Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates
Abstract
1. Introduction
2. Results
2.1. Characterization of DMC and R-I-Cad toward Different Transglutaminases
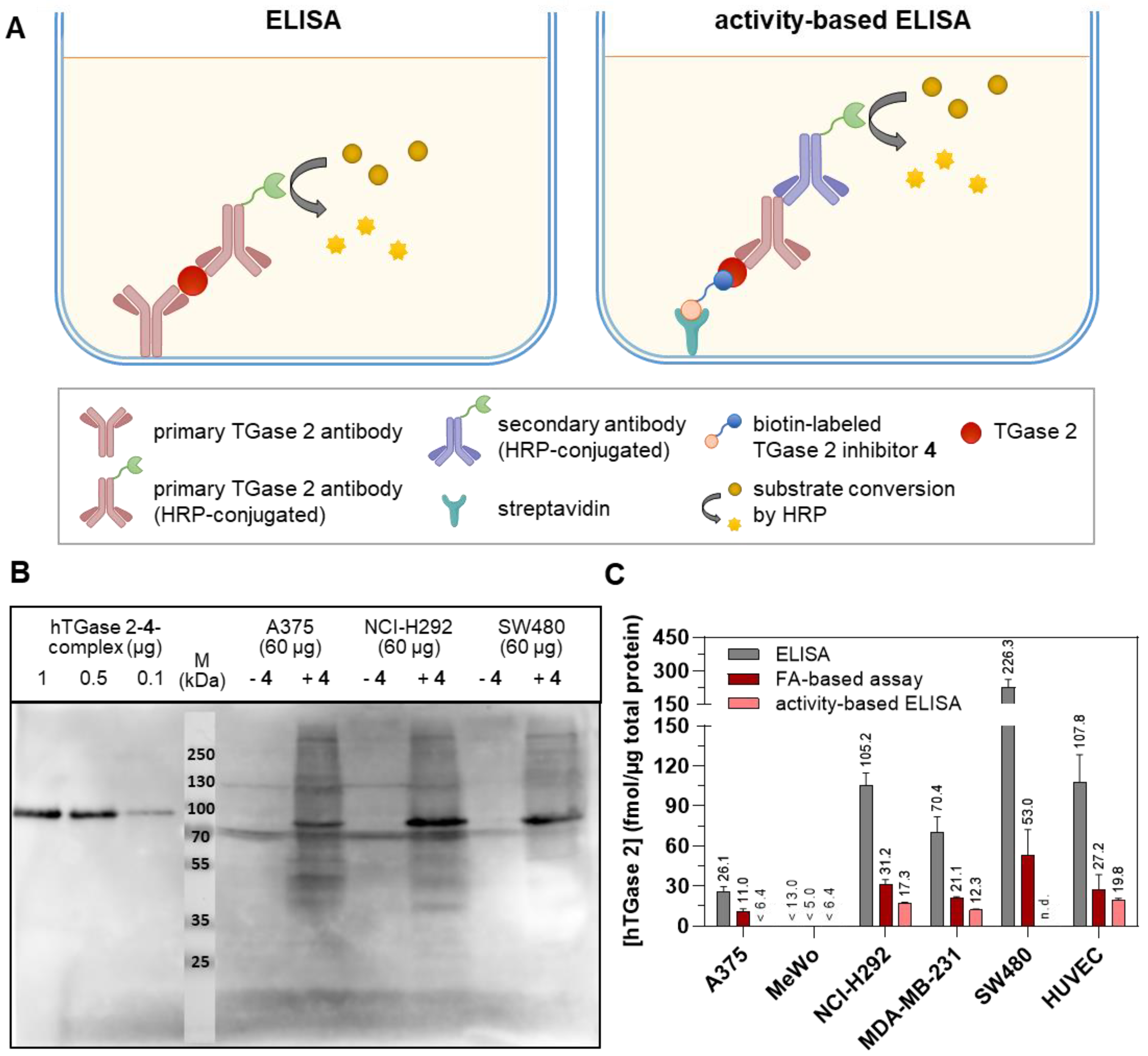
2.2. Quantification of TGase 2 Activity in Cell Lysates by the FA-Based Assay
2.3. Quantification of TGase 2 Activity in Cell Lysates by an Activity-Based ELISA
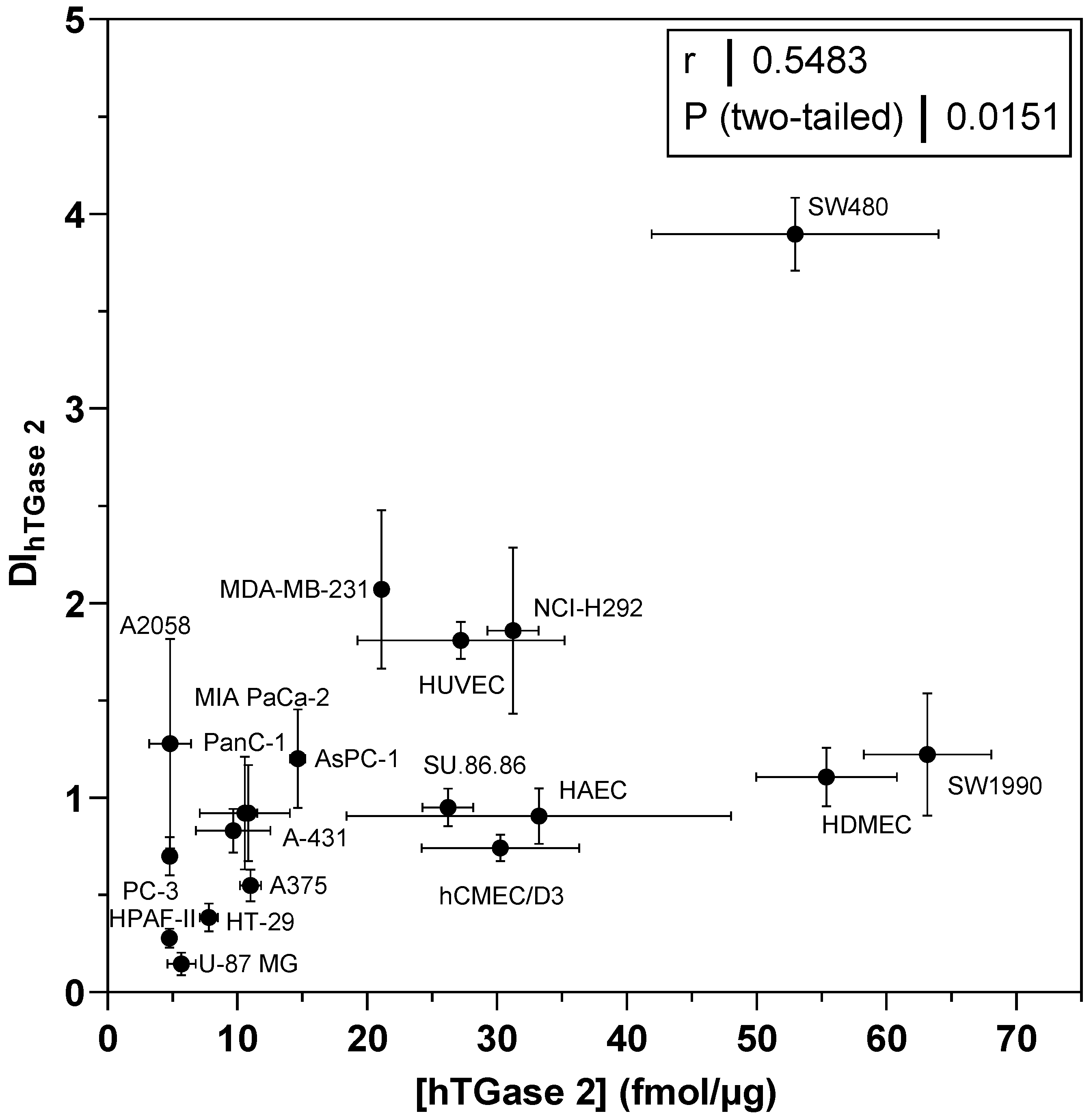
2.4. Quantification of the Cellular Expression of TGase 2 by a Two-Site Sandwich ELISA
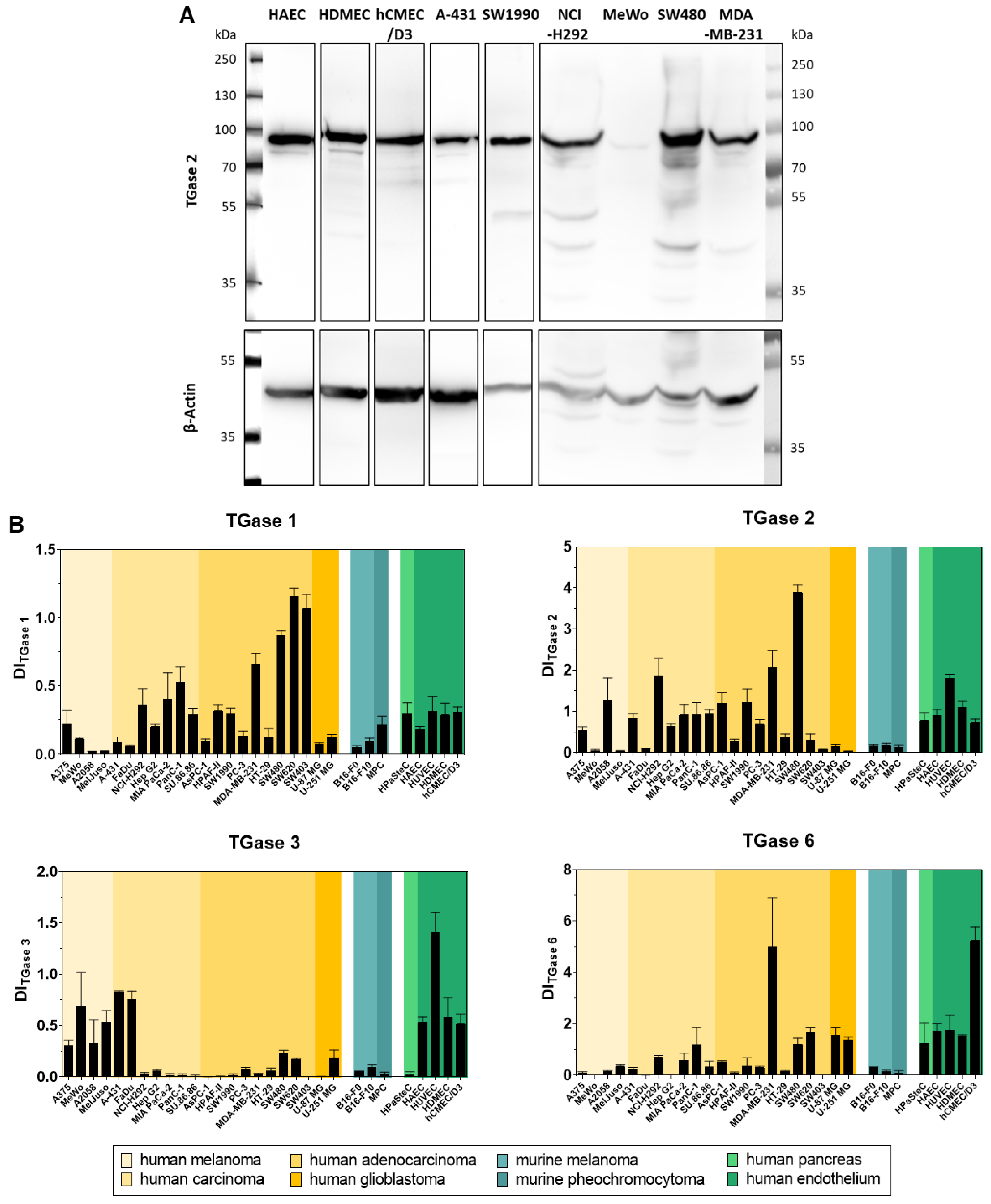
2.5. Analysis of the Cellular Expression of Different Transglutaminases by Western Blot
3. Discussion
4. Materials and Methods
4.1. General
4.2. Cell culture and Sample Preparation
4.3. Fluorescence Anisotropy Assay
4.3.1. Kinetic Characterization of the Acyl Donor DMC toward Human Transglutaminases Using the Acyl Acceptor R-I-Cad
4.3.2. Characterization of Irreversible Inhibitors toward Transglutaminases
4.3.3. Characterization of TGase 2 Activity in Cell Lysates
4.3.4. Separation and Visualization of R-I-Cad-Labeled DMC by SDS-PAGE and in-Gel Fluorescence Measurement
4.3.5. Assessing Noncovalent Binding of R-I-Cad to DMC with Ultrafiltration
4.4. Visualization of the hTGase 2–4 complex after SDS-PAGE and immunoblotting
4.5. Activity-Based ELISA Using Biotinylated Inhibitor 4
4.6. Cellular Expression of Different TGases and Propionyl-CoA Carboxylase
4.7. Two-Site Sandwich ELISA for Quantifying hTGase 2
4.8. Chemistry
4.8.1. General
4.8.2. Chromatography
4.8.3. Synthesis of 4-Boc-1-(6-ethinylpyridin-2-yl)piperazine (5)
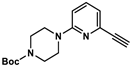
4.8.4. Synthesis of 6-ethinylpyridin-2-yl)piperazine (6)
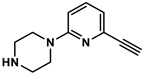
4.8.5. Synthesis of Nα-Boc-Nε-Acryloyl-l-lysine-4-(6-ethinylpyridin-2-yl)piperazide (7)

4.8.6. Synthesis of Nε-Acryloyl-l-lysine-4-(6-ethinylpyridin-2-yl)piperazide (8)
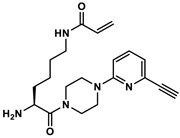
4.8.7. Synthesis of Nα-Phenylacetyl-Nε-acryloyl-l-lysine-4-(6-ethinylpyridin-2-yl)piperazide × TFA (3)
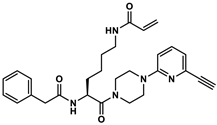
4.8.8. Synthesis of N-(2-(2-(2-(2-(4-(6-(4-(N6-acryloyl-N2-(2-phenylacetyl)-l-lysyl)piperazin-1-yl)pyridin-2-yl)-1H-1,2,3-triazol-1-yl)ethoxy)ethoxy)ethoxy)ethyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide × 2TFA (4)

Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisswanger, H. Practical Enzymology, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Poreba, M.; Groborz, K.M.; Rut, W.; Pore, M.; Snipas, S.J.; Vizovisek, M.; Turk, B.; Kuhn, P.; Drag, M.; Salvesen, G.S. Multiplexed probing of proteolytic enzymes using mass cytometry-compatible activity-based probes. J. Am. Chem. Soc. 2020, 142, 16704–16715. [Google Scholar] [CrossRef] [PubMed]
- Saghatelian, A.; Cravatt, B.F. Assignment of protein function in the postgenomic era. Nat. Chem. Biol. 2005, 1, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.M.; Niphakis, M.J.; Cravatt, B.F. Determining target engagement in living systems. Nat. Chem. Biol. 2013, 9, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Zotter, A.; Bauerle, F.; Dey, D.; Kiss, V.; Schreiber, G. Quantifying enzyme activity in living cells. J. Biol. Chem. 2017, 292, 15838–15848. [Google Scholar] [CrossRef]
- Stefaniak, J.; Huber, K.V.M. Importance of quantifying drug-target engagement in cells. ACS Med. Chem. Lett. 2020, 11, 403–406. [Google Scholar] [CrossRef]
- Evans, M.J.; Cravatt, B.F. Mechanism-based profiling of enzyme families. Chem. Rev. 2006, 106, 3279–3301. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Wright, A.T.; Kozarich, J.W. Activity-based protein profiling: From enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 2008, 77, 383–414. [Google Scholar] [CrossRef]
- Simon, G.M.; Cravatt, B.F. Activity-based proteomics of enzyme superfamilies: Serine hydrolases as a case study. J. Biol. Chem. 2010, 285, 11051–11055. [Google Scholar] [CrossRef]
- Van Rooden, E.J.; Bakker, A.T.; Overkleeft, H.S.; Van der Stelt, M. Activity-based protein profiling. In eLS; John Wiley & Sons: Chichester, UK, 2018. [Google Scholar] [CrossRef]
- Folk, J.E. Transglutaminases. Annu. Rev. Biochem. 1980, 49, 517–531. [Google Scholar] [CrossRef]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Mycek, M.J.; Neidle, A.; Waelsch, H. The incorporation of amines into protein. Arch. Biochem. Biophys. 1959, 79, 338–354. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Clarke, D.D.; Waelsch, H. An enzymatically catalyzed incorporation of amines into proteins. Biochim. Biophys. Acta 1957, 25, 451–452. [Google Scholar] [CrossRef]
- Pietsch, M.; Wodtke, R.; Pietzsch, J.; Löser, R. Tissue transglutaminase: An emerging target for therapy and imaging. Bioorg. Med. Chem. Lett. 2013, 23, 6528–6543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gundemir, S.; Colak, G.; Tucholski, J.; Johnson, G.V. Transglutaminase 2: A molecular Swiss army knife. Biochim. Biophys. Acta 2012, 1823, 406–419. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Greenberg, C.S. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J. Biol. Chem. 1987, 262, 1901–1906. [Google Scholar] [CrossRef]
- Nakaoka, H.; Perez, D.; Baek, K.; Das, T.; Husain, A.; Misono, K.; Im, M.; Graham, R. Gh: A GTP-binding protein with transglutaminase activity and receptor signaling function. Science 1994, 264, 1593–1596. [Google Scholar] [CrossRef]
- Lorand, L.; Dailey, J.E.; Turner, P.M. Fibronectin as a Carrier for the Transglutaminase from Human-Erythrocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 1057–1059. [Google Scholar] [CrossRef]
- Cardoso, I.; Osterlund, E.C.; Stamnaes, J.; Iversen, R.; Andersen, J.T.; Jorgensen, T.J.; Sollid, L.M. Dissecting the interaction between transglutaminase 2 and fibronectin. Amino Acids 2017, 49, 489–500. [Google Scholar] [CrossRef]
- Akimov, S.S.; Krylov, D.; Fleischman, L.F.; Belkin, A.M. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. 2000, 148, 825–838. [Google Scholar] [CrossRef]
- Belkin, A.M. Extracellular TG2: Emerging functions and regulation. FEBS J. 2011, 278, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Smethurst, P.A.; Griffin, M. Measurement of tissue transglutaminase activity in a permeabilized cell system: Its regulation by Ca2+ and nucleotides. Biochem. J. 1996, 313, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, R.; Demeny, M.; Fesüs, L. Protein transamidation by transglutaminase 2 in cells: A disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 2011, 278, 4717–4739. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Strnad, P.; Watts, R.E.; Choi, K.; Jabri, B.; Omary, M.B.; Khosla, C. Extracellular Transglutaminase 2 is Catalytically Inactive, but Is Transiently Activated upon Tissue Injury. PLoS ONE 2008, 3, e1861. [Google Scholar] [CrossRef]
- Stamnaes, J.; Pinkas, D.M.; Fleckenstein, B.; Khosla, C.; Sollid, L.M. Redox regulation of transglutaminase 2 activity. J. Biol. Chem. 2010, 285, 25402–25409. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, J.; Zhang, Z.; Johnson, G.V.; Cooper, A.J.; Feola, J.; Bank, A.; Shein, J.; Ruotsalainen, H.J.; Pihlajaniemi, T.A.; et al. Endostatin and transglutaminase 2 are involved in fibrosis of the aging kidney. Kidney Int. 2016, 89, 1281–1292. [Google Scholar] [CrossRef]
- Benn, M.C.; Weber, W.; Klotzsch, E.; Vogel, V.; Pot, S.A. Tissue transglutaminase in fibrosis—More than an extracellular matrix cross-linker. Curr. Opin. Biomed. Eng. 2019, 10, 156–164. [Google Scholar] [CrossRef]
- Fell, S.; Wang, Z.; Blanchard, A.; Nanthakumar, C.; Griffin, M. Transglutaminase 2: A novel therapeutic target for idiopathic pulmonary fibrosis using selective small molecule inhibitors. Amino Acids 2021, 53, 205–217. [Google Scholar] [CrossRef]
- Maamra, M.; Benayad, O.M.; Matthews, D.; Kettleborough, C.; Atkinson, J.; Cain, K.; Bon, H.; Brand, H.; Parkinson, M.; Watson, P.F.; et al. Transglutaminase 2: Development of therapeutic antibodies reveals four inhibitory epitopes and confirms extracellular function in fibrotic remodelling. Br. J. Pharmacol. 2022, 1–16. [Google Scholar] [CrossRef]
- Rauhavirta, T.; Hietikko, M.; Salmi, T.; Lindfors, K. Transglutaminase 2 and transglutaminase 2 autoantibodies in celiac disease: A review. Clin. Rev. Allergy Immunol. 2019, 57, 23–38. [Google Scholar] [CrossRef]
- Schuppan, D.; Maki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, O.; et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Condello, S.; Yakubov, B.; Emerson, R.; Caperell-Grant, A.; Hitomi, K.; Xie, J.; Matei, D. Tissue Transglutaminase Mediated Tumor-Stroma Interaction Promotes Pancreatic Cancer Progression. Clin. Cancer Res. 2015, 21, 4482–4493. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Antonyak, M.A.; Cerione, R.A. Simultaneously targeting tissue transglutaminase and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol. Pharm. 2015, 12, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Antonyak, M.A.; Cerione, R.A. The diamond anniversary of tissue transglutaminase: A protein of many talents. Drug Discov. Today 2018, 23, 575–591. [Google Scholar] [CrossRef]
- Tempest, R.; Guarnerio, S.; Maani, R.; Cooper, J.; Peake, N. The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment. Cancers 2021, 13, 2788. [Google Scholar] [CrossRef]
- Park, M.J.; Baek, H.W.; Rhee, Y.Y.; Lee, C.; Park, J.W.; Kim, H.W.; Moon, K.C. Transglutaminase 2 expression and its prognostic significance in clear cell renal cell carcinoma. J. Pathol. Transl. Med. 2015, 49, 37–43. [Google Scholar] [CrossRef]
- Fernandez-Acenero, M.J.; Torres, S.; Garcia-Palmero, I.; Diaz Del Arco, C.; Casal, J.I. Prognostic role of tissue transglutaminase 2 in colon carcinoma. Virchows Arch. 2016, 469, 611–619. [Google Scholar] [CrossRef]
- Eckert, R.L. Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target. Mol. Carcinog. 2019, 58, 837–853. [Google Scholar] [CrossRef]
- Keillor, J.W.; Apperley, K.Y.; Akbar, A. Inhibitors of tissue transglutaminase. Trends Pharmacol. Sci. 2015, 36, 32–40. [Google Scholar] [CrossRef]
- Van der Wildt, B.; Wilhelmus, M.M.M.; Beaino, W.; Kooijman, E.J.M.; Schuit, R.C.; Bol, J.; Breve, J.J.P.; Pasternack, R.; Lammertsma, A.A.; Windhorst, A.D.; et al. In vivo evaluation of two tissue transglutaminase PET tracers in an orthotopic tumour xenograft model. EJNMMI Res. 2018, 8, 39. [Google Scholar] [CrossRef]
- Folk, J.E.; Chung, S.I. Transglutaminases. Methods Enzymol. 1985, 113, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Nicholas, B.; Mian, S.; Davies, P.J.; Griffin, M. Reduced expression of tissue transglutaminase in a human endothelial cell line leads to changes in cell spreading, cell adhesion and reduced polymerisation of fibronectin. J. Cell Sci. 1997, 110, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-H.; Kim, C.-W.; Shin, D.-M.; Kim, K.-i.; Cho, S.-Y.; Kwon, J.-C.; Choi, K.-H.; Kang, H.-S.; Kim, I.-G. Differential incorporation of biotinylated polyamines by transglutaminase 2. FEBS Lett. 2003, 534, 180–184. [Google Scholar] [CrossRef]
- Choi, K.; Siegel, M.; Piper, J.L.; Yuan, L.; Cho, E.; Strnad, P.; Omary, B.; Rich, K.M.; Khosla, C. Chemistry and biology of dihydroisoxazole derivatives: Selective inhibitors of human transglutaminase 2. Chem. Biol. 2005, 12, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, T.F.; Achyuthan, K.E.; Lai, T.S.; Greenberg, C.S. A microtiter plate transglutaminase assay utilizing 5-(biotinamido)pentylamine as substrate. Anal. Biochem. 1992, 205, 166–171. [Google Scholar] [CrossRef]
- Almami, I.; Dickenson, J.M.; Hargreaves, A.J.; Bonner, P.L. Modulation of transglutaminase 2 activity in H9c2 cells by PKC and PKA signalling: A role for transglutaminase 2 in cytoprotection. Br. J. Pharmacol. 2014, 171, 3946–3960. [Google Scholar] [CrossRef]
- Algarni, A.S.; Hargreaves, A.J.; Dickenson, J.M. Activation of transglutaminase 2 by nerve growth factor in differentiating neuroblastoma cells: A role in cell survival and neurite outgrowth. Eur. J. Pharmacol. 2018, 820, 113–129. [Google Scholar] [CrossRef]
- Lorand, L.; Lockridge, O.M.; Campbell, L.K.; Myhrman, R.; Bruner-Lorand, J. Transamidating enzymes. II. A continuous fluorescent method suited for automating measurements of factor XIII in plasma. Anal. Biochem. 1971, 44, 221–231. [Google Scholar] [CrossRef]
- Henriksson, P.; Becker, S.; Lynch, G.; McDonagh, J. Identification of intracellular factor XIII in human monocytes and macrophages. J. Clin. Investig. 1985, 76, 528–534. [Google Scholar] [CrossRef]
- Prime, M.E.; Andersen, O.A.; Barker, J.J.; Brooks, M.A.; Cheng, R.K.; Toogood-Johnson, I.; Courtney, S.M.; Brookfield, F.A.; Yarnold, C.J.; Marston, R.W.; et al. Discovery and structure-activity relationship of potent and selective covalent inhibitors of transglutaminase 2 for Huntington’s disease. J. Med. Chem. 2012, 55, 1021–1046. [Google Scholar] [CrossRef]
- Hauser, C.; Wodtke, R.; Löser, R.; Pietsch, M. A fluorescence anisotropy-based assay for determining the activity of tissue transglutaminase. Amino Acids 2017, 49, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Wodtke, R.; Hauser, C.; Ruiz-Gomez, G.; Jäckel, E.; Bauer, D.; Lohse, M.; Wong, A.; Pufe, J.; Ludwig, F.A.; Fischer, S.; et al. Ne-Acryloyllysine piperazides as irreversible inhibitors of transglutaminase 2: Synthesis, structure-activity relationships, and pharmacokinetic profiling. J. Med. Chem. 2018, 61, 4528–4560. [Google Scholar] [CrossRef] [PubMed]
- Wityak, J.; Prime, M.E.; Brookfield, F.A.; Courtney, S.M.; Erfan, S.; Johnsen, S.; Johnson, P.D.; Li, M.; Marston, R.W.; Reed, L.; et al. SAR development of lysine-based irreversible inhibitors of transglutaminase 2 for huntington’s disease. ACS Med. Chem. Lett. 2012, 3, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Wodtke, R.; Schramm, G.; Pietzsch, J.; Pietsch, M.; Löser, R. Synthesis and kinetic characterisation of water-soluble fluorogenic acyl donors for transglutaminase 2. ChemBioChem 2016, 17, 1263–1281. [Google Scholar] [CrossRef]
- Tong, L. Structure and function of biotin-dependent carboxylases. Cell. Mol. Life Sci. 2013, 70, 863–891. [Google Scholar] [CrossRef]
- Kaschani, F.; Verhelst, S.H.; van Swieten, P.F.; Verdoes, M.; Wong, C.S.; Wang, Z.; Kaiser, M.; Overkleeft, H.S.; Bogyo, M.; van der Hoorn, R.A. Minitags for small molecules: Detecting targets of reactive small molecules in living plant tissues using ‘click chemistry’. Plant. J. 2009, 57, 373–385. [Google Scholar] [CrossRef]
- Lai, T.S.; Greenberg, C.S. TGM2 and implications for human disease: Role of alternative splicing. Front. Biosci. 2013, 18, 504–519. [Google Scholar]
- Jameson, D.M.; Ross, J.A. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem. Rev. 2010, 110, 2685–2708. [Google Scholar] [CrossRef]
- Lea, W.A.; Simeonov, A. Fluorescence polarization assays in small molecule screening. Expert Opin. Drug Discov. 2011, 6, 17–32. [Google Scholar] [CrossRef]
- Franklin, T.G.; Pruneda, J.N. A High-throughput assay for monitoring ubiquitination in real time. Front. Chem. 2019, 7, 816. [Google Scholar] [CrossRef]
- Yamane, A.; Fukui, M.; Sugimura, Y.; Itoh, M.; Alea, M.P.; Thomas, V.; El Alaoui, S.; Akiyama, M.; Hitomi, K. Identification of a preferred substrate peptide for transglutaminase 3 and detection of in situ activity in skin and hair follicles. FEBS J. 2010, 277, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Kuramoto, K.; Yamasaki, R.; Shimizu, Y.; Itoh, M.; Kawamoto, T.; Hitomi, K. Identification of a highly reactive substrate peptide for transglutaminase 6 and its use in detecting transglutaminase activity in the skin epidermis. FEBS J. 2013, 280, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Schaertl, S.; Prime, M.; Wityak, J.; Dominguez, C.; Muñoz-Sanjuan, I.; Pacifici, R.E.; Courtney, S.; Scheel, A.; Macdonald, D. A profiling platform for the characterization of transglutaminase 2 (TG2) inhibitors. J. Biomol. Screen. 2010, 15, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Case, A.; Stein, R.L. Kinetic analysis of the action of tissue transglutaminase on peptide and protein substrates. Biochemistry 2003, 42, 9466–9481. [Google Scholar] [CrossRef]
- Brandt, F.; Ullrich, M.; Laube, M.; Kopka, K.; Bachmann, M.; Löser, R.; Pietzsch, J.; Pietzsch, H.J.; van den Hoff, J.; Wodtke, R. “Clickable” albumin binders for modulating the tumor uptake of targeted radiopharmaceuticals. J. Med. Chem. 2022, 65, 710–733. [Google Scholar] [CrossRef]
- Dafik, L.; Khosla, C. Dihydroisoxazole analogs for labeling and visualization of catalytically active transglutaminase 2. Chem. Biol. 2011, 18, 58–66. [Google Scholar] [CrossRef][Green Version]
- Akbar, A.; McNeil, N.M.R.; Albert, M.R.; Ta, V.; Adhikary, G.; Bourgeois, K.; Eckert, R.L.; Keillor, J.W. Structure-activity relationships of potent, targeted covalent inhibitors that abolish both the transamidation and GTP binding activities of human tissue transglutaminase. J. Med. Chem. 2017, 60, 7910–7927. [Google Scholar] [CrossRef]
- McNeil, N.M.R.; Gates, E.W.J.; Firoozi, N.; Cundy, N.J.; Leccese, J.; Eisinga, S.; Tyndall, J.D.A.; Adhikary, G.; Eckert, R.L.; Keillor, J.W. Structure-activity relationships of N-terminal variants of peptidomimetic tissue transglutaminase inhibitors. Eur. J. Med. Chem. 2022, 232, 114172. [Google Scholar] [CrossRef]
- Wodtke, R.; Wodtke, J.; Hauser, S.; Laube, M.; Bauer, D.; Rothe, R.; Neuber, C.; Pietsch, M.; Kopka, K.; Pietzsch, J.; et al. Development of an 18F-labeled irreversible inhibitor of transglutaminase 2 as radiometric tool for quantitative expression profiling in cells and tissues. J. Med. Chem. 2021, 64, 3462–3478. [Google Scholar] [CrossRef]
- Kele, P.; Li, X.; Link, M.; Nagy, K.; Herner, A.; Lorincz, K.; Beni, S.; Wolfbeis, O.S. Clickable fluorophores for biological labeling--with or without copper. Org. Biomol. Chem. 2009, 7, 3486–3490. [Google Scholar] [CrossRef]
- Meyer, J.P.; Adumeau, P.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: The First 10 Years. Bioconjug. Chem. 2016, 27, 2791–2807. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.I.; van der Linden, W.A.; Li, N.; Li, K.Y.; Liu, N.; Hoogendoorn, S.; van der Marel, G.A.; Florea, B.I.; Overkleeft, H.S. Bioorthogonal chemistry: Applications in activity-based protein profiling. Acc. Chem. Res. 2011, 44, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Verhelst, S.H. Comparative analysis of click chemistry mediated activity-based protein profiling in cell lysates. Molecules 2013, 18, 12599–12608. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.; Buchold, C.; Pasternack, R.; Binder, N.B.; Moore, G.W. Clinical validation of an automated fluorogenic factor XIII activity assay based on isopeptidase activity. Int. J. Mol. Sci. 2021, 22, 1002. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; Xiao, H.; Wu, D.L.; Yang, Y.; Wang, P. A brief guide to good practices in pharmacological experiments: Western blotting. Acta Pharmacol. Sin. 2021, 42, 1015–1017. [Google Scholar] [CrossRef]
- Verma, A.; Wang, H.; Manavathi, B.; Fok, J.Y.; Mann, A.P.; Kumar, R.; Mehta, K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006, 66, 10525–10533. [Google Scholar] [CrossRef]
- Su, X.; He, X.; Ben, Q.; Wang, W.; Song, H.; Ye, Q.; Zang, Y.; Li, W.; Chen, P.; Yao, W.; et al. Effect of p53 on pancreatic cancer-glucose tolerance abnormalities by regulating transglutaminase 2 in resistance to glucose metabolic stress. Oncotarget 2017, 8, 74299–74311. [Google Scholar] [CrossRef][Green Version]
- Greenberg, C.S.; Achyuthan, K.E.; Borowitz, M.J.; Shuman, M.A. The transglutaminase in vascular cells and tissues could provide an alternate pathway for fibrin stabilization. Blood 1987, 70, 702–709. [Google Scholar] [CrossRef]
- Kojima, S.; Hagiwara, H.; Soga, W.; Shimonaka, M.; Saito, Y.; Inada, Y. Transglutaminase in endothelial-cells from bovine carotid-artery. Biomed. Res. 1987, 8, 25–29. [Google Scholar] [CrossRef]
- Thomazy, V.; Fesus, L. Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res. 1989, 255, 215–224. [Google Scholar] [CrossRef]
- Korner, G.; Schneider, D.E.; Purdon, M.A.; Bjornsson, T.D. Bovine aortic endothelial cell transglutaminase. Enzyme characterization and regulation of activity. Biochem. J. 1989, 262, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Hosono, M.; Wada, F.; Yoshimura, T.; Maki, M.; Hitomi, K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: Identification of peptide substrates for TGase 2 and Factor XIIIA. J. Biol. Chem. 2006, 281, 17699–17706. [Google Scholar] [CrossRef] [PubMed]
- Kenniston, J.A.; Conley, G.P.; Sexton, D.J.; Nixon, A.E. A homogeneous fluorescence anisotropy assay for measuring transglutaminase 2 activity. Anal. Biochem. 2013, 436, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Biri, B.; Schlosser, G.; Kiss, B.; Nyitray, L.; Fesus, L.; Kiraly, R. Real-time kinetic method to monitor isopeptidase activity of transglutaminase 2 on protein substrate. Anal. Biochem. 2016, 505, 36–42. [Google Scholar] [CrossRef]
- Reindl, W.; Graber, M.; Strebhardt, K.; Berg, T. Development of high-throughput assays based on fluorescence polarization for inhibitors of the polo-box domains of polo-like kinases 2 and 3. Anal. Biochem. 2009, 395, 189–194. [Google Scholar] [CrossRef]
- Banks, P.; Harvey, M. Considerations for using fluorescence polarization in the screening of G protein-coupled receptors. J. Biomol. Screen. 2002, 7, 111–117. [Google Scholar] [CrossRef][Green Version]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Wolf, S.; Haase-Kohn, C.; Lenk, J.; Hoppmann, S.; Bergmann, R.; Steinbach, J.; Pietzsch, J. Expression, purification and fluorine-18 radiolabeling of recombinant S100A4: A potential probe for molecular imaging of receptor for advanced glycation endproducts in vivo? Amino Acids 2011, 41, 809–820. [Google Scholar] [CrossRef]
- Glasel, J.A. A nuclear magnetic resonance investigation of biotin. The biotin sulfonium ion. Biochemistry 1966, 5, 1851–1855. [Google Scholar] [CrossRef]
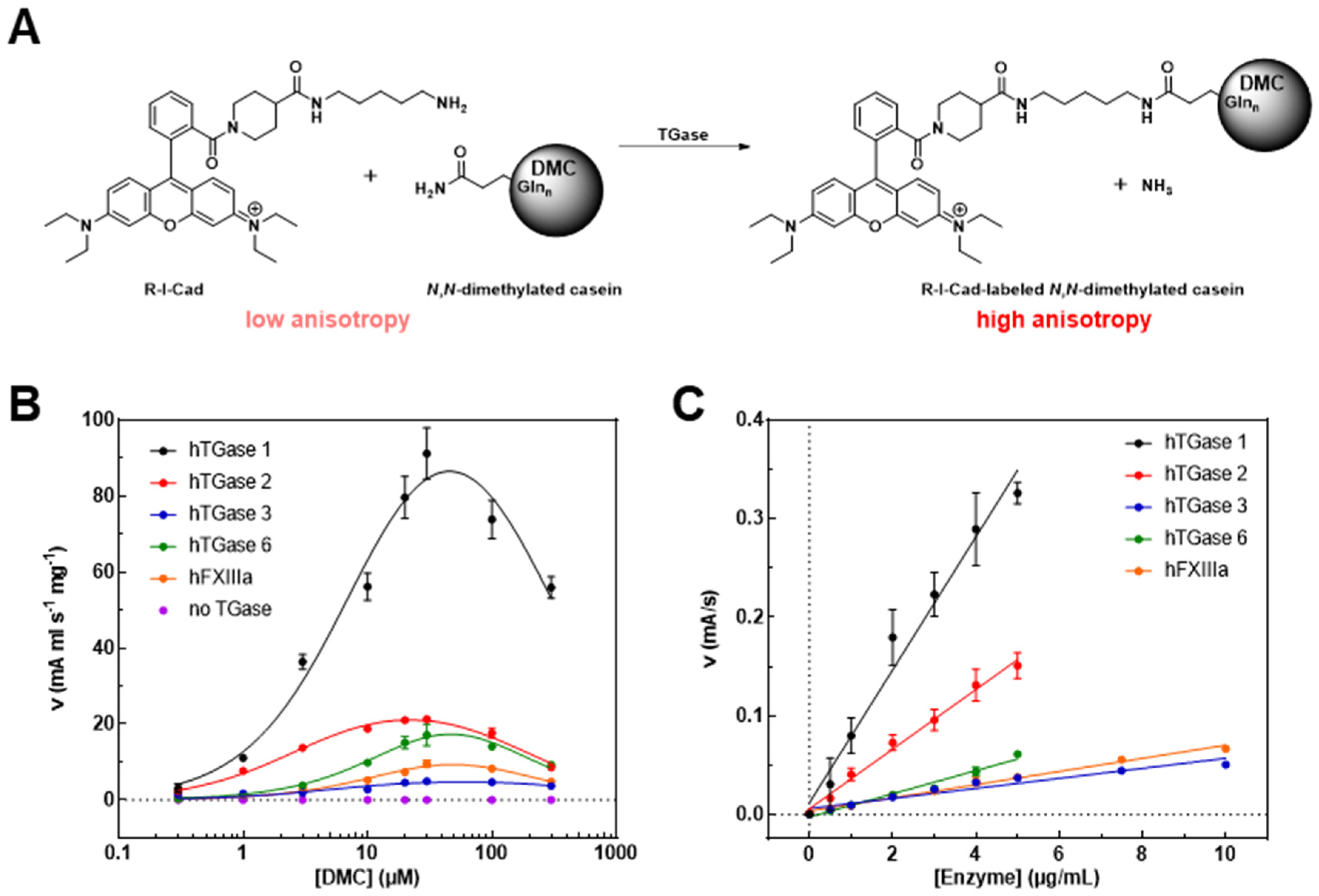
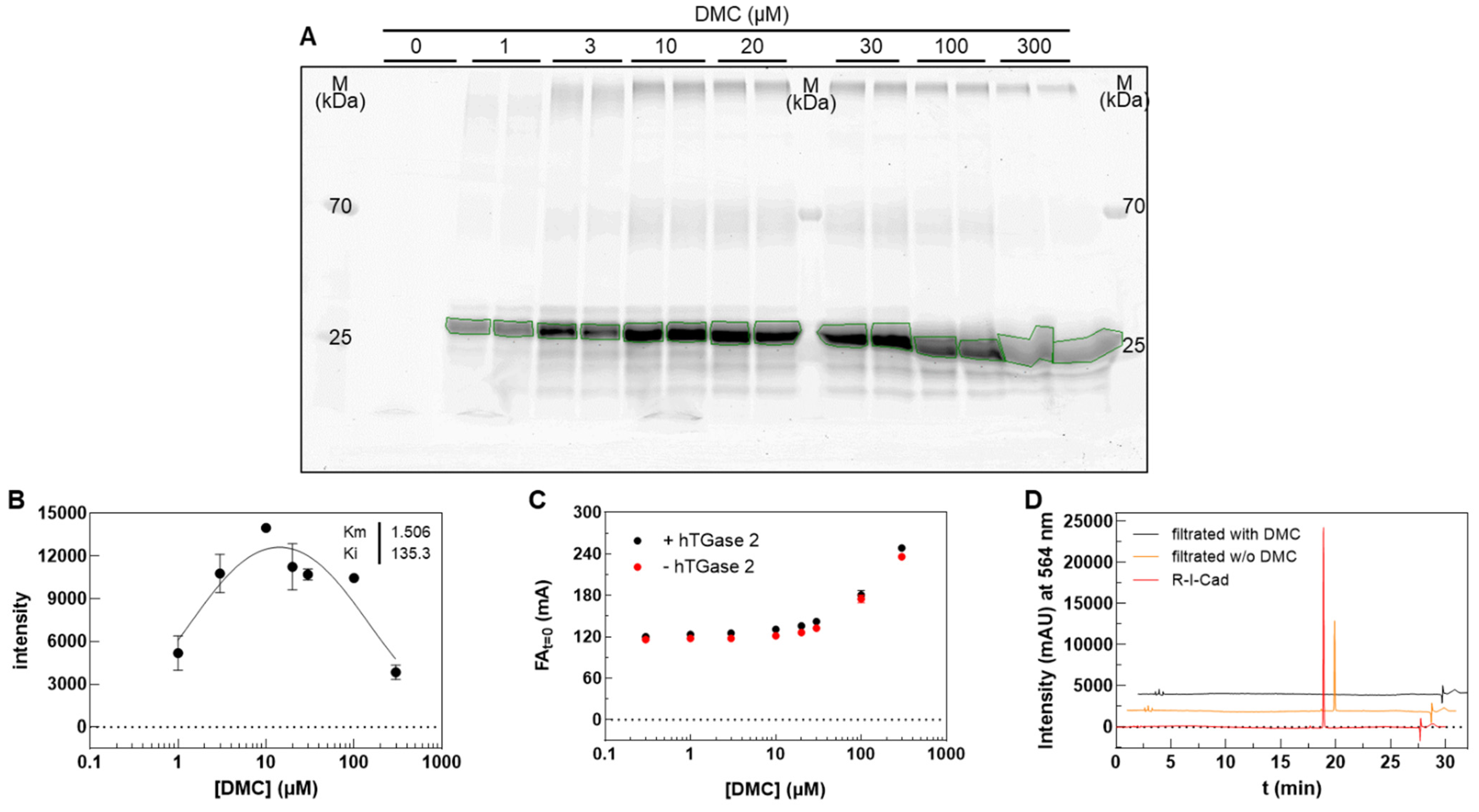
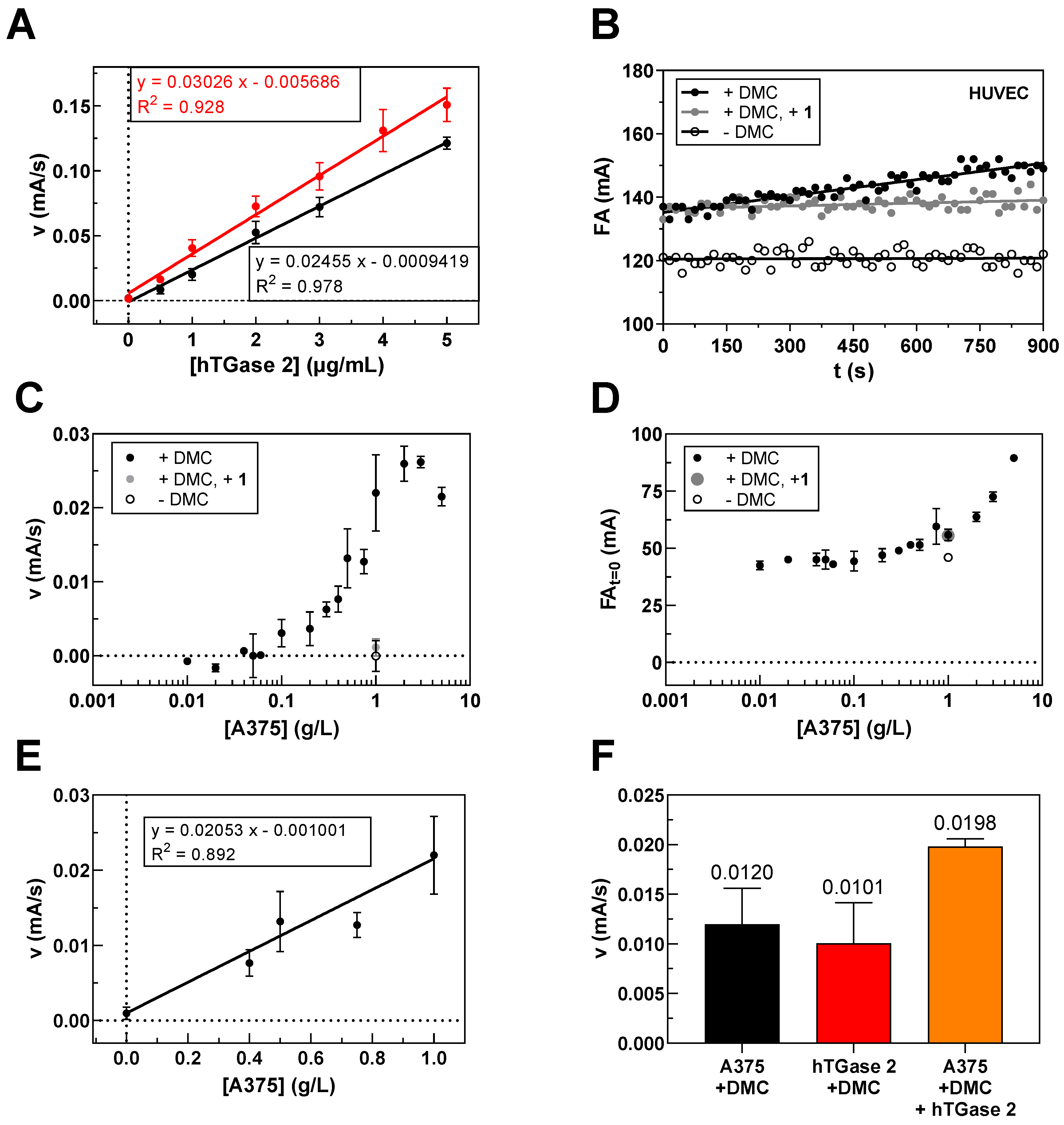
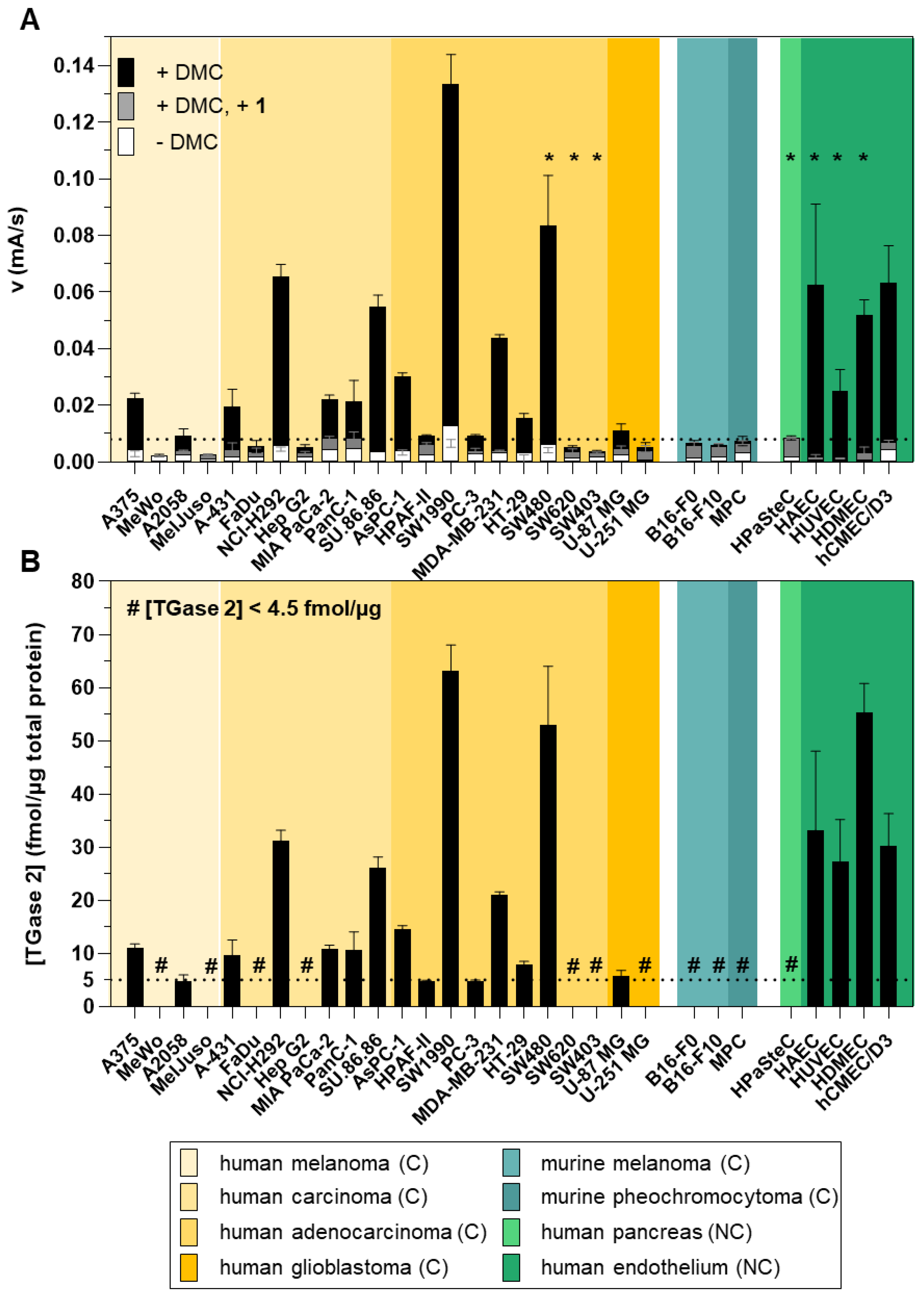
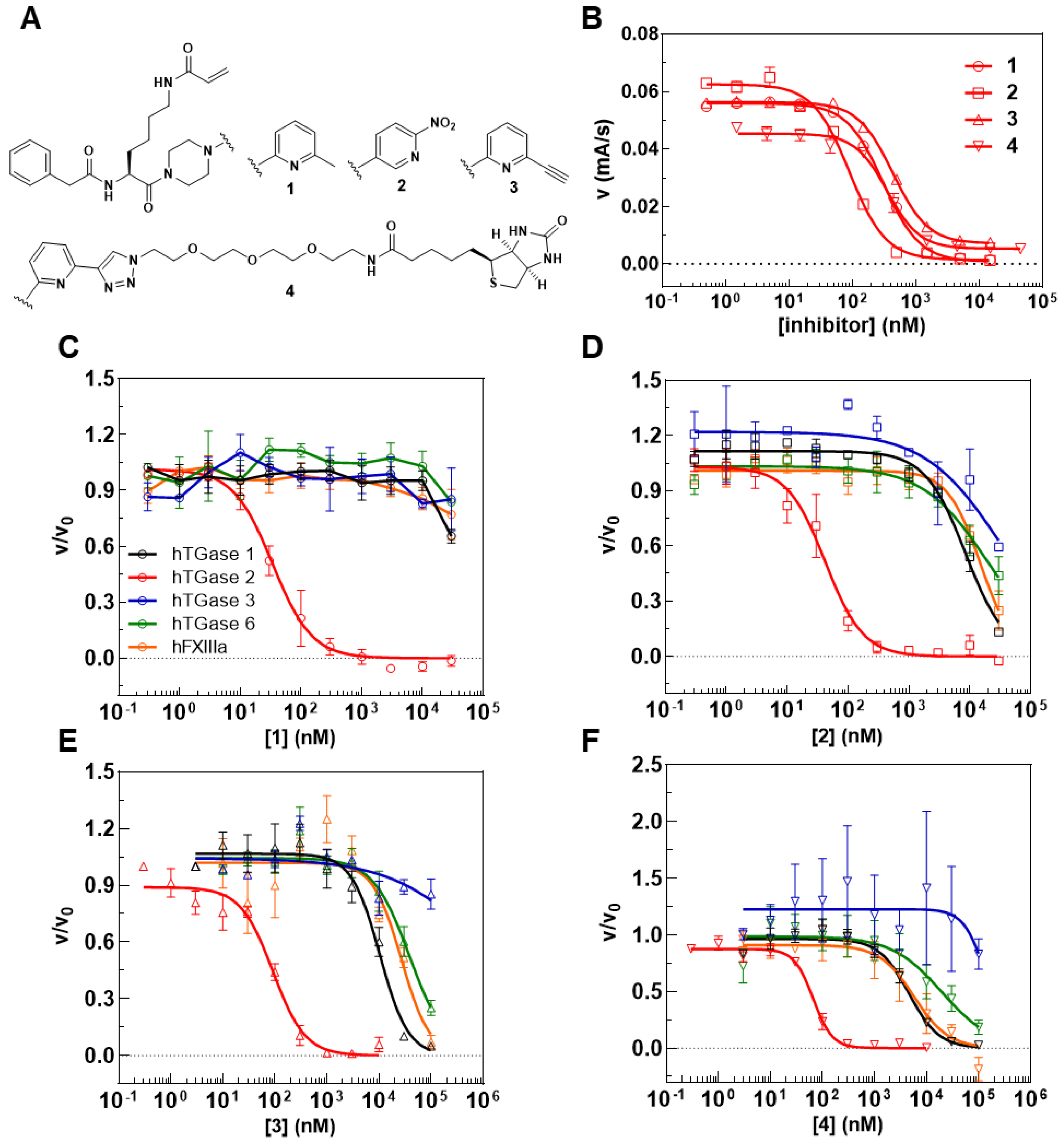
| hTGase 1 | hTGase 2 | hTGase 3 | hTGase 6 | hFXIIIa | |
|---|---|---|---|---|---|
| Vmax (mA mL s−1 mg−1) | 121 (13) | 26.4 (2.8) | 5.80 (0.09) | 27.1 (4.1) | 16.0 (1.1) |
| Km (µM) | 8.77 (1.70) | 2.78 (0.10) | 6.29 (1.52) | 16.6 (4.7) | 17.5 (0.7) |
| Ki (µM) | 270 (72) | 174 (16) | 571 (53) | 189 (61) | 135 (14) |
| Vmax/Km (mA mL s−1 mg−1 µM−1) | 13.8 | 9.50 | 0.92 | 1.63 | 0.91 |
| IC50 (nM) a | kinact/KI (M−1s−1) b | ||||||
|---|---|---|---|---|---|---|---|
| Time (min) | hTGase 1 | hTGase 2 | hTGase 3 | hTGase 6 | hFXIIIa | hTGase 2 | |
| 1 | 5 | n.d. | 310 c | n.d. | n.d. | n.d. | 4880 (20)c |
| 30 | >30,000 | 40 (12) | >30,000 | >30,000 | >30,000 | ||
| 2 | 5 | n.d. | 90 c | n.d. | n.d. | n.d. | 8460 (710) c |
| 30 | 8990 (1180) | 42 (15) | 32,800 (6600) | 19,500 (1300) | 17,600 (5600) | ||
| 3 | 5 | n.d. | 441 (24) | n.d. | n.d. | n.d. | 3450 (320) |
| 30 | 10,200 (900) | 96 (18) | >100,000 | 38,400 (6500) | 26,200 (4100) | ||
| 4 | 5 | n.d. | 249 (44) | n.d. | n.d. | n.d. | 7260 (1350) |
| 30 | 4560 (570) | 68 (8) | >100,000 | 21,800 (9700) | 7120 (3290) | ||
| [DMC]/[R-I-Cad] | 30/0.81 µM |
| Detection limit | ≥0.5 µg/mL hTGase 2 |
| Linear range | 0.5–5 µg/mL hTGase 2 |
| [cell lysate] | ≤1 g/L (otherwise nonspecific binding of R-I-Cad becomes limiting) |
| Selectivity | Order of enzymatic rate at 30 µM DMC: hTGase 1 > hTGase 2 ≈ hTGase 6 > hFXIIIa > hTGase 3 Control measurements with a selective hTGase 2 inhibitor are recommended. |
| Further applications | Inhibitor characterization is possible on recombinant TGases and in cell lysates [54]. |
| Assay buffer A | 100 mM MOPS pH 8.0, 3 mM CaCl2, 50 µM EDTA |
| Assay buffer B | 100 mM MOPS pH 8.0, 6 mM CaCl2, 50 µM EDTA |
| Enzyme buffer A | 100 mM MOPS pH 8.0, 3 mM CaCl2, 10 mM TCEP, 20% glycerol (v/v) |
| Enzyme buffer B | 100 mM MOPS pH 8.0, 3 mM CaCl2, 10 mM DTT, 20% glycerol (v/v) |
| RIPA lysis buffer | 150 mM NaCl, 50 mM Tris pH 8.0, 1% NP40, 0.5% SDS (w/v), 0.5% sodium deoxycholate (w/v), 7 µg/mL leupeptin, 1 mM PMSF, 1 mM Na3VO4, 1 mM DTT, 7 mM NaF |
| Modified RIPA buffer A | 150 mM NaCl, 50 mM Tris pH 8.0, 1 µg/mL leupeptin, 1 mM PMSF, 1 mM Na3VO4, 1 mM DTT, 5 mM NaF |
| Modified RIPA buffer B | 150 mM NaCl, 50 mM Tris pH 8.0, 7 µg/mL leupeptin, 1 mM PMSF, 1 mM Na3VO4, 1 mM DTT, 7 mM NaF |
| 5× SDS-PAGE protein loading buffer | 312.5 mM Tris pH 6.8, 40% glycerol, 10% SDS, 5% β-mercaptoethanol, 0.1% bromophenol blue |
| HEPES buffer A | 50 mM HEPES pH 8.0, 6 mM CaCl2 |
| HEPES buffer B | 50 mM HEPES pH 7.4 |
| Name | Species | Origin | Medium | |
|---|---|---|---|---|
| A375 | human | skin malignant melanoma | primary tumor | DMEM + 10% FCS |
| MeWo | human | skin malignant melanoma | lymph node metastasis | DMEM + 10% FCS |
| A2058 | human | skin melanoma | lymph node metastasis | DMEM + 10% FCS |
| MelJuso | human | skin melanoma | primary tumor | RPMI 1640 + 10% FCS |
| A-431 | human | skin epidermoid carcinoma | primary tumor | DMEM + 10% FCS |
| FaDu | human | pharynx squamous cell carcinoma | primary tumor | DMEM + 10% FCS |
| NCI-H292 | human | lung mucoepidermoid carcinoma | lymph node metastasis | RPMI 1640 + 10% FCS |
| Hep G2 | human | hepatocellular carcinoma | primary tumor | DMEM + 10% FCS |
| MIA PaCa-2 | human | pancreas epithelial cell carcinoma | primary tumor | DMEM + 10% FCS |
| PanC-1 | human | pancreas ductal epithelioid carcinoma | primary tumor | DMEM + 10% FCS |
| SU.86.86 | human | pancreas ductal carcinoma | liver metastasis | RPMI 1640 + 10% FCS |
| AsPC-1 | human | pancreas adenocarcinoma | ascites metastasis | RPMI 1640 + 2 mM glutamine + 1 mM sodium pyruvate + 10% FCS |
| HPAF-II | human | pancreas adenocarcinoma | ascites fluid | EMEM + 10% FCS |
| SW1990 | human | pancreas adenocarcinoma | spleen metastasis | L15 + 10% FCS |
| PC-3 | human | prostate adenocarcinoma | bone metastasis | F12 + 2 mM glutamine + 10% FCS |
| MDA-MB-231 | human | breast adenocarcinoma | pleural effusion | L15 + 2 mM glutamine + 15% FCS |
| HT-29 | human | colorectal adenocarcinoma | primary tumor | McCoy + 10% FCS |
| SW480 | human | Dukes’ type B, colorectal adenocarcinoma | primary tumor | L15 + 10% FCS |
| SW620 | human | Dukes’ type C, colorectal adenocarcinoma | lymph node metastasis | L15 + 10% FCS |
| SW403 | human | Dukes’ type C, colorectal adenocarcinoma | primary tumor | L15 + 10% FCS |
| U-87 MG | human | brain glioblastoma | primary tumor | DMEM + 10% FCS |
| U-251 MG | human | brain glioblastoma astrocytoma | primary tumor | DMEM + 2 mM glutamine + 1% nonessential amino acids + 1 mM sodium pyruvate + 10% FCS |
| B16-F0 | mouse | skin melanoma | primary tumor | DMEM + 10% FCS |
| B16-F10 | mouse | skin melanoma | lung metastasis, 10th generation | DMEM + 10% FCS |
| MPC | mouse | pheochromocytoma | primary tumor | RPMI 1640 + 10% horse serum + 5% FCS |
| HPaSteC | human | pancreatic stellate cells | SteCM | |
| HAEC | human | aortic endothelium | ECGM-2 | |
| HUVEC | human | umbilical vein endothelium | ECGM-2 | |
| HDMEC | human | dermal microvascular endothelium | ECGM-2 | |
| hCMEC/D3 | human | cerebral microvascular endothelium | ECGM-2 | |
| Primary Antibodies | Product No. | Company | Dilution |
| anti-TGase 1 polyclonal rabbit | ab103814 | Abcam | 1:100 |
| anti-TGase 2 monoclonal mouse | ab2386 | Abcam | 1:500 |
| anti-TGase 3 polyclonal rabbit | ab203229 | Abcam | 1:500 |
| anti-TGase 6 polyclonal rabbit | ab180959 | Abcam | 1:1000 |
| anti-factor XIIIa polyclonal rabbit | ab97636 | Abcam | 1:500 |
| anti-propionyl-CoA carboxylase polyclonal rabbit | ab187686 | Abcam | 1:5000 |
| anti-β-actin monoclonal mouse | A5316 | Sigma-Aldrich | 1:1000 |
| Secondary Antibodies | Product No. | Company | Dilution |
| anti-rabbit IgG POD polyclonal goat | A0545 | Sigma-Aldrich | 1:2000 |
| anti-mouse IgG POD polyclonal rabbit | A9044 | Sigma-Aldrich | 1:2000/1:10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauser, S.; Sommerfeld, P.; Wodtke, J.; Hauser, C.; Schlitterlau, P.; Pietzsch, J.; Löser, R.; Pietsch, M.; Wodtke, R. Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates. Int. J. Mol. Sci. 2022, 23, 4475. https://doi.org/10.3390/ijms23094475
Hauser S, Sommerfeld P, Wodtke J, Hauser C, Schlitterlau P, Pietzsch J, Löser R, Pietsch M, Wodtke R. Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates. International Journal of Molecular Sciences. 2022; 23(9):4475. https://doi.org/10.3390/ijms23094475
Chicago/Turabian StyleHauser, Sandra, Paul Sommerfeld, Johanna Wodtke, Christoph Hauser, Paul Schlitterlau, Jens Pietzsch, Reik Löser, Markus Pietsch, and Robert Wodtke. 2022. "Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates" International Journal of Molecular Sciences 23, no. 9: 4475. https://doi.org/10.3390/ijms23094475
APA StyleHauser, S., Sommerfeld, P., Wodtke, J., Hauser, C., Schlitterlau, P., Pietzsch, J., Löser, R., Pietsch, M., & Wodtke, R. (2022). Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates. International Journal of Molecular Sciences, 23(9), 4475. https://doi.org/10.3390/ijms23094475







