Hypoxia Regulates the Self-Renewal of Endometrial Mesenchymal Stromal/Stem-like Cells via Notch Signaling
Abstract
:1. Introduction
2. Results
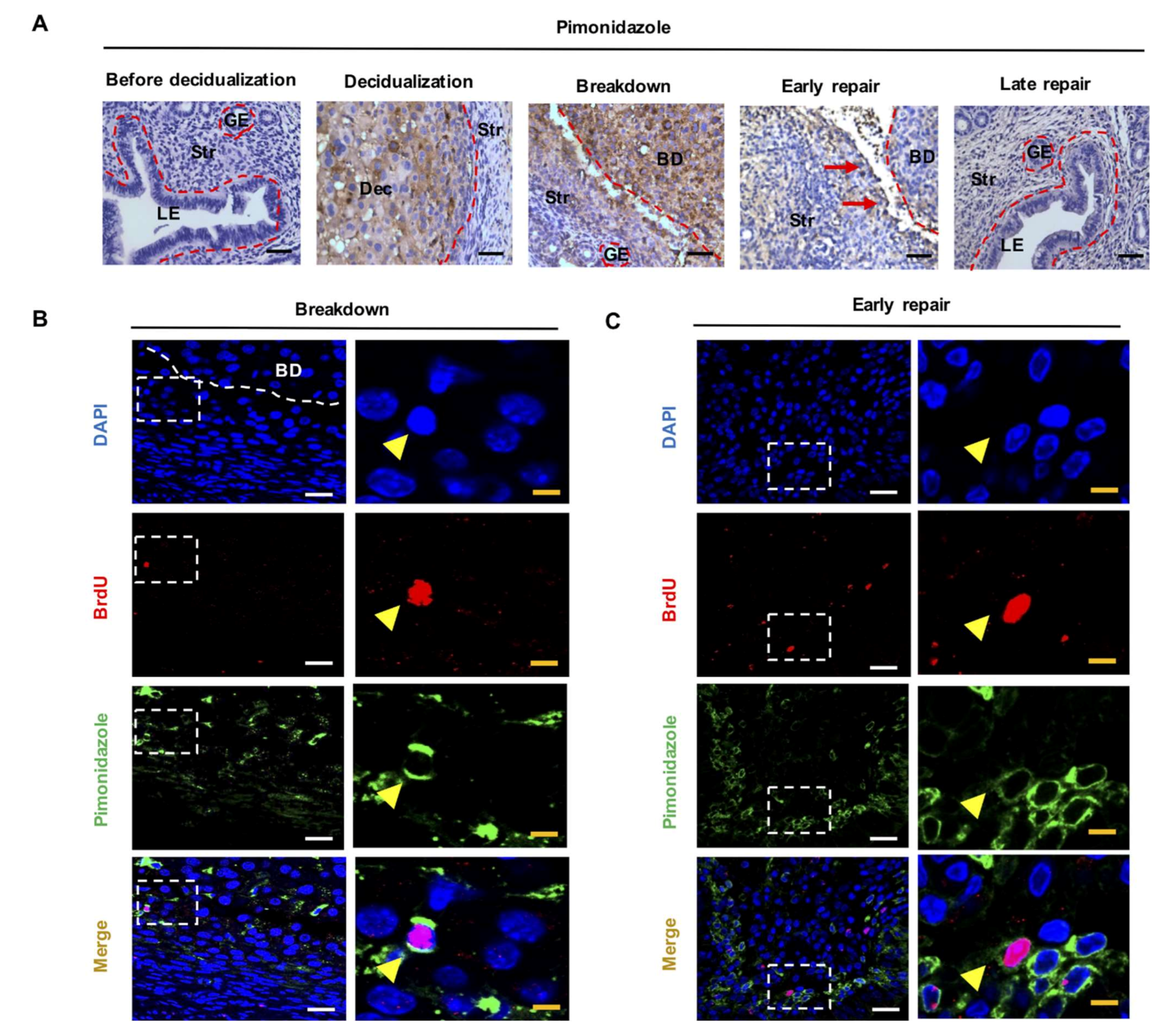
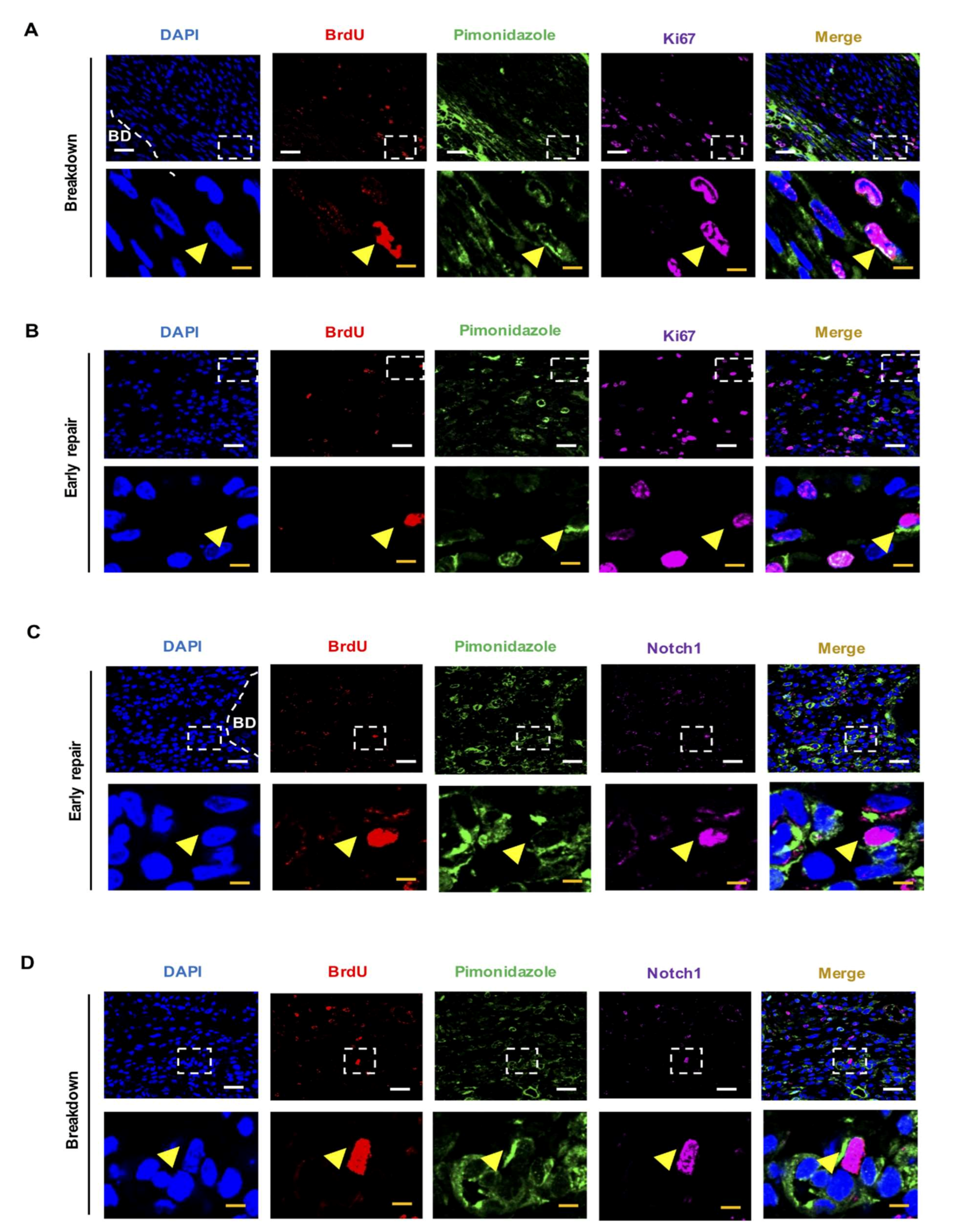
2.1. LRSC Reside in A Hypoxic Microenvironment during Endometrial Breakdown and Early Repair
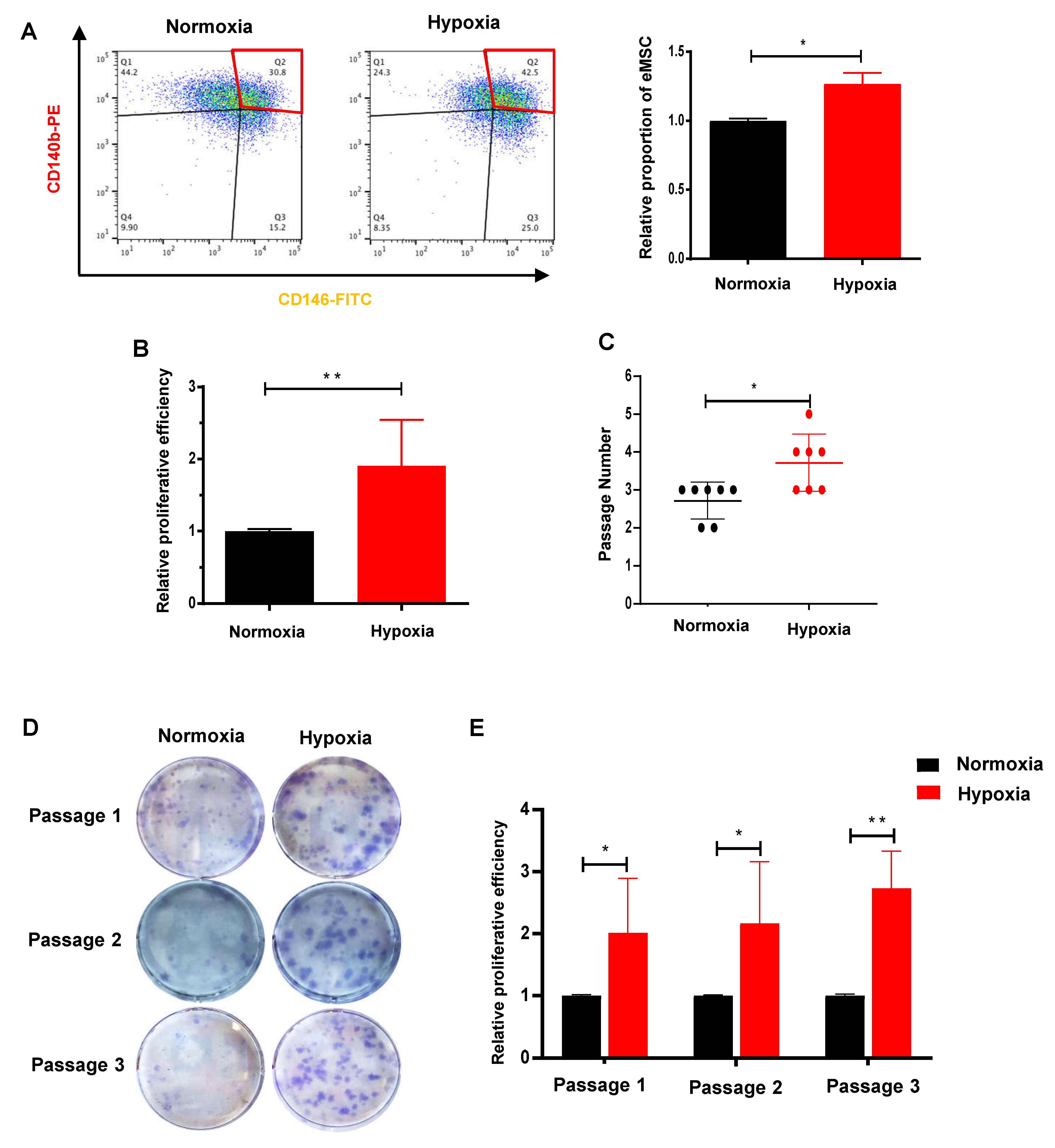
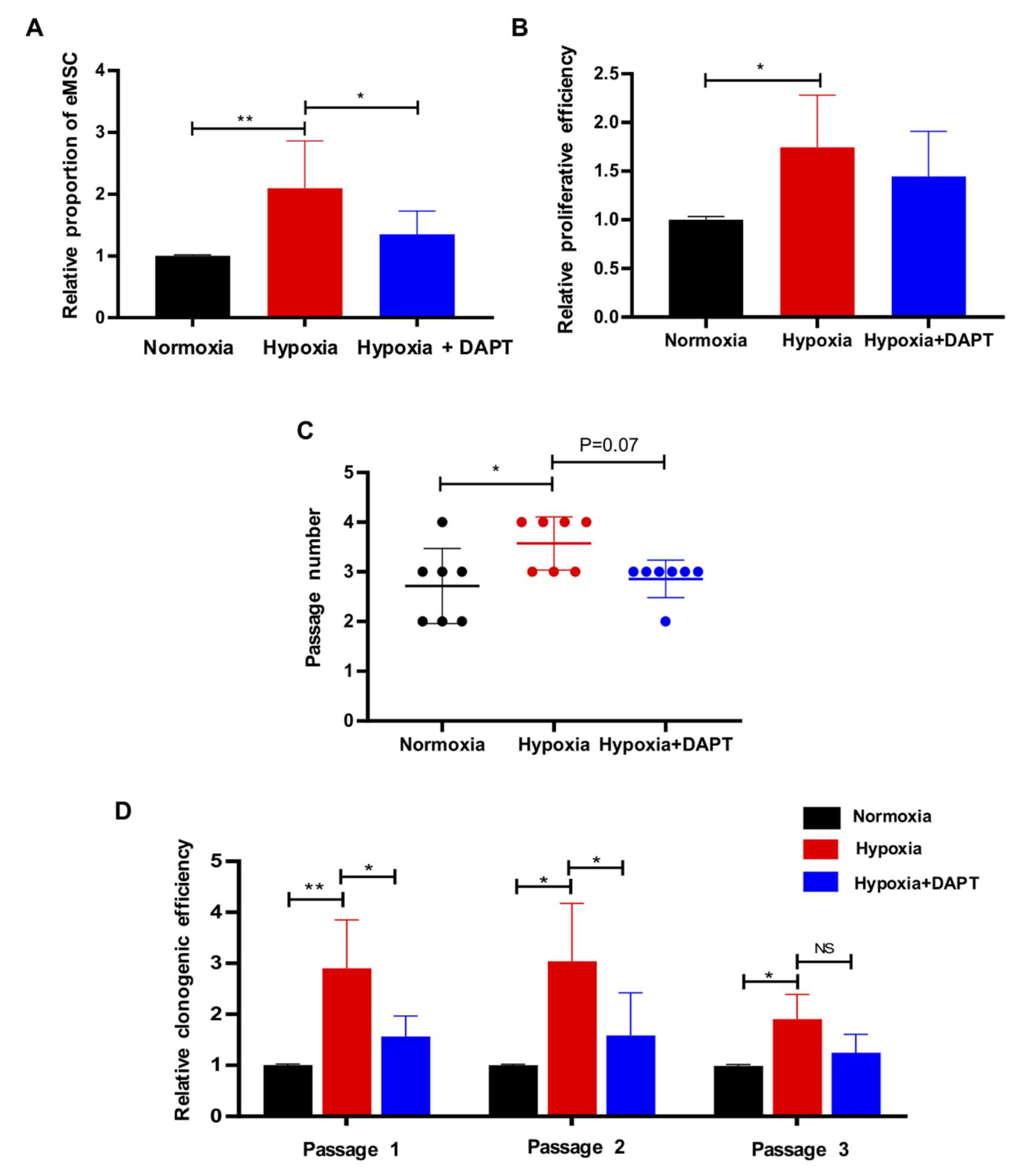
2.2. Hypoxia Facilitates the Proliferation and Self-Renewal Activities of eMSC
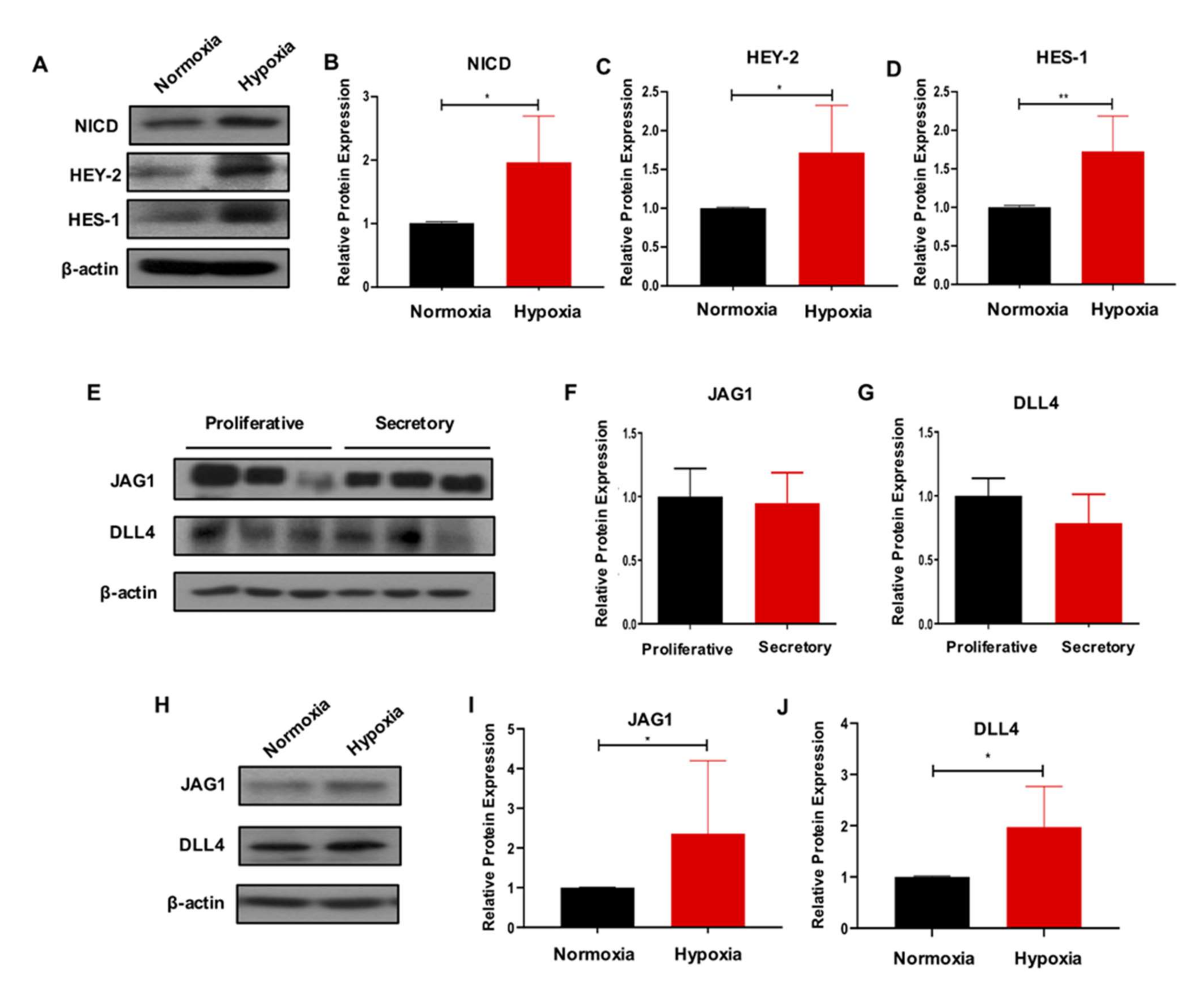
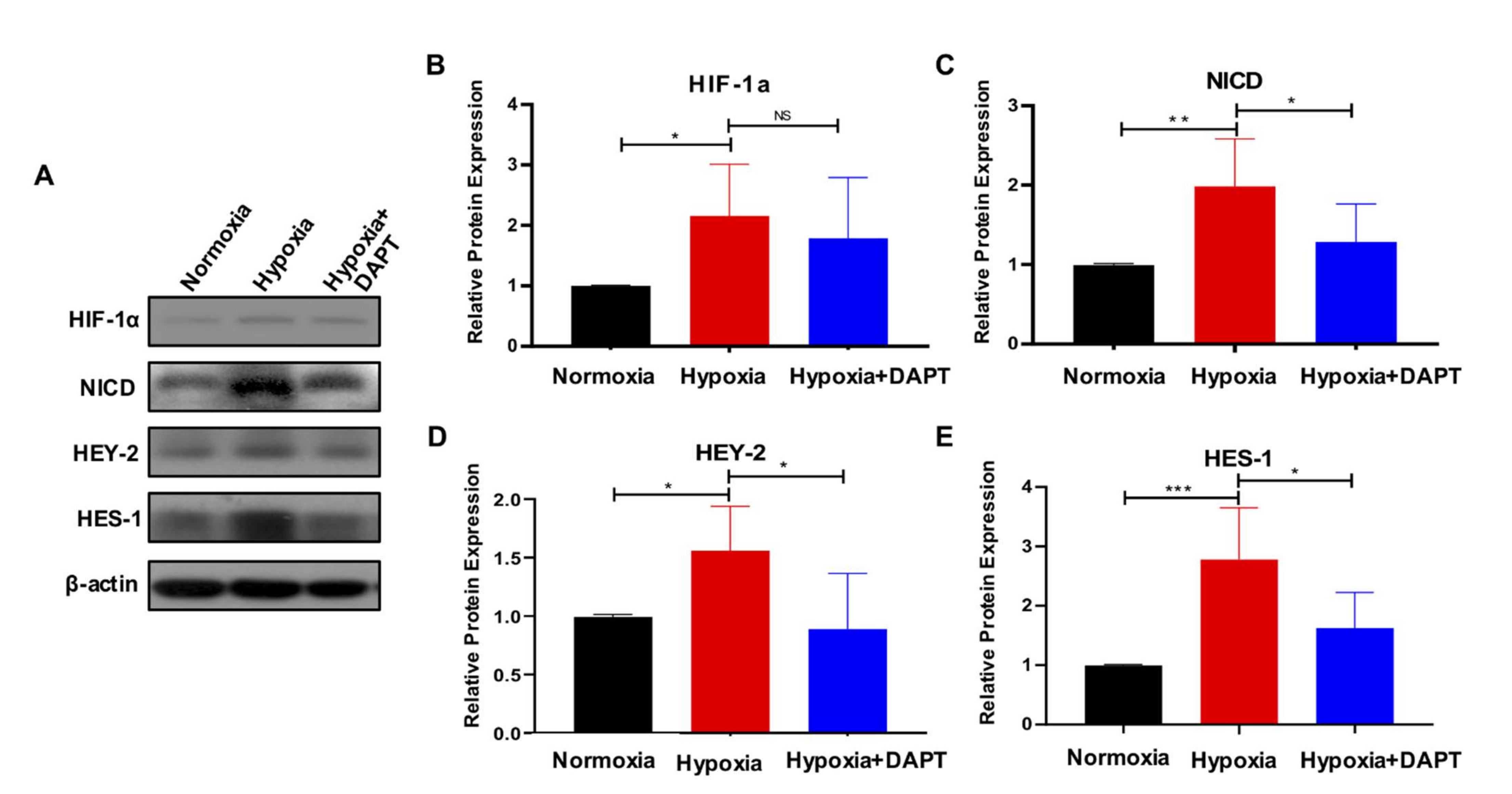
2.3. Hypoxia Activates the Notch Signaling in eMSC
2.4. Hypoxia Requires Notch Signaling to Drive the Maintenance of eMSC
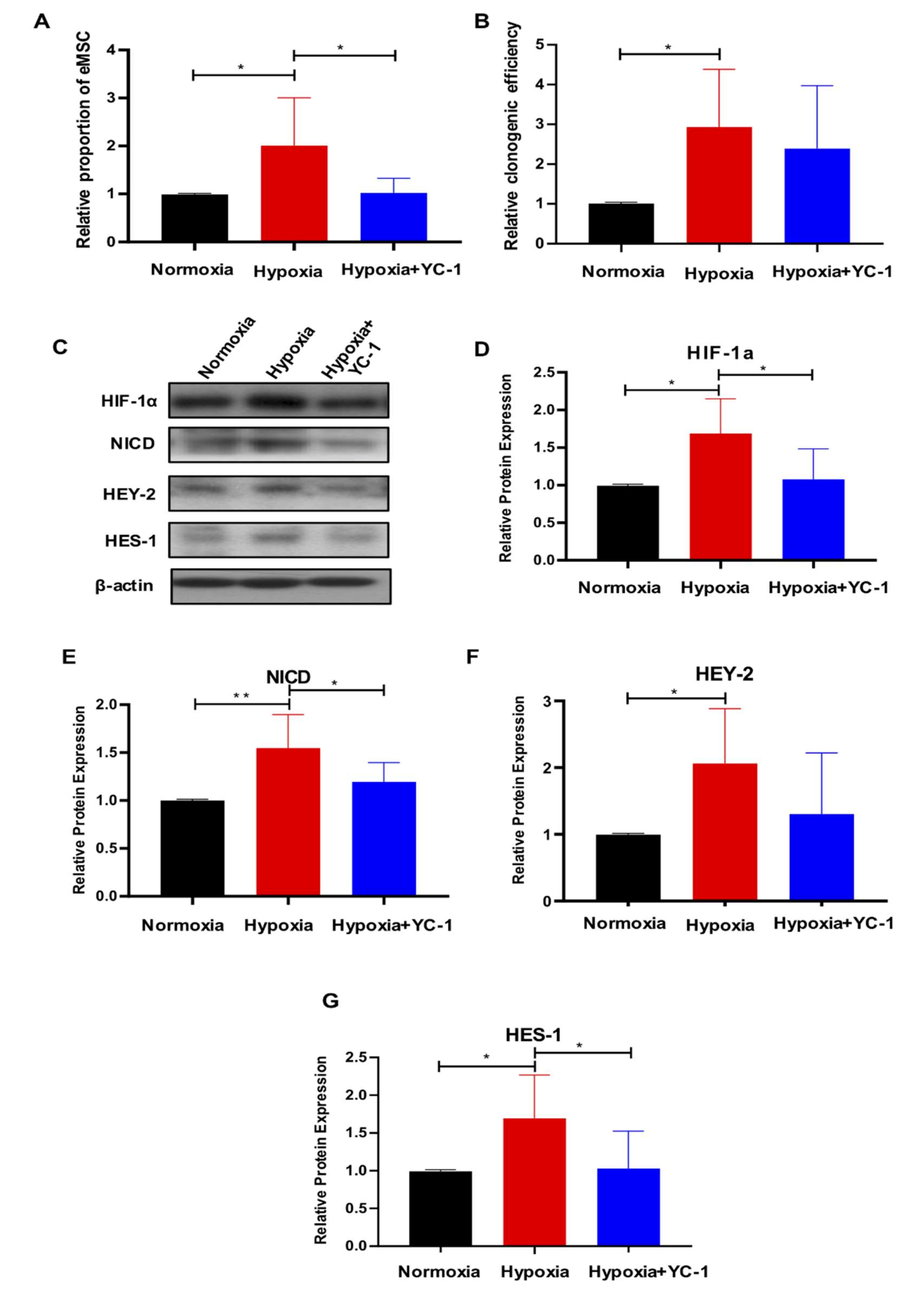
2.5. HIF-1a Is Essential for Hypoxia-Mediated Maintenance of eMSC by Activating Notch Signaling
3. Discussion
4. Materials and Methods
4.1. Animal and Housing Condition
4.2. Animal Study Design
4.3. Immunohistochemistry of Pimonidazole
4.4. Dual and Triple Immunofluorescence Staining
4.5. Human Endometrial Tissues
4.6. Isolation of Endometrial Stromal Cells
4.7. Magnetic Bead Selection for Endometrial Mesenchymal Stem-like Cells
4.8. Preparation of Human Endometrial Myometrial Cells
4.9. Preparation of Notch Signaling Inhibitor DAPT
4.10. Preparation of HIF-1α Inhibitor YC-1
4.11. Flow Cytometry
4.12. In Vitro Colony Forming Assay
4.13. In Vitro Serial Cloning
4.14. Cell Proliferation Assay
4.15. Western Blotting Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, G.M.; Jeffery, E.; Morrison, S.J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 2017, 17, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, A.N.; Lasry, A.; Austin, R.; Aifantis, I. Cell-by-Cell Deconstruction of Stem Cell Niches. Cell Stem Cell 2020, 27, 19–34. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalloul, A. Hypoxia and visualization of the stem cell niche. Methods Mol. Biol. 2013, 1035, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; van der Kooy, D. Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells 2009, 27, 1879–1886. [Google Scholar] [CrossRef] [Green Version]
- Eliasson, P.; Jönsson, J.I. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. J. Cell. Physiol. 2010, 222, 17–22. [Google Scholar] [CrossRef]
- Krinner, A.; Zscharnack, M.; Bader, A.; Drasdo, D.; Galle, J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009, 42, 471–484. [Google Scholar] [CrossRef]
- Liu, W.; Wen, Y.; Bi, P.; Lai, X.; Liu, X.S.; Liu, X.; Kuang, S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 2012, 139, 2857–2865. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Huang, G.; Chen, F.; Testroet, E.D.; Li, H.; Li, H.; Nong, T.; Yang, X.; Cui, J.; Shi, D.; et al. Hypoxia enhances buffalo adipose-derived mesenchymal stem cells proliferation, stemness, and reprogramming into induced pluripotent stem cells. J. Cell. Physiol. 2019, 234, 17254–17268. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical endometrial repair and regeneration. Development 2021, 148, dev199577. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markee, J.E. Menstruation in intraocular endometrial transplants in the Rhesus monkey. Am. J. Obstet. Gynecol. 1978, 131, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aguilar, R.; Kershaw, L.E.; Reavey, J.J.; Critchley, H.O.D.; Maybin, J.A. Hypoxia and Reproductive Health: The presence and role of hypoxia in the endometrium. Reproduction 2021, 161, F1–F17. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.A.; Murray, A.A.; Saunders, P.T.K.; Hirani, N.; Carmeliet, P.; Critchley, H.O.D. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wu, B.; Wang, S.; Liu, J.; Gao, H.; Zhou, F.; Nan, N.; Zhang, B.; Wang, J.; Xu, X.; et al. Hypoxia: Involved but not essential for endometrial breakdown in mouse menstural-like model. Reproduction 2020, 159, 133–144. [Google Scholar] [CrossRef]
- Coudyzer, P.; Lemoine, P.; Jordan, B.F.; Gallez, B.; Galant, C.; Nisolle, M.; Courtoy, P.J.; Henriet, P.; Marbaix, E. Hypoxia is not required for human endometrial breakdown or repair in a xenograft model of menstruation. FASEB J. 2013, 27, 3711–3719. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Chan, R.W.; Yeung, W.S. Label-retaining stromal cells in mouse endometrium awaken for expansion and repair after parturition. Stem Cells Dev. 2015, 24, 768–780. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, M.; Döcke, W.D.; Müller, A.; Menning, A.; Röse, L.; Zollner, T.M.; Gashaw, I. Induction of overt menstruation in intact mice. PLoS ONE 2012, 7, e32922. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sato, C.; Cerletti, M.; Wagers, A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010, 92, 367–409. [Google Scholar] [CrossRef]
- Qiang, L.; Wu, T.; Zhang, H.W.; Lu, N.; Hu, R.; Wang, Y.J.; Zhao, L.; Chen, F.H.; Wang, X.T.; You, Q.D.; et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012, 19, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, H.; Moriyama, M.; Isshi, H.; Ishihara, S.; Okura, H.; Ichinose, A.; Ozawa, T.; Matsuyama, A.; Hayakawa, T. Role of notch signaling in the maintenance of human mesenchymal stem cells under hypoxic conditions. Stem Cells Dev. 2014, 23, 2211–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, M.V.; Zheng, X.; Pereira, T.; Gradin, K.; Jin, S.; Lundkvist, J.; Ruas, J.L.; Poellinger, L.; Lendahl, U.; Bondesson, M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 2005, 9, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Main, H.; Lee, K.L.; Yang, H.; Haapa-Paananen, S.; Edgren, H.; Jin, S.; Sahlgren, C.; Kallioniemi, O.; Poellinger, L.; Lim, B.; et al. Interactions between Notch- and hypoxia-induced transcriptomes in embryonic stem cells. Exp. Cell Res. 2010, 316, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, J.A.; Calkins-Adams, D.P.; Rinker, L.H.; Ballenger, C.A.; Weissler, M.C.; Fowler, W.C., Jr.; Novotny, D.B.; Varia, M.A. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998, 58, 3765–3768. [Google Scholar] [PubMed]
- Aguilera, K.Y.; Brekken, R.A. Hypoxia Studies with Pimonidazole in vivo. Bio-Protocol 2014, 4, e1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottosen, L.D.; Hindkaer, J.; Husth, M.; Petersen, D.E.; Kirk, J.; Ingerslev, H.J. Observations on intrauterine oxygen tension measured by fibre-optic microsensors. Reprod. Biomed. Online 2006, 13, 380–385. [Google Scholar] [CrossRef]
- Cao, M.; Chan, R.W.S.; Cheng, F.H.C.; Li, J.; Li, T.; Pang, R.T.K.; Lee, C.L.; Li, R.H.W.; Ng, E.H.Y.; Chiu, P.C.N.; et al. Myometrial Cells Stimulate Self-Renewal of Endometrial Mesenchymal Stem-Like Cells Through WNT5A/β-Catenin Signaling. Stem Cells 2019, 37, 1455–1466. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Guzmán-Ocampo, D.C.; Dominguez, L. Toward the Characterization of DAPT Interactions with γ-Secretase. ChemMedChem 2019, 14, 1005–1010. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.A. HIF takes it up a notch. Sci. Signal. 2011, 4, pe33. [Google Scholar] [CrossRef]
- Zhang, J.; Salamonsen, L.A. Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum. Reprod. 2002, 17, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Krieg, S.; Kuo, C.J.; Wiegand, S.J.; Rabinovitch, M.; Druzin, M.L.; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008, 22, 3571–3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousins, F.L.; Murray, A.A.; Scanlon, J.P.; Saunders, P.T. Hypoxyprobe™ reveals dynamic spatial and temporal changes in hypoxia in a mouse model of endometrial breakdown and repair. BMC Res. Notes 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samal, J.R.K.; Rangasami, V.K.; Samanta, S.; Varghese, O.P.; Oommen, O.P. Discrepancies on the Role of Oxygen Gradient and Culture Condition on Mesenchymal Stem Cell Fate. Adv. Healthc. Mater. 2021, 10, e2002058. [Google Scholar] [CrossRef]
- Shivaraju, M.; Chitta, U.K.; Grange, R.M.H.; Jain, I.H.; Capen, D.; Liao, L.; Xu, J.; Ichinose, F.; Zapol, W.M.; Mootha, V.K.; et al. Airway stem cells sense hypoxia and differentiate into protective solitary neuroendocrine cells. Science 2021, 371, 52–57. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Sharp, T.V.; McKay, T.R. The role of hypoxia in stem cell potency and differentiation. Regen. Med. 2013, 8, 771–782. [Google Scholar] [CrossRef]
- Kheirandish, M.; Gavgani, S.P.; Samiee, S. The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. Transfus. Apher. Sci. 2017, 56, 392–399. [Google Scholar] [CrossRef]
- Boyette, L.B.; Creasey, O.A.; Guzik, L.; Lozito, T.; Tuan, R.S. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl. Med. 2014, 3, 241–254. [Google Scholar] [CrossRef]
- Liu, L.; Gao, J.; Yuan, Y.; Chang, Q.; Liao, Y.; Lu, F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol. Int. 2013, 37, 551–560. [Google Scholar] [CrossRef]
- Reavey, J.J.; Walker, C.; Nicol, M.; Murray, A.A.; Critchley, H.O.D.; Kershaw, L.E.; Maybin, J.A. Markers of human endometrial hypoxia can be detected in vivo and ex vivo during physiological menstruation. Hum. Reprod. 2021, 36, 941–950. [Google Scholar] [CrossRef]
- Itoh, M.; Okuhashi, Y.; Takahashi, Y.; Sonoda, Y.; Mohammad, S.; Saito, T.; Shiratori, E.; Tohda, S. Hypoxia Up-regulates HIF Expression While Suppressing Cell Growth and NOTCH Activity in Leukaemia Cells. Anticancer. Res. 2019, 39, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Park, J.S.; Manzanero, S.; Choi, Y.; Baik, S.H.; Okun, E.; Gelderblom, M.; Fann, D.Y.; Magnus, T.; Launikonis, B.S.; et al. Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol. Dis. 2014, 62, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, L.; Yu, M.; Yang, F.; Wu, B.; Lu, S.; Tu, M.; Xu, H. HIF-1α is Critical for the Activation of Notch Signaling in Neurogenesis During Acute Epilepsy. Neuroscience 2018, 394, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Martin, P.; Johnson, R.S. A new notch in the HIF belt: How hypoxia impacts differentiation. Dev. Cell 2005, 9, 575–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chan, R.W.S.; Ng, E.H.Y.; Yeung, W.S.B. Hypoxia Regulates the Self-Renewal of Endometrial Mesenchymal Stromal/Stem-like Cells via Notch Signaling. Int. J. Mol. Sci. 2022, 23, 4613. https://doi.org/10.3390/ijms23094613
Zhang S, Chan RWS, Ng EHY, Yeung WSB. Hypoxia Regulates the Self-Renewal of Endometrial Mesenchymal Stromal/Stem-like Cells via Notch Signaling. International Journal of Molecular Sciences. 2022; 23(9):4613. https://doi.org/10.3390/ijms23094613
Chicago/Turabian StyleZhang, Sisi, Rachel W.S. Chan, Ernest H.Y. Ng, and William S.B. Yeung. 2022. "Hypoxia Regulates the Self-Renewal of Endometrial Mesenchymal Stromal/Stem-like Cells via Notch Signaling" International Journal of Molecular Sciences 23, no. 9: 4613. https://doi.org/10.3390/ijms23094613
APA StyleZhang, S., Chan, R. W. S., Ng, E. H. Y., & Yeung, W. S. B. (2022). Hypoxia Regulates the Self-Renewal of Endometrial Mesenchymal Stromal/Stem-like Cells via Notch Signaling. International Journal of Molecular Sciences, 23(9), 4613. https://doi.org/10.3390/ijms23094613





