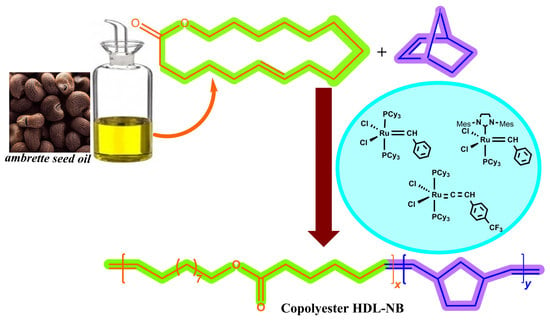
Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts
Abstract
1. Introduction
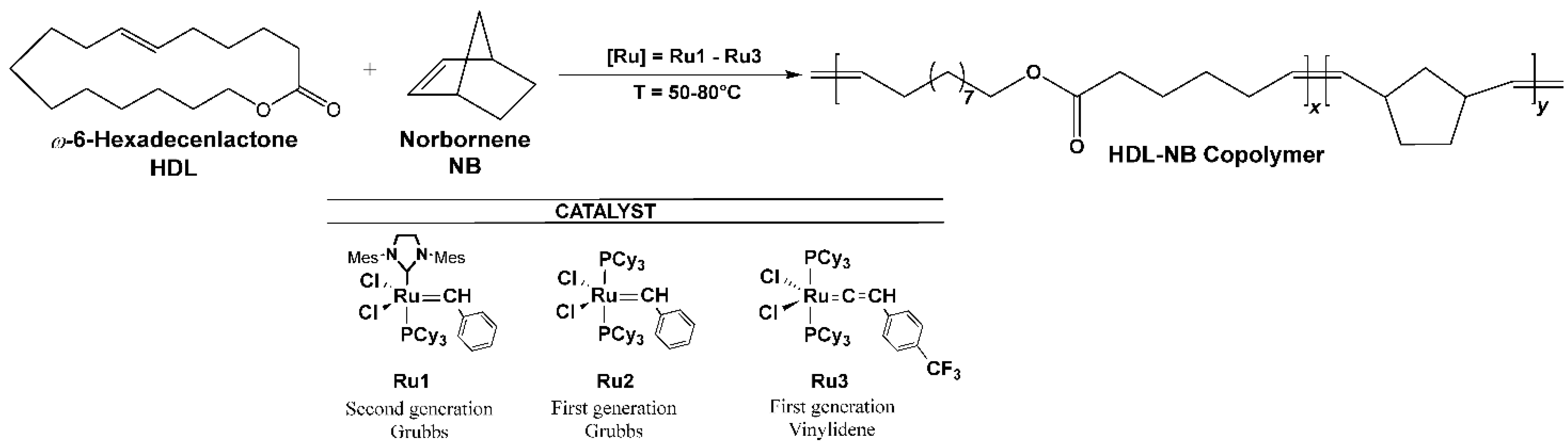
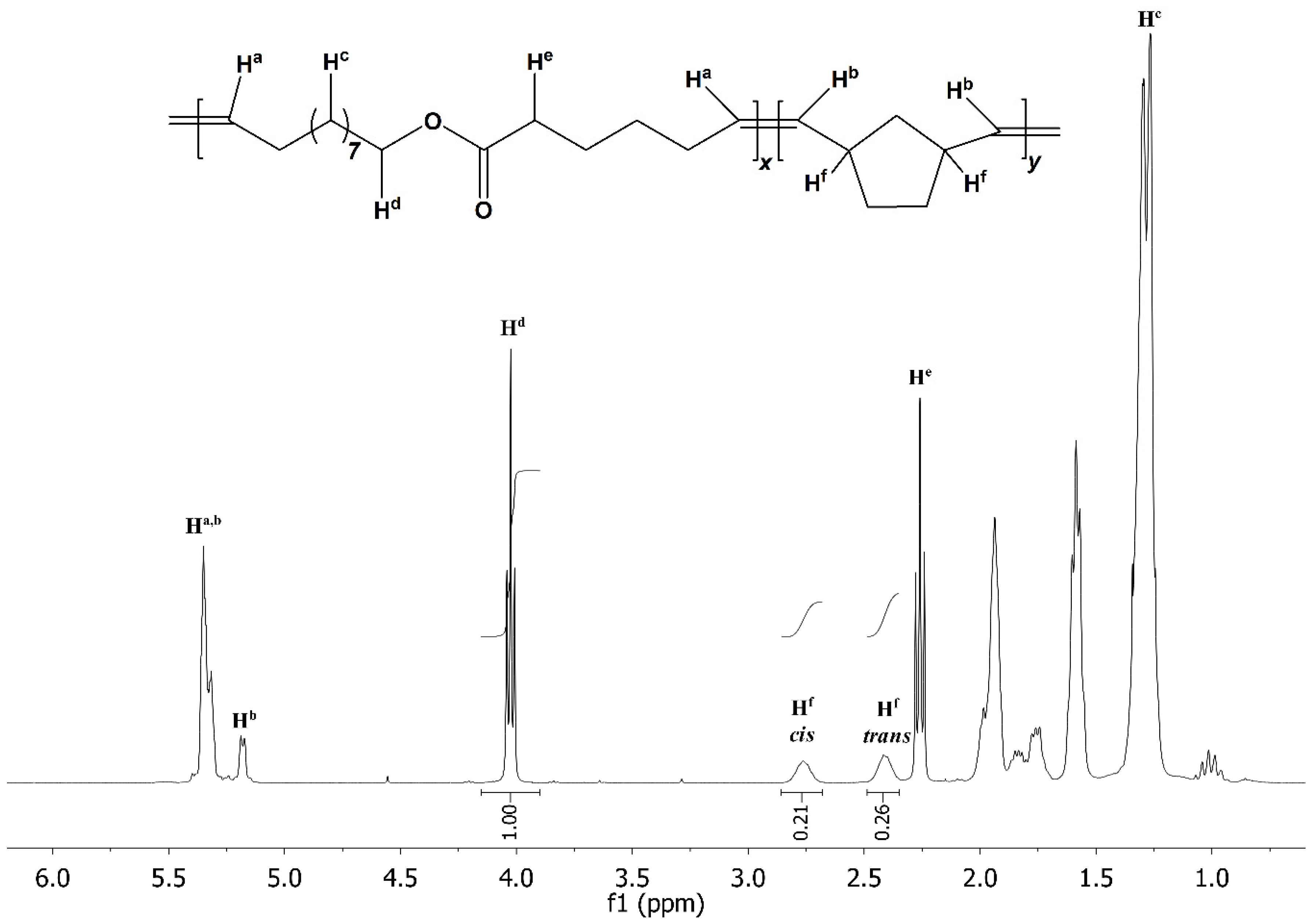
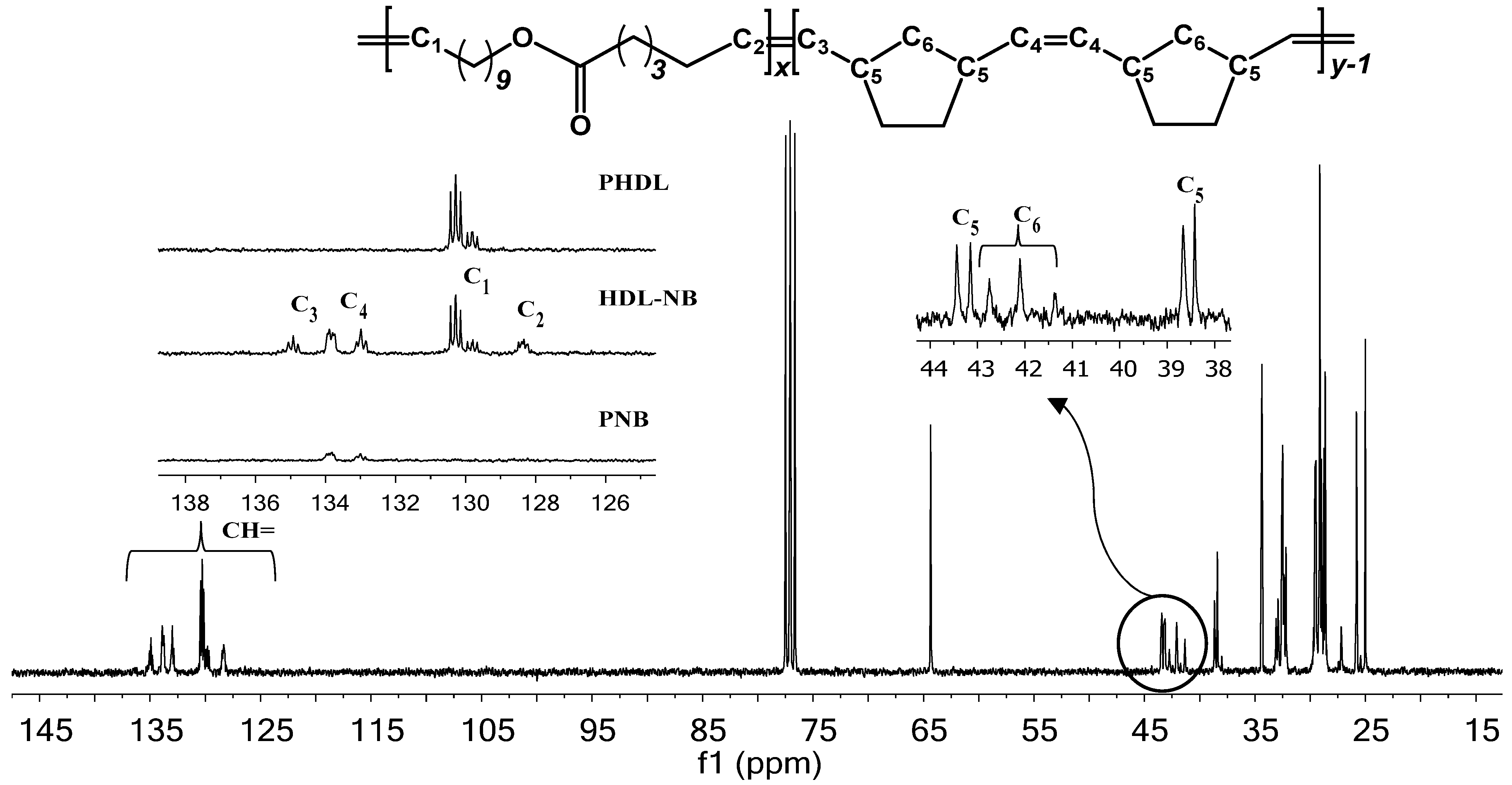
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Characterization Techniques
3.2. General Monomer Polymerization
3.2.1. Synthesis of Polynorbornene (PNB)
3.2.2. Synthesis of Poly(ω-6-Hexadecenlactone) (PHDL)
3.2.3. Synthesis of HDL-NB Copolymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pepels, M.P.F.; Govaert, L.E.; Duchateau, R. Influence of the Main-Chain Configuration on the Mechanical Properties of Linear Aliphatic Polyesters. Macromolecules 2015, 48, 5845–5854. [Google Scholar] [CrossRef]
- Stempfle, F.; Ortmann, P.; Mecking, S. Which polyesters can mimic polyethylene? Macromol. Rapid Commun. 2013, 34, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Hillmyer, M.A. High molar mass poly(ricinoleic acid): Via entropy-driven ring-opening metathesis polymerization. Polym. Chem. 2021, 12, 2253–2257. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Bouyahyi, M.; Heise, A.; Duchateau, R. Kinetic investigation on the catalytic ring-opening (co)polymerization of (macro)lactones using aluminum salen catalystsIt. Macromolecules 2012, 46, 4324–4334. [Google Scholar] [CrossRef]
- Fuoco, T.; Meduri, A.; Lamberti, M.; Venditto, V.; Pellecchia, C.; Pappalardo, D. Ring-opening polymerization of ω-6-hexadecenlactone by a salicylaldiminato aluminum complex: A route to semicrystalline and functional poly(ester)s. Polym. Chem. 2015, 6, 1727–1740. [Google Scholar] [CrossRef]
- Gong, S.; Du, P.; Ma, H. Binuclear aluminum complexes supported by linked bis(β-diketiminate) ligands for ring-opening polymerization of cyclic esters. Chin. J. Polym. Sci. 2018, 36, 190–201. [Google Scholar] [CrossRef]
- Bouyahyi, M.; Duchateau, R. Metal-Based Catalysts for Controlled Ring-Opening Polymerization of Macrolactones: High Molecular Weight and Well-Defined Copolymer Architectures. Macromolecules 2014, 47, 517–524. [Google Scholar] [CrossRef]
- D’Auria, I.; Santulli, F.; Ciccone, F.; Giannattasio, A.; Mazzeo, M.; Pappalardo, D. Synthesis of Semi-Aromatic Di-Block Polyesters by Terpolymerization of Macrolactones, Epoxides, and Anhydrides. ChemCatChem 2021, 13, 3303–3311. [Google Scholar] [CrossRef]
- Van der Meulen, I.; Li, Y.; Deumens, R.; Joosten, E.A.J.; Koning, C.E.; Heise, A. Copolymers from Unsaturated Macrolactones: Toward the Design of Cross-Linked Biodegradable Polyesters. Biomacromolecules 2011, 12, 837–843. [Google Scholar] [CrossRef]
- Witt, T.; Häußler, M.; Mecking, S. No Strain, No Gain? Enzymatic Ring-Opening Polymerization of Strainless Aliphatic Macrolactones. Macromol. Rapid Commun. 2017, 38, 1600638. [Google Scholar] [CrossRef]
- Tinajero-Díaz, E.; de Ilarduya, A.M.; Muñoz-Guerra, S. Copolymacrolactones Grafted with l-Glutamic Acid: Synthesis, Structure, and Nanocarrier Properties. Polymer 2020, 12, 995. [Google Scholar] [CrossRef]
- Tinajero-Díaz, E.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Synthesis and properties of diblock copolymers of ω-pentadecalactone and α-amino acids. Eur. Polym. J. 2019, 116, 169–179. [Google Scholar] [CrossRef]
- Martinez, A.; Tlenkopatchev, M.A.; Gutierrez, S. The Unsaturated Polyester Via Ring-Opening Metathesis Polymerization (ROMP) of ω-6-Hexadecenlactone. Curr. Org. Synth. 2018, 15, 566–571. [Google Scholar] [CrossRef]
- Wei, T.; Rempel, G.L.; Pan, Q.-M.; Jiang, B.; Zou, T.-T. A Novel Approval for Degradation of Polybutadiene and Synthesis of Diene-Based Telechelic Oligomers via Olefin Cross Metathesis. Macromol. React. Eng. 2015, 9, 480–489. [Google Scholar] [CrossRef]
- Fürstner, A.; Langemann, K. Conformationally unbiased macrocyclization reactions by ring closing metathesis. J. Org. Chem. 1996, 61, 3942–3943. [Google Scholar] [CrossRef]
- Manzini, B.; Hodge, P.; Ben-Haida, A. Entropically-driven ring-opening polymerization of macrocyclic esters with up to 84-membered rings catalysed by polymer-supported Candida antarctica lipase B. Polym. Chem. 2010, 1, 339–346. [Google Scholar] [CrossRef]
- Hodge, P.; Colquhoun, H.M. Recent work on entropically-driven ring-opening polymerizations: Some potential applications. Polym. Adv. Technol. 2005, 16, 84–94. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Hansen, M.R.; Goossens, H.; Duchateau, R. From polyethylene to polyester: Influence of ester groups on the physical properties. Macromolecules 2013, 46, 7668–7677. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Yang, J.; Ren, L.; Li, Y. Ring-opening metathesis polymerization of cis-5-norbornene-endo-2,3-dicarboxylic anhydride derivatives using the grubbs third generation catalyst. Chin. J. Polym. Sci. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Lyapkov, A.; Kiselev, S.; Bozhenkova, G.; Kukurina, O.; Yusubov, M.; Verpoort, F. Ring Opening Metathesis Polymerization. Recent Res. Polym. 2018, 2018, 43. [Google Scholar] [CrossRef][Green Version]
- Yasir, M.; Liu, P.; Markwart, J.C.; Suraeva, O.; Wurm, F.R.; Smart, M.; Lattuada, J.; Kilbinger, A.F.M. One-Step Ring Opening Metathesis Block-Like Copolymers and their Compositional Analysis by a Novel Retardation Technique. Angew. Chemie Int. Ed. 2020, 59, 13597–13601. [Google Scholar] [CrossRef] [PubMed]
- Gringolts, M.L.; Denisova, Y.I.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Olefin metathesis in multiblock copolymer synthesis, Beilstein. J. Org. Chem. 2019, 15, 218–235. [Google Scholar] [CrossRef]
- Fernandes, H.; Filgueiras, J.G.; de Azevedo, E.R.; Lima-Neto, B.S. Real time monitoring by time-domain NMR of ring opening metathesis copolymerization of norbornene-based red palm olein monomer with norbornene. Eur. Polym. J. 2020, 140, 110048. [Google Scholar] [CrossRef]
- Paradiso, V.; Grisi, F. Ruthenium-Catalyzed Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-ene with Cyclic Olefins. Adv. Synth. Catal. 2019, 361, 4133–4139. [Google Scholar] [CrossRef]
- Vasiuta, R.; Stockert, A.; Plenio, H. Alternating ring-opening metathesis polymerization by Grubbs-type catalysts with: N-pentiptycenyl, N-alkyl-NHC ligands. Chem. Commun. 2018, 54, 1706–1709. [Google Scholar] [CrossRef]
- Song, A.; Parker, K.A.; Sampson, N.S. Synthesis of Copolymers by Alternating ROMP (AROMP). J. Am. Chem. Soc. 2009, 131, 3444–3445. [Google Scholar] [CrossRef]
- Yasir, M.; Kilbinger, A.F.M. Cascade Ring-Opening/Ring-Closing Metathesis Polymerization of a Monomer Containing a Norbornene and a Cyclohexene Ring. ACS Macro Lett. 2021, 10, 210–214. [Google Scholar] [CrossRef]
- Gringolts, M.L.; Denisova, Y.I.; Shandryuk, G.A.; Krentsel, L.B.; Litmanovich, A.D.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Synthesis of norbornene-cyclooctene copolymers by the cross-metathesis of polynorbornene with polyoctenamer. RSC Adv. 2015, 5, 316–319. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Hawker, C.J. A General Approach to Sequence-Controlled Polymers Using Macrocyclic Ring Opening Metathesis Polymerization. J. Am. Chem. Soc. 2015, 137, 8038–8041. [Google Scholar] [CrossRef]
- Deng, L.L.; Guo, L.X.; Lin, B.P.; Zhang, X.Q.; Sun, Y.; Yang, H. An entropy-driven ring-opening metathesis polymerization approach towards main-chain liquid crystalline polymers. Polym. Chem. 2016, 7, 5265–5272. [Google Scholar] [CrossRef]
- Xue, Z.; Mayer, M.F. Entropy-driven ring-opening olefin metathesis polymerizations of macrocycles. Soft Matter. 2009, 5, 4600–4611. [Google Scholar] [CrossRef]
- Pearce, A.K.; Foster, J.C.; O’Reilly, R.K. Recent developments in entropy-driven ring-opening metathesis polymerization: Mechanistic considerations, unique functionality, and sequence control. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1621–1634. [Google Scholar] [CrossRef]
- Yang, Y.; Swager, T.M. Main-chain calix[4]arene elastomers by ring-opening metathesis polymerization. Macromolecules 2007, 40, 7437–7440. [Google Scholar] [CrossRef]
- Martínez, A.; Clark-Tapia, R.; Gutierrez, S.; Tlenkopatchev, M. Synthesis and Characterization of New Ruthenium Vinylidene Complexes. Lett. Org. Chem. 2014, 11, 748–754. [Google Scholar] [CrossRef]
- Justin, R.; Griffiths, J.R.; Diver, S.T. Factors Affecting Initiation Rates. In Handbook of Metathesis, 2nd ed.; Grubbs, R.H., Wenzel, A.G., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Berlin, Germany, 2015; Volume 2, pp. 273–279. [Google Scholar] [CrossRef]
- Mayo, F.R.; Lewis, F.M.; Copolymerization, I. A Basis for Comparing the Behavior of Monomers in Copolymerization; The Copolymerization of Styrene and Methyl Methacrylate. J. Am. Chem. Soc. 1944, 66, 1594–1601. [Google Scholar] [CrossRef]
- Fineman, M.; Ross, S.D. Linear Method for Determining Monomer Reactivity Ratios in Copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Erbil, C.; Özdemir, S.; Uyanik, N. Determination of the monomer reactivity ratios for copolymerization of itaconic acid and acrylamide by conductometric titration method. Polymer 2000, 41, 1391–1394. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Wiliiams, J.E.; Blanchard, K.R. Evaluation of strain in hydrocarbons. The strain in adamantane and its origin. J. Am. Chem. Soc. 1970, 92, 2377–2386. [Google Scholar] [CrossRef]
- Hlil, A.R.; Balogh, J.; Moncho, S.; Su, H.L.; Tuba, R.; Brothers, E.N.; Al-Hashimi, M.; Bazzi, H.S. Ring opening metathesis polymerization (ROMP) of five- to eight-membered cyclic olefins: Computational, thermodynamic, and experimental approach. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3137–3145. [Google Scholar] [CrossRef]
- Hodge, P. Entropically driven ring-opening polymerization of strainless organic macrocycles. Chem. Rev. 2014, 114, 2278–2312. [Google Scholar] [CrossRef] [PubMed]
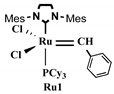
| Entry a | Molar Ratio b | [Ru] | [C=C] c [Ru] | Time (h) | Temp (°C) | Yield (%) d | Mne (g mol−1) | MWD e | HDL/NB Expected wt. % | HDL/NB Measured f wt. % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HDL NB | |||||||||||
| 1 | 1 | 0 | Ru1 | 500 | 3 | 50 | 96 | 109,600 | 1.15 | -- | -- |
| 2 | 0 | 1 | Ru1 | 1000 | 40 min | 25 | 99 | 134,000 | 1.10 | -- | -- |
| 3 | 1 | 1 | Ru1 | 500 | 4 min | 50 | 22 | 78,876 | 2.80 | -- | 39/61 |
| 4 | 1 | 1 | Ru1 | 500 | 30 min | 50 | 36 | 81,500 | 2.90 | -- | 42/58 |
| 5 | 1 | 1 | Ru1 | 500 | 3 | 50 | 49 | 88,677 | 2.78 | -- | 57/43 |
| 6 | 1 | 1 | Ru1 | 500 | 8 | 50 | 72 | 91,981 | 2.84 | -- | 61/39 |
| 7 | 1 | 1 | Ru1 | 500 | 20 | 50 | 97 | 104,424 | 2.10 | 74/26 | 72/28 |
| 8 | 1 | 1 | Ru2 | 250 | 20 | 50 | 66 | 94,210 | 2.00 | 74/26 | 70/30 |
| 9 | 1 | 1 | Ru3 | 250 | 20 | 80 | 57 | 92,005 | 2.09 | 74/26 | 69/31 |
| 10 | 1 | 5 | Ru1 | 500 | 20 | 50 | 96 | 110,500 | 2.20 | 35/65 | 32/68 |
| 11 | 1 | 10 | Ru1 | 500 | 20 | 50 | 96 | 118,080 | 2.00 | 20/80 | 17/83 |
| 12 | 2 | 1 | Ru1 | 500 | 20 | 50 | 98 | 114,600 | 2.10 | 84/16 | 82/18 |
| 13 | 2 | 1 | Ru2 | 250 | 20 | 50 | 53 | 90,600 | 2.10 | 84/16 | 79/21 |
| 14 | 2 | 1 | Ru3 | 250 | 20 | 80 | 42 | 88,500 | 2.38 | 84/16 | 77/23 |
| 15 | 3 | 1 | Ru1 | 500 | 20 | 50 | 97 | 115,000 | 2.10 | 89/11 | 87/13 |
| 16 | 10 | 1 | Ru1 | 500 | 20 | 50 | 98 | 113,790 | 2.00 | 91/9 | 89/11 |
| Entry | Mass of HDL in the Feed (g) | [HDL] a [NB] | Mol % of HDL in the Feed b | Incorporation of HDL in Copolymer (%) c | Time (h) | Yield d (%) |
|---|---|---|---|---|---|---|
| Second-Generation Grubbs (Ru1) | ||||||
| 1 | 0.48 | 1:1 | 50 | 39.00 | 4 min | 22.10 |
| 2 | 0.48 | 1.5:1 | 60 | 48.00 | 4 min | 19.60 |
| 3 | 0.48 | 2:1 | 67 | 62.00 | 6 min | 16.50 |
| 4 | 0.48 | 3:1 | 75 | 74.00 | 6 min | 8.40 |
| 5 | 0.48 | 10:1 | 91 | 86.00 | 10 min | 5.30 |
| First-Generation Grubbs (Ru2) | ||||||
| 6 | 0.48 | 1:1 | 50 | 35.40 | 2 | 19.50 |
| 7 | 0.48 | 1.5:1 | 60 | 42.90 | 2 | 15.40 |
| 8 | 0.48 | 2:1 | 67 | 48.00 | 4 | 13.40 |
| 9 | 0.48 | 3:1 | 75 | 61.30 | 4 | 10.20 |
| 10 | 0.48 | 10:1 | 91 | 78.90 | 7 | 7.60 |
| First-Generation Vinylidene (Ru3) | ||||||
| 11 | 0.48 | 1:1 | 50 | 27.50 | 2 | 15.70 |
| 12 | 0.48 | 1.5:1 | 60 | 43.40 | 2 | 13.30 |
| 13 | 0.48 | 2:1 | 67 | 47.10 | 4 | 12.30 |
| 14 | 0.48 | 3:1 | 75 | 59.40 | 4 | 10.10 |
| 15 | 0.48 | 10:1 | 91 | 77.60 | 7 | 7.20 |
| Mayo–Lewis Method | Finemann–Ross Method | |||
|---|---|---|---|---|
| Catalyst | rHDA | rNB | rHDA | rNB |
 | 0.10 | 5.60 | 0.12 | 5.81 |
 | 0.24 | 3.78 | 0.28 | 4.02 |
 | 0.06 | 4.47 | 0.07 | 4.30 |
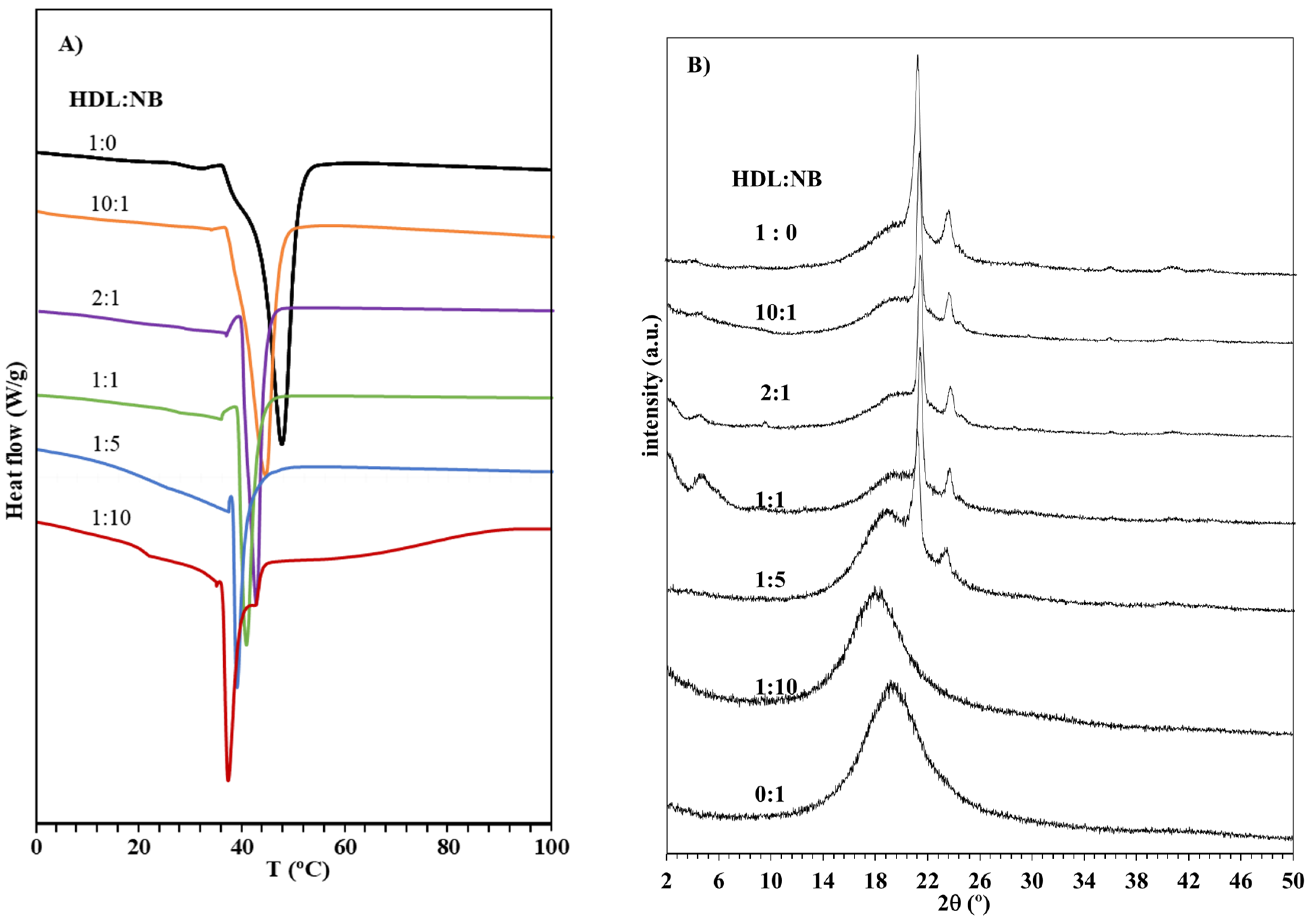
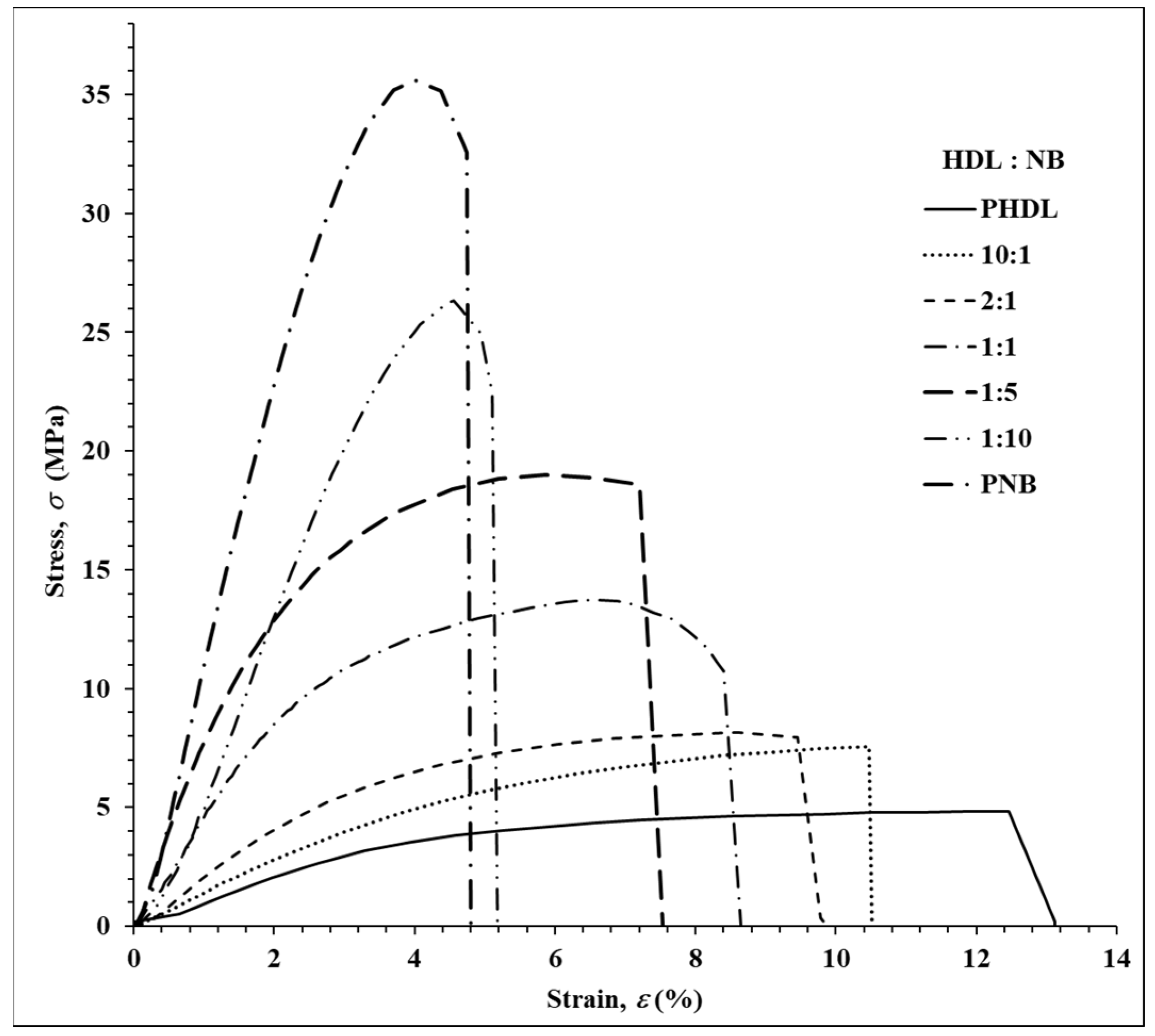
| Entry | Molar Ratio a | Thermal Properties | Crystallinity X-ray e | Mechanical Properties f | |||||
|---|---|---|---|---|---|---|---|---|---|
| HDL | NB | Tm b (°C) | Td c (°C) | ΔHm d (J/g) | (%) | E (MPa) | σ (MPa) | ε (%) | |
| 1 | 0 | 1 | -- | 418 | -- | -- | 1280 | 35.60 | 4.00 |
| 2 | 1 | 0 | 47.60 | 384 | 73.00 | 31.00 | 119 | 4.84 | 12.50 |
| 3 | 10 | 1 | 44.34 | 390 | 53.50 | 26.50 | 156 | 7.55 | 10.47 |
| 4 | 2 | 1 | 42.10 | 397 | 47.10 | 23.00 | 229 | 8.13 | 8.637 |
| 5 | 1 | 1 | 40.50 | 400 | 43.30 | 19.80 | 464 | 13.73 | 6.40 |
| 6 | 1 | 5 | 38.10 | 409 | 23.20 | 15.90 | 695 | 19.01 | 5.87 |
| 7 | 1 | 10 | 37.20 | 411 | 12.00 | -- | 775 | 26.33 | 4.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Zárate-Saldaña, D.; Vargas, J.; Santiago, A.A. Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts. Int. J. Mol. Sci. 2022, 23, 4521. https://doi.org/10.3390/ijms23094521
Martínez A, Zárate-Saldaña D, Vargas J, Santiago AA. Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts. International Journal of Molecular Sciences. 2022; 23(9):4521. https://doi.org/10.3390/ijms23094521
Chicago/Turabian StyleMartínez, Araceli, Daniel Zárate-Saldaña, Joel Vargas, and Arlette A. Santiago. 2022. "Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts" International Journal of Molecular Sciences 23, no. 9: 4521. https://doi.org/10.3390/ijms23094521
APA StyleMartínez, A., Zárate-Saldaña, D., Vargas, J., & Santiago, A. A. (2022). Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts. International Journal of Molecular Sciences, 23(9), 4521. https://doi.org/10.3390/ijms23094521