Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance
Abstract
1. Introduction
1.1. Diabetes and Skeletal Muscle Insulin Resistance
1.2. Current Therapies for Prediabetes and Disease Management
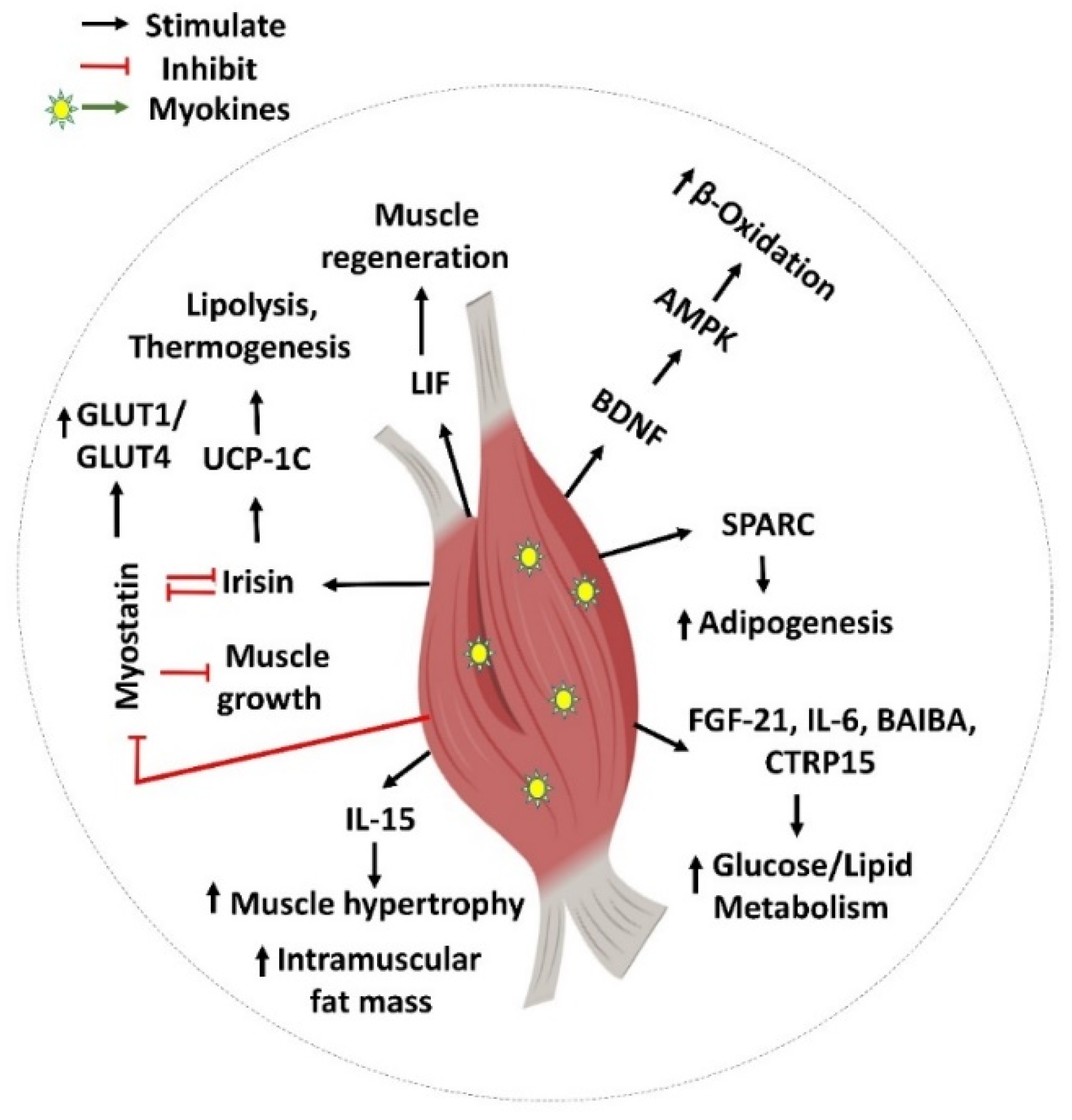
1.3. Skeletal Muscle Myokine-Mediated Regulatory Actions
2. Myokine-Mediated Muscle-to-Muscle and Muscle-to-Pancreas Communication
Myokines Mediate Muscle-to-Muscle Cross Talk
3. Muscle-to-Pancreas Cross-Talk
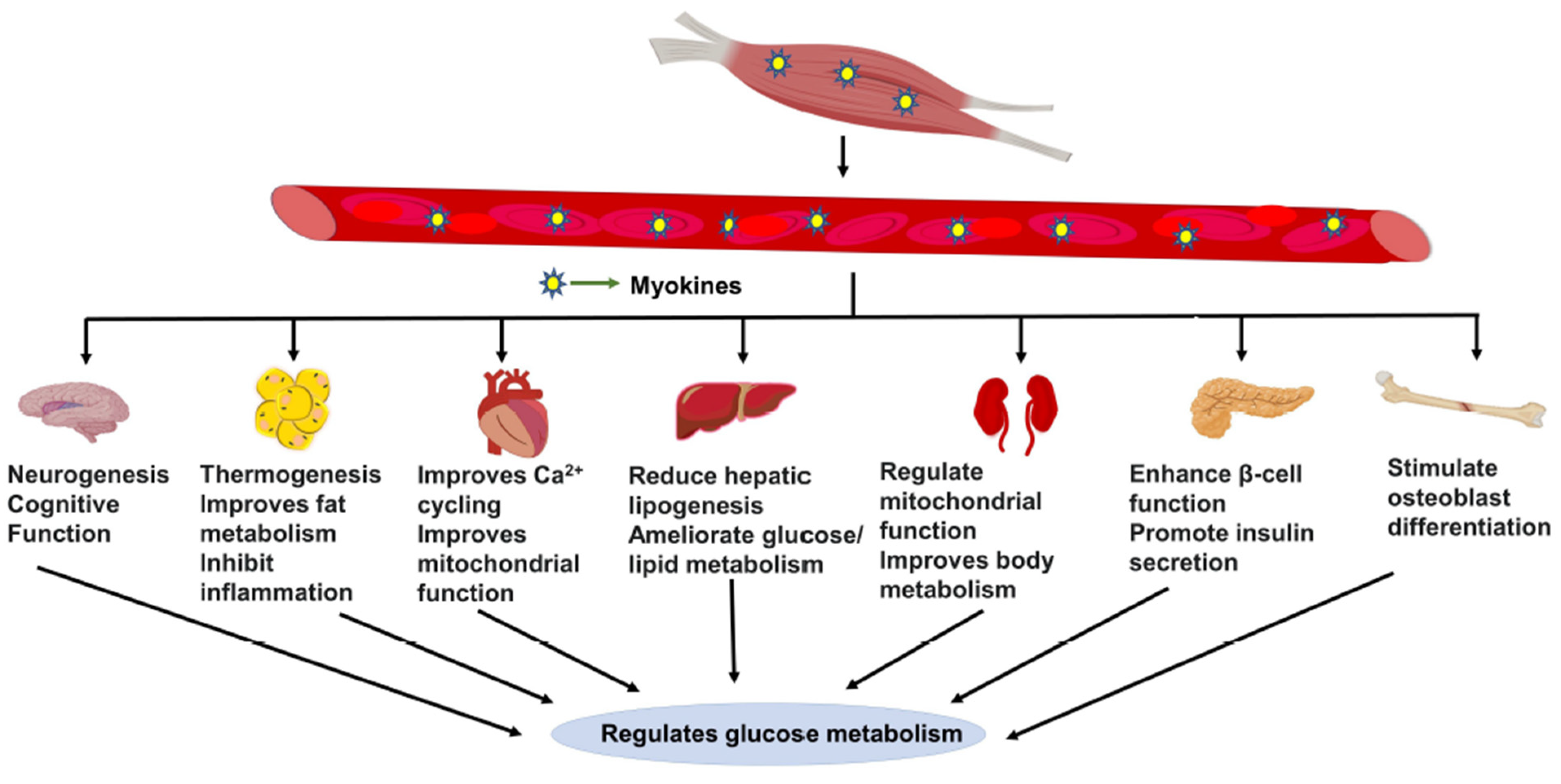
4. Myokine Cross-Talk with Other Major Metabolic Organs
4.1. Muscle-to-Adipose Tissue Cross-Talk
4.2. Muscle-to-Brain Cross-Talk
4.3. Muscle-to-Liver Cross-Talk
4.4. Muscle-to-Heart/Kidney/Bone Tissue Cross-Talk
5. Perspectives: Myokines as Therapeutic Targets for T2D
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, C.S.; Golden, S.H.; Anderson, C.; Bray, G.A.; Burke, L.E.; de Boer, I.H.; Deedwania, P.; Eckel, R.H.; Ershow, A.G.; Fradkin, J.; et al. Update on Prevention of Cardiovascular Disease in Adults with Type 2 Diabetes Mellitus in Light of Recent Evidence. Circulation 2015, 132, 691–718. [Google Scholar] [CrossRef] [PubMed]
- Ariza, L.; Pages, G.; García-Lareu, B.; Cobianchi, S.; Otaegui, P.; Ruberte, J.; Chillon, M.; Navarro, X.; Bosch, A. Experimental diabetes in neonatal mice induces early peripheral sensorimotor neuropathy. Neuroscience 2014, 274, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xie, T.; Li, D.; Du, X.; Wang, T.; Li, C.; Song, X.; Xu, L.; Yi, F.; Liang, X.; et al. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway. Mol. Metab. 2019, 23, 24–36. [Google Scholar] [CrossRef]
- Saadane, A.; Lessieur, E.M.; Du, Y.; Liu, H.; Kern, T.S. Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS ONE 2020, 15, e0238727. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G.; Bevilacqua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. Available online: https://dev.diabetes.org/sites/default/files/2019-06/cdc-statistics-report-2017.pdf (accessed on 12 March 2021).
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pr. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 12 March 2021).
- Bagnasco, A.; Di Giacomo, P.; Mora, R.D.R.D.; Catania, G.; Turci, C.; Rocco, G.; Sasso, L. Factors influencing self-management in patients with type 2 diabetes: A quantitative systematic review protocol. J. Adv. Nurs. 2014, 70, 187–200. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Pot, G.K.; Battjes-Fries, M.C.; Patijn, O.N.; van der Zijl, N.; Pijl, H.; Voshol, P. Lifestyle medicine for type 2 diabetes: Practice-based evidence for long-term efficacy of a multicomponent lifestyle intervention (Reverse Diabetes2 Now). BMJ Nutr. Prev. Health 2020, 3, 188–195. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Phung, O.J.; Scholle, J.M.; Talwar, M.; Coleman, C. Effect of Noninsulin Antidiabetic Drugs Added to Metformin Therapy on Glycemic Control, Weight Gain, and Hypoglycemia in Type 2 Diabetes. JAMA 2010, 303, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Carson, B.P. The Potential Role of Contraction-Induced Myokines in the Regulation of Metabolic Function for the Prevention and Treatment of Type 2 Diabetes. Front. Endocrinol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 Increases Insulin-Stimulated Glucose Disposal in Humans and Glucose Uptake and Fatty Acid Oxidation In Vitro via AMP-Activated Protein Kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Toth, K.G.; McKay, B.R.; De Lisio, M.; Little, J.P.; Tarnopolsky, M.A.; Parise, G. IL-6 Induced STAT3 Signalling Is Associated with the Proliferation of Human Muscle Satellite Cells Following Acute Muscle Damage. PLoS ONE 2011, 6, e17392. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a Secretory Organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Åkerström, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Itakura, Y.; Inoue, T.; Tsuchida, A.; Nakagawa, T.; Noguchi, H.; Taiji, M. Protective effect of brain-derived neurotrophic factor on pancreatic islets in obese diabetic mice. Metabolism 2006, 55, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Raschke, S.; Eckardt, K.; Holven, K.B.; Jensen, J.; Eckel, J. Identification and Validation of Novel Contraction-Regulated Myokines Released from Primary Human Skeletal Muscle Cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef]
- Hartwig, S.; Raschke, S.; Knebel, B.; Scheler, M.; Irmler, M.; Passlack, W.; Muller, S.; Hanisch, F.-G.; Franz, T.; Li, X.; et al. Secretome profiling of primary human skeletal muscle cells. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 1011–1017. [Google Scholar] [CrossRef]
- Norheim, F.; Raastad, T.; Thiede, B.; Rustan, A.C.; Drevon, C.A.; Haugen, F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am. J. Physiol. Metab. 2011, 301, E1013–E1021. [Google Scholar] [CrossRef]
- Chan, X.C.Y.; McDermott, J.C.; Siu, K.W.M. Identification of Secreted Proteins during Skeletal Muscle Development. J. Proteome Res. 2007, 6, 698–710. [Google Scholar] [CrossRef]
- Chan, C.Y.X.; Masui, O.; Krakovska, O.; Belozerov, V.E.; Voisin, S.; Ghanny, S.; Chen, J.; Moyez, D.; Zhu, P.; Evans, K.R.; et al. Identification of Differentially Regulated Secretome Components During Skeletal Myogenesis. Mol. Cell. Proteom. 2011, 10, M110.004804. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Who would have thought—Myokines two decades on. Nat. Rev. Endocrinol. 2020, 16, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.; MacNeil, L.G.; Lally, J.S.; Ford, R.J.; Bujak, A.L.; Brar, I.K.; Kemp, B.; Raha, S.; Steinberg, G.; Tarnopolsky, M.A. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015, 14, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradère, J.-P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Vuillermoz, B.; Wegrowski, Y.; Contet-Audonneau, J.-L.; Danoux, L.; Pauly, G.; Maquart, F.-X. Influence of aging on glycosaminoglycans and small leucine-rich proteoglycans production by skin fibroblasts. Mol. Cell. Biochem. 2005, 277, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Topolcan, O.; Pecen, L.; Kinkorova, J.; Svobodova, S.; Windrichová, J.; Fuchsova, R. Reference values of IGF1, IGFBP3 and IGF1/IGFBP3 ratio in adult population in the Czech Republic. Clin. Chim. Acta 2015, 444, 271–277. [Google Scholar] [CrossRef]
- Li, L.; Yang, G.; Li, Q.; Tang, Y.; Yang, M.; Yang, H.; Li, K. Changes and Relations of Circulating Visfatin, Apelin, and Resistin Levels in Normal, Impaired Glucose Tolerance, and Type 2 Diabetic Subjects. Exp. Clin. Endocrinol. Diabetes 2006, 114, 544–548. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Wolden-Hanson, T. Serum and muscle interleukin-15 levels decrease in aging mice: Correlation with declines in soluble interleukin-15 receptor alpha expression. Exp. Gerontol. 2010, 45, 106–112. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Silvennoinen, M.; Lehti, M.; Kivelä, R.; Kainulainen, H. Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am. J. Physiol. Metab. 2012, 302, E307–E315. [Google Scholar] [CrossRef]
- Efthymiadou, A.; Vasilakis, I.-A.; Giannakopoulos, A.; Chrysis, D. Myostatin serum levels in children with type 1 diabetes mellitus. Hormones 2021, 20, 777–782. [Google Scholar] [CrossRef]
- Wang, F.; Liao, Y.; Li, X.; Ren, C.; Cheng, C.; Ren, Y. Increased circulating myostatin in patients with type 2 diabetes mellitus. J. Huazhong Univ. Sci. Technol. 2012, 32, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Moon, K.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ahn, C.W.; Park, J.S.; Kim, Y.; Nam, J.S. Circulating myokine levels in different stages of glucose intolerance. Medicine 2020, 99, e19235. [Google Scholar] [CrossRef] [PubMed]
- Bouzakri, K.; Plomgaard, P.; Berney, T.; Donath, M.Y.; Pedersen, B.K.; Halban, P.A. Bimodal Effect on Pancreatic β-Cells of Secretory Products from Normal or Insulin-Resistant Human Skeletal Muscle. Diabetes 2011, 60, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Ciaraldi, T.P.; Ryan, A.J.; Mudaliar, S.R.; Henry, R.R. Altered Myokine Secretion Is an Intrinsic Property of Skeletal Muscle in Type 2 Diabetes. PLoS ONE 2016, 11, e0158209. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Kuro-o, M. Ageing-related receptors resolved. Nature 2018, 553, 409–410. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Mohanty, J.; Sousa, L.P.; Tome, F.; Pardon, E.; Steyaert, J.; Lemmon, M.; Lax, I.; Schlessinger, J. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 2018, 553, 501–505. [Google Scholar] [CrossRef]
- Adams, A.C.; Yang, C.; Coskun, T.; Cheng, C.C.; Gimeno, R.E.; Luo, Y.; Kharitonenkov, A. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab. 2012, 2, 31–37. [Google Scholar] [CrossRef]
- Kolumam, G.; Chen, M.Z.; Tong, R.; Zavala-Solorio, J.; Kates, L.; van Bruggen, N.; Ross, J.; Wyatt, S.K.; Gandham, V.D.; Carano, R.A.; et al. Sustained Brown Fat Stimulation and Insulin Sensitization by a Humanized Bispecific Antibody Agonist for Fibroblast Growth Factor Receptor 1/βKlotho Complex. EBioMedicine 2015, 2, 730–743. [Google Scholar] [CrossRef]
- Lan, T.; Morgan, D.A.; Rahmouni, K.; Sonoda, J.; Fu, X.; Burgess, S.C.; Holland, W.L.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19, FGF21, and an FGFR1/β-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab. 2017, 26, 709–718.e3. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Pedersen, M.; Nielsen, A.R.; Krogh-Madsen, R.; Yfanti, C.; Åkerstrom, T.; Nielsen, S.; Pedersen, B.K. Fibroblast Growth Factor-21 Is Induced in Human Skeletal Muscles by Hyperinsulinemia. Diabetes 2009, 58, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.-S.; Lindberg, R.A.; et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.-F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The Metabolic State of Diabetic Monkeys Is Regulated by Fibroblast Growth Factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef]
- Foltz, I.N.; Hu, S.; King, C.; Wu, X.; Yang, C.; Wang, W.; Weiszmann, J.; Stevens, J.; Chen, J.S.; Nuanmanee, N.; et al. Treating Diabetes and Obesity with an FGF21-Mimetic Antibody Activating the βKlotho/FGFR1c Receptor Complex. Sci. Transl. Med. 2012, 4, 162ra153. [Google Scholar] [CrossRef]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The Effects of LY2405319, an FGF21 Analog, in Obese Human Subjects with Type 2 Diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef]
- Kim, C.-S.; Joe, Y.; Choi, H.-S.; Back, S.H.; Park, J.W.; Chung, H.T.; Roh, E.; Kim, M.-S.; Ha, T.Y.; Yu, R. Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J. Inflamm. 2019, 16, 17. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.-H.; Chen, M.M.; Hancock, C.; Lehman, J.J.; Huss, J.M.; McClain, D.; et al. A Role for the Transcriptional Coactivator PGC-1α in Muscle Refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Xiang, L.; Xiang, G.; Yue, L.; Zhang, J.; Zhao, L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis 2014, 235, 328–333. [Google Scholar] [CrossRef]
- Alis, R.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Hernández-Mijares, A.; Romagnoli, M.; Víctor, V.M.; Rocha, M. Association between irisin and homocysteine in euglycemic and diabetic subjects. Clin. Biochem. 2014, 47, 333–335. [Google Scholar] [CrossRef]
- Yano, N.; Zhang, L.; Wei, D.; Dubielecka, P.M.; Wei, L.; Zhuang, S.; Zhu, P.; Qin, G.; Liu, P.Y.; Chin, Y.E.; et al. Irisin counteracts high glucose and fatty acid-induced cytotoxicity by preserving the AMPK-insulin receptor signaling axis in C2C12 myoblasts. Am. J. Physiol. Metab. 2020, 318, E791–E805. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes Through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise Training of Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Mice Suggests That Exercise-Induced Muscle Phenotype Changes Are SPARC-Dependent. Appl. Sci. 2020, 10, 9108. [Google Scholar] [CrossRef]
- Aoi, W.; Hirano, N.; Lassiter, D.G.; Björnholm, M.; Chibalin, A.V.; Sakuma, K.; Tanimura, Y.; Mizushima, K.; Takagi, T.; Naito, Y.; et al. Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. FASEB J. 2019, 33, 10551–10562. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D.; Graves, D.C.; Motamed, K.; Sage, E.H. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc. Natl. Acad. Sci. USA 2003, 100, 6045–6050. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.J.; Kim, E.J.; Lee, S.J.; Kim, H.D.; Shin, H.J.; Lim, W.K. Involvement of SPARC in in Vitro Differentiation of Skeletal Myoblasts. Biochem. Biophys. Res. Commun. 2000, 271, 630–634. [Google Scholar] [CrossRef]
- Motamed, K.; Blake, D.J.; Angello, J.C.; Allen, B.L.; Rapraeger, A.C.; Hauschka, S.D.; Sage, E.H. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: A role for protein kinase A. J. Cell. Biochem. 2003, 90, 408–423. [Google Scholar] [CrossRef]
- Nakamura, S.K.; Nakano, S.-I.; Miyoshi, T.; Yamanouchi, K.; Matsuwaki, T.; Nishihara, M. Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging 2012, 4, 40–48. [Google Scholar] [CrossRef]
- Shi, C.-X.; Zhao, M.-X.; Shu, X.-D.; Xiong, X.-Q.; Wang, J.-J.; Gao, X.-Y.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. β-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci. Rep. 2016, 6, 21924. [Google Scholar] [CrossRef]
- Jung, T.W.; Hwang, H.-J.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Choi, K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK–PPARδ-dependent pathway in mice. Diabetologia 2015, 58, 2096–2105. [Google Scholar] [CrossRef]
- Roberts, L.; Boström, P.; O’Sullivan, J.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric Acid Induces Browning of White Fat and Hepatic β-Oxidation and Is Inversely Correlated with Cardiometabolic Risk Factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Abbey-Toby, A.; Igoudjil, A.; Lettéron, P.; Fromenty, B. β-Aminoisobutyric Acid Prevents Diet-induced Obesity in Mice with Partial Leptin Deficiency. Obesity 2008, 16, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Åström, M.-B.; Chan, S.; Bruce, C.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Takada, S.; Furihata, T.; Nambu, H.; Kakutani, N.; Maekawa, S.; Mizushima, W.; Nakano, I.; Fukushima, A.; Yokota, T.; et al. Brain-Derived Neurotrophic Factor Improves Impaired Fatty Acid Oxidation Via the Activation of Adenosine Monophosphate-Activated Protein Kinase-α—Proliferator-Activated Receptor-r Coactivator-1α Signaling in Skeletal Muscle of Mice with Heart Failure. Circ. Heart Fail. 2021, 14, e005890. [Google Scholar] [CrossRef]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [CrossRef]
- Yang, X.; Brobst, D.; Chan, W.S.; Tse, M.C.L.; Herlea-Pana, O.; Ahuja, P.; Bi, X.; Zaw, A.M.; Kwong, Z.S.W.; Jia, W.-H.; et al. Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Sci. Signal. 2019, 12, eaau1468. [Google Scholar] [CrossRef]
- Delezie, J.; Weihrauch, M.; Maier, G.; Tejero, R.; Ham, D.J.; Gill, J.F.; Karrer-Cardel, B.; Rüegg, M.A.; Tabares, L.; Handschin, C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 16111–16120. [Google Scholar] [CrossRef]
- Yamanaka, M.; Tsuchida, A.; Nakagawa, T.; Nonomura, T.; Ono-Kishino, M.; Sugaru, E.; Noguchi, H.; Taiji, M. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes. Metab. 2007, 9, 59–64. [Google Scholar] [CrossRef]
- Kim, H.-J.; Higashimori, T.; Park, S.-Y.; Choi, H.; Dong, J.; Kim, Y.-J.; Noh, H.-L.; Cho, Y.-R.; Cline, G.; Kim, Y.-B.; et al. Differential Effects of Interleukin-6 and -10 on Skeletal Muscle and Liver Insulin Action In Vivo. Diabetes 2004, 53, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Keller, C.; Richard, A.-M.; Saha, A.K.; Luo, Z.; Xiang, X.; Giralt, M.; Ritov, V.B.; Menshikova, E.V.; Kelley, D.E.; et al. Interleukin-6 Regulation of AMP-Activated Protein Kinase: Potential Role in the Systemic Response to Exercise and Prevention of the Metabolic Syndrome. Diabetes 2006, 55 (Suppl. S2), S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, E.; Mygind, H.; Grøndahl, T.S.; Pedersen, B.K.; van Hall, G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am. J. Physiol. Metab. 2010, 299, E832–E840. [Google Scholar] [CrossRef]
- Senn, J.J. Toll-like Receptor-2 Is Essential for the Development of Palmitate-induced Insulin Resistance in Myotubes. J. Biol. Chem. 2006, 281, 26865–26875. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Planavila, A.; Sánchez, R.M.; Merlos, M.; Laguna, J.C.; Vázquez-Carrera, M. Palmitate Induces Tumor Necrosis Factor-α Expression in C2C12 Skeletal Muscle Cells by a Mechanism Involving Protein Kinase C and Nuclear Factor-κB Activation. Endocrinology 2006, 147, 552–561. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Foss, N.T.; Donadi, E.; Foss, M.C. In Vitro TNF- and IL-6 Production by Adherent Peripheral Blood Mononuclear Cells Obtained from Type 1 and Type 2 Diabetic Patients Evaluated according to the Metabolic Control. Ann. N. Y. Acad. Sci. 2006, 1079, 177–180. [Google Scholar] [CrossRef]
- Carey, A.L.; Bruce, C.R.; Sacchetti, M.; Anderson, M.; Olsen, D.B.; Saltin, B.; Hawley, J.; Febbraio, M.A. Interleukin-6 and tumor necrosis factor-? are not increased in patients with Type 2 diabetes: Evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia 2004, 47, 1029–1037. [Google Scholar] [CrossRef]
- Broholm, C.; Pedersen, B.K. Leukaemia inhibitory factor--an exercise-induced myokine. Exerc. Immunol. Rev. 2010, 16, 77–85. [Google Scholar]
- Broholm, C.; Laye, M.J.; Brandt, C.; Vadalasetty, R.; Pilegaard, H.; Pedersen, B.K.; Schéele, C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 2011, 111, 251–259. [Google Scholar] [CrossRef]
- Brandt, N.; O’Neill, H.M.; Kleinert, M.; Schjerling, P.; Vernet, E.; Steinberg, G.R.; Richter, E.A.; Jorgensen, S.B. Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am. J. Physiol. Metab. 2015, 309, E142–E153. [Google Scholar] [CrossRef][Green Version]
- Broholm, C.; Brandt, C.; Schultz, N.S.; Nielsen, A.R.; Pedersen, B.K.; Scheele, C. Deficient leukemia inhibitory factor signaling in muscle precursor cells from patients with type 2 diabetes. Am. J. Physiol. Metab. 2012, 303, E283–E292. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Corral, C.M.; Banner, L.R. Early changes of LIFR and gp130 in sciatic nerve and muscle of diabetic mice. Acta Histochem. 2012, 114, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Grit, E.; Legård, B.K.P. Muscle and Exercise Physiology; Academic Press: Cambridge, MA, USA, 2019; pp. 285–307. ISBN 9780128145937. [Google Scholar]
- Tamura, Y.; Watanabe, K.; Kantani, T.; Hayashi, J.; Ishida, N.; Kaneki, M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: Is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr. J. 2011, 58, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.R.; Maples, J.; Hickner, R.C. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: Local effects of IL-15 on adipose tissue lipolysis. Am. J. Physiol. Metab. 2015, 308, E1131–E1139. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, B.; Salesi, M.; Koushki, M.; Amirghofran, Z. Effects of Eccentric and Concentric Emphasized Resistance Exercise on IL-15 Serum Levels and Its Relation to Inflammatory Markers in Athletes and Non-Athletes. Asian J. Sports Med. 2015, 6, e27980. [Google Scholar] [CrossRef] [PubMed]
- Barra, N.G.; Reid, S.; MacKenzie, R.; Werstuck, G.; Trigatti, B.L.; Richards, C.; Holloway, A.C.; Ashkar, A.A. Interleukin-15 Contributes to the Regulation of Murine Adipose Tissue and Human Adipocytes. Obesity 2010, 18, 1601–1607. [Google Scholar] [CrossRef]
- Almendro, V.; Fuster, G.; Busquets, S.; Ametller, E.; Figueras, M.; Argiles, J.M.; López-Soriano, F.J. Effects of IL-15 on Rat Brown Adipose Tissue: Uncoupling Proteins and PPARs. Obesity 2008, 16, 285–289. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Conner, J.D.; Pistilli, E.E.; Wolden-Hanson, T. Overexpression of interleukin-15 in mice promotes resistance to diet-induced obesity, increased insulin sensitivity, and markers of oxidative skeletal muscle metabolism. Int. J. Interf. Cytokine Mediat. Res. 2011, 3, 29–42. [Google Scholar] [CrossRef]
- Sun, H.; Liu, D. Hydrodynamic delivery of interleukin 15 gene promotes resistance to high fat diet-induced obesity, fatty liver and improves glucose homeostasis. Gene Ther. 2015, 22, 341–347. [Google Scholar] [CrossRef]
- Gray, S.R.; Kamolrat, T. The effect of exercise induced cytokines on insulin stimulated glucose transport in C2C12 cells. Cytokine 2011, 55, 221–228. [Google Scholar] [CrossRef]
- Krolopp, J.E.; Thornton, S.M.; Abbott, M.J. IL-15 Activates the Jak3/STAT3 Signaling Pathway to Mediate Glucose Uptake in Skeletal Muscle Cells. Front. Physiol. 2016, 7, 626. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.S.; Anderson, B.G.; Conner, J.D.; Wolden-Hanson, T. IL-15 Overexpression Promotes Endurance, Oxidative Energy Metabolism, and Muscle PPARδ, SIRT1, PGC-1α, and PGC-1β Expression in Male Mice. Endocrinology 2013, 154, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.; Wang, J.; Hug, C.; Tsao, T.-S.; Lodish, H.F. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl. Acad. Sci. USA 2004, 101, 10302–10307. [Google Scholar] [CrossRef]
- Peterson, J.M.; Aja, S.; Wei, Z.; Wong, G.W. CTRP1 Protein Enhances Fatty Acid Oxidation via AMP-activated Protein Kinase (AMPK) Activation and Acetyl-CoA Carboxylase (ACC) Inhibition. J. Biol. Chem. 2012, 287, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Seldin, M.M.; Wei, Z.; Aja, S.; Wong, G.W. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am. J. Physiol. Liver Physiol. 2013, 305, G214–G224. [Google Scholar] [CrossRef]
- Lim, S.; Choi, S.H.; Koo, B.K.; Kang, S.M.; Yoon, J.W.; Jang, H.C.; Choi, S.M.; Lee, M.G.; Lee, W.; Shin, H.; et al. Effects of Aerobic Exercise Training on C1q Tumor Necrosis Factor α-Related Protein Isoform 5 (Myonectin): Association with Insulin Resistance and Mitochondrial DNA Density in Women. J. Clin. Endocrinol. Metab. 2012, 97, E88–E93. [Google Scholar] [CrossRef]
- Seldin, M.M.; Lei, X.; Tan, S.Y.; Stanson, K.P.; Wei, Z.; Wong, G.W. Skeletal Muscle-derived Myonectin Activates the Mammalian Target of Rapamycin (mTOR) Pathway to Suppress Autophagy in Liver. J. Biol. Chem. 2013, 288, 36073–36082. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef]
- Li, K.; Liao, X.; Wang, K.; Mi, Q.; Zhang, T.; Jia, Y.; Xu, X.; Luo, X.; Zhang, C.; Liu, H.; et al. Myonectin Predicts the Development of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 139–147. [Google Scholar] [CrossRef]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef]
- Pourranjbar, M.; Arabnejad, N.; Naderipour, K.; Rafie, F. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J. Med. Life 2018, 11, 381–386. [Google Scholar] [CrossRef]
- Lenk, K.; Schur, R.; Linke, A.; Erbs, S.; Matsumoto, Y.; Adams, V.; Schuler, G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur. J. Heart Fail. 2009, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Joulia, D.; Bernardi, H.; Garandel, V.; Rabenoelina, F.; Vernus, B.; Cabello, G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 2003, 286, 263–275. [Google Scholar] [CrossRef]
- Amthor, H.; Macharia, R.; Navarrete, R.; Schuelke, M.; Brown, S.C.; Otto, A.; Voit, T.; Muntoni, F.; Vrbóva, G.; Partridge, T.; et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc. Natl. Acad. Sci. USA 2007, 104, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef]
- McPherron, A.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. Suppression of body fat accumulation in myostatin-deficient mice. J. Clin. Investig. 2002, 109, 595–601. [Google Scholar] [CrossRef]
- Lehr, S.; Hartwig, S.; Sell, H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl. 2012, 6, 91–101. [Google Scholar] [CrossRef]
- Lin, J.; Arnold, H.B.; Della-Fera, M.A.; Azain, M.; Hartzell, D.L.; Baile, C.A. Myostatin Knockout in Mice Increases Myogenesis and Decreases Adipogenesis. Biochem. Biophys. Res. Commun. 2002, 291, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.J.; Lloyd, D.J.; Gekakis, N. Loss-of-Function Mutation in Myostatin Reduces Tumor Necrosis Factor α Production and Protects Liver Against Obesity-Induced Insulin Resistance. Diabetes 2009, 58, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wall, R.J.; Yang, J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem. Biophys. Res. Commun. 2005, 337, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin Inhibition in Muscle, but Not Adipose Tissue, Decreases Fat Mass and Improves Insulin Sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Pennington, C.; Webb, C.N.; Isales, C.M. Resistance to body fat gain in ‘double-muscled’ mice fed a high-fat diet. Int. J. Obes. 2006, 30, 868–870. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jarmin, S.; Eilers, W.; Elashry, M.; Andersen, D.K.; Dickson, G.; Foster, K. Local overexpression of the myostatin propeptide increases glucose transporter expression and enhances skeletal muscle glucose disposal. Am. J. Physiol. Metab. 2014, 306, E814–E823. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Zeman-Meier, D.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Handschin, C.; Choi, C.S.; Chin, S.; Kim, S.; Kawamori, D.; Kurpad, A.J.; Neubauer, N.; Hu, J.; Mootha, V.K.; Kim, Y.-B.; et al. Abnormal glucose homeostasis in skeletal muscle–specific PGC-1α knockout mice reveals skeletal muscle–pancreatic β cell crosstalk. J. Clin. Investig. 2007, 117, 3463–3474. [Google Scholar] [CrossRef]
- Hirner, S.; Krohne, C.; Schuster, A.; Hoffmann, S.; Witt, S.; Erber, R.; Sticht, C.; Gasch, A.; Labeit, S.; Labeit, D. MuRF1-dependent Regulation of Systemic Carbohydrate Metabolism as Revealed from Transgenic Mouse Studies. J. Mol. Biol. 2008, 379, 666–677. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Scheler, M.; Irmler, M.; Lehr, S.; Hartwig, S.; Staiger, H.; Al-Hasani, H.; Beckers, J.; de Angelis, M.H.; Häring, H.-U.; Weigert, C. Cytokine response of primary human myotubes in an in vitro exercise model. Am. J. Physiol. Physiol. 2013, 305, C877–C886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Oh, E.; Clapp, D.W.; Chernoff, J.; Thurmond, D.C. Inhibition or Ablation of p21-activated Kinase (PAK1) Disrupts Glucose Homeostatic Mechanisms in Vivo. J. Biol. Chem. 2011, 286, 41359–41367. [Google Scholar] [CrossRef] [PubMed]
- Tunduguru, R.; Zhang, J.; Aslamy, A.; Salunkhe, V.A.; Brozinick, J.T.; Elmendorf, J.S.; Thurmond, D.C. The actin-related p41ARC subunit contributes to p21-activated kinase-1 (PAK1)–mediated glucose uptake into skeletal muscle cells. J. Biol. Chem. 2017, 292, 19034–19043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Du, L.; Tang, D.D.; Gunst, S.J. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am. J. Physiol. Physiol. 2005, 288, C1145–C1160. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Tunduguru, R.; Ahn, M.; Salunkhe, V.A.; Veluthakal, R.; Hwang, J.; Bhattacharya, S.; McCown, E.M.; Garcia, P.A.; Zhou, C.; et al. Changes in Skeletal Muscle PAK1 Levels Regulate Tissue Crosstalk to Impact Whole Body Glucose Homeostasis. Front. Endocrinol. 2022, 13, 821849. [Google Scholar] [CrossRef]
- Ryan, A.J.; Ciaraldi, T.P.; Henry, R.R. Myokine Regulation of Insulin Secretion: Impact of Inflammation and Type 2 Diabetes. Front. Physiol. 2019, 10, 1608. [Google Scholar] [CrossRef]
- Schulthess, F.T.; Paroni, F.; Sauter, N.S.; Shu, L.; Ribaux, P.; Haataja, L.; Strieter, R.M.; Oberholzer, J.; King, C.C.; Maedler, K. CXCL10 Impairs β Cell Function and Viability in Diabetes through TLR4 Signaling. Cell Metab. 2009, 9, 125–139. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, Z.-H.; Song, Y.W. CXCL10 and autoimmune diseases. Autoimmun. Rev. 2009, 8, 379–383. [Google Scholar] [CrossRef]
- Nigi, L.; Brusco, N.; Grieco, G.E.; Licata, G.; Krogvold, L.; Marselli, L.; Gysemans, C.; Overbergh, L.; Marchetti, P.; Mathieu, C.; et al. Pancreatic Alpha-Cells Contribute Together with Beta-Cells to CXCL10 Expression in Type 1 Diabetes. Front. Endocrinol. 2020, 11, 630. [Google Scholar] [CrossRef]
- Nicoletti, F.; Conget, I.; Di Mauro, M.; Di Marco, R.; Mazzarino, M.C.; Bendtzen, K.; Messina, A.; Gomis, R. Serum concentrations of the interferon-γ-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia 2002, 45, 1107–1110. [Google Scholar] [CrossRef]
- Rhode, A.; Pauza, M.E.; Barral, A.M.; Rodrigo, E.; Oldstone, M.B.A.; Von Herrath, M.G.; Christen, U. Islet-Specific Expression of CXCL10 Causes Spontaneous Islet Infiltration and Accelerates Diabetes Development. J. Immunol. 2005, 175, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J. Quadrupling Muscle Mass in Mice by Targeting TGF-ß Signaling Pathways. PLoS ONE 2007, 2, e789. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.F.; Phelps, M.P.; Fuentes, F.D.; Bradley, T.M. Overexpression of follistatin in trout stimulates increased muscling. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R235–R242. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Rinnov, A.; Krogh-Madsen, R.; Fischer, C.P.; Andreasen, A.S.; Berg, R.M.G.; Møller, K.; Pedersen, B.K.; Plomgaard, P. Plasma follistatin is elevated in patients with type 2 diabetes: Relationship to hyperglycemia, hyperinsulinemia, and systemic low-grade inflammation. Diabetes/Metabolism Res. Rev. 2013, 29, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Haukeland, J.W.; Dahl, T.B.; Bjøro, K.; Gladhaug, I.P.; Berge, C.; Damås, J.K.; Haaland, T.; Løberg, E.M.; Linnestad, P.; et al. A Complex Role of Activin A in Non-Alcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2009, 104, 2196–2205. [Google Scholar] [CrossRef]
- Plomgaard, P.; Halban, P.A.; Bouzakri, K. Bimodal impact of skeletal muscle on pancreatic β-cell function in health and disease. Diabetes Obes. Metab. 2012, 14 (Suppl. S3), 78–84. [Google Scholar] [CrossRef]
- Hansen, J.S.; Rutti, S.; Arous, C.; Clemmesen, J.O.; Secher, N.H.; Drescher, A.; Gonelle-Gispert, C.; Halban, P.A.; Pedersen, B.K.; Weigert, C.; et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J. Clin. Endocrinol. Metab. 2016, 101, 550–560. [Google Scholar] [CrossRef]
- Bertolino, P.; Holmberg, R.; Reissmann, E.; Andersson, O.; Berggren, P.-O.; Ibáñez, C.F. Activin B receptor ALK7 is a negative regulator of pancreatic β-cell function. Proc. Natl. Acad. Sci. USA 2008, 105, 7246–7251. [Google Scholar] [CrossRef]
- Ripoche, D.; Charbord, J.; Hennino, A.; Teinturier, R.; Bonnavion, R.; Jaafar, R.; Goehrig, D.; Cordier-Bussat, M.; Ritvos, O.; Zhang, C.X.; et al. ActivinB Is Induced in Insulinoma to Promote Tumor Plasticity through a β-Cell-Induced Dedifferentiation. Mol. Cell. Biol. 2015, 36, 756–764. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Sreekumaran Nair, K.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Morinaga, H.; Kim, J.J.; Lagakos, W.; Taylor, S.; Keshwani, M.; Perkins, G.; Dong, H.; Kayali, A.G.; Sweet, I.R.; et al. The Fractalkine/CX3CR1 System Regulates β Cell Function and Insulin Secretion. Cell 2013, 153, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Riopel, M.; Seo, J.B.; Bandyopadhyay, G.K.; Li, P.; Wollam, J.; Chung, H.; Jung, S.-R.; Murphy, A.; Wilson, M.; De Jong, R.; et al. Chronic fractalkine administration improves glucose tolerance and pancreatic endocrine function. J. Clin. Investig. 2018, 128, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Rutti, S.; Arous, C.; Schvartz, D.; Timper, K.; Sanchez, J.-C.; Dermitzakis, E.; Donath, M.Y.; Halban, P.A.; Bouzakri, K. Fractalkine (CX3CL1), a new factor protecting β-cells against TNFα. Mol. Metab. 2014, 3, 731–741. [Google Scholar] [CrossRef]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fang, Q.; Christ-Roberts, C.Y.; Hong, J.Y.; Kim, R.Y.; et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006, 8, 516–523. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kadowaki, T. Adiponectin Receptor as a Key Player in Healthy Longevity and Obesity-Related Diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef]
- Schinzari, F.; Veneziani, A.; Mores, N.; Barini, A.; Di Daniele, N.; Cardillo, C.; Tesauro, M. Beneficial Effects of Apelin on Vascular Function in Patients with Central Obesity. Hypertension 2017, 69, 942–949. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Wang, J.; Zhang, Y. Hypoxia exposure alleviates impaired muscular metabolism, glucose tolerance, and aerobic capacity in apelin-knockout mice. FEBS Open Bio 2019, 9, 498–509. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, F.; Li, W.; Cao, Y.; Wang, C.; Wang, Y.; Liang, D.; Zhang, R.; Zhang, S.; Wang, H.; et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol. Cell. Biochem. 2011, 353, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ceylan-Isik, A.F.; Kandadi, M.R.; Xu, X.; Hua, Y.; Chicco, A.J.; Ren, J.; Nair, S. Apelin administration ameliorates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell. Cardiol. 2013, 63, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Olsen, C.H.; Pedersen, B.K.; Hojman, P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am. J. Physiol. Metab. 2012, 302, E831–E840. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, Y.; Xiao, J.; Liu, L.; Chen, S.; Mohammadi, M.; McClain, C.J.; Li, X.; Feng, W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res. 2015, 56, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.D.L.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef]
- Gimeno, R.E.; Moller, D.E. FGF21-based pharmacotherapy—Potential utility for metabolic disorders. Trends Endocrinol. Metab. 2014, 25, 303–311. [Google Scholar] [CrossRef]
- Nadeau, L.; Patten, D.; Caron, A.; Garneau, L.; Pinault-Masson, E.; Foretz, M.; Haddad, P.; Anderson, B.; Quinn, L.; Jardine, K.; et al. IL-15 improves skeletal muscle oxidative metabolism and glucose uptake in association with increased respiratory chain supercomplex formation and AMPK pathway activation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1863, 395–407. [Google Scholar] [CrossRef]
- Timper, K.; Denson, J.L.; Steculorum, S.; Heilinger, C.; Ruud, L.E.; Wunderlich, C.M.; Rose-John, S.; Wunderlich, F.T.; Brüning, J.C. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans -Signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Duque-Guimaraes, D.E.; Machado, U.F.; Zierath, J.R.; Krook, A. Altered Response of Skeletal Muscle to IL-6 in Type 2 Diabetic Patients. Diabetes 2013, 62, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalili, L.; Bouzakri, K.; Glund, S.; Lönnqvist, F.; Koistinen, H.; Krook, A. Signaling Specificity of Interleukin-6 Action on Glucose and Lipid Metabolism in Skeletal Muscle. Mol. Endocrinol. 2006, 20, 3364–3375. [Google Scholar] [CrossRef]
- Hong, E.-G.; Ko, H.J.; Cho, Y.-R.; Kim, H.-J.; Ma, Z.; Yu, T.Y.; Friedline, R.H.; Kurt-Jones, E.; Finberg, R.; Fischer, M.A.; et al. Interleukin-10 Prevents Diet-Induced Insulin Resistance by Attenuating Macrophage and Cytokine Response in Skeletal Muscle. Diabetes 2009, 58, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, S.; Jung, D.Y.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Inashima, K.; Tran, D.A.; Hu, X.; et al. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017, 31, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Liu, J.; Zhang, J.D.; Zhu, D.; Wang, H.; Xiong, L.; Lee, Y.; Ye, J.; Lian, K.; Xu, C.; et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016, 40, 443–451. [Google Scholar] [CrossRef]
- Lee, J.O.; Byun, W.S.; Kang, M.J.; Han, J.A.; Moon, J.; Shin, M.; Lee, H.J.; Chung, J.H.; Lee, J.; Son, C.; et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKα2. FEBS J. 2020, 287, 2087–2104. [Google Scholar] [CrossRef]
- Hu, W.; Wang, R.; Sun, B. Meteorin-Like Ameliorates β Cell Function by Inhibiting β Cell Apoptosis of and Promoting β Cell Proliferation via Activating the WNT/β-Catenin Pathway. Front. Pharmacol. 2021, 12, 627147. [Google Scholar] [CrossRef]
- Wei, Z.; Peterson, J.M.; Lei, X.; Cebotaru, L.; Wolfgang, M.J.; Baldeviano, G.C.; Wong, G.W. C1q/TNF-related Protein-12 (CTRP12), a Novel Adipokine That Improves Insulin Sensitivity and Glycemic Control in Mouse Models of Obesity and Diabetes. J. Biol. Chem. 2012, 287, 10301–10315. [Google Scholar] [CrossRef]
- Liu, X.-H.; Bauman, W.A.; Cardozo, C.P. Myostatin inhibits glucose uptake via suppression of insulin-dependent and -independent signaling pathways in myoblasts. Physiol. Rep. 2018, 6, e13837. [Google Scholar] [CrossRef]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin Decreases with Aerobic Exercise and Associates with Insulin Resistance. Med. Sci. Sports Exerc. 2010, 42, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Cleary, A.S.; Speaker, K.J.; Lindsay, S.F.; Uyenishi, J.; Reed, J.M.; Madden, M.C.; Mehan, R.S. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am. J. Physiol. Metab. 2008, 294, E918–E927. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Ali, N.; Zhang, L.; Qi, Y.; Clarke, I.; Enriquez, R.; Brzozowska, M.; Lee, I.; Rogers, M.; Laybutt, D.; et al. Osteoglycin, a novel coordinator of bone and glucose homeostasis. Mol. Metab. 2018, 13, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Li, Z.; Zoch, M.L.; Frey, J.L.; Bowman, C.E.; Kushwaha, P.; Ryan, K.A.; Goh, B.; Scafidi, S.; Pickett, J.E.; et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight 2017, 2, e92704. [Google Scholar] [CrossRef]
- Nie, J.; Sage, E.H. SPARC Inhibits Adipogenesis by Its Enhancement of β-Catenin Signaling. J. Biol. Chem. 2009, 284, 1279–1290. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in Exercise Training- and Cold-Induced UCP1 Expression in Subcutaneous White Adipose Tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef]
- Wan, Z.; Ritchie, I.; Beaudoin, M.-S.; Castellani, L.; Chan, C.B.; Wright, D.C. IL-6 Indirectly Modulates the Induction of Glyceroneogenic Enzymes in Adipose Tissue during Exercise. PLoS ONE 2012, 7, e41719. [Google Scholar] [CrossRef]
- Van Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Moller, K.; Saltin, B.; Febbraio, M.A.; et al. Interleukin-6 Stimulates Lipolysis and Fat Oxidation in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef]
- Wueest, S.; Konrad, D. The role of adipocyte-specific IL-6-type cytokine signaling in FFA and leptin release. Adipocyte 2018, 7, 226–228. [Google Scholar] [CrossRef]
- Javaid, H.M.A.; Sahar, N.E.; ZhuGe, D.-L.; Huh, J.Y. Exercise Inhibits NLRP3 Inflammasome Activation in Obese Mice via the Anti-Inflammatory Effect of Meteorin-like. Cells 2021, 10, 3480. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pervin, S.; Lee, S.-J.; Kuo, A.; Grijalva, V.; David, J.; Vergnes, L.; Reddy, S.T. Metabolic profiling of follistatin overexpression: A novel therapeutic strategy for metabolic diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Liu, Y.; Han, F.; Wang, D.; Hou, X.; Hou, S.; Sun, X. Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese mice. J. Mol. Cell. Cardiol. 2016, 99, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Still, C.D.; Argyropoulos, G. Does IRISIN Have a BRITE Future as a Therapeutic Agent in Humans? Curr. Obes. Rep. 2014, 3, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Nikolova, V.; Vyagova, D.; Chaldakov, G.N.; Aloe, L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int. J. Cardiol. 2005, 102, 169–171. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Ono, M.; Ichihara, J.; Nonomura, T.; Itakura, Y.; Taiji, M.; Nakayama, C.; Noguchi, H. Brain-Derived Neurotrophic Factor Reduces Blood Glucose Level in Obese Diabetic Mice but Not in Normal Mice. Biochem. Biophys. Res. Commun. 1997, 238, 633–637. [Google Scholar] [CrossRef]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional Deletion of Brain-Derived Neurotrophic Factor in the Postnatal Brain Leads to Obesity and Hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Oelmann, S.; Nauck, M.; Völzke, H.; Bahls, M.; Friedrich, N. Circulating Irisin Concentrations Are Associated with a Favourable Lipid Profile in the General Population. PLoS ONE 2016, 11, e0154319. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Hiscock, N.; Sacchetti, M.; Fischer, C.P.; Pedersen, B.K. Interleukin-6 Is a Novel Factor Mediating Glucose Homeostasis During Skeletal Muscle Contraction. Diabetes 2004, 53, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Peppler, W.T.; Townsend, L.K.; Meers, G.M.; Panasevich, M.R.; MacPherson, R.E.K.; Rector, R.S.; Wright, D.C. Acute administration of IL-6 improves indices of hepatic glucose and insulin homeostasis in lean and obese mice. Am. J. Physiol. Liver Physiol. 2019, 316, G166–G178. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Pang, S.; Wang, M.; Xiao, X.; Rong, Y.; Wang, H.; Zang, Y.Q. TLR4 Activation Is Required for IL-17–Induced Multiple Tissue Inflammation and Wasting in Mice. J. Immunol. 2010, 185, 2563–2569. [Google Scholar] [CrossRef]
- Duzova, H.; Karakoc, Y.; Emre, M.H.; Dogan, Z.Y.; Kilinc, E. Effects of Acute Moderate and Strenuous Exercise Bouts on IL-17 Production and Inflammatory Response in Trained Rats. J. Sports Sci. Med. 2009, 8, 219–224. [Google Scholar] [PubMed]
- Harley, I.T.; Stankiewicz, T.E.; Giles, D.A.; Softic, S.; Flick, L.M.; Cappelletti, M.; Sheridan, R.; Xanthakos, S.A.; Steinbrecher, K.A.; Sartor, R.B.; et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 2014, 59, 1830–1839. [Google Scholar] [CrossRef]
- Tarantino, G.; Costantini, S.; Finelli, C.; Capone, F.; Guerriero, E.; La Sala, N.; Gioia, S.; Castello, G. Is serum Interleukin-17 associated with early atherosclerosis in obese patients? J. Transl. Med. 2014, 12, 214. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Ding, Y.-J.; Fu, Q.-Q.; Xie, J.-J.; Tang, T.-T.; Yao, R.; Chen, Y.; Liao, Y.-H. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 2008, 127, 89–97. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Yuan, C.; Li, R.; Ma, Y.; Tang, X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: A cross-sectional study. Sci. Rep. 2020, 10, 16093. [Google Scholar] [CrossRef]
- Hu, J.; Ke, Y.; Wu, F.; Liu, S.; Ji, C.; Zhu, X.; Zhang, Y. Circulating Irisin Levels in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pr. 2020, 2020, 8818191. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Shi, C.-X.; Gao, R.; Sun, H.-J.; Xiong, X.-Q.; Ding, L.; Chen, Q.; Li, Y.-H.; Wang, J.-J.; Kang, Y.-M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver Plays a Major Role in FGF-21 Mediated Glucose Homeostasis. Cell. Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Lee, Y.-H.; Yi, H.-K. Gradual downhill running improves age-related skeletal muscle and bone weakness: Implication of autophagy and bone morphogenetic proteins. Exp. Physiol. 2016, 101, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Ohashi, K.; Shibata, R.; Murohara, T. Protective Roles of Adipocytokines and Myokines in Cardiovascular Disease. Circ. J. 2016, 80, 2073–2080. [Google Scholar] [CrossRef]
- Ebert, T.; Kralisch, S. Newly discovered myokines in chronic kidney disease. Pol. Arch. Intern. Med. 2016, 126, 457–458. [Google Scholar] [CrossRef][Green Version]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.D.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart from Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Q.; Lou, T.; Qin, J.; Jung, S.; Shetty, V.; Li, F.; Wang, Y.; Feng, X.-H.; Mitch, W.E.; et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat. Commun. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Effects of myokines on bone. BoneKEy Rep. 2016, 5, 826. [Google Scholar] [CrossRef] [PubMed]
- Narendran, P.; Jackson, N.; Daley, A.; Thompson, D.; Stokes, K.; Greenfield, S.; Charlton, M.; Curran, M.; Solomon, T.; Nouwen, A.; et al. Exercise to preserve β-cell function in recent-onset Type 1 diabetes mellitus (EXTOD)—A randomized controlled pilot trial. Diabet. Med. 2017, 34, 1521–1531. [Google Scholar] [CrossRef]
- Paula, F.M.M.; Leite, N.C.; Vanzela, E.C.; Kurauti, M.A.; Freitas-Dias, R.; Carneiro, E.M.; Boschero, A.C.; Zoppi, C.C. Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J. 2015, 29, 1805–1816. [Google Scholar] [CrossRef]
- Camporez, J.P.G.; Jornayvaz, F.; Petersen, M.C.; Pesta, D.; Guigni, B.; Serr, J.; Zhang, D.; Kahn, M.; Samuel, V.T.; Jurczak, M.; et al. Cellular Mechanisms by Which FGF21 Improves Insulin Sensitivity in Male Mice. Endocrinology 2013, 154, 3099–3109. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Aguilar-Salinas, C.A.; Gómez-Pérez, F.J. Metabolic actions of fibroblast growth factor 21. Curr. Opin. Pediatr. 2012, 24, 523–529. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.-L.; Zhu, Y.-J.; Li, C.-G.; Tang, Y.-Z.; Ni, C.-L.; Chen, L.-M.; Niu, W.-Y. Circulating Serum Myonectin Levels in Obesity and Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2021, 129, 528–534. [Google Scholar] [CrossRef]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef]
- Barlow, J.P.; Solomon, T.P. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am. J. Physiol. Metab. 2018, 314, E297–E307. [Google Scholar] [CrossRef]
- Ying, L.; Zhang, Q.; Yang, Y.-M.; Zhou, J.-Y. A Combination of Serum Biomarkers in Elderly Patients with Sarcopenia: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2022, 2022, 4026940. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisloff, U.; Tjonna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Erickson, H.P. Irisin and FNDC5 in retrospect. Adipocyte 2013, 2, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Kaufman, A.; Abuqayyas, L.; Denney, W.; Tillman, E.; Rolph, T. AKR-001, an Fc-FGF21 Analog, Showed Sustained Pharmacodynamic Effects on Insulin Sensitivity and Lipid Metabolism in Type 2 Diabetes Patients. Cell Rep. Med. 2020, 1, 100057. [Google Scholar] [CrossRef]
| Myokines | Organ Cross-Talk | Role in Energy Metabolism |
|---|---|---|
| Adiponectin | Adipose tissue, Pancreas | ↑ Glucose metabolism [170,171] |
| Apelin | Heart, Pancreas | ↑Insulin sensitivity [172] ↑Glucose uptake [173,174] ↑ β-oxidation [175] |
| BAIBA | Fat, Liver, Bone | ↑ Mitochondrial metabolism [81,83] ↑ Insulin sensitivity [82] |
| CX3CL1/Fractaline | Pancreas | ↑ Fatty acid oxidation [176] |
| FGF21 | Adipose tissue, Liver | ↑ Insulin sensitivity [59,177] ↑ Lipolysis [178,179] ↑ Oxidative capacities [180] ↓ Triglycerides l [181,182] |
| IL-15 | Adipose tissue, Bone | ↑ Glucose uptake [183] ↑ Fatty acid oxidation [115] ↑ Mitochondrial function [183] ↓Oxidative stress and lipid accumulation [183] |
| IL-6 | Liver, Adipose tissue, Pancreas, Bone | ↑ Insulin sensitivity [184] ↑ Glucose uptake [18,184,185] ↑ Fatty acid oxidation [186] ↑ Glycogen synthesis [186] |
| IL-10 | Adipose tissue | ↑ Glucose metabolism [187,188] |
| Irisin | Adipose tissue, Brain, Bone, Heart, Blood, Kidney | ↑Glucose uptake [64,189] ↑ β-oxidation and mitochondrial biogenesis [63,189] |
| METRNL | Adipose tissue | ↑ Glucose metabolism [190,191] |
| Musclin (osteocrin) | Heart, Bone, Brain | ↓Decrease glucose uptake and insulin sensitivity |
| Myonectin | Heart, Liver, Adipocytes | ↑ Glucose uptake [120] ↑ β-oxidation [123,192] |
| Myostatin | Adipose tissue, Liver, Bone, Muscle | ↓Decrease glucose uptake and insulin sensitivity [193,194,195] |
| Osteoglycin | Muscle, Bone | ↑ Glucose metabolism [196] ↑ Fatty acid oxidation [197] |
| SPARC | Adipose tissue, Muscle | ↑ Glucose tolerance [76] inhibits adipogenesis [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakrishnan, R.; Thurmond, D.C. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. Int. J. Mol. Sci. 2022, 23, 4636. https://doi.org/10.3390/ijms23094636
Balakrishnan R, Thurmond DC. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. International Journal of Molecular Sciences. 2022; 23(9):4636. https://doi.org/10.3390/ijms23094636
Chicago/Turabian StyleBalakrishnan, Rekha, and Debbie C. Thurmond. 2022. "Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance" International Journal of Molecular Sciences 23, no. 9: 4636. https://doi.org/10.3390/ijms23094636
APA StyleBalakrishnan, R., & Thurmond, D. C. (2022). Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. International Journal of Molecular Sciences, 23(9), 4636. https://doi.org/10.3390/ijms23094636







