
- Plinta, R.; Olszanecka-Glinianowicz, M.; Drosdzol-Cop, A.; Chudek, J.; Skrzypulec-Plinta, V. The effect of three-month pre-season preparatory period and short-termexercise on plasma leptin, adiponectin, visfatin, and ghrelin levels in young female handball and basketball players. J. Endocrinol. Investig. 2012, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of Montmorency tart cherry (L. Prunus Cerasus) consumption on nitric oxide biomarkers and exercise performance. Scand. J. Med. Sci. Sport. 2018, 28, 1746–1756. [Google Scholar] [CrossRef]
- Morgan, P.T.; Barton, M.J.; Bowtell, J.L. Montmorency cherry supplementation improves 15-km cycling time- trial performance. Eur. J. Appl. Physiol. 2019, 119, 675–684. [Google Scholar] [CrossRef]
- Visioli, F. Polyphenols in sport: Facts or fads? In Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2015; pp. 1–7. [Google Scholar]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef]
- Mehri, A.; Hosseinpour Delaware, S.; Azizi, M.; Azarbaijani, M.A.; Farzangi, P. The effect of aerobic training and resveratrol on some regulatory and executive factors of cardiomyocytes apoptosis in STZ-diabetic male rats. Med. Sci. J. 2020, 30, 59–66. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Gonçalves, A.C.; Jesus, F.; Simões, M.; Silva, L.R. Phenolic compounds: Sources, properties and applications. In Bioactive Compounds: Sources, Properties and Applications; Porter, R., Parker, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 271–299. ISBN 6312317269. [Google Scholar]
- Li, S.; Liu, J.; Li, Z.; Wang, L.; Gao, W.; Zhang, Z.; Guo, C. Sodium-dependent glucose transporter 1 and glucose transporter 2 mediate intestinal transport of quercetrin in Caco-2 cells. Food Nutr. Res. 2020, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1; US Department of Agriculture: Beltsville, MD, USA, 2013. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav3-1.pdf (accessed on 26 November 2021).
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Ock, K.C.; Sang, J.C.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989–1009. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Služek, V.B.; Cindrić, I.; Molnar, M. Coumarins in food and methods of their determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Costa, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Anti-inflammatory and antiproliferative properties of sweet cherry phenolic-rich extracts. Molecules 2022, 27, 268. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2019, 70, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Montesano, D.; Masoero, F.; Trevisan, M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res. Int. 2017, 100, 69–77. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic compounds and bioaccessibility thereof in functional pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Reports 2019, 24, e00370. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Flores-Félix, D.; Costa, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Hepatoprotective effects of sweet cherry extracts (cv. Saco). Foods 2021, 10, 2623. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Martinez-Negrin, G.; Acton, J.P.; Cocksedge, S.P.; Bailey, S.J.; Clifford, T. The effect of dietary (poly) phenols on exercise-induced physiological adaptations: A systematic review and meta-analysis of human intervention trials adaptations: A systematic review and meta-analysis of human intervention trials. Crit. Rev. Food Sci. Nutr. 2020, 1–16, 2872–2887. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2010, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Swiderski, G.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Swisłocka, R. Plant-derived and dietary hydroxybenzoic acids—A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in MDA-MB-231 and MCF-7 cell lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 5666. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Ratty, A.K.; Das, N.P. Effects of flavonoids on nonenzymatic lipid peroxidation: Structure-activity relationship. Biochem. Med. Metab. Biol. 1988, 39, 69–79. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; De Groot, M.J.; van den Berg, D.-J.; Tromp, M.N.J.L.; Donné-Op den Kelder, G.; Van Der Vijgh, W.J.F.; Bast, A. A quantum chemical explanation of the antioxidant activity of flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and flavonols’ antiradical structure—Activity relationship—A quantum chemical study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Quindry, J.C.; Hosick, P.A.; Hudson, M.H.; Still, L.; Henson, D.A.; Milne, G.L.; Morrow, J.D.; et al. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl. Physiol. Nutr. Metab. 2008, 33, 254–262. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Rühlmann, A.; Antovic, D.; Müller, T.J.J.; Urlacher, V.B. Regioselective hydroxylation of stilbenes by engineered cytochrome P450 from Thermobifida fusca YX. Adv. Synth. Catal. 2017, 359, 984–994. [Google Scholar] [CrossRef]
- Shi, Y.W.; Wang, C.P.; Liu, L.; Liu, Y.L.; Wang, X.; Hong, Y.; Li, Z.; Kong, L.D. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol. Nutr. Food Res. 2012, 56, 1433–1444. [Google Scholar] [CrossRef]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef]
- Yamauchi, S.; Hayashi, Y.; Nakashima, Y.; Kirikihira, T.; Yamada, K.; Masuda, T. Effect of benzylic oxygen on the antioxidant activity of phenolic lignans. J. Nat. Prod. 2005, 68, 1459–1470. [Google Scholar] [CrossRef]
- Dugas, A.J.; Castañeda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure-activity relationships. J. Nat. Prod. 2000, 63, 327–331. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Alov, P.; Tsakovska, I.; Pajeva, I. Computational studies of free radical-scavenging properties of phenolic compounds. Curr. Top. Med. Chem. 2015, 15, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Burdyliuk, N.I.; Lushchak, V.I. Effects of pH on antioxidant and prooxidant properties of common medicinal herbs. Open Life Sci. 2016, 11, 298–307. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on i. Mediators Inflamm. 2007, 2007, 045673. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. PI-103 and quercetin attenuate PI3K-AKT signaling pathway in T-cell lymphoma exposed to hydrogen peroxide. PLoS ONE 2016, 11, e0160686. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Jung, W.K.; Choi, I.; Lee, D.Y.; Yea, S.S.; Choi, Y.H.; Kim, M.M.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; et al. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-κB pathways. Int. J. Biochem. Cell Biol. 2008, 40, 2572–2582. [Google Scholar] [CrossRef]
- Chao, P.C.; Hsu, C.C.; Yin, M.C. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr. Metab. 2009, 6, 33. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef]
- WHO World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 15 December 2021).
- Baumgartner, L.; Schulz, T.; Oberhoffer, R.; Weberruß, H. Influence of vigorous physical activity on structure and function of the cardiovascular system in young athletes—The MuCAYA-study. Front. Cardiovasc. Med. 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, A. Aerobic training induced structural changes of the heart. Turkish J. Sport Exerc. 2012, 14, 1–5. [Google Scholar]
- Zhang, K.; Liu, Y.; Liu, J.; Liu, R.; Cao, C. Detecting structural and functional neuroplasticity in elite ice-skating athletes. Hum. Mov. Sci. 2021, 78, 102795. [Google Scholar] [CrossRef] [PubMed]
- Paruk, T.; Rauch, L.; Jankiewicz, M.; Van Breda, K.; Stein, D.J.; King, M. Structural brain differences between ultra-endurance athletes and sedentary persons. Sport. Med. Health Sci. 2020, 2, 89–94. [Google Scholar] [CrossRef]
- Duru, A.D.; Balcioglu, T.H. Functional and structural plasticity of brain in elite karate athletes. J. Healthc. Eng. 2018, 2018, 8310975. [Google Scholar] [CrossRef]
- Schlaffke, L.; Lissek, S.; Lenz, M.; Brüne, M.; Juckel, G.; Hinrichs, T.; Platen, P.; Tegenthoff, M.; Schmidt-Wilcke, T. Sports and brain morphology—A voxel-based morphometry study with endurance athletes and martial artists. Neuroscience 2014, 259, 35–42. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Bragazzi, N.L.; Boukhris, O.; Bouaziz, M.; Ayadi, F.; Abed, K.E.; Driss, T.; Souissi, N.; Chtourou, H.; et al. Effects of natural polyphenol-rich pomegranate juice on the acute and delayed response of homocysteine and steroidal hormones following weightlifting exercises: A double-blind, placebo- controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 15. [Google Scholar] [CrossRef]
- Beaton, L.J.; Allan, D.A.; Tarnopolsky, M.A.; Tiidus, P.M.; Phillips, S.M. Contraction-induced muscle damage is unaffected by vitamin E supplementation. Med. Sci. Sports Exerc. 2002, 34, 798–805. [Google Scholar] [CrossRef]
- Hooper, D.R.; Orange, T.; Gruber, M.T.; Darakjian, A.A.; Conway, K.L.; Hausenblas, H.A. Broad spectrum polyphenol supplementation from tart cherry extract on tarkers of tecovery from intense resistance exercise. J. Int. Soc. Sports Nutr. 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Tanabe, Y.; Chino, K.; Ohnishi, T.; Ozawa, H.; Sagayama, H.; Maeda, S.; Takahashi, H. Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation. Scand. J. Med. Sci. Sport. 2019, 29, 524–534. [Google Scholar] [CrossRef]
- Roelofs, E.J.; Smith-Ryan, A.E.; Trexler, E.T.; Hirsch, K.R.; Mock, M.G. Effects of pomegranate extract on blood flow and vessel diameter after high-intensity exercise in young, healthy adults. Eur. J. Sport Sci. 2017, 17, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.; Santana, A.A.; Santamarina, A.B.; Oyama, L.M.; Caperuto, É.C.; De Souza, C.T.; Barboza, C.D.A.; Rocha, L.Y.; Figueroa, D.; Mostarda, C.; et al. Role of training and detraining on inflammatory and metabolic profile in infarcted rats: Influences of cardiovascular autonomic nervous system. Mediators Inflamm. 2014, 2014, 207131. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, S.; Parissis, J.; Kroupis, C.; Georgiadis, M.; Karatzas, D.; Karavolias, G.; Koniavitou, K.; Coats, A.J.S.; Kremastinos, D.T. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur. Heart J. 2001, 22, 791–797. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Marques, V.; Cyrne, L.; Marinho, H.S.; Antunes, F. A quantitative study of NF-κB activation by H2O2: Relevance in inflammation and synergy with TNF-α. J. Immunol. 2007, 178, 3893–3902. [Google Scholar] [CrossRef]
- Thompson, D.; Williams, C.; McGregor, S.J.; Nicholas, C.W.; McArdle, F.; Jackson, M.J.; Powell, J.R. Prolonged vitamin C supplementation and recovery from demanding exercise. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 466–481. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Bechke, E.; Williamson, C.; Green, Z.; Bailey, P.; McLester, J.; McLester, C. The influence of citrus aurantium and caffeine complex versus placebo on the cardiac autonomic response: A double blind crossover design. J. Int. Soc. Sports Nutr. 2019, 16, 34. [Google Scholar] [CrossRef]
- Jówko, E.; Sacharuk, J.; Balasinska, B.; Wilczak, J.; Charmas, M.; Ostaszewski, P.; Charmas, R. Effect of a single dose of green tea polyphenols on the blood markers of exercise-induced oxidative stress in soccer players. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 486–496. [Google Scholar] [CrossRef]
- D’Unienville, N.M.A.; Hill, A.; Coates, A.; Yandell, C.; Nelson, M.; Buckley, J. Effects of almond, dried grape and dried cranberry consumption on endurance exercise performance, recovery and psychomotor speed: Protocol of a randomised controlled trial. BMJ Open Sport Exerc. Med. 2019, 5, e000560. [Google Scholar] [CrossRef]
- Council for Responsible Nutrition Council for Responsible Nutrition. Available online: https://www.crnusa.org/ (accessed on 17 December 2021).
- Davison, G.; Gleeson, M. The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. Eur. J. Appl. Physiol. 2006, 97, 454–461. [Google Scholar] [CrossRef]
- McBride, J.M.; Kraemer, W.J.; Triplett-McBride, T.; Sebastianelli, W. Effect of resistance exercise on free radical production. Med. Sci. Sport. Exerc. 1998, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bakker, G.C.M.; Van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.P.; Kooistra, T.; Van Ommen, B.; Hendriks, H.F.J. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: A nutrigenomics approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Crum, E.M.; Che Muhamed, A.M.; Barnes, M.; Stannard, S.R. The effect of acute pomegranate extract supplementation on oxygen uptake in highly-trained cyclists during high-intensity exercise in a high altitude environment. J. Int. Soc. Sports Nutr. 2017, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef] [PubMed]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; Woude, H.; Zanden, J.; Bladeren, P.J.; Vervoort, J.; Rietjens, I.M.C.M. Identification of o-quinone/quinone methide metabolites of quercetin in a cellular in vitro system. FEBS Lett. 2002, 520, 30–34. [Google Scholar] [CrossRef]
- Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Nakamura, A.; Akagi, K.; Shirai, T. Green tea catechins enhance tumor development in the colon without effects in the lung or thyroid after pretreatment with 1,2-dimethylhydrazine or 2,2′-dihydroxy-di-n-propylnitrosamine in male F344 rats. Cancer Lett. 2001, 168, 23–29. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and kidney carcinogenicity of caffeic acid in F344 Rats and C57BL/6N × C3H/HeN F1 mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar]
- Mendez, M.A.; Anthony, M.S.; Arab, L. Soy-based formulae and infant growth and development: A review. J. Nutr. 2002, 132, 2127–2130. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Gosselin, S.J.; Welsh, M.B.; Johnston, J.O.; Balistreri, W.F.; Kramer, L.W.; Dresser, B.L.; Tarr, M.J. Dietary estrogens-A probable cause of infertility and liver disease in captive cheetahs. Gastroenterology 1987, 93, 225–233. [Google Scholar] [CrossRef]
- Hughes, C.L. Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ. Health Perspect. 1988, 78, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Bennetau-Pelissero, C.; Breton, B.B.; Bennetau, B.; Corraze, G.; Le Menn, F.; Davail-Cuisset, B.; Helou, C.; Kaushik, S.J. Effect of genistein-enriched diets on the endocrine process of gametogenesis and on reproduction efficiency of the rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2001, 121, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997, 350, 23–27. [Google Scholar] [CrossRef]
- Cassidv, A.; Bingham, S.; Setchell, K.D.R. Biological effects on the menstrual of a diet of soy protein cycle of premenopausal. Am. J. Clin. Nutr. 1994, 60, 333–340. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Gross, S.J.; Jenkins, D.P.; Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Dumke, C.L.; Utter, A.C.; Mcanulty, S.R.; et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007, 39, 1561–1569. [Google Scholar] [CrossRef]
- Overman, A.; Chuang, C.C.; McIntosh, M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2011, 35, 1165–1172. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; De Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef]
- Ungvari, Z.; Orosz, Z.; Rivera, A.; Labinskyy, N.; Xiangmin, Z.; Olson, S.; Podlutsky, A.; Csiszar, A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 2417–2424. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Ota, N.; Hase, T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 2009, 10, 423–434. [Google Scholar] [CrossRef]
- Menzies, K.J.; Singh, K.; Saleem, A.; Hood, D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. 2013, 288, 6968–6979. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Rimbaud, S.; Ruiz, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Veksler, V.; Garnier, A.; Ventura-Clapier, R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS ONE 2011, 6, e26391. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarei, M.; Hoxha, B.; Talley, N.A.; Anderson, M.R.; Alkhouli, M.F.; Squire, M.A.; Eckman, D.M.; Babu, J.R.; Lopaschuk, G.D.; Broderick, T.L. Beneficial effects of resveratrol and exercise training on cardiac and aortic function and structure in the 3xTg mouse model of Alzheimer’s disease. Drug Des. Dev. Ther. 2019, 13, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Broderick, T.L.; Rasool, S.; Li, R.; Zhang, Y.; Anderson, M.; Al-Nakkash, L.; Plochocki, J.H.; Geetha, T.; Babu, J.R. Neuroprotective effects of chronic resveratrol treatment and exercise training in the 3xtg-ad mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 7337. [Google Scholar] [CrossRef]
- Coban, F.; Akil, M.; Lİman, R.; Ciğerci, İ. Effects of resveratrol on oxidative DNA damage induced by the acute swimming exercise. J. Pharm. Res. Int. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Bicer, M.; Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K.; Avunduk, M.C. Effect of resveratrol administration on muscle glycogen levels in rats subjected to acute swimming exercise. Cell. Mol. Biol. 2005, 65, 28–31. [Google Scholar] [CrossRef]
- Amirazodi, F.; Mehrabi, A.; Amirazodi, M.; Parsania, S.; Rajizadeh, M.A.; Esmaeilpour, K. The combination effects of resveratrol and swimming HIIT exercise on novel object recognition and open-field tasks in aged rats. Exp. Aging Res. 2020, 46, 336–358. [Google Scholar] [CrossRef]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Arslangil, D.; Mogulkoc, R.; Patlar, S. Effect of resveratrol administration on the element metabolism in the blood and brain tissues of rats subjected to acute swimming exercise. Biol. Trace Elem. Res. 2017, 175, 421–427. [Google Scholar] [CrossRef]
- Hr, N.; Azizi, M.; Ma, A.; Farzangi, P. The effect of combination of regular continuous exercise and resveratrol supplementation on some regulatory and executive factors of hepatocytic apoptosis in male diabetic rats. Feys 2020, 23, 605–614. [Google Scholar]
- Yen, C.C.; Chang, C.W.; Hsu, M.C.; Wu, Y.T. Self-Nanoemulsifying drug delivery system for resveratrol: Enhanced oral bioavailability and reduced physical fatigue in rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bal, N.B.; Sadi, G.; Usanmaz, S.E.; Uludag, M.O.; Demirel-Yilmaz, E. The effects of resveratrol and exercise on age and gender-dependent alterations of vascular functions and biomarkers. Exp. Gerontol. 2018, 110, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H.; Tang, C.-F.; Ouyang, J.-Q. Influence of salidroside from Rhodiola sachalinensis A. Bor on some related indexes of free radical and energy metabolism after exercise in mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2012, 28, 53–56. [Google Scholar] [PubMed]
- Cao, J.; Zhang, Y.; Chen, W.; Zhao, X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br. J. Nutr. 2010, 103, 249–255. [Google Scholar] [CrossRef]
- Quindry, J.C.; McAnulty, S.R.; Hudson, M.B.; Hosick, P.; Dumke, C.; McAnulty, L.S.; Henson, D.; Morrow, J.D.; Nieman, D. Oral quercetin supplementation and blood oxidative capacity in response to ultramarathon competition. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 601–616. [Google Scholar] [CrossRef]
- Utter, A.C.; Nieman, D.C.; Kang, J.; Dumke, C.L.; Quindry, J.C.; McAnulty, S.R.; McAnulty, L.S. Quercetin does not affect rating of perceived exertion in athletes during the western states endurance run. Res. Sport. Med. 2009, 17, 71–83. [Google Scholar] [CrossRef]
- Sharp, M.A.; Hendrickson, N.R.; Staab, J.S.; Mcclung, H.L.; Nindl, B.C.; Michniak-Kohn, B.B. Effects of short-term quercetin supplementation on soldier performance. J. Strength Cond. Res. 2012, 26, 53–60. [Google Scholar] [CrossRef]
- Ganio, M.S.; Armstrong, L.E.; Johnson, E.C.; Klau, J.F.; Ballard, K.D.; Michniak-Kohn, B.; Kaushik, D.; Maresh, C.M. Effect of quercetin supplementation on maximal oxygen uptake in men and women. J. Sports Sci. 2010, 28, 201–208. [Google Scholar] [CrossRef]
- Abbey, E.L.; Rankin, J.W. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 91–96. [Google Scholar] [CrossRef]
- Bigelman, K.A.; Chapman, D.P.; Freese, E.C.; Trilk, J.L.; Cureton, K.J. Effects of 6 weeks of quercetin supplementation on energy, fatigue, and sleep in ROTC cadets. Mil. Med. 2011, 176, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Dumke, C.L.; Gross, S.J.; Jenkins, D.P.; Murphy, E.A.; Carmichael, M.D.; Quindry, J.C.; McAnulty, S.R.; et al. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States endurance run. J. Interf. Cytokine Res. 2007, 27, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- MacRae, H.S.H.; Mefferd, K.M. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L.; Sacchetti, M.; Felici, F. The effects of quercetin supplementation on eccentric exercise-induced muscle damage. Nutrients 2019, 11, 205. [Google Scholar] [CrossRef]
- Pelletier, D.M.; Lacerte, G.; Goulet, E.D.B. Effects of quercetin supplementation on endurance performance and maximal oxygen consumption: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 73–82. [Google Scholar] [CrossRef]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO2max and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef]
- Konrad, M.; Nieman, D.C.; Henson, D.A.; Kennerly, K.M.; Jin, F.; Wallner-Liebmann, S.J. The acute effect of ingesting a quercetin-based supplement on exercise-induced inflammation and immune changes in runners. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 338–346. [Google Scholar] [CrossRef]
- Macedo, R.C.S.; Vieira, A.; Marin, D.P.; Otton, R. Effects of chronic resveratrol supplementation in military firefighters undergo a physical fitness test—A placebo-controlled, double blind study. Chem. Biol. Interact. 2015, 227, 89–95. [Google Scholar] [CrossRef]
- Ozemek, C.; Hildreth, K.L.; Blatchford, P.J.; Joseph Hurt, K.; Bok, R.; Seals, D.R.; Kohrt, W.M.; Moreau, K.L. Effects of resveratrol or estradiol on postexercise endothelial function in estrogen-deficient postmenopausal women. J. Appl. Physiol. 2020, 128, 739–747. [Google Scholar] [CrossRef]
- Tsao, J.P.; Liu, C.C.; Wang, H.F.; Bernard, J.R.; Huang, C.C.; Cheng, I.S. Oral resveratrol supplementation attenuates exercise-induced interleukin-6 but not oxidative stress after a high intensity cycling challenge in adults. Int. J. Med. Sci. 2021, 18, 2137–2145. [Google Scholar] [CrossRef]
- Zahedi, H.S.; Jazayeri, S.; Ghiasvand, R.; Djalali, M.; Eshraghian, M.R. Effects of Polygonum cuspidatum containing resveratrol on inflammation in male professional basketball players. Int. J. Prev. Med. 2013, 4, S1–S4. [Google Scholar] [PubMed]
- Eschle, T.M.; Goodall, S.; Kennedy, D.O.; Wightman, E.L. Acute resveratrol administration increases neural effort but not whole body metabolism or cognitive performance in healthy, young participants. J. Cogn. Enhanc. 2020, 4, 315–322. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Ho, C.S.; Lee, M.C.; Ho, C.S.; Huang, C.C.; Kan, N.W. Protective effects of resveratrol supplementation on contusion induced muscle injury. Int. J. Med. Sci. 2020, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Polley, K.R.; Jenkins, N.; O’Connor, P.; McCully, K. Influence of exercise training with resveratrol supplementation on skeletal muscle mitochondrial capacity. Appl. Physiol. Nutr. Metab. 2016, 41, 26–32. [Google Scholar] [CrossRef]
- Mcanulty, L.S.; Miller, L.E.; Hosick, P.A.; Utter, A.C.; Quindry, J.C.; Mcanulty, S.R. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 760–765. [Google Scholar] [CrossRef]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Kuwahata, M.; Higashi, A. Astaxanthin-, β-carotene-, and resveratrol-rich foods support resistance training-induced adaptation. Antioxidants 2021, 10, 113. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2184. [Google Scholar] [CrossRef]
- Trombold, J.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin consumption improves strength recovery 2-3 d after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef]
- Overdevest, E.; Wouters, J.A.; Wolfs, K.H.M.; Van Leeuwen, J.J.M.; Possemiers, S. Citrus flavonoid supplementation improves exercise performance in trained athletes. J. Sport. Sci. Med. 2018, 17, 24–30. [Google Scholar]
- Jajtner, A.R.; Hoffman, J.R.; Townsend, J.R.; Beyer, K.S.; Varanoske, A.N.; Church, D.D.; Oliveira, L.P.; Herrlinger, K.A.; Radom-Aizik, S.; Fukuda, D.H.; et al. The effect of polyphenols on cytokine and granulocyte response to resistance exercise. Physiol. Rep. 2016, 4, e13058. [Google Scholar] [CrossRef]
- Kim, H.; Suzuki, T.; Saito, K.; Yoshida, H.; Kojima, N.; Kim, M.; Sudo, M.; Yamashiro, Y.; Tokimitsu, I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M.; Kapoor, M.P.; Nishimura, A.; Okubo, T. Influence of green tea catechins on oxidative stress metabolites at rest and during exercise in healthy humans. Nutrition 2016, 32, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Haramizu, S.; Ota, N.; Hase, T.; Murase, T. Catechins attenuate eccentric exercise-induced inflammation and loss of force production in muscle in senescence-accelerated mice. J. Appl. Physiol. 2011, 111, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, K.A.; Chirouzes, D.M.; Ceddia, M.A. Supplementation with a polyphenolic blend improves post-exercise strength recovery and muscle soreness. Food Nutr. Res. 2015, 59, 30034. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglan, S.; Afousi, A.G.; Gaeini, A.A. Epicatechin supplementation and resistance training-induced improvement of muscle strength and circulatory levels of plasma follistatin and myostatin in sarcopenic older adults. J. Aging Phys. Act. Run. Head Train. Suppl. 2018, 27, 384–391. [Google Scholar] [CrossRef]
- Hill, A.M.; Coates, A.M.; Buckley, J.D.; Ross, R.; Thielecke, F.; Howe, P.R.C. Can EGCG reduce abdominal fat in obese subjects? J. Am. Coll. Nutr. 2007, 26, 396S–402S. [Google Scholar] [CrossRef]
- Richards, J.C.; Lonac, M.C.; Johnson, T.K.; Schweder, M.M.; Bell, C. Epigallocatechin-3-gallate increases maximal oxygen uptake in adult humans. Med. Sci. Sports Exerc. 2010, 42, 739–744. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Kreider, R.B.; Willoughby, D.S. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids 2010, 39, 219–232. [Google Scholar] [CrossRef]
- Lima, L.C.R.; Barreto, R.V.; Bassan, N.M.; Greco, C.C.; Denadai, B.S. Consumption of an anthocyanin-rich antioxidant juice accelerates recovery of running economy and indirect markers of exercise-induced muscle damage following downhill running. Nutrients 2019, 11, 2274. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: A randomized, double-blind study. Menopause 2007, 14, 624–629. [Google Scholar] [CrossRef]
- Lebon, J.; Riesco, E.; Tessier, D.; Dionne, I.J. Additive effects of isoflavones and exercise training on inflammatory cytokines and body composition in overweight and obese postmenopausal women: A randomized controlled trial. Menopause 2014, 21, 869–875. [Google Scholar] [CrossRef]
- Barsalani, R.; Riesco, E.; Lavoie, J.M.; Dionne, I.J. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric 2013, 16, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Nakhostin-Roohi, B.; Nasirvand Moradlou, A.; Mahmoodi Hamidabad, S.; Ghanivand, B. The effect of curcumin supplementation on selected markers of delayed onset muscle soreness (DOMS). Ann. Appl. Sport Sci. 2016, 4, 25–31. [Google Scholar] [CrossRef]
- Tanabe, Y.; Chino, K.; Sagayama, H.; Lee, H.J.; Ozawa, H.; Maeda, S.; Takahashi, H. Effective timing of curcumin ingestion to attenuate eccentric exercise-induced muscle soreness in men. J. Nutr. Sci. Vitaminol. 2019, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Kerksick, C.M. Eight weeks of a high dose of curcumin supplementation may attenuate performance decrements following muscle-damaging exercise. Nutrients 2019, 11, 1692. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Basham, S.A.; Waldman, H.S.; Smith, J.E.W.; Butawan, M.B.; Bloomer, R.J. Effects of curcumin on the oxidative stress response to a dual stress challenge in trained men. J. Diet. Suppl. 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Basham, S.A.; Waldman, H.S.; Krings, B.M.; Lamberth, J.; Smith, J.E.W.; McAllister, M.J. Effect of curcumin supplementation on exercise-induced oxidative stress, inflammation, muscle damage, and muscle soreness. J. Diet. Suppl. 2019, 17, 401–414. [Google Scholar] [CrossRef]
- Chen, Y.J.; Huang, A.C.; Chang, H.H.; Liao, H.F.; Jiang, C.M.; Lai, L.Y.; Chan, J.T.; Chen, Y.Y.; Chiang, J. Caffeic acid phenethyl ester, an antioxidant from propolis, protects peripheral blood mononuclear cells of competitive cyclists against hyperthermal stress. J. Food Sci. 2009, 74, 162–167. [Google Scholar] [CrossRef]
- Shen, Y.C.; Yen, J.C.; Liou, K.T. Ameliorative effects of caffeic acid phenethyl ester on an eccentric exercise-induced skeletal muscle injury by down-regulating NF-κB mediated inflammation. Pharmacology 2013, 91, 219–228. [Google Scholar] [CrossRef]
- Sae-Tan, S.; Grove, K.A.; Kennett, M.J.; Lambert, J.D. (-)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct. 2011, 2, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Gangemi, J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Jones, K.E.; Sidhu, R.S.; Haykowsky, M.; Czubryt, M.P.; Gordon, T.; Dyck, J.R.B. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J. Physiol. 2012, 590, 2783–2799. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Duran, M.O.; Mogulkoc, R.; Oltulu, P.; Avunduk, M.C. Resveratrol does not affect leptin while it has regulatory effects on liver glycogen levels in exercised and non-exercised rats. Int. J. Vitam. Nutr. Res. 2019, 89, 303–308. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadizad, S.; Hedayati, M.; Zarekar, T.; Seydyousefi, M.; Faghfoori, Z. Trans-resveratrol supplement lowers lipid peroxidation responses of exercise in male Wistar rats. Int. J. Vitam. Nutr. Res. 2021, 91, 507–512. [Google Scholar] [CrossRef]
- Ringholm, S.; Olesen, J.; Pedersen, J.T.; Brandt, C.T.; Halling, J.F.; Hellsten, Y.; Prats, C.; Pilegaard, H. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1α. Exp. Gerontol. 2013, 48, 1311–1318. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.; Kim, M.J.; Su, Y.; Qin, L.; Dong, J.; Zhou, Y.; Ding, S. Early potential effects of resveratrol supplementation on skeletal muscle adaptation involved in exercise-induced weight loss in obese mice. BMB Rep. 2018, 51, 200–205. [Google Scholar] [CrossRef]
- Jackson, J.R.; Ryan, M.J.; Hao, Y.; Alway, S.E. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 1572–1581. [Google Scholar] [CrossRef]
- Xiao, N.N. Effects of resveratrol supplementation on oxidative damage and lipid peroxidation induced by strenuous exercise in rats. Biomol. Ther. 2015, 23, 374–378. [Google Scholar] [CrossRef]
- Ryan, M.J.; Jackson, J.R.; Hao, Y.; Williamson, C.L.; Dabkowski, E.R.; Hollander, J.M.; Alway, S.E. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 815–831. [Google Scholar] [CrossRef]
- Tung, B.T.; Rodríguez-Bies, E.; Ballesteros-Simarro, M.; Motilva, V.; Navas, P.; López-Lluch, G. Modulation of endogenous antioxidant activity by resveratrol and exercise in mouse liver is age dependent. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Vafaee, R.; Soori, H.; Hedayati, M.; Ainy, E.; Hatamabadi, H. Effects of resveratrol supplementation in male Wistar rats undergoing an endurance exercise and acute exercise training. Hum. Antibodies 2019, 27, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Vafaee, R.; Hatamabadi, H.; Soori, H.; Hedayati, M. The impact of resveratrol supplementation on inflammation induced by acute exercise in rats: IL6 responses to exercise. Iran. J. Pharm. Res. 2019, 18, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Asadmanesh, E.; Koushkie Jahromi, M.; Samadi, M.; Daryanoosh, F.; Neamati, J. Effect of resistance training and Resveratrol supplementation on muscle regeneration of MyoD and eMHC in CT-26 colon cancer mice. J. Gorgan Univ. Med. Sci. 2020, 22, 40–48. [Google Scholar]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Kan, N.W.; Ho, C.S.; Chiu, Y.S.; Huang, W.C.; Chen, P.Y.; Tung, Y.T.; Huang, C.C. Effects of resveratrol supplementation and exercise training on exercise performance in middle-aged mice. Molecules 2016, 21, 611. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, I.K.; Song, W.; Lee, J.; Park, S. The synergic effect of regular exercise and resveratrol on kainate-induced oxidative stress and seizure activity in mice. Neurochem. Res. 2013, 38, 117–122. [Google Scholar] [CrossRef]
- Tung, B.T.; Rodriguez-Bies, E.; Thanh, H.N.; Le-Thi-Thu, H.; Navas, P.; Sanchez, V.M.; López-Lluch, G. Organ and tissue-dependent effect of resveratrol and exercise on antioxidant defenses of old mice. Aging Clin. Exp. Res. 2015, 27, 775–783. [Google Scholar] [CrossRef]
- Alkhouli, M.F.; Hung, J.; Squire, M.; Anderson, M.; Castro, M.; Babu, J.R.; Al-Nakkash, L.; Broderick, T.L.; Plochocki, J.H. Exercise and resveratrol increase fracture resistance in the 3xTg-AD mouse model of Alzheimer’s disease. BMC Complement. Altern. Med. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Rodríguez-Bies, E.; Tung, B.T.; Navas, P.; López-Lluch, G. Resveratrol primes the effects of physical activity in old mice. Br. J. Nutr. 2016, 116, 979–988. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, Z.; Jia, J.; Chen, J.L.; Xiao, Q. The effects of resveratrol feeding and exercise training on the skeletal muscle function and transcriptome of aged rats. PeerJ 2019, 2019, e7199. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Allam, M.M. Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J. Physiol. Sci. 2018, 68, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The synergistic effects of resveratrol combined with resistant training on exercise performance and physiological adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.; Sarga, L.; Csende, Z.; Koltai, E.; Koch, L.G.; Britton, S.L.; Davies, K.J.A.; Kouretas, D.; Wessner, B.; Radak, Z. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem. Toxicol. 2013, 61, 53–59. [Google Scholar] [CrossRef]
- Qin, L.; Lu, T.; Qin, Y.; He, Y.; Cui, N.; Du, A.; Sun, J. In vivo effect of resveratrol-loaded solid lipid nanoparticles to relieve physical fatigue for sports nutrition supplements. Molecules 2020, 25, 5302. [Google Scholar] [CrossRef]
- Hajighasem, A.; Farzanegi, P.; Mazaheri, Z.; Naghizadeh, M.; Salehi, G. Effects of resveratrol, exercises and their combination on Farnesoid X receptor, Liver X receptor and Sirtuin 1 gene expression and apoptosis in the liver of elderly rats with nonalcoholic fatty liver. PeerJ 2018, 2018, e5522. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Roberts, M.D.; Dalbo, V.J.; Kreider, R.B.; Willoughby, D.S. Changes in skeletal muscle proteolytic gene expression after prophylactic supplementation of EGCG and NAC and eccentric damage. Food Chem. Toxicol. 2013, 61, 47–52. [Google Scholar] [CrossRef]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Paknahad, Z.; Karimian, J.; Rabiee, K.; Sharifirad, G.; Feizi, A. The effects of quercetin supplementation on body composition, exercise performance and muscle damage indices in athletes. Int. J. Prev. Med. 2013, 4, 21–26. [Google Scholar]
- Askari, G.; Ghiasvand, R.; Feizi, A.; Ghanadian, S.M.; Karimian, J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J. Res. Med. Sci. 2012, 17, 637–641. [Google Scholar]
- Askari, G.; Ghiasvand, R.; Karimian, J.; Feizi, A.; Paknahad, Z.; Sharifirad, G.; Hajishafiei, M. Does quercetin and vitamin C improve exercise performance, muscle damage, and body composition in male athletes? J. Res. Med. Sci. 2012, 17, 328–331. [Google Scholar] [PubMed]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; Mcanulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of quercetin and egcg on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, S.S.; Christensen, A.J.; Oliveira, A.S. Resveratrol administration reduces pain perception but does not attenuate force loss following exercise-induced muscle damage. Sport Sci. Health 2022, 13, 3217. [Google Scholar] [CrossRef]
- Gliemann, L.; Olesen, J.; Biensø, R.S.; Schmidt, J.F.; Akerstrom, T.; Nyberg, M.; Lindqvist, A.; Bangsbo, J.; Hellsten, Y. Resveratrol modulates the angiogenic response to exercise training in skeletal muscles of aged men. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1111–H1119. [Google Scholar] [CrossRef]
- Huang, C.C.; Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Ho, C.C.; Kan, N.W. Protective and recovery effects of resveratrol supplementation on exercise performance and muscle damage following acute plyometric exercise. Nutrients 2021, 13, 3217. [Google Scholar] [CrossRef]
- Jo, E.; Bartosh, R.; Auslander, A.T.; Directo, D.; Osmond, A.; Wong, M.W. Post-exercise recovery following 30-day supplementation of trans-resveratrol and polyphenol-enriched extracts. Sports 2019, 7, 226. [Google Scholar] [CrossRef]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.B.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef]
- Falgiano, P.A.; Gillum, T.L.; Schall, Z.J.; Strag, H.R.; Kuennen, M.R. Dietary curcumin supplementation does not alter peripheral blood mononuclear cell responses to exertional heat stress. Eur. J. Appl. Physiol. 2018, 118, 2707–2717. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35, 469–475. [Google Scholar] [CrossRef]
- Szymanski, M.C.; Gillum, T.L.; Gould, L.M.; Morin, D.S.; Kuennen, M.R. Short-term dietary curcumin supplementation reduces gastrointestinal barrier damage and physiological strain responses during exertional heat stress. J. Appl. Physiol. 2018, 124, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Maeda, S.; Akazawa, N.; Zempo-Miyaki, A.; Choi, Y.; Ra, S.G.; Imaizumi, A.; Otsuka, Y.; Nosaka, K. Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin. Eur. J. Appl. Physiol. 2015, 115, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Caldwell, A.R.; Sanders, E.; Mitchell, J.B.; Rogers, J.; Purpura, M.; Oliver, J.M. Curcumin reduces muscle damage and soreness following muscle-damaging exercise. FASEB J. 2018, 31, lb766. [Google Scholar]
- Sciberras, J.N.; Galloway, S.D.R.; Fenech, A.; Grech, G.; Farrugia, C.; Duca, D.; Mifsud, J. The effect of turmeric (Curcumin) supplementation on cytokine and inflammatory marker responses following 2 h of endurance cycling. J. Int. Soc. Sports Nutr. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Delecroix, B.; Abaïdia, A.E.; Leduc, C.; Dawson, B.; Dupont, G. Curcumin and piperine supplementation and recovery following exercise induced muscle damage: A randomized controlled trial. J. Sport. Sci. Med. 2017, 16, 147–153. [Google Scholar]
- Chilelli, N.C.; Ragazzi, E.; Valentini, R.; Cosma, C.; Ferraresso, S.; Lapolla, A.; Sartore, G. Curcumin and Boswellia serrata modulate the glyco-oxidative status and lipo-oxidation in master athletes. Nutrients 2016, 8, 745. [Google Scholar] [CrossRef]
- Wu, J.; Oka, J.; Tabata, I.; Higuchi, M.; Toda, T.; Fuku, N.; Ezaki, J.; Sugiyama, F.; Uchiyama, S.; Yamada, K.; et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: A 1-year randomized placebo-controlled trial. J. Bone Miner. Res. 2006, 21, 780–789. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Nagasawa, A.; Tokimitsu, I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 708–715. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Tokimitsu, I.; Hase, T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, K.; Ochi, E.; Waga, T. Dietary apple polyphenols have preventive effects against lengthening contraction-induced muscle injuries. Mol. Nutr. Food Res. 2010, 54, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ros, S.; Zoll, J.; Lang, A.L.; Auger, C.; Keller, N.; Bronner, C.; Geny, B.; Schini-Kerth, V.B. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: Role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011, 404, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Effects of honey supplementation combined with different jumping exercise intensities on bone mass, serum bone metabolism markers and gonadotropins in female rats. BMC Complement. Altern. Med. 2014, 14, 126. [Google Scholar] [CrossRef]
- Amozadeh, H.; Shabani, R.; Nazari, M. The effect of aerobic training and green tea supplementation on cardio metabolic risk factors in overweight and obese females: A randomized trial. Int. J. Endocrinol. Metab. 2018, 16, e60738. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 13. [Google Scholar] [CrossRef]
- Jówko, E.; Długołęcka, B.; Makaruk, B.; Cieśliński, I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur. J. Nutr. 2015, 54, 783–791. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, J.C.; Bernard, J.R.; Liao, Y.H. Green tea extract supplementation does not hamper endurance-training adaptation but improves antioxidant capacity in sedentary men. Appl. Physiol. Nutr. Metab. 2015, 40, 990–996. [Google Scholar] [CrossRef]
- Machado, Á.S.; Silva, W.; Souza, M.A.; Carpes, F.P. Green tea extract preserves neuromuscular activation and muscle damage markers in athletes under cumulative fatigue. Front. Physiol. 2018, 9, 1137. [Google Scholar] [CrossRef]
- da Silva, W.; Machado, Á.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Arent, S.M.; Senso, M.; Golem, D.L.; McKeever, K.H. The effects of theaflavin-enriched black tea extract on muscle soreness, oxidative stress, inflammation, and endocrine responses to acute anaerobic interval training: A randomized, double-blind, crossover study. J. Int. Soc. Sports Nutr. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The effects of Montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Romain, C.; Marín-Pagán, C.; Chung, L.H.; Rubio-Pérez, J.M.; Laurent, C.; Gaillet, S.; Prost-Camus, E.; Prost, M.; Alcaraz, P.E. Supplementation with a polyphenol-rich extract, PerfLoad®, improves physical performance during high-intensity exercise: A randomized, double blind, crossover trial. Nutrients 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J. Strength Cond. Res. 2011, 25, 1782–1788. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B.; Mooren, F.C.; Krüger, K.; FitzGerald, L.Z.; Chehrazi, M. A randomized controlled trial examining the effects of 16 weeks of moderate-to-intensive cycling and honey supplementation on lymphocyte oxidative DNA damage and cytokine changes in male road cyclists. Cytokine 2016, 88, 222–231. [Google Scholar] [CrossRef]
- Godwin, C.; Cook, M.D.; Willems, M.E.T. Effect of New Zealand blackcurrant extract on performance during the running based anaerobic sprint test in trained youth and recreationally active male football players. Sports 2017, 5, 69. [Google Scholar] [CrossRef]
- Strauss, J.A.; Willems, M.E.T.; Shepherd, S.O. New Zealand blackcurrant extract enhances fat oxidation during prolonged cycling in endurance-trained females. Eur. J. Appl. Physiol. 2018, 118, 1265–1272. [Google Scholar] [CrossRef]
- Murphy, C.A.; Cook, M.D.; Willems, M.E.T. Effect of New Zealand blackcurrant extract on repeated cycling time trial performance. Sports 2017, 5, 25. [Google Scholar] [CrossRef]
- Perkins, I.C.; Vine, S.V.; Blacker, S.D.; Willems, M.E. New Zealand extract improves high-intensity intermittent running. Int. J. Sport Nutr. Metab. 2013, 53, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.T.; Cousins, L.; Williams, D.; Blacker, S.D. Beneficial effects of New Zealand blackcurrant extract on maximal sprint speed during the loughborough intermittent shuttle test. Sports 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Flieller, E.B.; Dillon, K.J.; Leverett, B.D. Black currant nectar reduces muscle damage and inflammation following a bout of high-intensity eccentric contractions. J. Diet. Suppl. 2016, 13, 1–15. [Google Scholar] [CrossRef]
- Hurst, R.D.; Wells, R.W.; Hurst, S.M.; McGhie, T.K.; Cooney, J.M.; Jensen, D.J. Blueberry fruit polyphenolics suppress oxidative stressinduced skeletal muscle cell damage in vitro. Mol. Nutr. Food Res. 2010, 54, 353–363. [Google Scholar] [CrossRef]
- Lynn, A.; Garner, S.; Nelson, N.; Simper, T.N.; Hall, A.C.; Ranchordas, M.K. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Ahmed, M.; Henson, D.A.; Sanderson, M.C.; Nieman, D.C.; Gillitt, N.D.; Lila, M.A. The protective effects of a polyphenol-enriched protein powder on exercise-induced susceptibility to virus infection. Phyther. Res. 2014, 28, 1829–1836. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef]
- Ives, S.J.; Bloom, S.; Matias, A.; Morrow, N.; Martins, N.; Roh, Y.; Ebenstein, D.; O’Brien, G.; Escudero, D.; Brito, K.; et al. Effects of a combined protein and antioxidant supplement on recovery of muscle function and soreness following eccentric exercise. J. Int. Soc. Sports Nutr. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2012, 9, 19. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Meaney, M.P.; John, C.; Pappan, K.L.; Kinchen, J.M. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J. Proteome Res. 2015, 14, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Esposito, D.; Ramamoorthy, S. Metabolic recovery from heavy exertion following banana compared to sugar beverage or water only ingestion: A randomized, crossover trial. PLoS ONE 2018, 13, e0194843. [Google Scholar] [CrossRef] [PubMed]
- Deley, G.; Guillemet, D.; Allaert, F.-A.; Babault, N. An acute dose of specific grape and apple polyphenols improves endurance performance: A randomized, crossover, double-blind versus placebo controlled study. Nutrients 2017, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Kłapcińska, B.; Kimsa, E.; Karpiński, R. Effects of supplemetation with red grape skin polyphenolic extract and interval swimming test on the blood antioxidant status in healthy men. Med. Sport. 2008, 12, 1–7. [Google Scholar] [CrossRef]
- Swamy, M.S.L.; Naveen, S.; Singsit, D.; Naika, M.; Khanum, F. Anti-fatigue effects of polyphenols extracted from pomegranate peel. Int. J. Integr. Biol. 2011, 11, 69–72. [Google Scholar]
- Crum, E.M.; Barnes, M.J.; Stannard, S.R. Multiday pomegranate extract supplementation decreases oxygen uptake during submaximal cycling exercise, but cosupplementation with n-acetylcysteine negates the effect. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 586–592. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Melvin, M.N.; Roelofs, E.J.; Wingfield, H.L. The effects of pomegranate extract on blood flow and running time to exhaustion. Appl. Physiol. Nutr. Metab. 2014, 39, 1038–1042. [Google Scholar] [CrossRef]
- Al-Dujaili, E.A.S.; Good, G.; Tsang, C. Consumption of pomegranate juice attenuates exercise-induced oxidative stress, blood pressure and urinary cortisol/cortisone ratio in human adults. EC Nutr. 2016, 4, 982–995. [Google Scholar]
- Machin, D.R.; Christmas, K.M.; Chou, T.-H.; Hill, S.C.; Van Pelt, D.W.; Trombold, J.R.; Coyle, E.F. Effects of differing dosages of pomegranate juice supplementation after eccentric exercise. Physiol. J. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of pomegranate juice supplementation on oxidative stress biomarkers following weightlifting exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef]
- Romain, C.; Freitas, T.T.; Martínez-noguera, F.J.; Laurent, C.; Gaillet, S.; Chung, L.H.; Alcaraz, P.E.; Cases, J. Supplementation with a polyphenol-rich extract, TensLess®, attenuates delayed onset muscle soreness and improves nuscle recovery from damages after eccentric exercise. Phyther. Res. 2017, 31, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Morehen, J.C.; Clarke, J.; Batsford, J.; Barrow, S.; Brown, A.D.; Stewart, C.E.; Morton, J.P.; Close, G.L. Montmorency tart cherry juice does not reduce markers of muscle soreness, function and inflammation following professional male Rugby league match-play. Eur. J. Sport Sci. 2020, 21, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.; Brashill, C.; Brett, A.; Clifford, T. Tart cherry juice: No effect on muscle function loss or muscle soreness in professional soccer players after a match. Int. J. Sports Physiol. Perform. 2020, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kupusarevic, J.; McShane, K.; Clifford, T. Cherry gel supplementation does not attenuate subjective muscle soreness or alter wellbeing following a match in a team of professional rugby union players: A pilot study. Sports 2019, 7, 6–11. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef]
- Keuhl, K.; Perrier, E.; Elliot, D.L.; Chesnutt, J.C. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 17. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; Goodenough, C.; O’Connor, A.; Simbo, S.; Barringer, N.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr. 2015, 12, 41. [Google Scholar] [CrossRef]
- Quinlan, R.; Hill, J.A. The efficacy of tart cherry juice in aiding recovery after intermittent exercise. Int. J. Sports Physiol. Perform. 2019, 15, 368–374. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2016, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci. 2019, 19, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sport. 2009, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Ferreira, P.R.; Marins, J.C.B.; de Oliveira, L.L.; Bastos, D.S.S.; Soares Júnior, D.T.; da Silva, C.D.; Fontes, E.A.F. Beverage based on whey permeate with phenolic extract of jabuticaba peel: A pilot study on effects on muscle and oxidative stress in trained individuals. J. Funct. Foods 2020, 65, 103749. [Google Scholar] [CrossRef]
- Buchwald-Werner, S.; Naka, I.; Wilhelm, M.; Schütz, E.; Schoen, C.; Reule, C. Effects of lemon verbena extract (Recoverben®) supplementation on muscle strength and recovery after exhaustive exercise: A randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Kang, S.W.; Hahn, S.; Kim, J.K.; Yang, S.M.; Park, B.J.; Lee, S.C. Oligomerized lychee fruit extract (OLFE) and a mixture of vitamin C and vitamin E for endurance capacity in a double blind randomized controlled trial. J. Clin. Biochem. Nutr. 2012, 50, 106–113. [Google Scholar] [CrossRef][Green Version]
- Jurcău, R.; Jurcău, I. Effect of Manuka honey administration on malondialdehyde, in intense exercise. Palestrica Third Millenn. Civiliz. Sport 2017, 18, 201–205. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H. The effects of honey supplementation on seminal plasma cytokines, oxidative stress biomarkers, and antioxidants during 8 weeks of intensive cycling training. J. Androl. 2012, 33, 449–461. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Aziz, A.A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose-response effect of Tualang honey on postprandial antioxidant activity and oxidative stress in female athletes: A pilot study. J. Altern. Complement. Med. 2017, 23, 989–995. [Google Scholar] [CrossRef]
- Soleimani, D.; Miryan, M.; Hadi, V.; Navashenaq, J.G.; Moludi, J.; Sayedi, S.M.; Bagherniya, M.; Askari, G.; Nachvak, S.M.; Sadeghi, E.; et al. Effect of propolis supplementation on athletic performance, body composition, inflammation, and oxidative stress following intense exercise: A triple-blind randomized clinical trial. Food Sci. Nutr. 2021, 9, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Brouner, J.; Allgrove, J.E.; Spendiff, O. The influence of different concentrations of flavanol chocolate bars under acute supplement conditions on exercise and performance. Eur. J. Appl. Physiol. 2020, 120, 2075–2082. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Patel, M.; Higginbotham, E.; Ulloa, A.M.-; Román-Pintos, L.M.; Phillips, P.; Perkins, G.; Ceballos, G.; Villarreal, F. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: Underlying mechanisms: A double blind, randomized, placebo controlled trial. Food Funct. 2016, 7, 3686–3693. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Brouner, J.; Spendiff, O. Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. J. Int. Soc. Sports Nutr. 2015, 12, 7–14. [Google Scholar] [CrossRef]
- González-Garrido, J.A.; García-Sánchez, J.R.; Garrido-Llanos, S.; Olivares-Corichi, I.M. An association of cocoa consumption with improved physical fitness and decreased muscle damage and oxidative stress in athletes. J. Sports Med. Phys. Fitness 2017, 57, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Cavarretta, E.; Peruzzi, M.; Vescovo, R.D.; Pilla, F.D.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark chocolate intake positively modulates redox status and markers of muscular damage in elite football athletes: A randomized controlled study. Oxid. Med. Cell. Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, A.U.; Wong, T.S.; Bandegan, A.; Lemon, P.W.R. Cycling time trial performance 4 h after glycogen-lowering exercise is similarly enhanced by recovery nondairy chocolate beverages versus chocolate milk. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 65–70. [Google Scholar] [CrossRef]
- Spaccarotella, K.J.; Andzel, W.D. The effects of low fat chocolate milk on postexercise recovery in collegiate athletes. J. Strength Cond. Res. 2011, 25, 3456–3460. [Google Scholar] [CrossRef]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin. Dev. Immunol. 2005, 12, 11–17. [Google Scholar] [CrossRef]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef]
- Davison, G.; Callister, R.; Williamson, G.; Cooper, K.A.; Gleeson, M. The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur. J. Nutr. 2012, 51, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.; Fuller, B.J. The effectiveness of chocolate milk as a post-climbing recovery aid. J. Sports Med. Phys. Fitness 2015, 55, 1438–1444. [Google Scholar] [PubMed]
- Thomas, K.; Morris, P.; Stevenson, E. Improved endurance capacity following chocolate milk consumption compared with 2 commercially available sport drinks. Appl. Physiol. Nutr. Metab. 2009, 34, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ferguson-Stegall, L.; McCleave, E.L.; Ding, Z.; Doerner, P.G.; Wang, B.; Liao, Y.-H.; Kammer, L.; Liu, Y.; Hwang, J.; Dessard, B.M.; et al. Postexercise carbohydrate-protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. J. Strength Cond. Res. 2011, 25, 1210–1214. [Google Scholar] [CrossRef]
- Papacosta-Kokkinou, E.; Nassis, G.P.; Gleeson, M. Effects of acute post-exercise chocolate milk consumption during intensive judo training on the recovery of salivary hormones, salivary IgA, mood state, muscle soreness and judo-related performance. Appl. Physiol. Nutr. Metab. 2015, 40, 1116–1122. [Google Scholar] [CrossRef]
- Morgan, P.T.; Wollman, P.M.; Jackman, S.R.; Bowtell, J.L. Flavanol-rich cacao mucilage juice enhances recovery of power but not strength from intensive exercise in healthy, young men. Sports 2018, 6, 159. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute cocoa flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 28. [Google Scholar] [CrossRef]
- Sadler, D.G.; Draijer, R.; Stewart, C.E.; Jones, H.; Marwood, S.; Thijssen, D.H.J. Cocoa-flavanols enhance moderate-intensity pulmonary VO2 kinetics but not exercise tolerance in sedentary middle-aged adults. Eur. J. Appl. Physiol. 2021, 121, 2285–2294. [Google Scholar] [CrossRef]
- Wiswedel, I.; Hirsch, D.; Kropf, S.; Gruening, M.; Pfister, E.; Schewe, T.; Sies, H. Flavanol-rich cocoa drink lowers plasma F2-isoprostane concentrations in humans. Free Radic. Biol. Med. 2004, 37, 411–421. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Lespagnol, E.; Balestra, C.; Descat, A.; Drittij-Reijnders, M.J.; Blackwell, J.R.; Stahl, W.; Jones, A.M.; Weseler, A.R.; et al. One-week cocoa flavanol intake increases prefrontal cortex oxygenation at rest and during moderate-intensity exercise in normoxia and hypoxia. J. Appl. Physiol. 2018, 125, 8–18. [Google Scholar] [CrossRef]
- Areta, J.L.; Austarheim, I.; Wangensteen, H.; Capelli, C. Metabolic and performance effects of Yerba Mate on well-trained cyclists. Med. Sci. Sports Exerc. 2018, 50, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Gaamouri, N.; Zouhal, H.; Hammami, M.; Hackney, A.C.; Abderrahman, A.B.; Saeidi, A.; El Hage, R.; Ounis, O. Ben Effects of polyphenol (carob) supplementation on body composition and aerobic capacity in taekwondo athletes. Physiol. Behav. 2019, 205, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Kłapcińska, B.; Pokora, I.; Domaszewski, P.; Kempa, K.; Podgórski, T. Effects of six-week Ginkgo biloba supplementation on aerobic performance, blood Pro/antioxidant balance, and serum brain-derived neurotrophic factor in physically active men. Nutrients 2017, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Shin, Y.O.; Yoon, J.H.; Kim, S.H.; Shin, H.C.; Hwang, H.J. Effect of supplementation with Ecklonia cava polyphenol on endurance performance of college students. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 72–79. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. Beetroot juice is more beneficial than sodium nitrate for attenuating muscle pain after strenuous eccentric-bias exercise. Phys. Today 2007, 44, 1–25. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic beetroot juice supplementation accelerates recovery kinetics following simulated match play in soccer players. J. Am. Coll. Nutr. 2021, 40, 61–69. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef]
- Karp, J.R.; Johnston, J.D.; Tecklenburg, S.; Mickleborough, T.D.; Fly, A.D.; Stager, J.M. Chocolate milk as a post-exercise recovery aid. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 78–91. [Google Scholar] [CrossRef]
- Jówko, E.; Sacharuk, J.; Balasińska, B.; Ostaszewski, P.; Charmas, M.; Charmas, R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr. Res. 2011, 31, 813–821. [Google Scholar] [CrossRef]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef]
- Eichenberger, P.; Colombani, P.C.; Mettler, S. Effects of 3-week consumption of green tea extracts on whole-body metabolism during cycling exercise in endurance-trained men. Int. J. Vitam. Nutr. Res. 2009, 79, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Panza, V.S.P.; Wazlawik, E.; Ricardo Schütz, G.; Comin, L.; Hecht, K.C.; da Silva, E.L. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition 2008, 24, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.S.; Stout, J.R.; Fukuda, D.H.; Jajtner, A.R.; Townsend, J.R.; Church, D.D.; Wang, R.; Riffe, J.J.; Muddle, T.W.D.; Herrlinger, K.A.; et al. Impact of polyphenol supplementation on acute and chronic response to resistance training. J. Strength Cond. Res. 2017, 31, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Takahashi, M.; Li, C.Y.; Lin, S.P.; Tomari, M.; Shing, C.M.; Fang, S.H. The acute effects of green tea and carbohydrate coingestion on systemic inflammation and oxidative stress during sprint cycling. Appl. Physiol. Nutr. Metab. 2015, 40, 997–1003. [Google Scholar] [CrossRef]
- Ichinose, T.; Nomura, S.; Someya, Y.; Akimoto, S.; Tachiyashiki, K.; Imaizumi, K. Effect of endurance training supplemented with green tea extract on substrate metabolism during exercise in humans. Scand. J. Med. Sci. Sport. 2011, 21, 598–605. [Google Scholar] [CrossRef]
- Urbaniak, A.; Basta, P.; Ast, K.; Wołoszyn, A.; Kuriańska-Wołoszyn, J.; Latour, E.; Skarpańska-Stejnborn, A. The impact of supplementation with pomegranate fruit (Punica granatum L.) juice on selected antioxidant parameters and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2018, 15, 35. [Google Scholar] [CrossRef]
- Fuster-Muñoz, E.; Roche, E.; Funes, L.; Martínez-Peinado, P.; Sempere, J.M.; Vicente-Salar, N. Effects of pomegranate juice in circulating parameters, cytokines, and oxidative stress markers in endurance-based athletes: A randomized controlled trial. Nutrition 2016, 32, 539–545. [Google Scholar] [CrossRef]
- Clifford, T.; Berntzen, B.; Davison, G.W.; West, D.J.; Howatson, G.; Stevenson, E.J. Effects of beetroot juice on recovery of muscle function and performance between bouts of repeated sprint exercise. Nutrients 2016, 8, 506. [Google Scholar] [CrossRef]
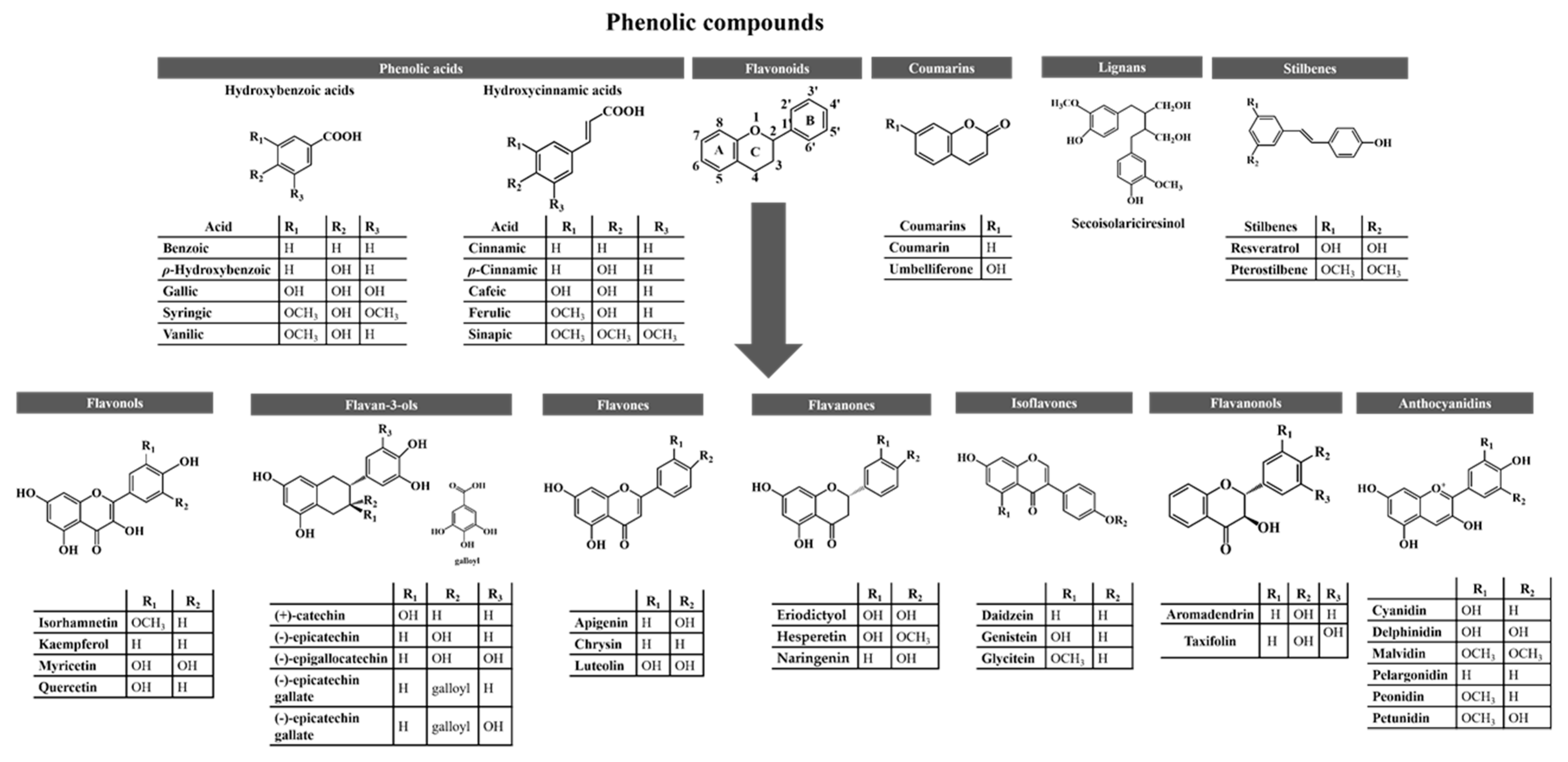
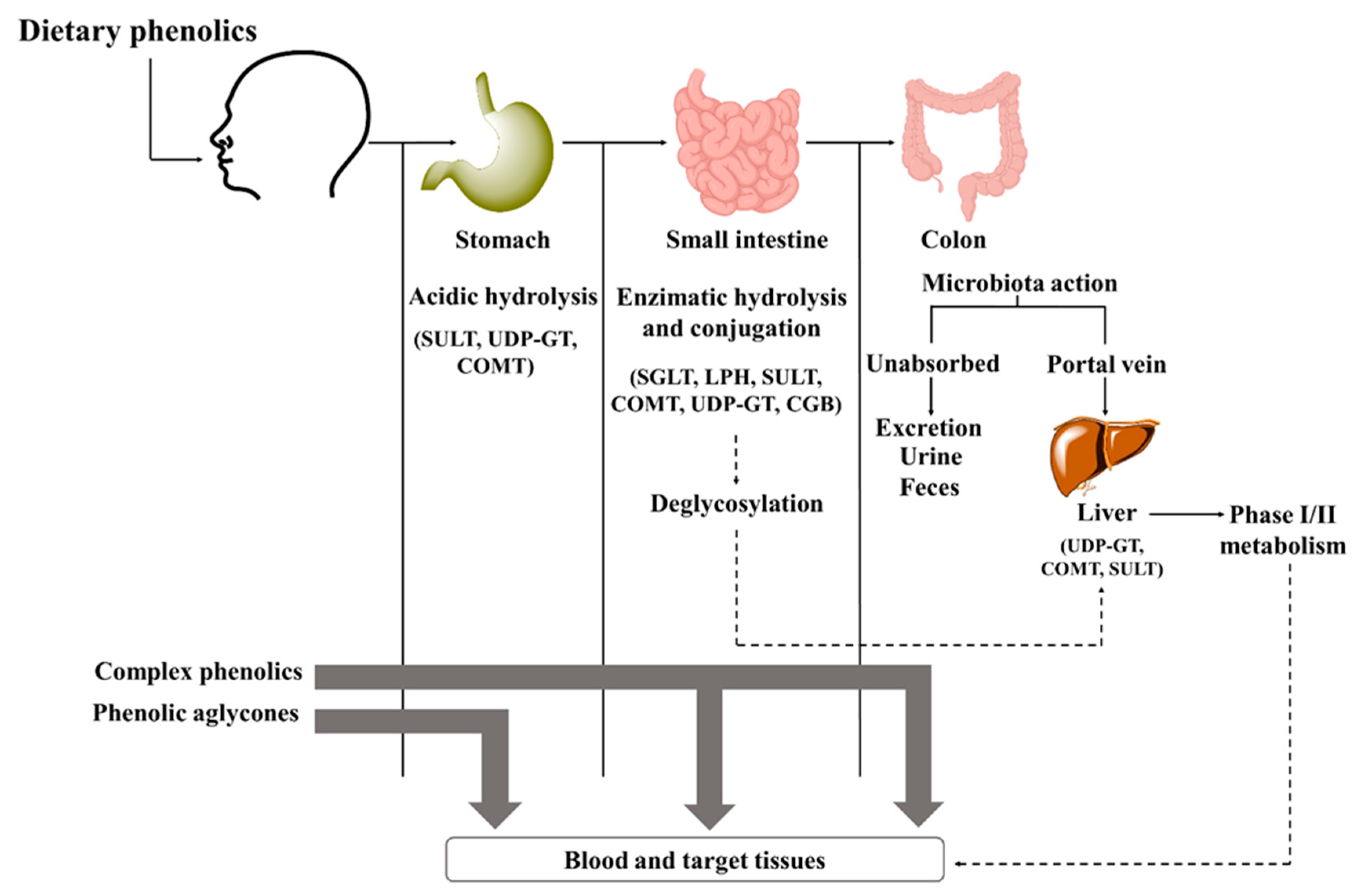
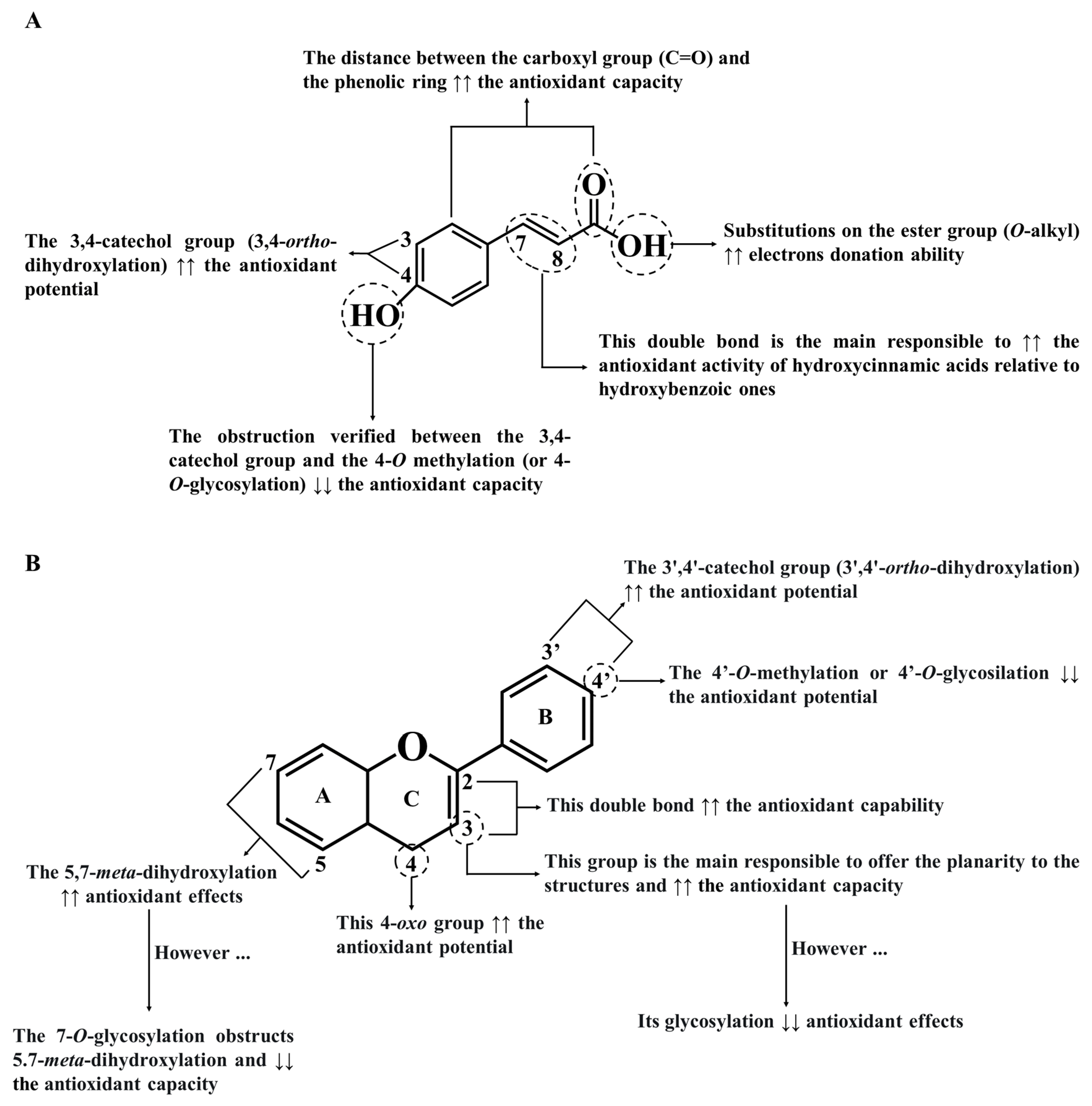
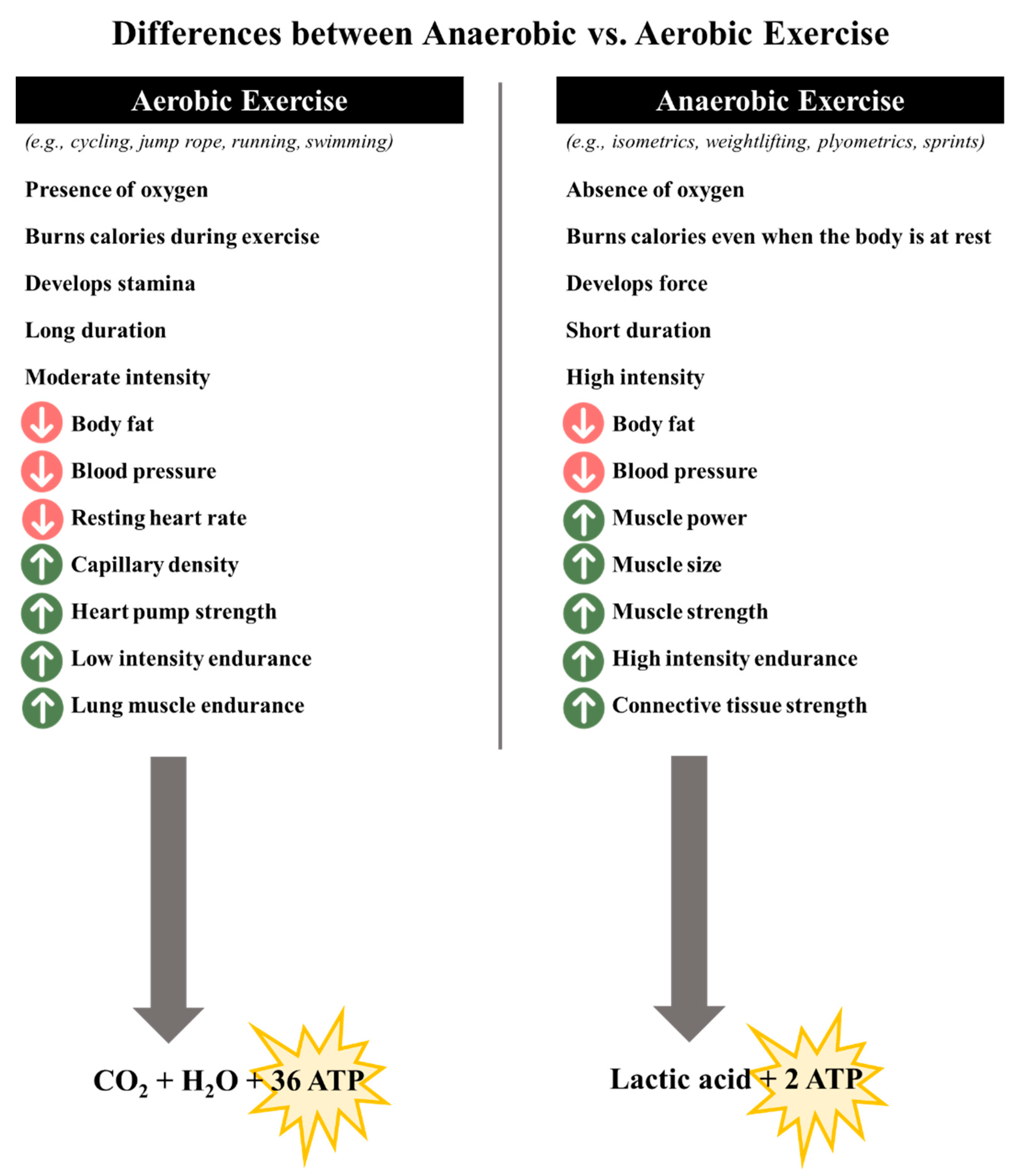
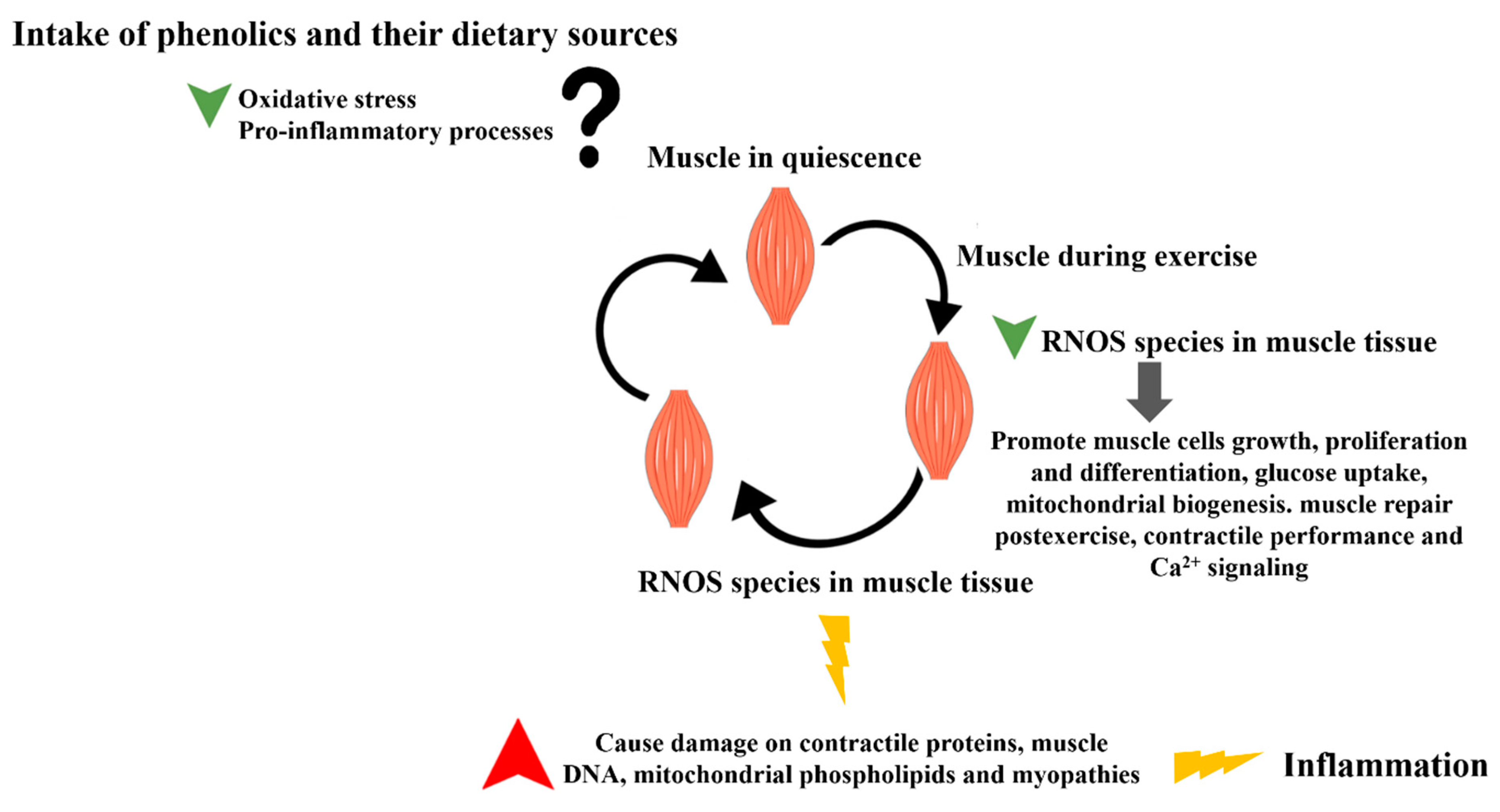
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.C.; Gaspar, D.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review. Int. J. Mol. Sci. 2022, 23, 4652. https://doi.org/10.3390/ijms23094652
Gonçalves AC, Gaspar D, Flores-Félix JD, Falcão A, Alves G, Silva LR. Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review. International Journal of Molecular Sciences. 2022; 23(9):4652. https://doi.org/10.3390/ijms23094652
Chicago/Turabian StyleGonçalves, Ana C., Dário Gaspar, José David Flores-Félix, Amílcar Falcão, Gilberto Alves, and Luís R. Silva. 2022. "Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review" International Journal of Molecular Sciences 23, no. 9: 4652. https://doi.org/10.3390/ijms23094652
APA StyleGonçalves, A. C., Gaspar, D., Flores-Félix, J. D., Falcão, A., Alves, G., & Silva, L. R. (2022). Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review. International Journal of Molecular Sciences, 23(9), 4652. https://doi.org/10.3390/ijms23094652










