Nickel(II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption
Abstract
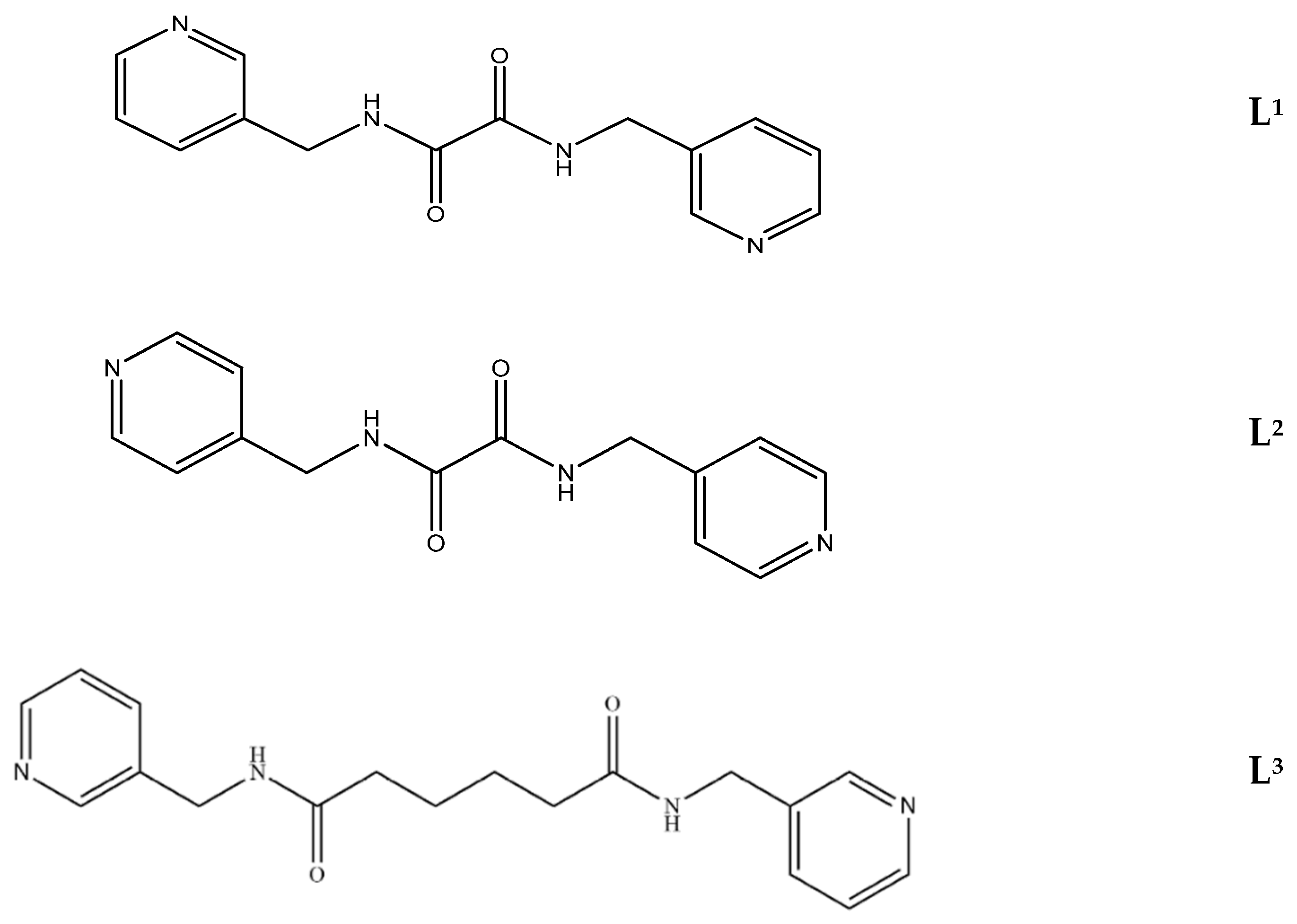
:1. Introduction
2. Results and Discussion
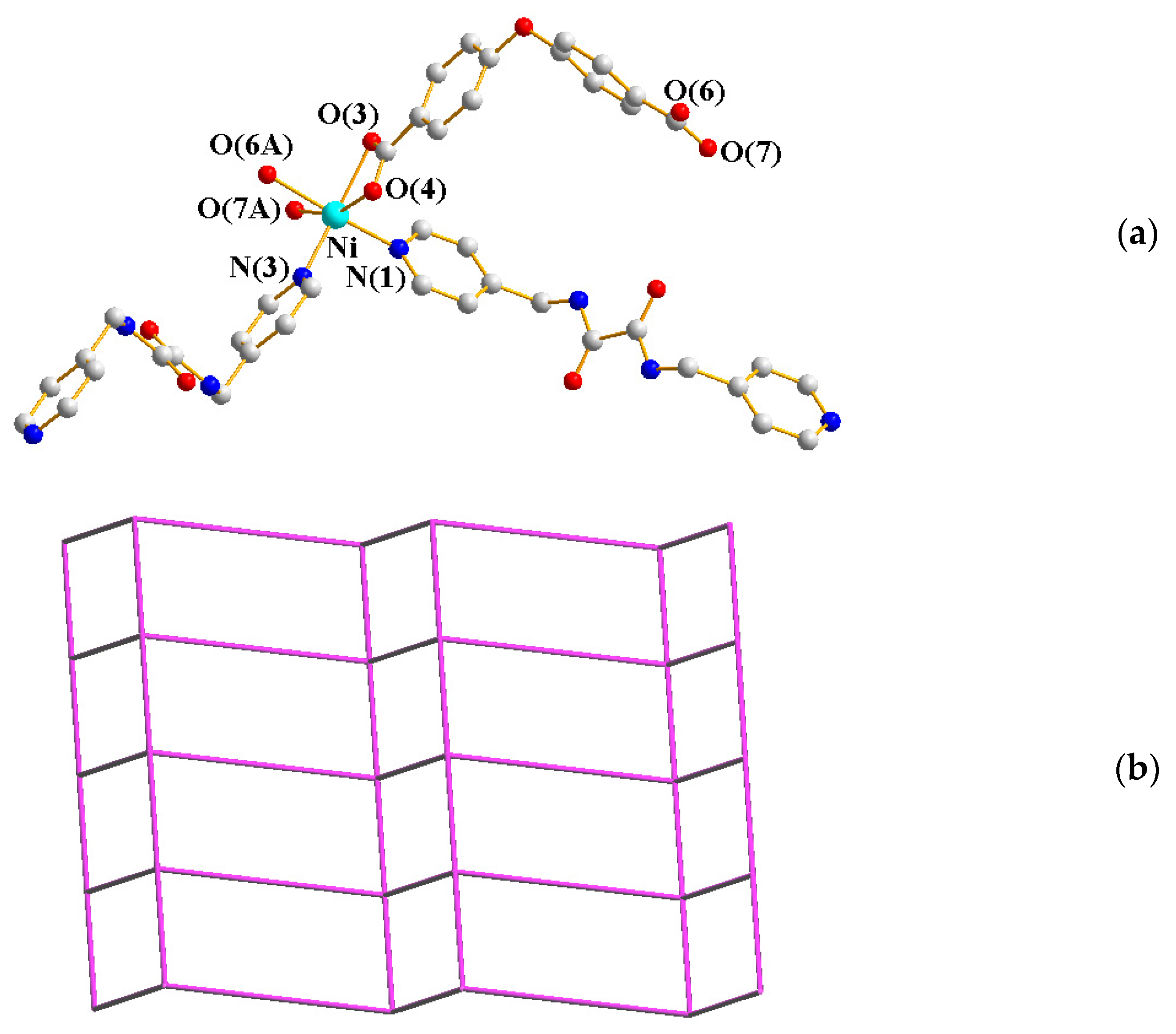
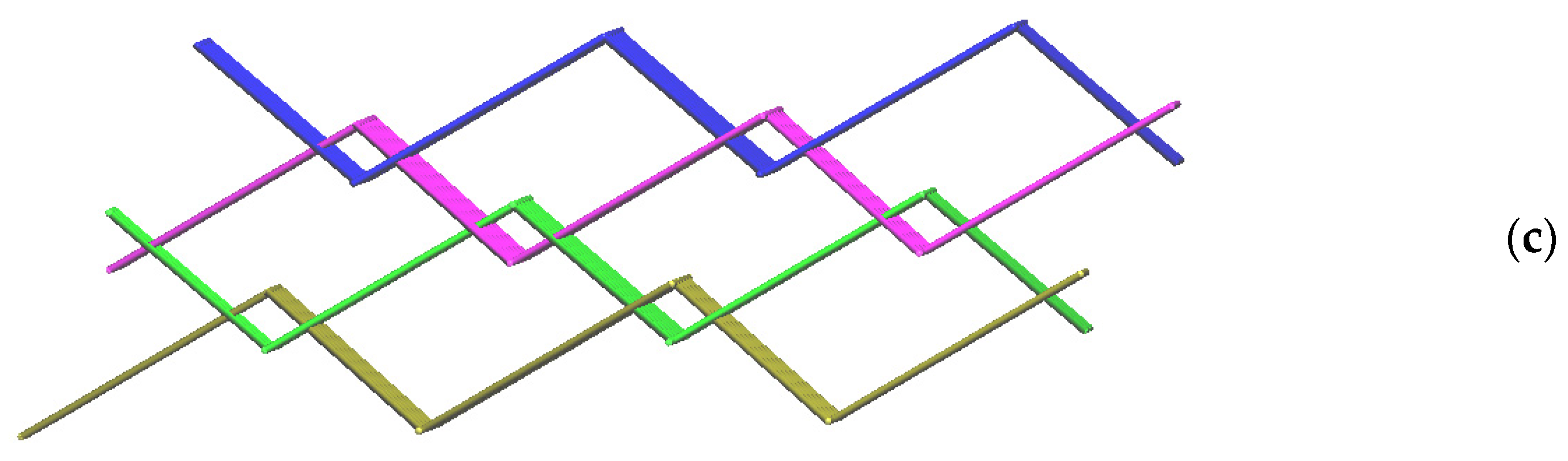
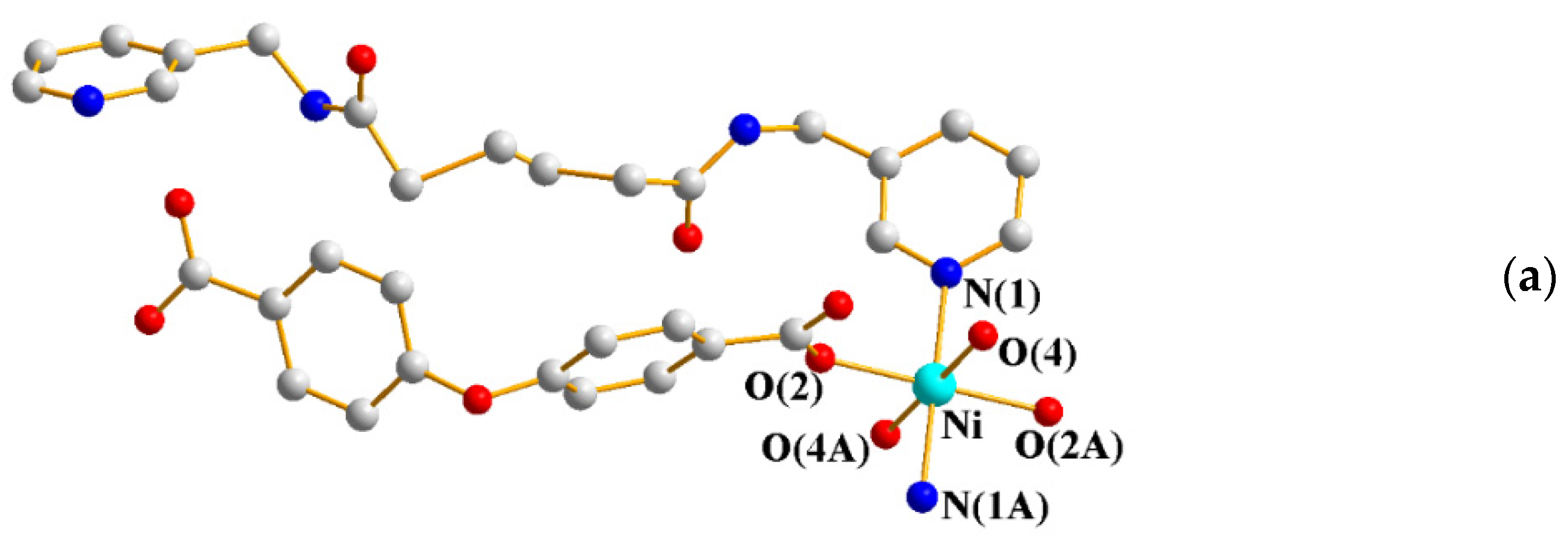
2.1. Crystal Structure of 1
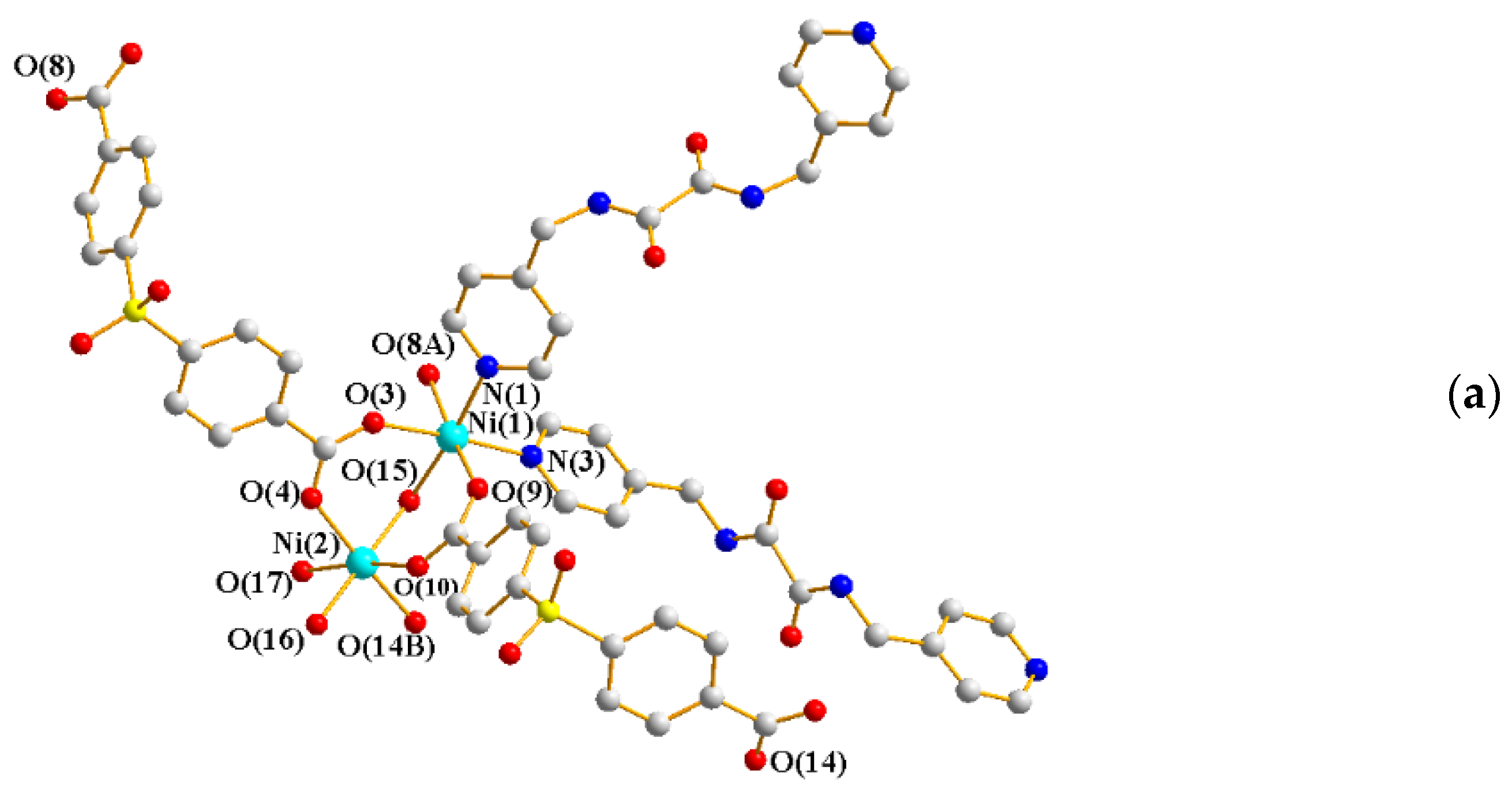
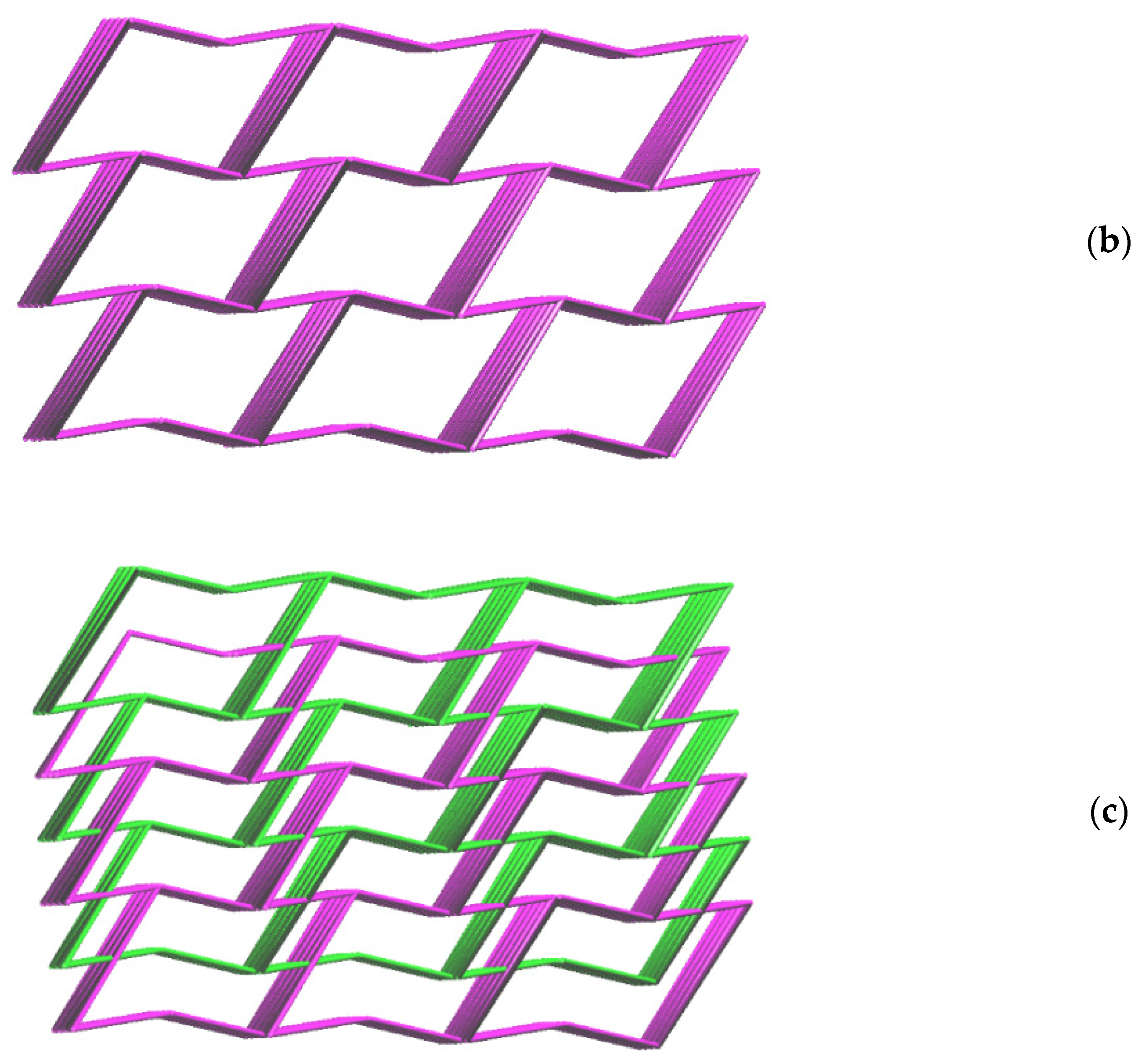
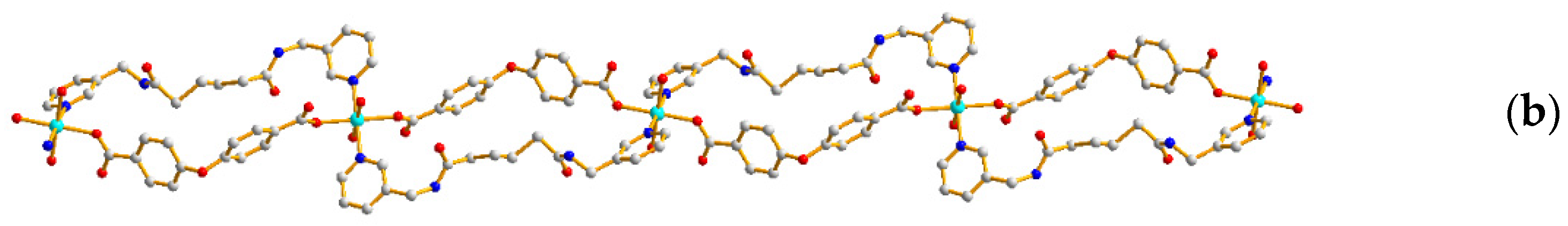
2.2. Structure of 2
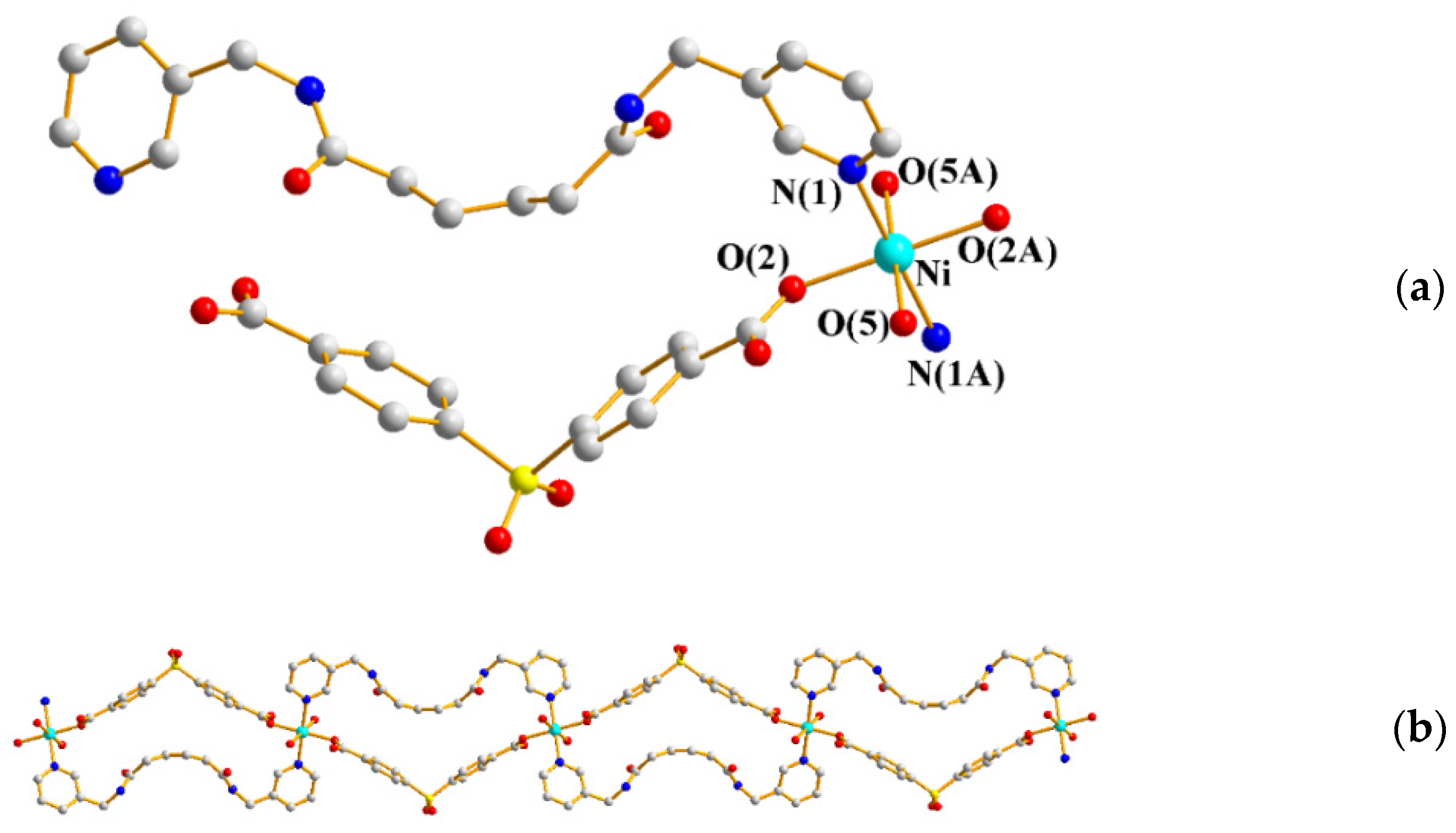
2.3. Structures of 3 and 4
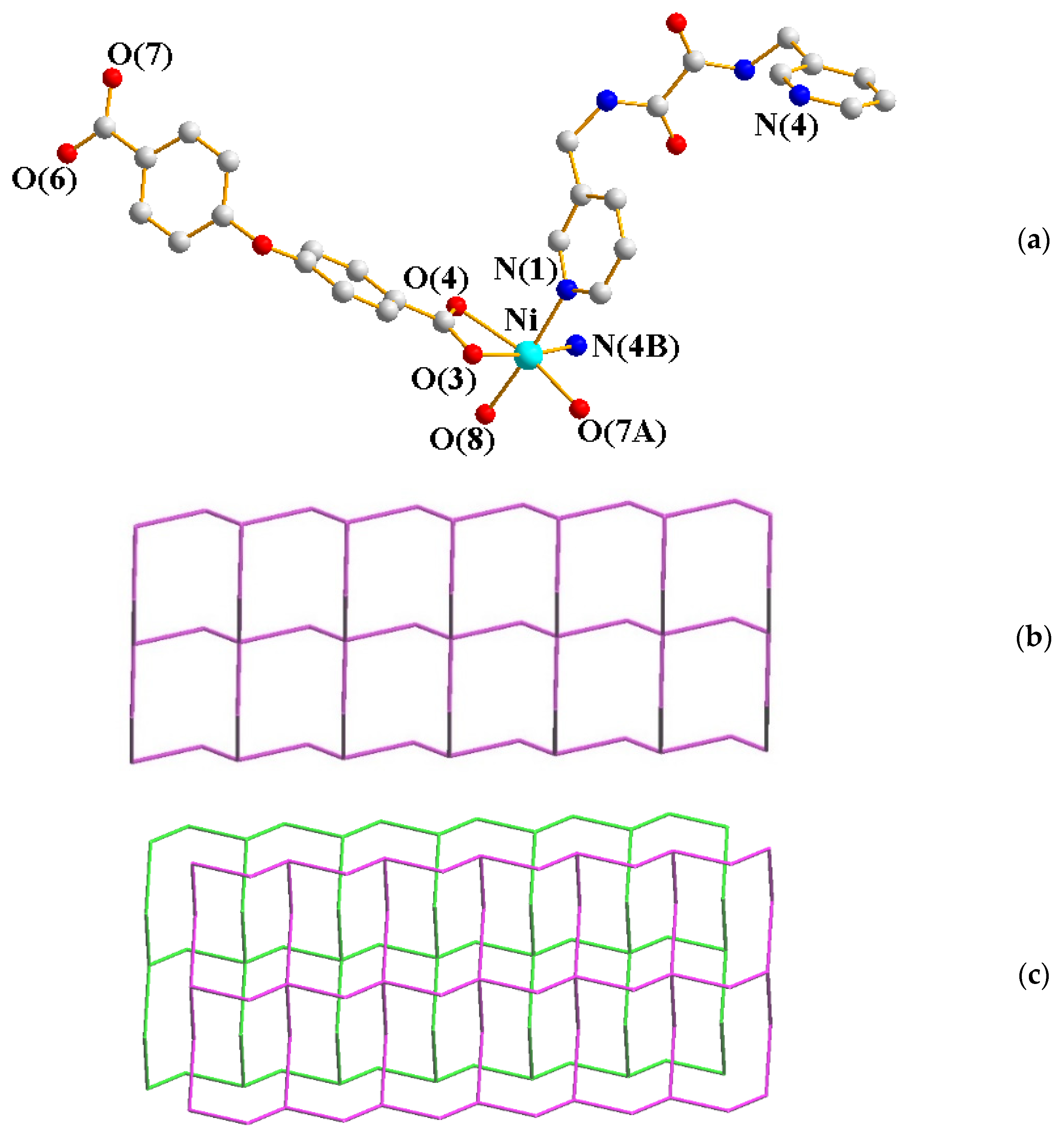
2.4. Structure of 5 and 6
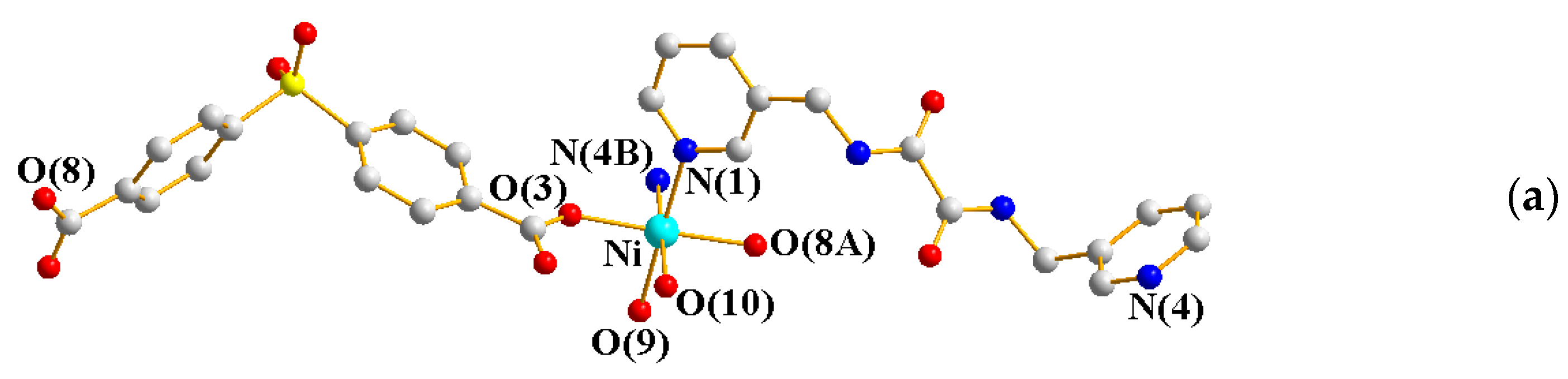
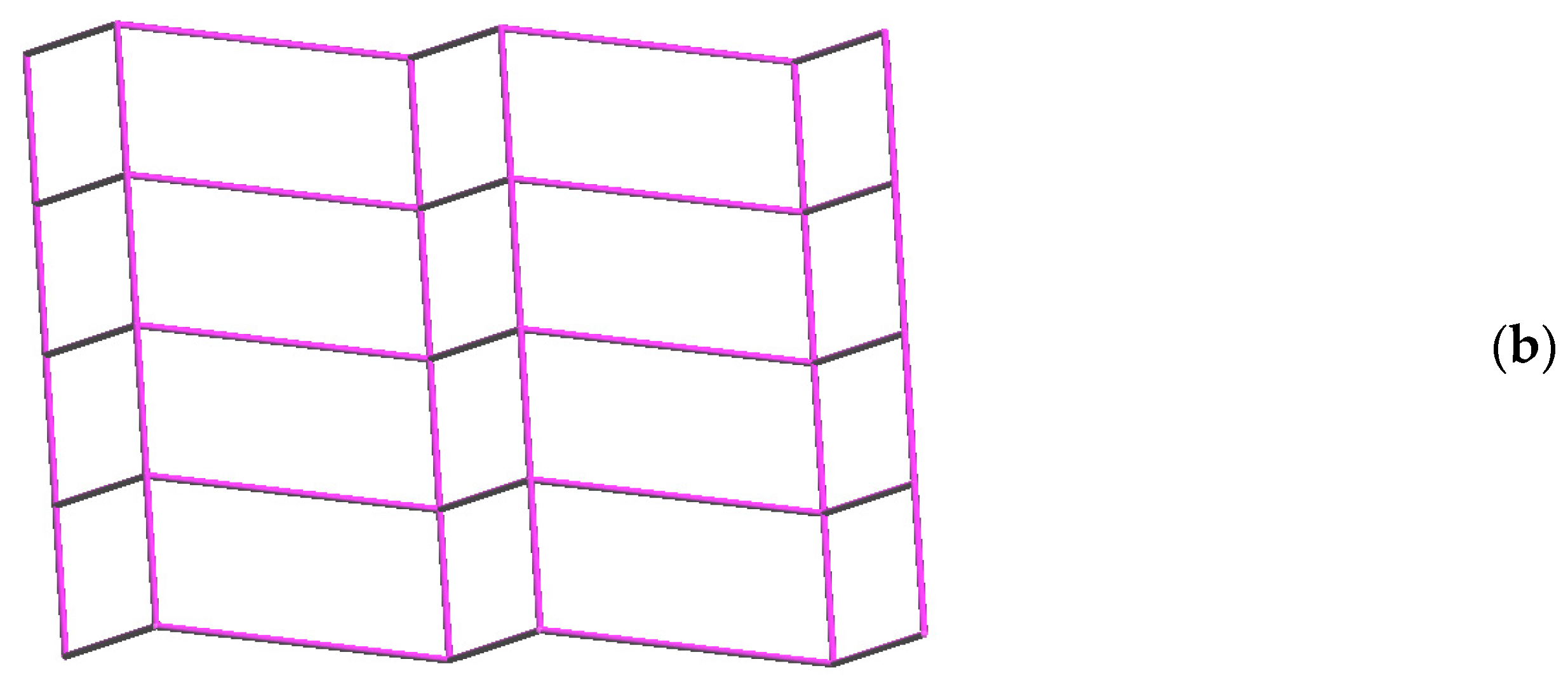
2.5. Structure of 7
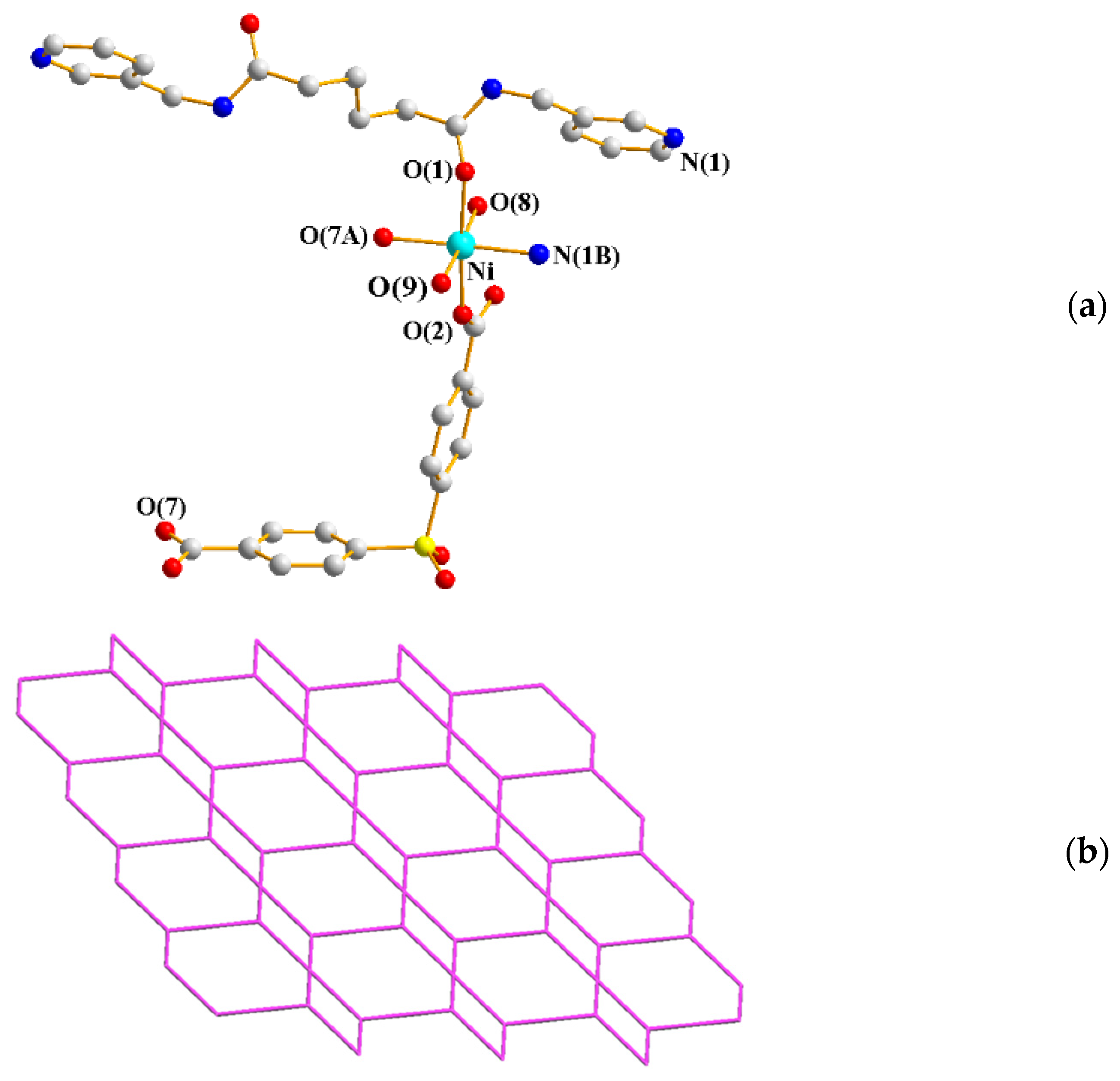
2.6. Structure of 8
2.7. Structure of 9
2.8. Ligand Conformations and Coordination Modes
2.9. PXRD Patterns and Thermal Analysis
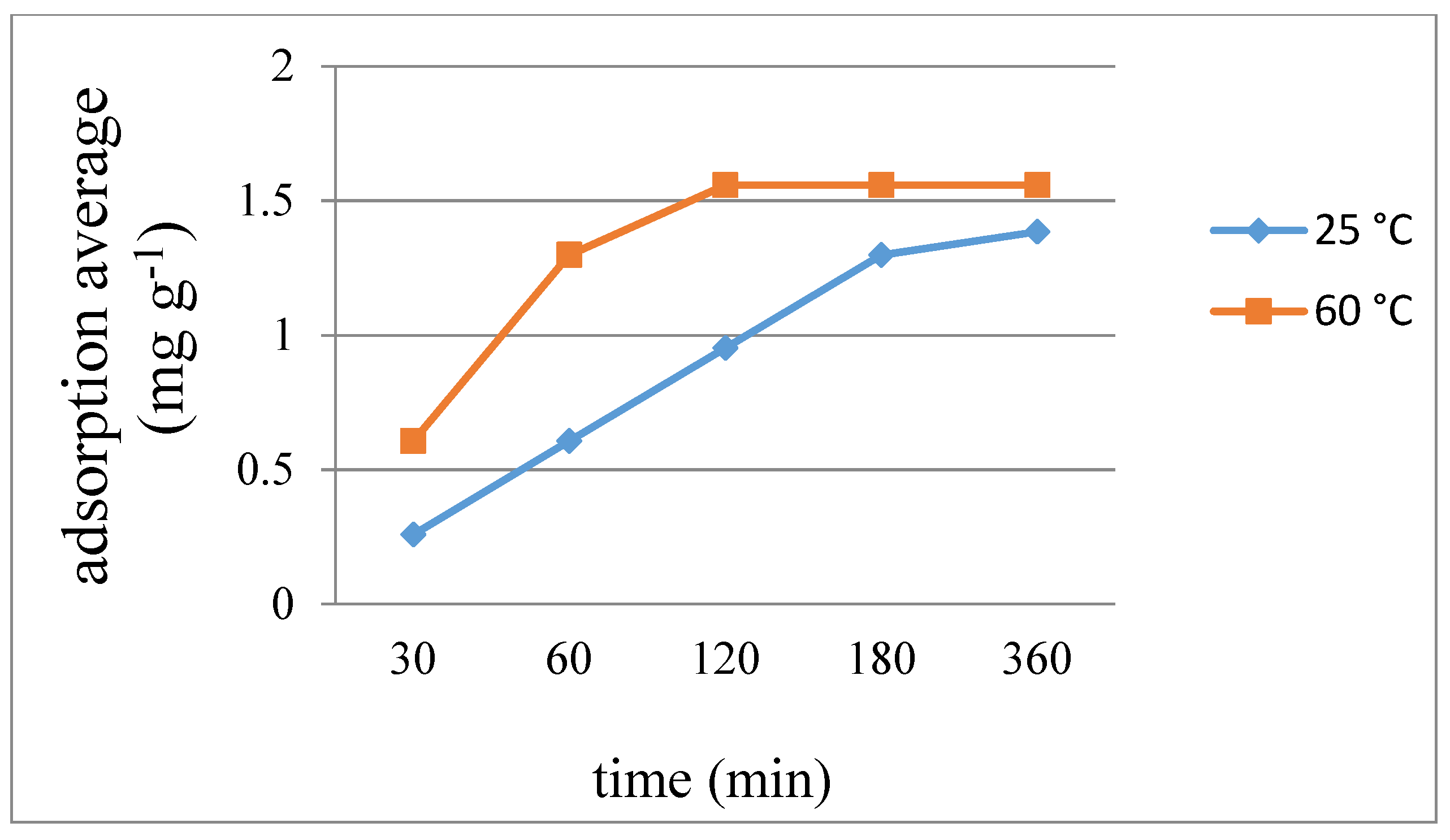
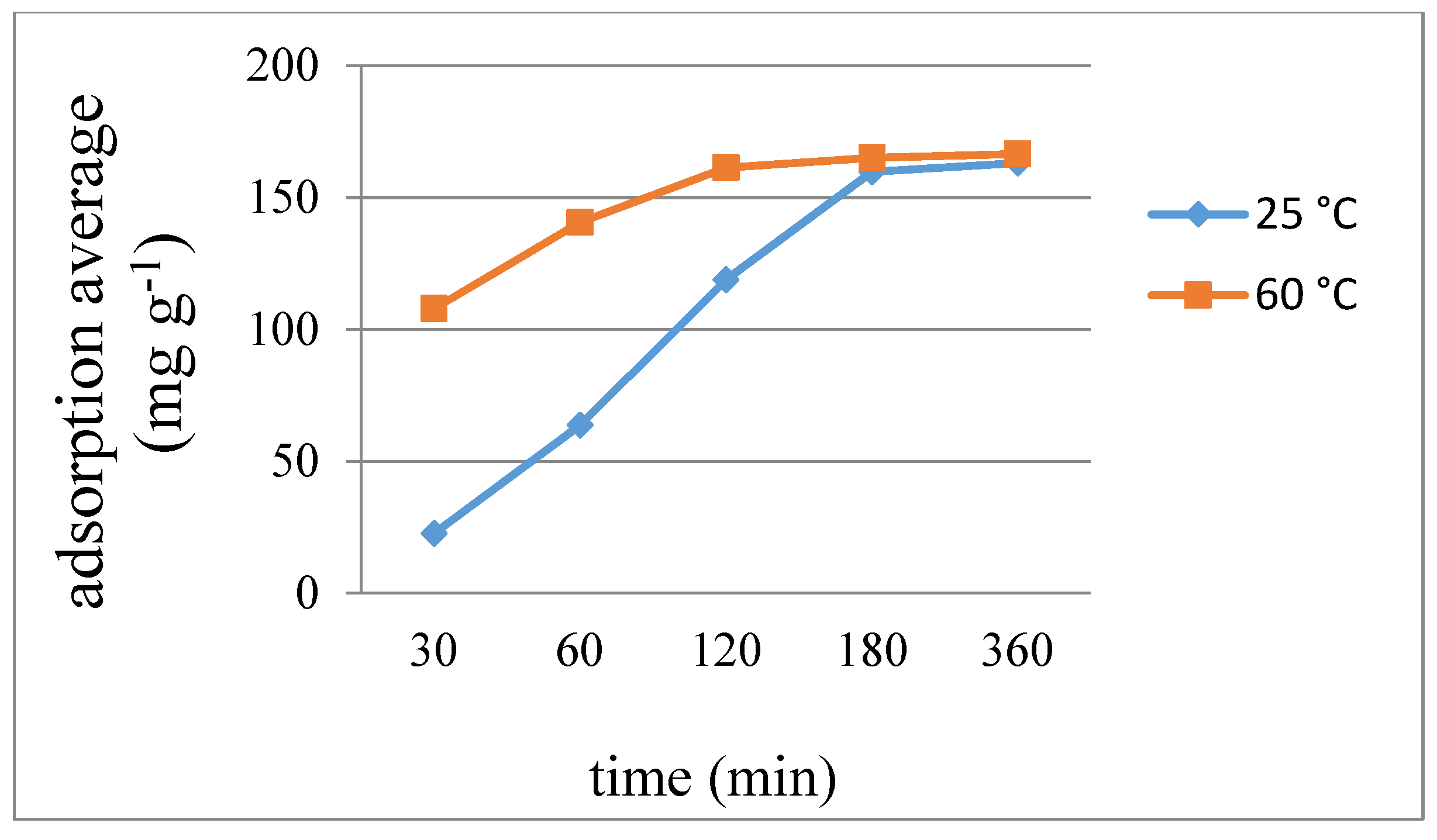
2.10. Iodine Adsorption
2.11. Gas Adsorption
3. Experimental Section
3.1. General Procedures
3.2. Materials
3.3. Preparations
3.3.1. {[Ni(L1)(OBA)(H2O)]·H2O}n, 1
3.3.2. {[Ni(L1)(SDA)(H2O)2]·H2O·CH3OH}n, 2
3.3.3. {[Ni(L2)(OBA)]·C2H5OH}n, 3
3.3.4. {[Ni(L2)(OBA)]·CH3OH }n, 4
3.3.5. {[Ni2(L2)(SDA)2(H2O)3]·5H2O}n, 5
3.3.6. {[Ni2(L2)(SDA)2(H2O)3]·H2O·2C2H5OH}n, 6
3.3.7. {[Ni(L3)(OBA)(H2O)2]·2H2O}n, 7
3.3.8. {[Ni(L3)(SDA)(H2O)2]·2H2O}n, 8
3.3.9. {[Ni(L3)0.5(SDA)(H2O)2]·0.5C2H5OH}n, 9
3.4. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, L.; Ciani, G.; Proserpio, D.M. Polycatenation, polythreading and polyknotting in coordination network chemistry. Coord. Chem. Rev. 2003, 246, 247–289. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Influence of an amine group on the highly efficient reversible adsorption of iodine in two novel isoreticular interpenetrated pillared-layer microporous metal–organic frameworks. CrystEngComm 2014, 16, 8660–8663. [Google Scholar] [CrossRef]
- Guo, B.; Li, F.; Wang, C.; Zhang, L.; Sun, D. A rare (3,12)-connected zirconium metal–organic framework with efficient iodine adsorption capacity and pH sensing. J. Mater. Chem. A 2019, 7, 13173–13179. [Google Scholar] [CrossRef]
- Arici, M.; Arici, T.A.; Demiral, H.; Taşd, M.; Yeşilel, O.Z. A porous Zn(II)-coordination polymer based on a tetracarboxylic acid exhibiting selective CO2 adsorption and iodine uptake. Dalton Trans. 2020, 49, 10824–10831. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Huang, W.-C.; Yang, X.-K.; Yang, C.-T.; Chhetri, P.M.; Chen, J.-D. Entanglement and irreversible structural transformation in Co (II) Coordination Polymers Based on Isomeric Bis-pyridyl-bis-amide Ligands. Cryst. Growth Des. 2019, 19, 1728–1737. [Google Scholar] [CrossRef]
- Wu, T.-T.; Hsu, W.; Yang, X.-K.; He, H.-Y.; Chen, J.-D. Entanglement in Co (II) coordination networks: Polycatenation from single net to 2-fold and 3-fold interpenetrated nets. CrystEngComm 2015, 17, 916–924. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Hu, J.-H.; Lee, W.-T.; Yang, X.-K.; Chen, J.-D. Structural Transformations of Cobalt(II) Coordination Polymers Constructed from N, N′-Di-3-pyridyladipoamide and Tetracarboxylic Acids: Disentanglement upon Water Coordination. Cryst. Growth Des. 2020, 20, 7211–7218. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Lai, Y.-T.; Yang, X.-K.; Govindaraj, M.; Lin, C.-H.; Chen, J.-D. Eight-Fold Interpenetrating Diamondoid Coordination Polymers for Sensing Volatile Organic Compounds and Metal Ions. Polymers 2021, 13, 3018. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Thapa, K.B.; Chen, J.-D. Crystal engineering of coordination polymers containing flexible bis-pyridyl-bis-amide ligands. CrystEngComm 2015, 17, 4611–4626. [Google Scholar] [CrossRef]
- Yang, X.-K.; Chen, J.-D. Crystal-to-crystal transformation and linker exchange in Cd(II) coordination polymers based on flexible bis-pyridyl-bis-amide and 1,4-naphthalenedicarboxylate. CrystEngComm 2019, 21, 7437–7446. [Google Scholar] [CrossRef]
- Mohanambe, L.; Vasudevan, S. Insertion of Iodine in a Functionalized Inorganic Layered Solid. Inorg. Chem. 2004, 43, 6421–6425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, L.-X.; Bai, F.-Y.; Xing, Y.-H. Thorium−Organic Framework Constructed with a Semirigid Triazine Hexacarboxylic Acid Ligand: Unique Structure with Thorium Oxide Wheel Clusters and Iodine Adsorption Behavior. Inorg. Chem. 2020, 59, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Qi, Y.-J.; Zhao, D.; Li, H.-H.; Zheng, S.-T. Heterometallic Organic Frameworks Built from Trinuclear Indium and Cuprous Halide Clusters: Ligand-Oriented Assemblies and Iodine Adsorption Behavior. Inorg. Chem. 2019, 58, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Cryst. 2020, 76, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruker AXS. APEX2, V2008.6, SADABS V2008/1, SAINT V7.60A, SHELXTL V6.14; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Complexes | Conformation | Coordination Mode |
|---|---|---|
| 1 | 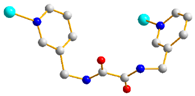 trans-anti-anti | 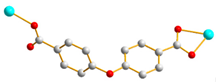 μ2-κOκO’:κO’’ |
| 2 | 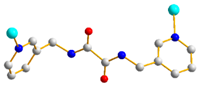 trans-syn-anti | 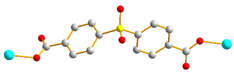 μ2-κO:κO’ |
| 3, 4 | 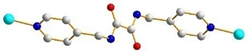 trans |  μ2-κOκO’:κO’’κO’’’ |
| 5, 6 | 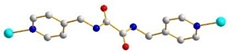 trans | 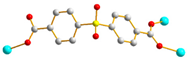 μ3-κO:κO’:κO’’ |
| 7 | 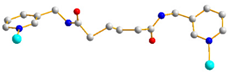 AAA-trans-syn-syn | 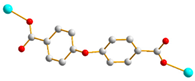 μ2-κO:κO’ |
| 8 | 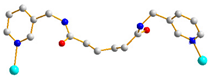 AAA-trans-syn-syn | 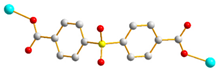 μ2-κO:κO’ |
| 9 | 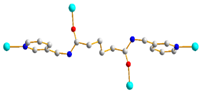 GAG-trans-syn-anti | 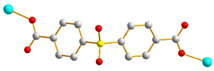 μ2-κO:κO’ |
| Complex | Weight Loss of Solvent °C (Calc/Found), % | Weight Loss of Ligand °C (Calc/Found), % |
|---|---|---|
| 1 | 2 H2O | L1 + (OBA2−) |
| ~220 (5.79/6.71) | 240 − 800 (85.31/84.28) | |
| 2 | MeOH + H2O ~220 (11.96/10.56) | L1 + (SDA2−) 240 − 800 (80.07/81.29) |
| 3 | EtOH | L2 + (OBA2−) |
| ~300 (7.29/4.52) | 310 − 800 (85.86/84.77) | |
| 4 | MeOH + H2O ~130 (5.11/5.62) | L2 + (OBA2−) 290 − 800 (90.82/88.14) |
| 5 | 8 H2O | L2 + 2 (SDA−2) |
| ~180 (12.67/10.20) | 270 − 800 (77.34/78.28) | |
| 6 | 2 EtOH + H2O ~170 (10.44/13.13) | L2 + 2 (SDA−2) 200 − 800 (76.00/78.63) |
| 7 | 4 H2O ~200 (10.09/9.97) | L3 + (OBA2−) 260 − 800 (82.14/82.12) |
| 8 | 4 H2O ~160 (9.40/9.71) | L3 + (SDA2−) 230 − 800 (77.57/76.96) |
| 9 | 0.5 EtOH + 2 H2O ~220 (10.08/9.26) | 0.5 L3 + (SDA2−) 240 − 900 (80.13/83.25) |
| 7 | 8 | 9 | ||||
|---|---|---|---|---|---|---|
| Time | 25 °C | 60 °C | 25 °C | 60 °C | 25 °C | 60 °C |
| 30 | 0.208 | 0.379 | 0.258 | 0.606 | 22.73 | 107.92 |
| 60 | 0.379 | 0.549 | 0.606 | 1.298 | 63.72 | 140.64 |
| 120 | 0.521 | 0.587 | 0.952 | 1.558 | 118.93 | 161.30 |
| 180 | 0.587 | 0.606 | 1.298 | 1.558 | 159.75 | 165.15 |
| 360 | 0.606 | 0.606 | 1.385 | 1.558 | 163.14 | 166.55 |
| Element | 7 | 8 | 9 |
|---|---|---|---|
| C | 56.93 | 55.70 | 49.45 |
| O | 23.54 | 22.04 | 25.66 |
| Ni | 11.34 | 11.07 | 9.68 |
| I | 0.42 | 0.01 | 7.03 |
| Compound | 1 | 2 | 3 |
| Formula | C28H26NiN4O9 | C29H32NiN4O12S | C30H28NiN4O8 |
| Formula weight | 621.24 | 719.35 | 631.27 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | Pī | Pī | P21/c |
| a, Å | 10.2625(9) | 12.0618(3) | 10.4220(4) |
| b, Å | 10.7071(9) | 12.5407(3) | 13.7045(5) |
| c, Å | 13.8202(12) | 12.6199(3) | 19.3529(7) |
| α, ° | 70.272(5) | 110.7299(12) | 90 |
| β, ° | 89.128(5) | 99.6766(12) | 91.645(2) |
| γ,° | 81.387(5) | 108.9309(12) | 90 |
| V, Å3 | 1412.3(2) | 1595.49(7) | 2763.00(18) |
| Z | 2 | 2 | 4 |
| Dcalc, Mg/m3 | 1.461 | 1.497 | 1.518 |
| F(000) | 644 | 748 | 1312 |
| µ(Mo Kα), mm−1 | 0.748 | 0.743 | 0.763 |
| Range(2θ) for data collection,deg | 1.566 ≤≤ 2θ ≤ 25.999 | 1.821 ≤ 2θ ≤ 28.308 | 1.821 ≤ 2θ ≤ 26.000 |
| Independent reflections | 5528 [R(Int) = 0.0234] | 7907 [R(Int) = 0.0235] | 5427 [R(Int) = 0.0300] |
| Data/restraints/parameters | 5528/0/379 | 7907/1/442 | 5427/6/385 |
| quality-of-fit indicatorc | 1.045 | 1.045 | 1.053 |
| Final R indices [I > 2σ(I)] a,b | R1 = 0.0384, wR2 = 0.0978 | R1 = 0.0408, wR2 = 0.1196 | R1 = 0.0407, wR2 = 0.1109 |
| R indices (all data) | R1 = 0.0500, wR2 = 0.1039 | R1 = 0.0510, wR2 = 0.1271 | R1 = 0.0494, wR2 = 0.1163 |
| Compound | 4 | 5 | 6 |
| Formula | C29H26NiN4O8 | C42H46Ni2N4O22S2 | C46H50Ni2N4O20S2 |
| Formula weight | 617.25 | 1140.37 | 1160.44 |
| Crystal system | Monoclinic | Triclinic | Triclinic |
| Space group | P21/c | Pī | Pī |
| a, Å | 10.3888(3) | 11.6188(10) | 11.5864(5) |
| b, Å | 13.6881(4) | 12.3844(10) | 12.3663(5) |
| c, Å | 19.0647(7) | 19.5572(16) | 19.3944(8) |
| α, ° | 90 | 80.487(5) | 82.136(2) |
| β, ° | 92.4267(17) | 87.616(5) | 87.810(2) |
| γ,° | 90 | 63.355(4) | 63.543(2) |
| V, Å3 | 2708.63(15) | 2479.0(4) | 2463.61(18) |
| Z | 4 | 2 | 2 |
| Dcalc, Mg/m3 | 1.514 | 1.528 | 1.564 |
| F(000) | 1280 | 1180 | 1204 |
| µ(Mo Kα), mm−1 | 0.777 | 0.929 | 0.743 |
| Range(2θ) for data collection,deg | 1.8332 ≤ 2θ ≤ 28.287 | 1.865 ≤ 2θ ≤ 28.434 | 1.821 ≤ 2θ ≤ 26.000 |
| Independent reflections | 6700 [R(Int) = 0.0197] | 12315 [R(Int) = 0.0317] | 12260 [R(Int) = 0.0216] |
| Data/restraints/parameters | 6700/0/379 | 12315/0/651 | 12260/6/667 |
| quality-of-fit indicator c | 1.047 | 1.030 | 1.048 |
| Final R indices [I > 2σ(I)] a,b | R1 = 0.0395, wR2 = 0.1102 | R1 = 0.0448, wR2 = 0.1244 | R1 = 0.0412, wR2 = 0.1239 |
| R indices (all data) | R1 = 0.0440, wR2 = 0.1132 | R1 = 0.0616, wR2 = 0.1345 | R1 = 0.0455, wR2 = 0.1273 |
| Compound | 7 | 8 | 9 |
| Formula | C32H38NiN4O11 | C32H38NiN4O12S | C24H26NiN2O9.5S |
| Formula weight | 713.37 | 761.43 | 585.24 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | C2/c | Pī |
| a, Å | 20.2189(10) | 19.5355(6) | 7.1192(2) |
| b, Å | 9.4113(5) | 9.5220(3) | 11.0064(3) |
| c, Å | 18.7496(9) | 19.2870(5) | 17.0108(5) |
| α, ° | 90 | 90 | 89.7201(17) |
| β, ° | 102.6301(18) | 103.1949(11) | 78.8806(18) |
| γ,° | 90 | 90 | 81.4570(19) |
| V, Å3 | 3481.5(3) | 3492.99(18) | 1292.99(6) |
| Z | 4 | 4 | 2 |
| Dcalc, Mg/m3 | 1.361 | 1.448 | 1.503 |
| F(000) | 1496 | 1592 | 608 |
| µ(Mo Kα), mm−1 | 0.620 | 0.683 | 0.888 |
| Range(2θ) for data collection,deg | 2.226 ≤ 2θ ≤ 25.998 | 2.169 ≤ 2θ ≤ 28.286 | 1.872 ≤ 2θ ≤ 28.356 |
| Independent reflections | 3424 [R(Int) = 0.0465] | 4322 [R(Int) = 0.0292] | 6421 [R(Int) = 0.0431] |
| Data/restraints/parameters | 3424/0/236 | 4322/1/236 | 6421/3/353 |
| quality-of-fit indicator c | 1.036 | 1.069 | 1.039 |
| Final R indices [I > 2σ(I)] a,b | R1 = 0.0532, wR2 = 0.1488 | R1 = 0.0371, wR2 = 0.0976 | R1 = 0.0440, wR2 = 0.0977 |
| R indices (all data) | R1 = 0.0646, wR2 = 0.1645 | R1 = 0.0439, wR2 = 0.1038 | R1 = 0.0760, wR2 = 0.1089 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-T.; Liao, T.-T.; Chen, J.-D. Nickel(II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption. Int. J. Mol. Sci. 2022, 23, 3603. https://doi.org/10.3390/ijms23073603
Lee W-T, Liao T-T, Chen J-D. Nickel(II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption. International Journal of Molecular Sciences. 2022; 23(7):3603. https://doi.org/10.3390/ijms23073603
Chicago/Turabian StyleLee, Wei-Te, Tsung-Te Liao, and Jhy-Der Chen. 2022. "Nickel(II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption" International Journal of Molecular Sciences 23, no. 7: 3603. https://doi.org/10.3390/ijms23073603
APA StyleLee, W.-T., Liao, T.-T., & Chen, J.-D. (2022). Nickel(II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption. International Journal of Molecular Sciences, 23(7), 3603. https://doi.org/10.3390/ijms23073603






