Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 Pathways
Abstract
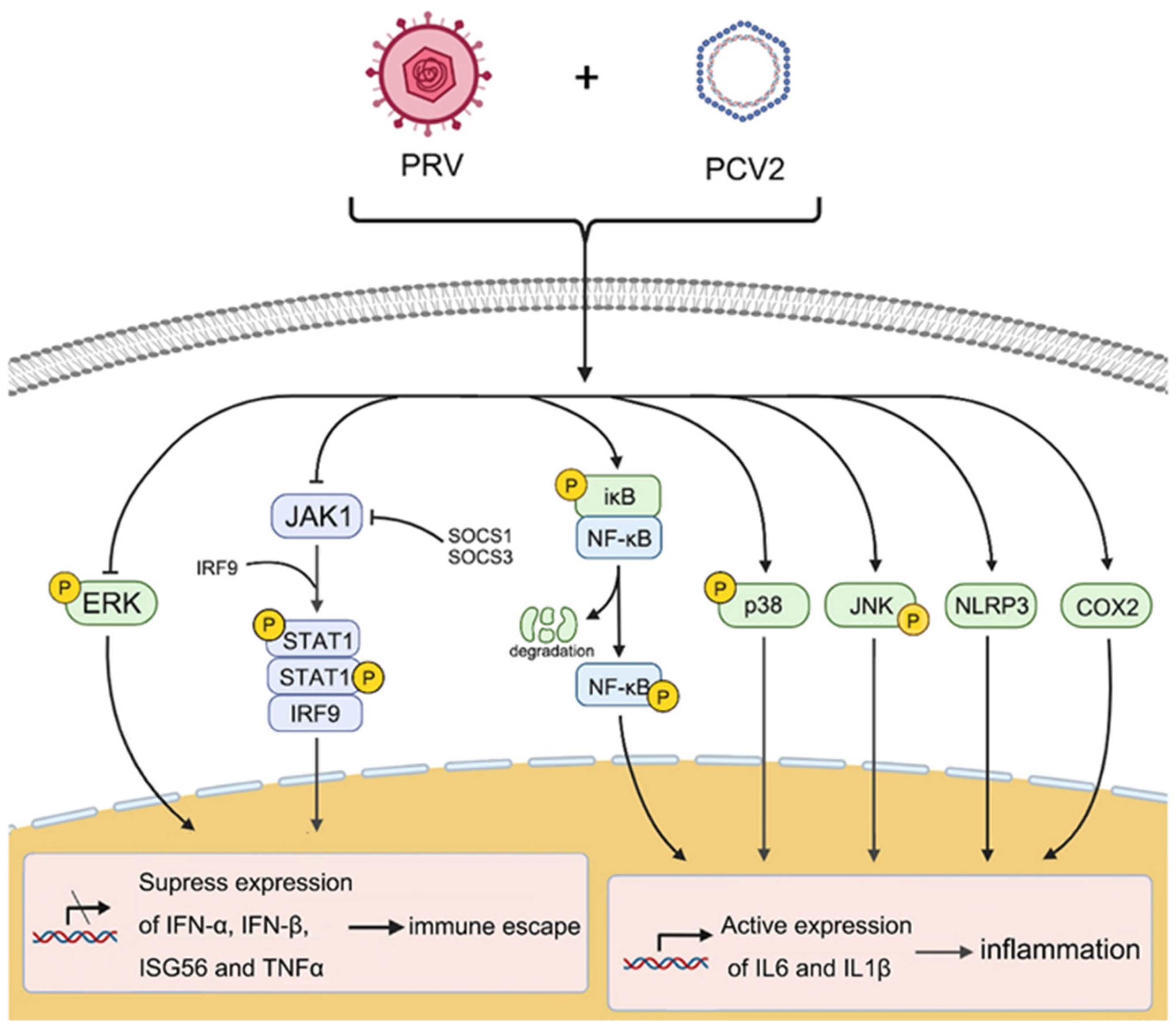
:1. Introduction
2. Results
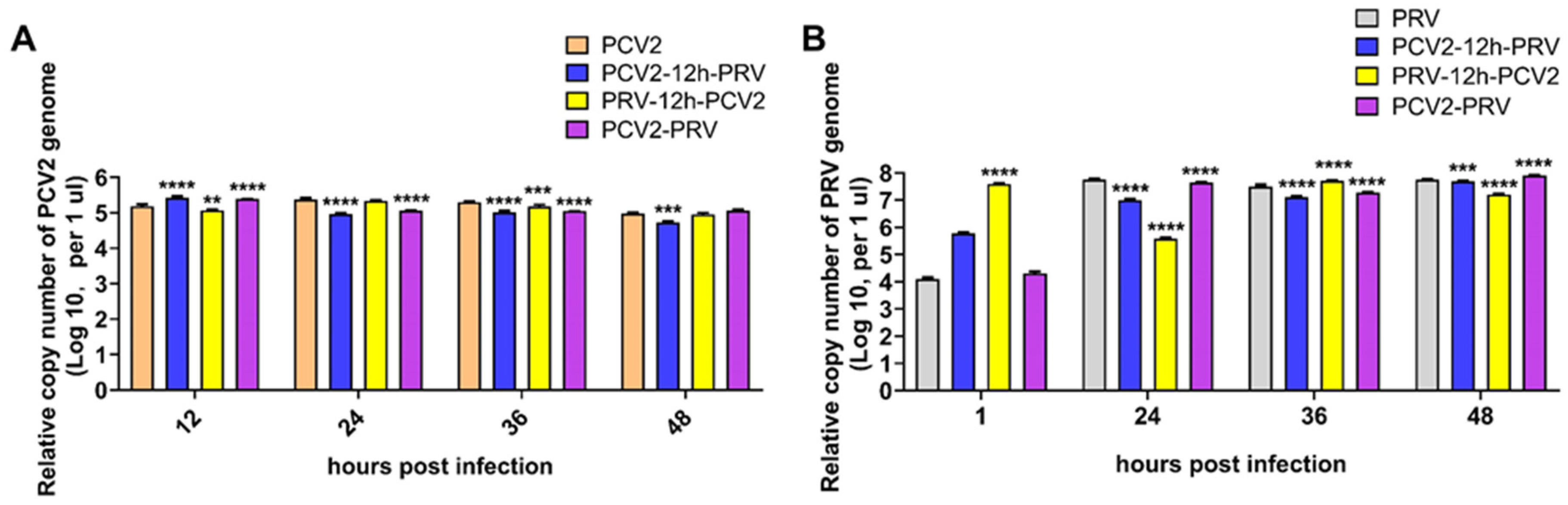
2.1. Coinfection of PCV2 and PRV Inhibit Each Other
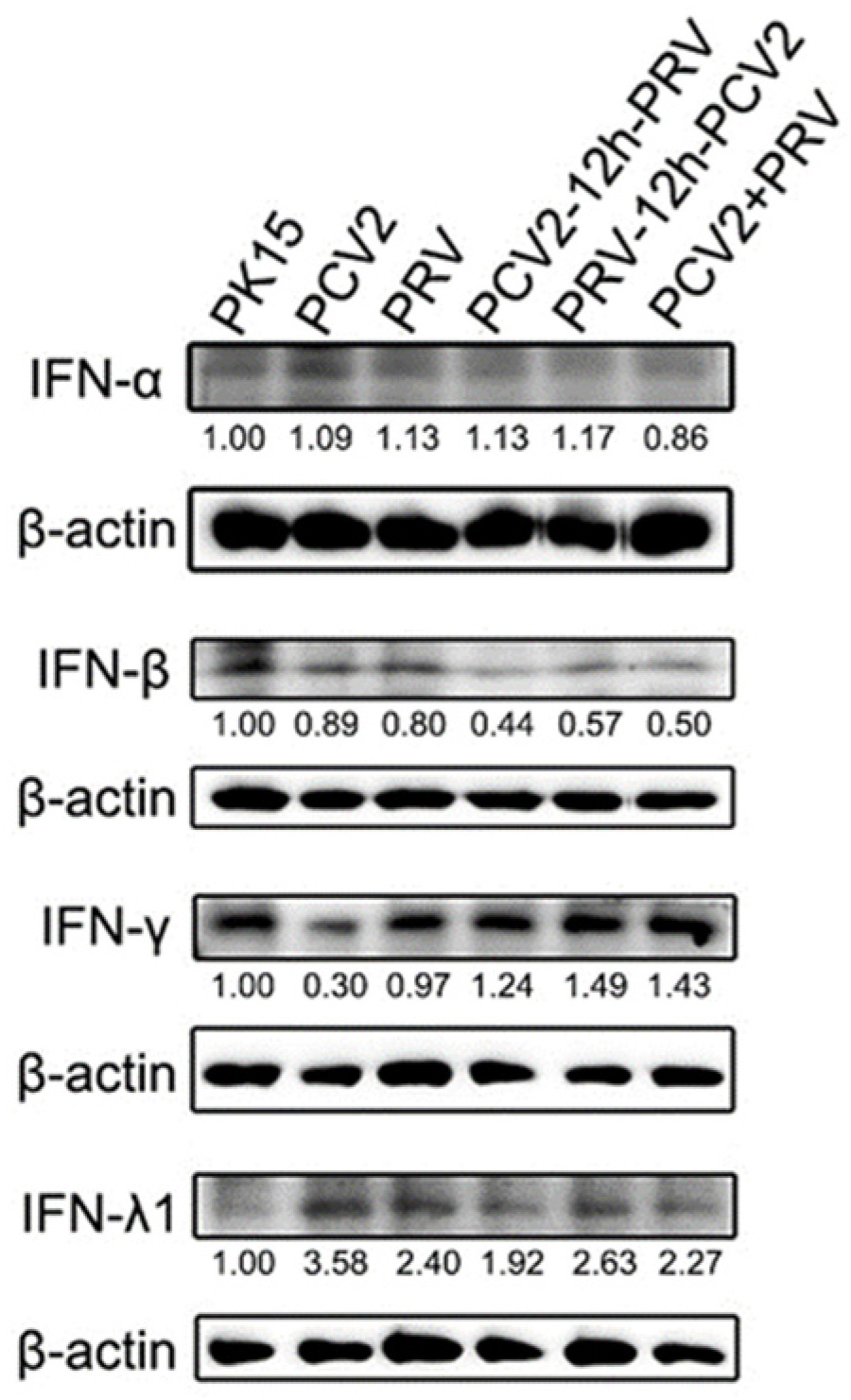
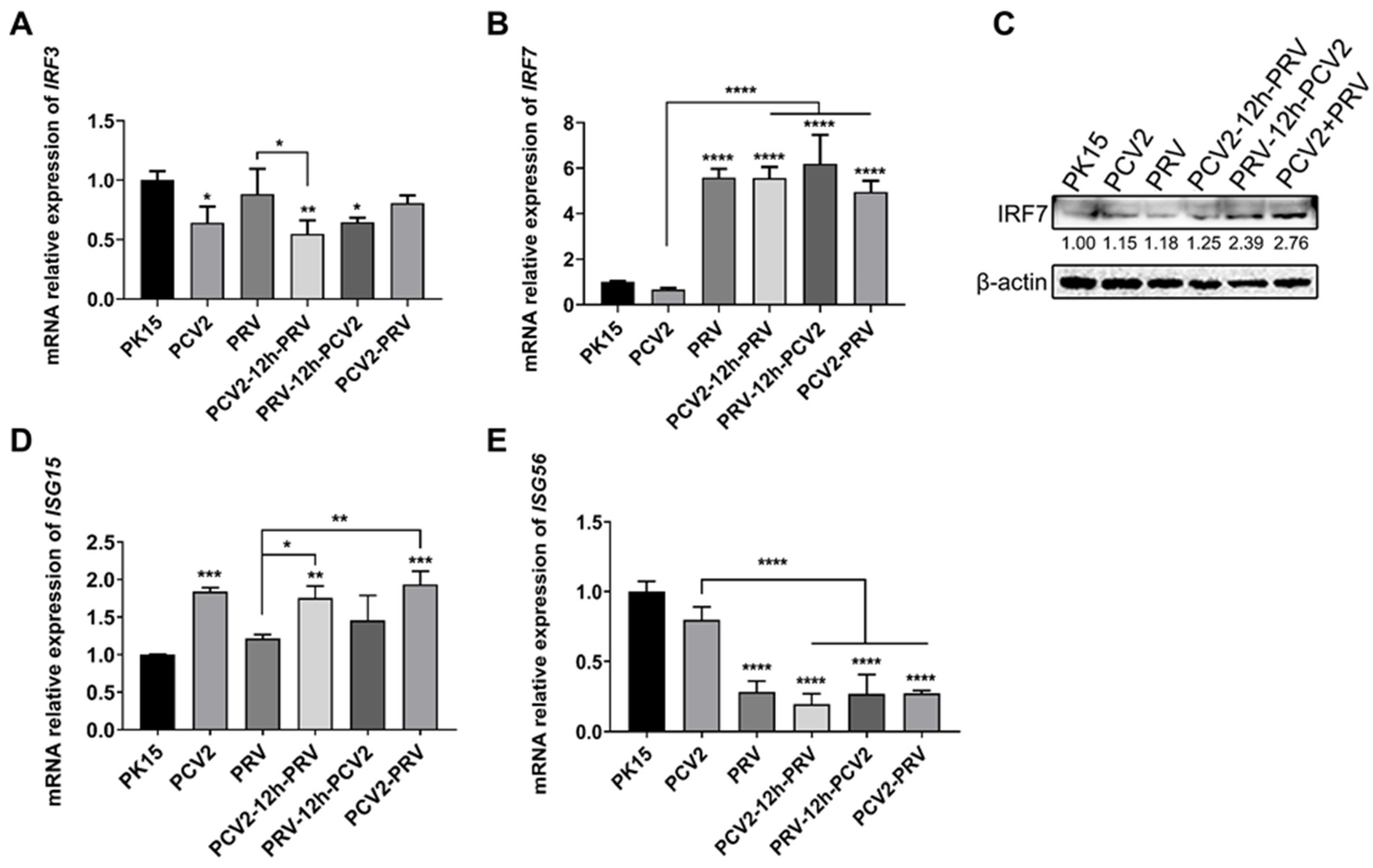
2.2. Coinfection of PCV2 and PRV Inhibits the Expression of IFN-β, IRF3, and ISG56/IFIT1 but Promotes the Expression of IFN-γ, IFN-λ1, IRF7, and ISG15
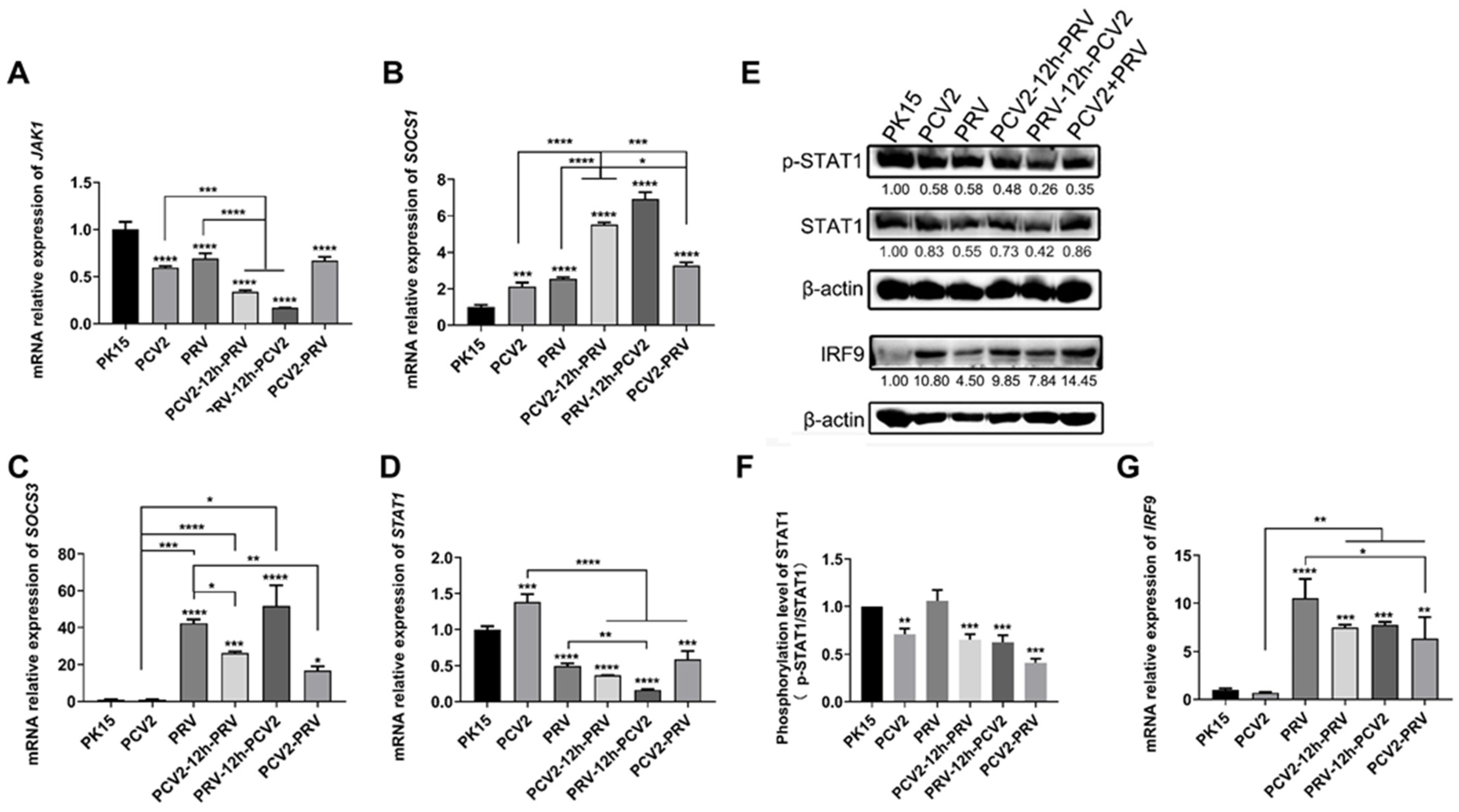
2.3. Coinfection of PCV2 and PRV Suppress JAK1- and STAT1-Related JAK/STAT Pathways
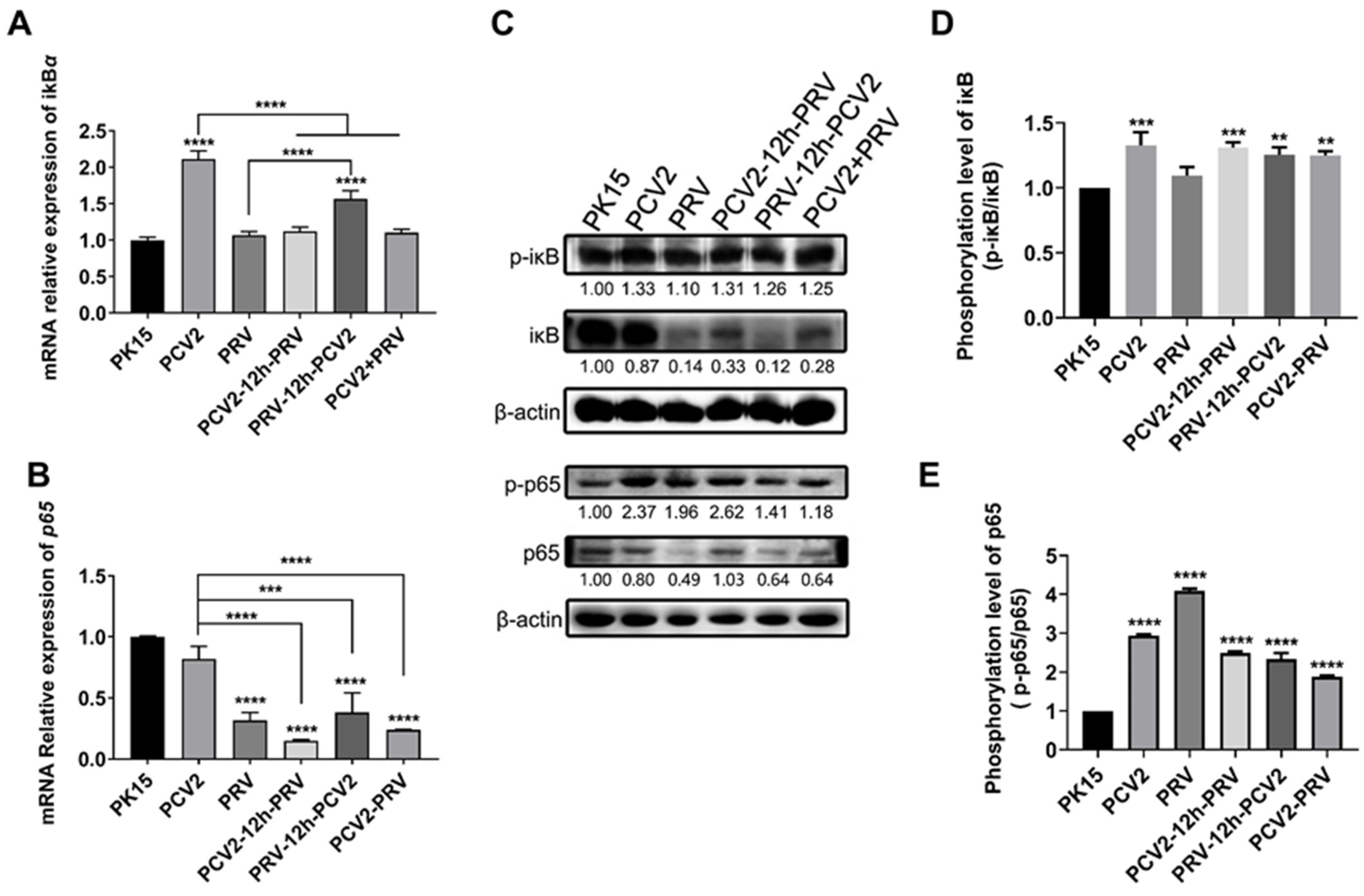
2.4. Coinfection of PCV2 and PRV Modulates NF-κB Signal Pathway
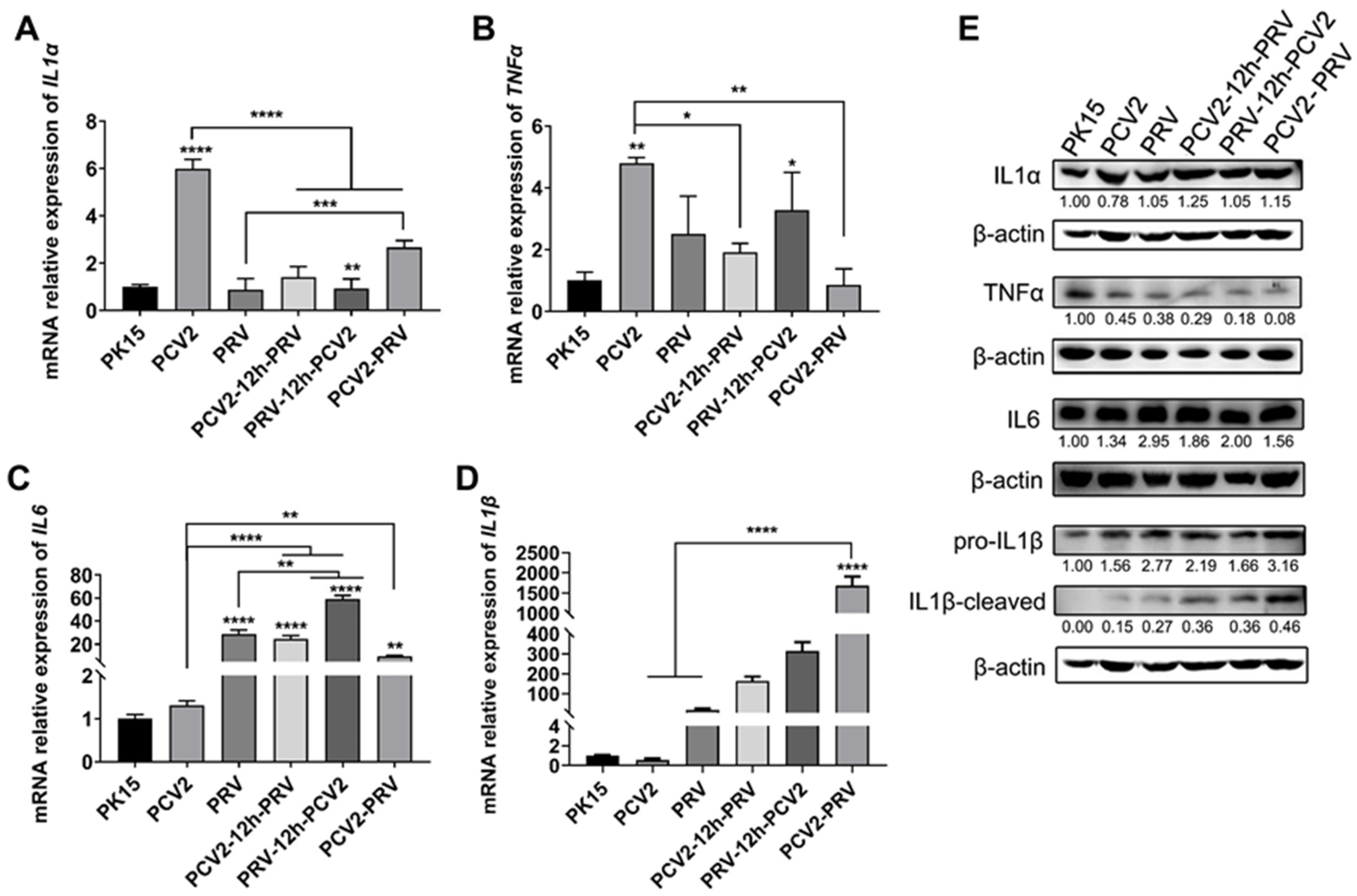
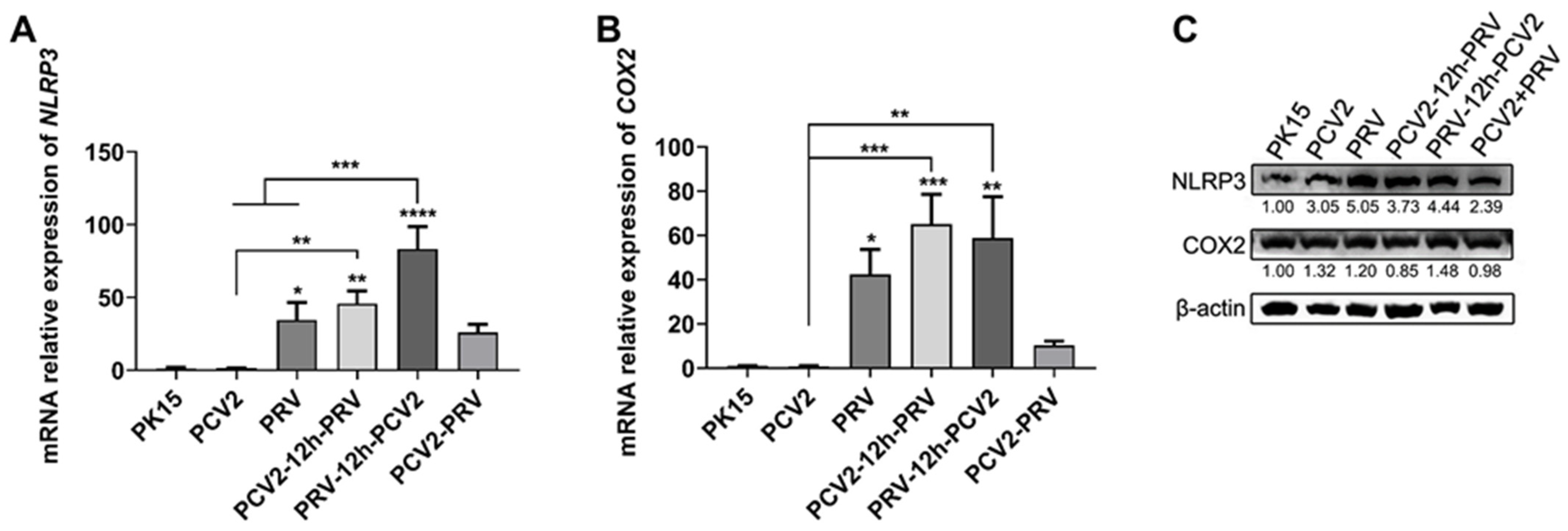
2.5. Coinfection of PCV2 and PRV Modulates Expressions of Host Pro-Inflammatory Factors
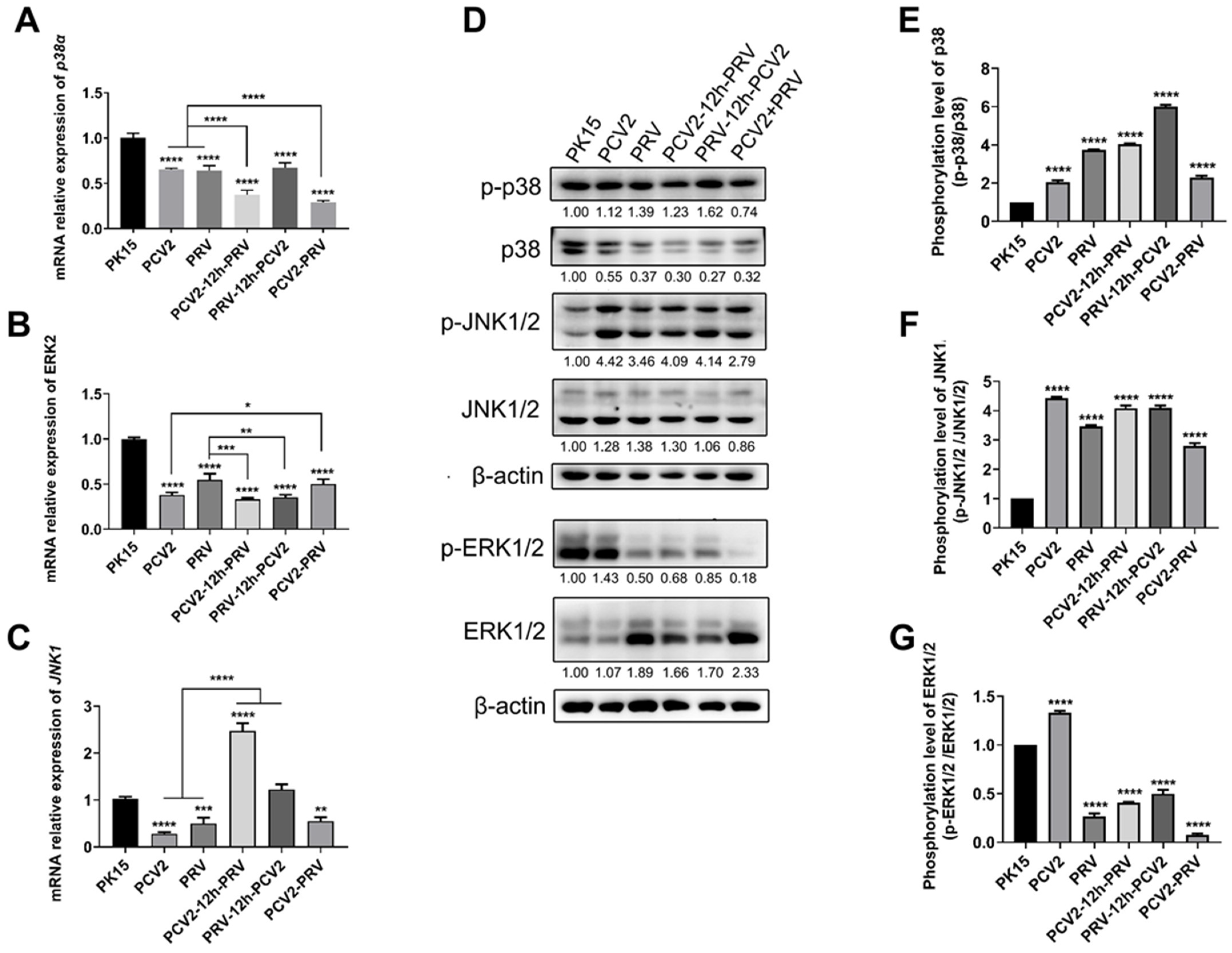
2.6. Coinfection of PCV2 and PRV Activates Inflammatory and Immune via p38 and JNK1/2
3. Materials and Methods
3.1. Cells and Virus
3.2. Viral Infection
3.3. Real-Time PCR
3.4. Western Blotting
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Han, Z.; Liu, Z.; Meng, F.; Wang, H.; Cao, L.; Li, Y.; Jiao, Q.; Liu, S.; Liu, M. Epidemiological investigation of porcine pseudorabies virus and its coinfection rate in Shandong Province in China from 2015 to 2018. J. Vet. Sci. 2020, 21, e36. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shu, X.; Bai, H.; Li, W.; Li, X.; Wu, C.; Gao, Y.; Wang, Y.; Yang, K.; Song, C. Effect of porcine circovirus type 2 on the severity of lung and brain damage in piglets infected with porcine pseudorabies virus. Vet. Microbiol. 2019, 237, 108394. [Google Scholar] [CrossRef]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Crehan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef]
- Kim, D.; Ha, Y.; Oh, Y.; Chae, C. Prevalence of porcine circovirus types 2a and b in pigs with and without post-weaning multi-systemic wasting syndrome. Vet. J. 2011, 188, 115–117. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Liu, Z.; Meng, F.; Wang, H.; Cao, L.; Li, Y.; Jiao, Q.; Han, Z.; Liu, S. Epidemiological investigation of porcine circovirus type 2 and its coinfection rate in Shandong province in China from 2015 to 2018. BMC Vet. Res. 2021, 17, 17. [Google Scholar] [CrossRef]
- Eclercy, J.; Larcher, T.; Andraud, M.; Renson, P.; Bernard, C.; Bigault, L.; Ledevin, M.; Paboeuf, F.; Grasland, B.; Rose, N.; et al. PCV2 co-infection does not impact PRRSV MLV1 safety but enhances virulence of a PRRSV MLV1-like strain in infected SPF pigs. Vet. Microbiol. 2020, 244, 108656. [Google Scholar] [CrossRef]
- Fan, P.; Wei, Y.; Guo, L.; Wu, H.; Huang, L.; Liu, J.; Liu, C. Synergistic effects of sequential infection with highly pathogenic porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Virol. J. 2013, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhuang, D.; Li, H.; Zhao, M.; Zhu, E.; Xie, B.; Chen, J.; Zhao, M. Recombinant pseudorabies virus with gI/gE deletion generated by overlapping polymerase chain reaction and homologous recombination technology induces protection against the PRV variant PRV-GD2013. BMC Vet. Res. 2021, 17, 164. [Google Scholar] [CrossRef]
- Zhou, H.; Pan, Y.; Liu, M.; Han, Z. Prevalence of Porcine Pseudorabies Virus and Its Coinfection Rate in Heilongjiang Province in China from 2013 to 2018. Viral Immunol. 2020, 33, 550–554. [Google Scholar] [CrossRef]
- He, W.; Auclert, L.Z.; Zhai, X.; Wong, G.; Zhang, C.; Zhu, H.; Xing, G.; Wang, S.; He, W.; Li, K.; et al. Interspecies Transmission, Genetic Diversity, and Evolutionary Dynamics of Pseudorabies Virus. J. Infect Dis. 2019, 219, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Rho, S.B.; Kim, H.S.; Han, J.; Bae, J.; Lee, S.J.; Jung, W.W.; Chun, T. The ORF3 protein of porcine circovirus type 2 promotes secretion of IL-6 and IL-8 in porcine epithelial cells by facilitating proteasomal degradation of regulator of G protein signalling 16 through physical interaction. J. Gen. Virol. 2015, 96 Pt 5, 1098–1108. [Google Scholar] [CrossRef]
- Meng, X.J. Porcine circovirus type 2 (PCV2): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Jiang, C.; Cao, H.; Zeng, W.; Zhang, X.; Li, Z.; He, Q. Porcine circovirus type 2 infection activates NF-kappaB pathway and cellular inflammatory responses through circPDCD4/miR-21/PDCD4 axis in porcine kidney 15 cell. Virus Res. 2021, 298, 198385. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.; Qiao, D.; Yuan, Y.; Han, C.; Yang, N.; Li, R.; Du, Q.; Tong, D.; Huang, Y. Porcine circovirus type 2 infection attenuates the K63-linked ubiquitination of STING to inhibit IFN-beta induction via p38-MAPK pathway. Vet. Microbiol. 2021, 258, 109098. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.M.T.; Puvanendiran, S.; Murtaugh, M.P. Porcine circovirus 2 infection induces IFNbeta expression through increased expression of genes involved in RIG-I and IRF7 signaling pathways. Virus Res. 2018, 253, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.; Van Waesberghe, C.; Favoreel, H.W. Pseudorabies Virus Infection of Epithelial Cells Leads to Persistent but Aberrant Activation of the NF-kappaB Pathway, Inhibiting Hallmark NF-kappaB-Induced Proinflammatory Gene Expression. J. Virol. 2020, 94, e00196-20. [Google Scholar] [CrossRef]
- Romero, N.; Favoreel, H.W. Pseudorabies Virus Infection Triggers NF-kappaB Activation via the DNA Damage Response but Actively Inhibits NF-kappaB-Dependent Gene Expression. J. Virol. 2021, 95, e0166621. [Google Scholar] [CrossRef]
- Sun, W.; Liu, S.; Huang, X.; Yuan, R.; Yu, J. Cytokine storms and pyroptosis are primarily responsible for the rapid death of mice infected with pseudorabies virus. R. Soc. Open Sci. 2021, 8, 210296. [Google Scholar] [CrossRef]
- Laval, K.; Vernejoul, J.B.; Van Cleemput, J.; Koyuncu, O.O.; Enquist, L.W. Virulent Pseudorabies Virus Infection Induces a Specific and Lethal Systemic Inflammatory Response in Mice. J. Virol. 2018, 92, e01614-18. [Google Scholar] [CrossRef] [Green Version]
- Kekarainen, T.; Montoya, M.; Dominguez, J.; Mateu, E.; Segales, J. Porcine circovirus type 2 (PCV2) viral components immunomodulate recall antigen responses. Vet. Immunol. Immunopathol. 2008, 124, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kekarainen, T.; Montoya, M.; Mateu, E.; Segales, J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J. Gen. Virol. 2008, 89 Pt 3, 760–765. [Google Scholar] [CrossRef]
- Gao, F.; Xie, J.L.; Jia, C.W.; Ren, H.Y.; Zhou, S.H. Effects of porcine circovirus type 2 and pseudorabies vaccine co-inoculation on regulatory cytokine mRNA expression in pig peripheral blood mononuclear cells. Genet. Mol. Res. 2014, 13, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Faurez, F.; Grasland, B.; Beven, V.; Cariolet, R.; Keranflec’h, A.; Henry, A.; Jestin, A.; Dory, D. The protective immune response against Pseudorabies virus induced by DNA vaccination is impaired if the plasmid harbors a functional Porcine circovirus type 2 rep and origin of replication. Antiviral Res. 2012, 96, 271–279. [Google Scholar] [CrossRef]
- Honda, K.; Takaoka, A.; Taniguchi, T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 2006, 25, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Yanai, H.; Negishi, H.; Asagiri, M.; Sato, M.; Mizutani, T.; Shimada, N.; Ohba, Y.; Takaoka, A.; Yoshida, N.; et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005, 434, 772–777. [Google Scholar] [CrossRef]
- Imada, K.; Leonard, W.J. The Jak-STAT pathway. Mol. Immunol. 2000, 37, 1–11. [Google Scholar] [CrossRef]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-kappaB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Jiang, Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome-A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.C.A.; Parker, L.C.; Zhang, Y. Emerging Regulatory Roles of Dual-Specificity Phosphatases in Inflammatory Airway Disease. Int. J. Mol. Sci. 2019, 20, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Yang, X.; Pang, D.; Peng, Z.; Ma, T.; Ouyang, H.; Ren, L. A dark-to-bright reporter cell for classical swine fever virus infection. Antiviral Res. 2015, 117, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, F.; Cao, Y.; Pang, D.; Ouyang, H.; Ren, L. Complete genome sequence of porcine circovirus 2b strain CC1. J. Virol. 2012, 86, 9536. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.G.; Zhou, J.Y.; Huang, Z.Y.; Guo, J.Q.; Xing, G.; He, J.L.; Yan, Y.; Gong, L.Y. Protective immunity against porcine circovirus 2 by vaccination with ORF2-based DNA and subunit vaccines in mice. J. Gen. Virol. 2008, 89 Pt 8, 1857–1865. [Google Scholar] [CrossRef]
- Peng, Z.; Ouyang, T.; Pang, D.; Ma, T.; Chen, X.; Guo, N.; Chen, F.; Yuan, L.; Ouyang, H.; Ren, L. Pseudorabies virus can escape from CRISPR-Cas9-mediated inhibition. Virus Res. 2016, 223, 197–205. [Google Scholar] [CrossRef]
- Ouyang, T.; Niu, G.; Zhang, Y.; Liu, X.; Zhang, X.; Zhang, S.; Geng, Y.; Pang, D.; Ouyang, H.; Ren, L. Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins. Viruses 2019, 11, 544. [Google Scholar] [CrossRef] [Green Version]
- Shahriar, S.; Araf, Y.; Ahmad, R.; Kattel, P.; Sah, G.S.; Rahaman, T.I.; Sadiea, R.Z.; Sultana, S.; Islam, M.S.; Zheng, C.; et al. Insights Into the Coinfections of Human Immunodeficiency Virus-Hepatitis B Virus, Human Immunodeficiency Virus-Hepatitis C Virus, and Hepatitis B Virus-Hepatitis C Virus: Prevalence, Risk Factors, Pathogenesis, Diagnosis, and Treatment. Front. Microbiol. 2021, 12, 780887. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alshawi, A.M.; Alomran, S.A.; Almuhanna, M.S.; Almuslim, A.A.; Bu Shafia, A.H.; Alotaibi, A.M.; Ahmed, G.Y.; et al. Coinfections with Bacteria, Fungi, and Respiratory Viruses in Patients with SARS-CoV-2: A Systematic Review and Meta-Analysis. Pathogens 2021, 10, 809. [Google Scholar] [CrossRef]
- Kim, E.H.; Nguyen, T.Q.; Casel, M.A.B.; Rollon, R.; Kim, S.M.; Kim, Y.I.; Yu, K.M.; Jang, S.G.; Yang, J.; Poo, H.; et al. Coinfection with SARS-CoV-2 and Influenza A Virus Increases Disease Severity and Impairs Neutralizing Antibody and CD4(+) T Cell Responses. J. Virol. 2022, 96, e0187321. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Zhang, Q.G.; Huang, K.; Ma, D.; Du, Q.; Tong, D.; Huang, Y. Porcine circovirus type 2 infection inhibits the activation of type I interferon signaling via capsid protein and host gC1qR. Vet. Microbiol. 2022, 266, 109354. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, M.; Huang, B.; Lv, Y. Porcine circovirus type 2 inhibits inter-beta expression by targeting Karyopherin alpha-3 in PK-15 cells. Virology 2018, 520, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rao, Y.; Tian, M.; Zhang, S.; Feng, P. Modulation of Innate Immune Signaling Pathways by Herpesviruses. Viruses 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, Y.; Wu, C.; Zhang, Y.J. Interplay between Janus Kinase/Signal Transducer and Activator of Transcription Signaling Activated by Type I Interferons and Viral Antagonism. Front. Immunol. 2017, 8, 1758. [Google Scholar] [CrossRef]
- Mallampalli, R.K.; Adair, J.; Elhance, A.; Farkas, D.; Chafin, L.; Long, M.E.; De, M.; Mora, A.L.; Rojas, M.; Peters, V.; et al. Interferon Lambda Signaling in Macrophages Is Necessary for the Antiviral Response to Influenza. Front. Immunol. 2021, 12, 735576. [Google Scholar] [CrossRef]
- Nelli, R.K.; Mora-Diaz, J.C.; Gimenez-Lirola, L.G. The Betacoronavirus PHEV Replicates and Disrupts the Respiratory Epithelia and Upregulates Key Pattern Recognition Receptor Genes and Downstream Mediators, Including IL-8 and IFN-lambda. mSphere 2021, 6, e0082021. [Google Scholar] [CrossRef]
- Yin, Y.; Favoreel, H.W. Herpesviruses and the Type III Interferon System. Virol. Sin. 2021, 36, 577–587. [Google Scholar] [CrossRef]
- Pervolaraki, K.; Stanifer, M.L.; Munchau, S.; Renn, L.A.; Albrecht, D.; Kurzhals, S.; Senis, E.; Grimm, D.; Schroder-Braunstein, J.; Rabin, R.L.; et al. Type I and Type III Interferons Display Different Dependency on Mitogen-Activated Protein Kinases to Mount an Antiviral State in the Human Gut. Front. Immunol. 2017, 8, 459. [Google Scholar] [CrossRef]
- Wei, L.; Kwang, J.; Wang, J.; Shi, L.; Yang, B.; Li, Y.; Liu, J. Porcine circovirus type 2 induces the activation of nuclear factor kappa B by IkappaBalpha degradation. Virology 2008, 378, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, S.; Zhang, L.; Niu, G.; Zhang, X.; Yang, L.; Ji, W.; Ren, L. Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 Pathways. Int. J. Mol. Sci. 2022, 23, 4469. https://doi.org/10.3390/ijms23084469
Li X, Chen S, Zhang L, Niu G, Zhang X, Yang L, Ji W, Ren L. Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 Pathways. International Journal of Molecular Sciences. 2022; 23(8):4469. https://doi.org/10.3390/ijms23084469
Chicago/Turabian StyleLi, Xue, Si Chen, Liying Zhang, Guyu Niu, Xinwei Zhang, Lin Yang, Weilong Ji, and Linzhu Ren. 2022. "Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 Pathways" International Journal of Molecular Sciences 23, no. 8: 4469. https://doi.org/10.3390/ijms23084469
APA StyleLi, X., Chen, S., Zhang, L., Niu, G., Zhang, X., Yang, L., Ji, W., & Ren, L. (2022). Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 Pathways. International Journal of Molecular Sciences, 23(8), 4469. https://doi.org/10.3390/ijms23084469






