A Central Contribution of TG2 Activity to the Antiproliferative and Pro-Apoptotic Effects of Caffeic Acid in K562 Cells of Human Chronic Myeloid Leukemia
Abstract
1. Introduction
2. Results
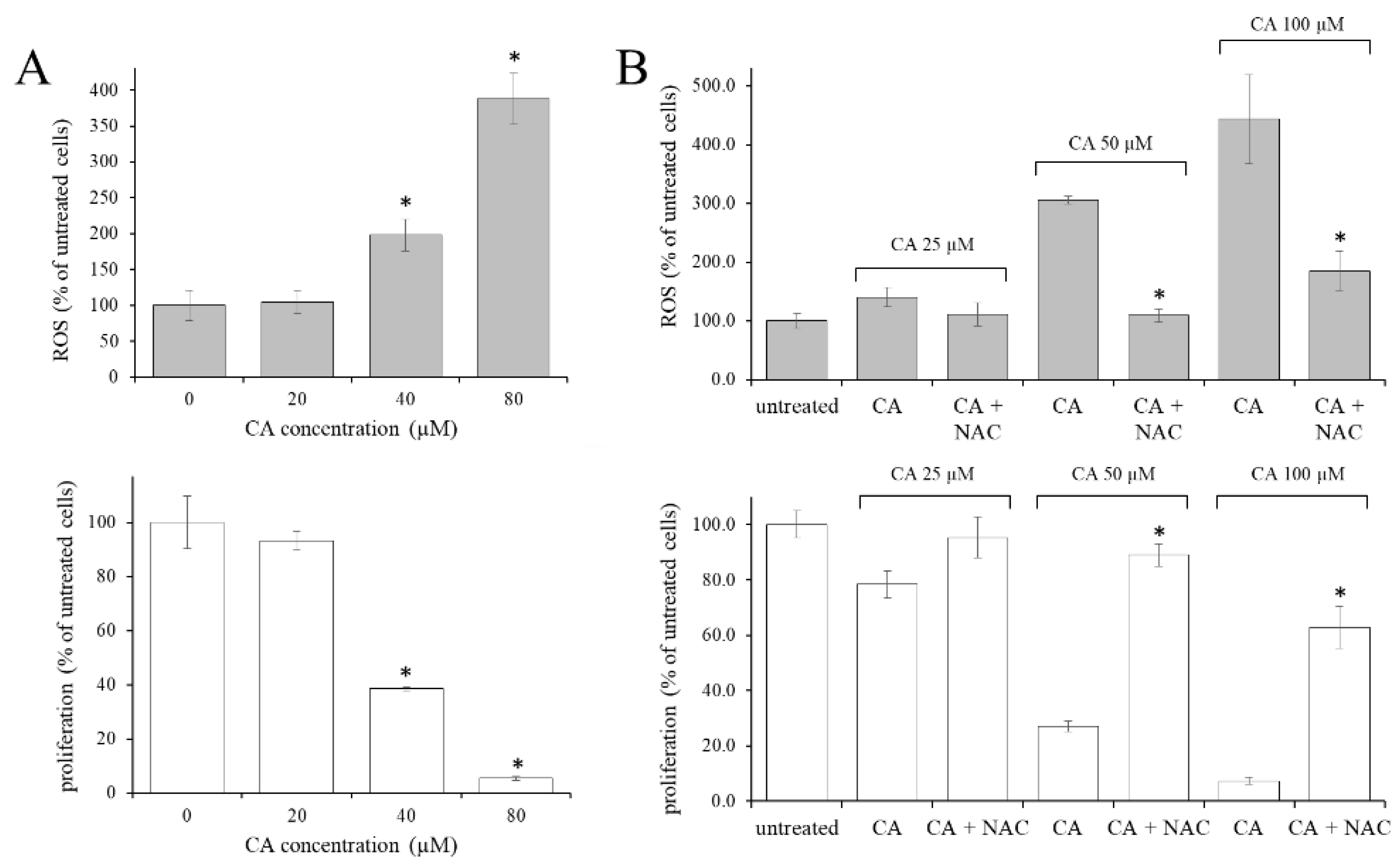
2.1. The Increase in the Intracellular Oxidative State Was Responsible for the Antiproliferative Effect of CA in CML Cells
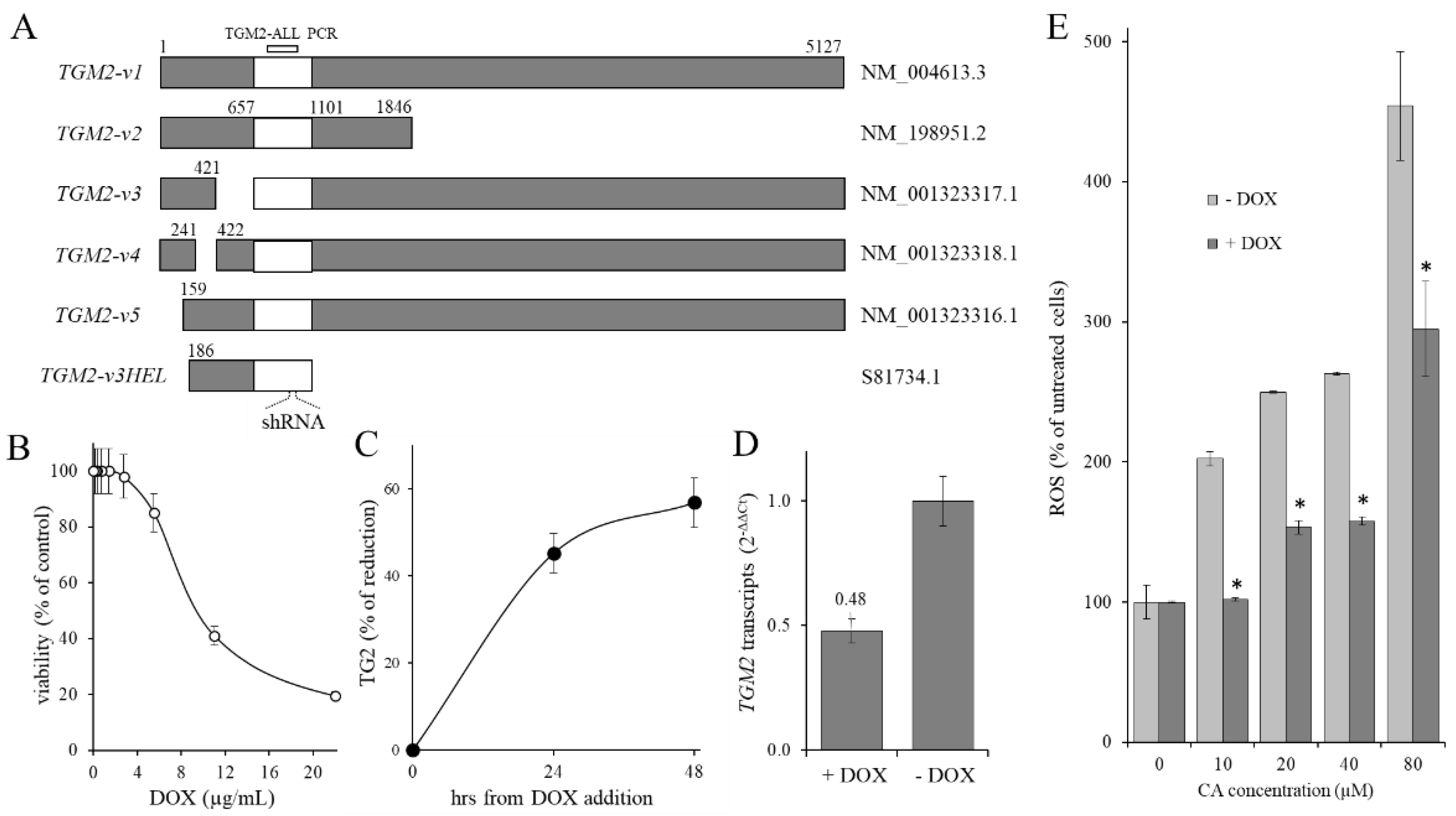
2.2. TG2 Activity Supported the CA-Increased Level of ROS
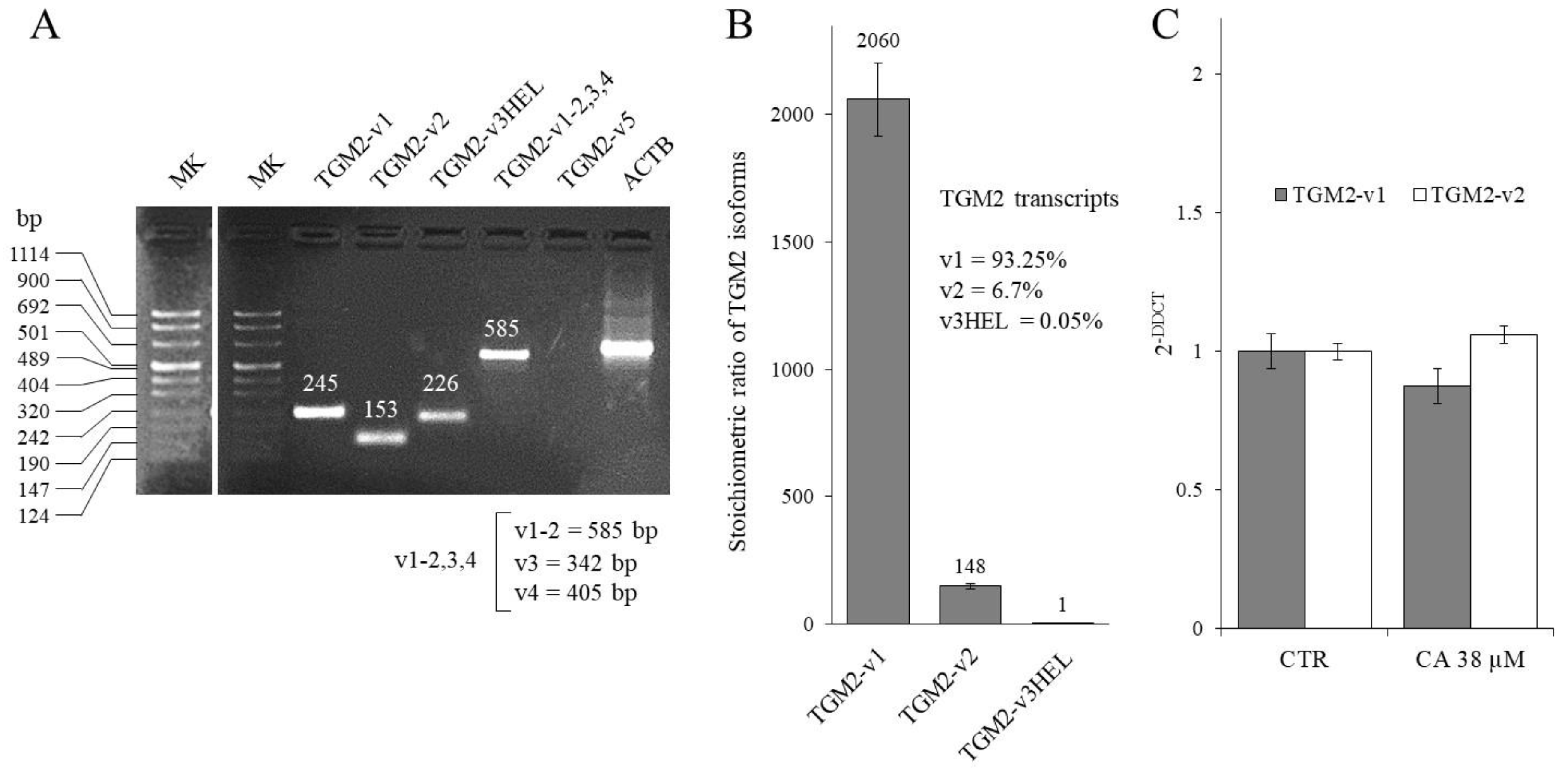
2.3. Effect of CA Treatment on TGM2 Gene Transcription
2.4. Contribution of TG2 on CA-Induced ROS Level Increase
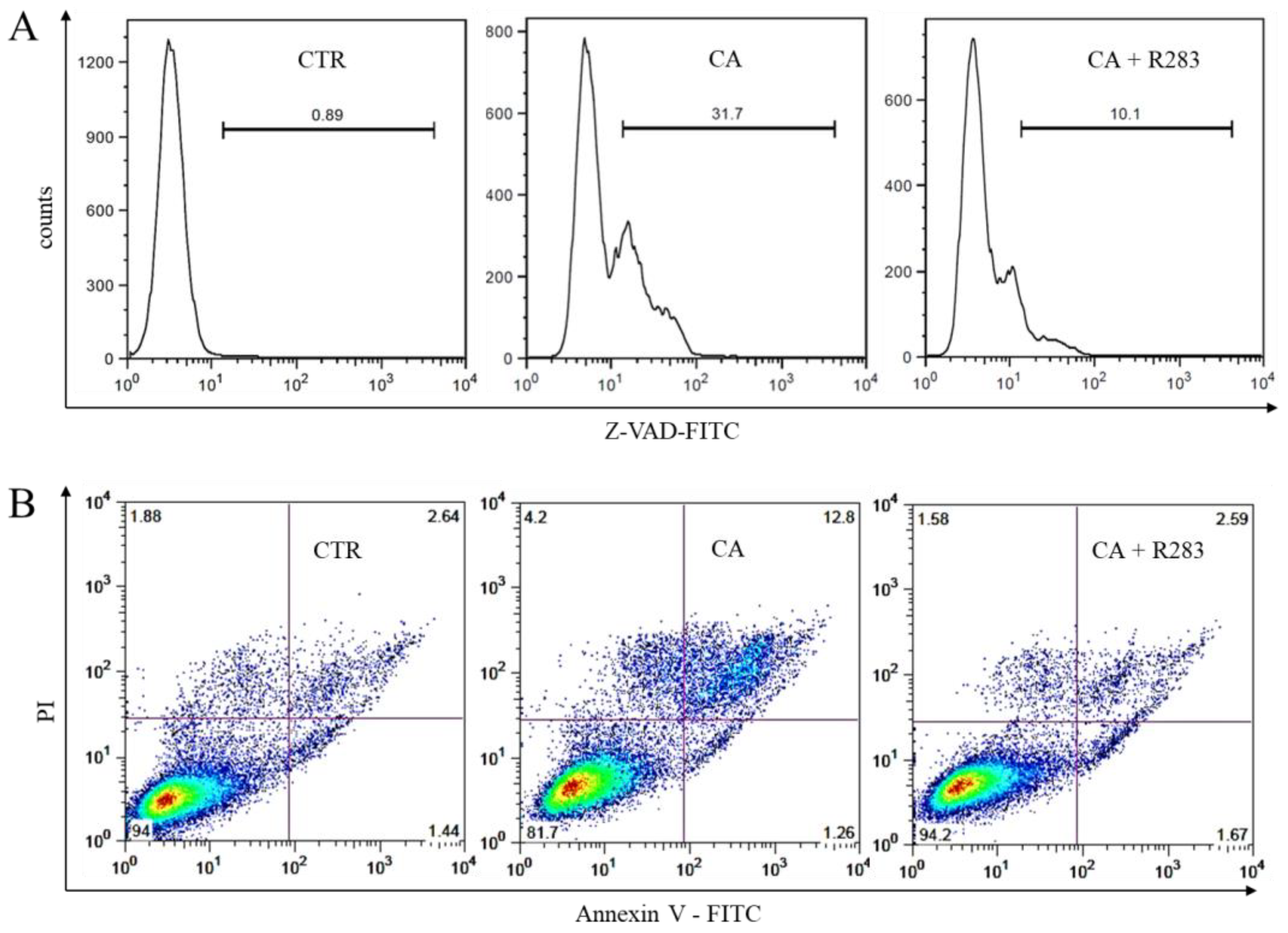
2.5. TG2 Activity Sustained the CA-Triggered Apoptotic Mechanism
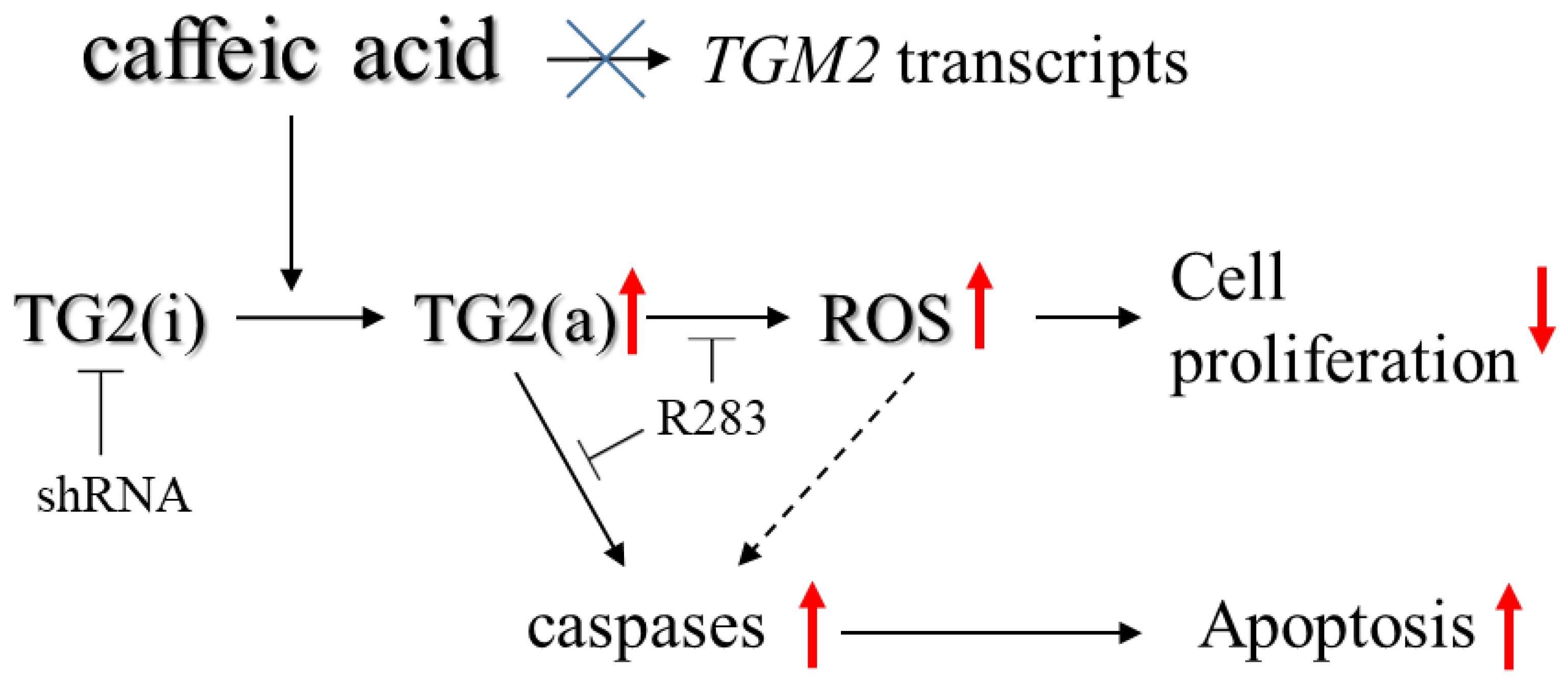
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Viability Test
4.3. RNA Isolation and RT-PCR
4.4. TG2 Activity Assay
4.5. KD-TGM2 Cells Preparation
4.6. Apoptosis Measurement
4.7. Caspase Activity Assay
4.8. Assessment of Intracellular Oxygen-Derived Free Radicals
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yung, Y.; Lee, E.; Chu, H.T.; Yip, P.K.; Gill, H. Targeting Abnormal Hematopoietic Stem Cells in Chronic Myeloid Leukemia and Philadelphia Chromosome-Negative Classical Myeloproliferative Neoplasms. Int. J. Mol. Sci. 2021, 22, 659. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, C.; De Martino, A.; Mischiati, C.; Feriotto, G.; Beninati, S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med. Sci. 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Lentini, A.; Abbruzzese, A.; Provenzano, B.; Tabolacci, C.; Beninati, S. Transglutaminases: Key regulators of cancer metastasis. Amino Acids. 2013, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, K.; Kojima, S.; Fesus, L. Transglutaminases, Multiple Functional Modifiers and Targets for New Drug Discovery, 1st ed.; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Tatsukawa, H.; Sano, T.; Fukaya, Y.; Ishibashi, N.; Watanabe, M.; Okuno, M.; Moriwaki, H.; Kojima, S. Dual induction of caspase 3- and transglutaminase-dependent apoptosis by acyclic retinoid in hepatocellular carcinoma cells. Mol. Cancer 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Antonyak, M.A.; Cerione, R.A. The diamond anniversary of tissue transglutaminase: A protein of many talents. Drug Discov. Today 2018, 23, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Birckbichler, P.J.; Orr, G.R.; Conway, E.; Patterson, M.K., Jr. Transglutaminase activity in normal and transformed cells. Cancer Res. 1977, 37, 1340–1344. [Google Scholar]
- Barnes, R.N.; Bungay, P.J.; Elliott, B.M.; Walton, P.L.; Griffin, M. Alterations in the distribution and activity of transglutam-inase during tumour growth and metastasis. Carcinogenesis 1985, 6, 459–463. [Google Scholar] [CrossRef]
- Mangala, L.S.; Mehta, K. Tissue transglutaminase (TG2) in cancer biology. Prog. Exp. Tumor Res. 2005, 38, 125–138. [Google Scholar]
- Delaine-Smith, R.; Wright, N.; Hanley, C.; Hanwell, R.; Bhome, R.; Bullock, M.; Drifka, C.; Eliceiri, K.; Thomas, G.; Knight, M.; et al. Transglutaminase-2 Mediates the Biomechanical Properties of the Colorectal Cancer Tissue Microenvironment that Contribute to Disease Progression. Cancers 2019, 11, 701. [Google Scholar] [CrossRef]
- Seo, S.; Moon, Y.; Choi, J.; Yoon, S.; Jung, K.H.; Cheon, J.; Kim, W.; Kim, D.; Lee, C.H.; Kim, S.W.; et al. The GTP binding activity of transglutaminase 2 promotes bone metastasis of breast cancer cells by downregulating mi-croRNA-205. Am. J. Cancer Res. 2019, 9, 597–607. [Google Scholar]
- Roncoroni, L.; Elli, L.; Braidotti, P.; Tosi, D.; Vaira, V.; Tacchini, L.; Lombardo, V.; Branchi, F.; Scricciolo, A.; Doneda, L. Tran-sglutaminase 2 Mediates the Cytotoxicity of Resveratrol in a Human Cholangiocarcinoma and Gallbladder Cancer Cell Lines. Nutr. Cancer 2018, 70, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Suedhoff, T.; Birckbichler, P.J.; Lee, K.N.; Conway, E.; Patterson, M.K., Jr. Differential expression of transglutaminase in human erythroleukemia cells in response to retinoic acid. Cancer Res. 1990, 50, 7830–7834. [Google Scholar] [PubMed]
- Berntorp, E.; Seiving, B.; Stenberg, P. Characterization of transglutaminases in normal and malignant human leukocytes. Scand. J. Haematol. 1985, 34, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.O.; Lim, Y.C.; Kim, Y.M.; Ha, K.S. Transglutaminase 2 promotes both caspase-dependent and caspase-independent apoptotic cell death via the calpain/Bax protein signaling pathway. J. Biol. Chem. 2012, 287, 14377–14388. [Google Scholar] [CrossRef]
- Caccamo, D.; Currò, M.; Ferlazzo, N.; Condello, S.; Ientile, R. Monitoring of transglutaminase 2 under different oxidative stress conditions. Amino Acids 2012, 42, 1037–1043. [Google Scholar] [CrossRef]
- Fraij, B.M.; Birckbichler, P.J.; Patterson, M.K.; Lee, K.N.; Gonzales, R.G. A retinoic acid-inducible mRNA from human erythroleukemia cells encodes a novel tissue transglutaminase homologue. J. Biol. Chem. 1992, 267, 22616–22673. [Google Scholar] [CrossRef]
- Fraij, B.M.; Gonzales, R.A. A third human tissue transglutaminase homologue as a result of alternative gene transcripts. Biochim. Biophys. Acta 1996, 1306, 63–74. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Liu, H.; Guo, J.; Long, S. Hybrid molecules based on caffeic acid as potential therapeutics: A focused review. Eur. J. Med. Chem. 2022, 243, 114745. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef]
- Meshram, D.D.; Pike, C.V.S.; Coussons, P.J. Inhibition of Transglutaminase 2 activity increases cisplatin cytotoxicity in a model of human hepatocarcinoma chemotherapy. Eur. J. Pharmacol. 2017, 815, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Fraij, B.M. The 55 kDa tissue transglutaminase cross-linking active isoform TG induces cell death. Mol. Carcinog. 2015, 54, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.M.; Kim, I. Regulation of Transglutaminase 2 by Oxidative Stress. In Transglutaminases; Springer: Berlin/Heidelberg, Germany, 2015; pp. 315–331. [Google Scholar]
- Phatak, V.M.; Croft, S.M.; Setty, S.G.R.; Scarpellini, A.; Hughes, D.C.; Rees, R.; McArdle, S.; Verderio, E.A. Expres-sion of transglutaminase-2 isoforms in normal human tissues and cancer cell lines: Dysregulation of alternative splicing in cancer. Amino Acids 2013, 44, 33–44. [Google Scholar] [CrossRef]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.N. Synergistic anticancer activity of combined use of caffeic acid with paclitaxel enhances apoptosis of non-small-cell lung cancer H1299 cells in vivo and in vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Camarasa, J.; Escubedo, E.; Adzet, T. Pharmacokinetics of caffeic acid in rats by a high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 1988, 6, 503–510. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; De Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Chen, I.L.; Chou, C.T.; Liang, W.Z.; Kuo, D.H.; Shieh, P.; Jan, C.R. Effect of caffeic acid on Ca(2+) homeostasis and apoptosis in SCM1 human gastric cancer cells. Arch. Toxicol. 2013, 87, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Shin, D.H.; Zheng, H.; Kang, J.S.; Kim, W.K.; Kim, S.J. Inhibition of store-operated Ca2+ entry channels and K+ channels by caffeic acid phenethylester in T lymphocytes. Eur. J. Pharmacol. 2009, 612, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.S.; Gangan, V.; Jain, S.K.; Manna, S.K. Novel caffeic acid ester derivative induces apoptosis by expressing FasL and downregulating NF-KappaB: Potentiation of cell death mediated by chemotherapeutic agents. J. Cell. Physiol. 2009, 218, 653–662. [Google Scholar] [CrossRef]
- Slaughter, T.F.; Achyuthan, K.E.; Lai, T.S.; Greenberg, C.S. A microtiter plate transglutaminase assay utilizing 5-(biotinamido)pentylamine as substrate. Anal. Biochem. 1992, 205, 166–171. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feriotto, G.; Tagliati, F.; Brunello, A.; Beninati, S.; Tabolacci, C.; Mischiati, C. A Central Contribution of TG2 Activity to the Antiproliferative and Pro-Apoptotic Effects of Caffeic Acid in K562 Cells of Human Chronic Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 15004. https://doi.org/10.3390/ijms232315004
Feriotto G, Tagliati F, Brunello A, Beninati S, Tabolacci C, Mischiati C. A Central Contribution of TG2 Activity to the Antiproliferative and Pro-Apoptotic Effects of Caffeic Acid in K562 Cells of Human Chronic Myeloid Leukemia. International Journal of Molecular Sciences. 2022; 23(23):15004. https://doi.org/10.3390/ijms232315004
Chicago/Turabian StyleFeriotto, Giordana, Federico Tagliati, Arianna Brunello, Simone Beninati, Claudio Tabolacci, and Carlo Mischiati. 2022. "A Central Contribution of TG2 Activity to the Antiproliferative and Pro-Apoptotic Effects of Caffeic Acid in K562 Cells of Human Chronic Myeloid Leukemia" International Journal of Molecular Sciences 23, no. 23: 15004. https://doi.org/10.3390/ijms232315004
APA StyleFeriotto, G., Tagliati, F., Brunello, A., Beninati, S., Tabolacci, C., & Mischiati, C. (2022). A Central Contribution of TG2 Activity to the Antiproliferative and Pro-Apoptotic Effects of Caffeic Acid in K562 Cells of Human Chronic Myeloid Leukemia. International Journal of Molecular Sciences, 23(23), 15004. https://doi.org/10.3390/ijms232315004







