Characterization of Stem Nodes Associated with Carbon Partitioning in Maize in Response to Nitrogen Availability
Abstract
:1. Introduction
2. Results
2.1. Maize Growth, N Uptake, and Photosynthesis
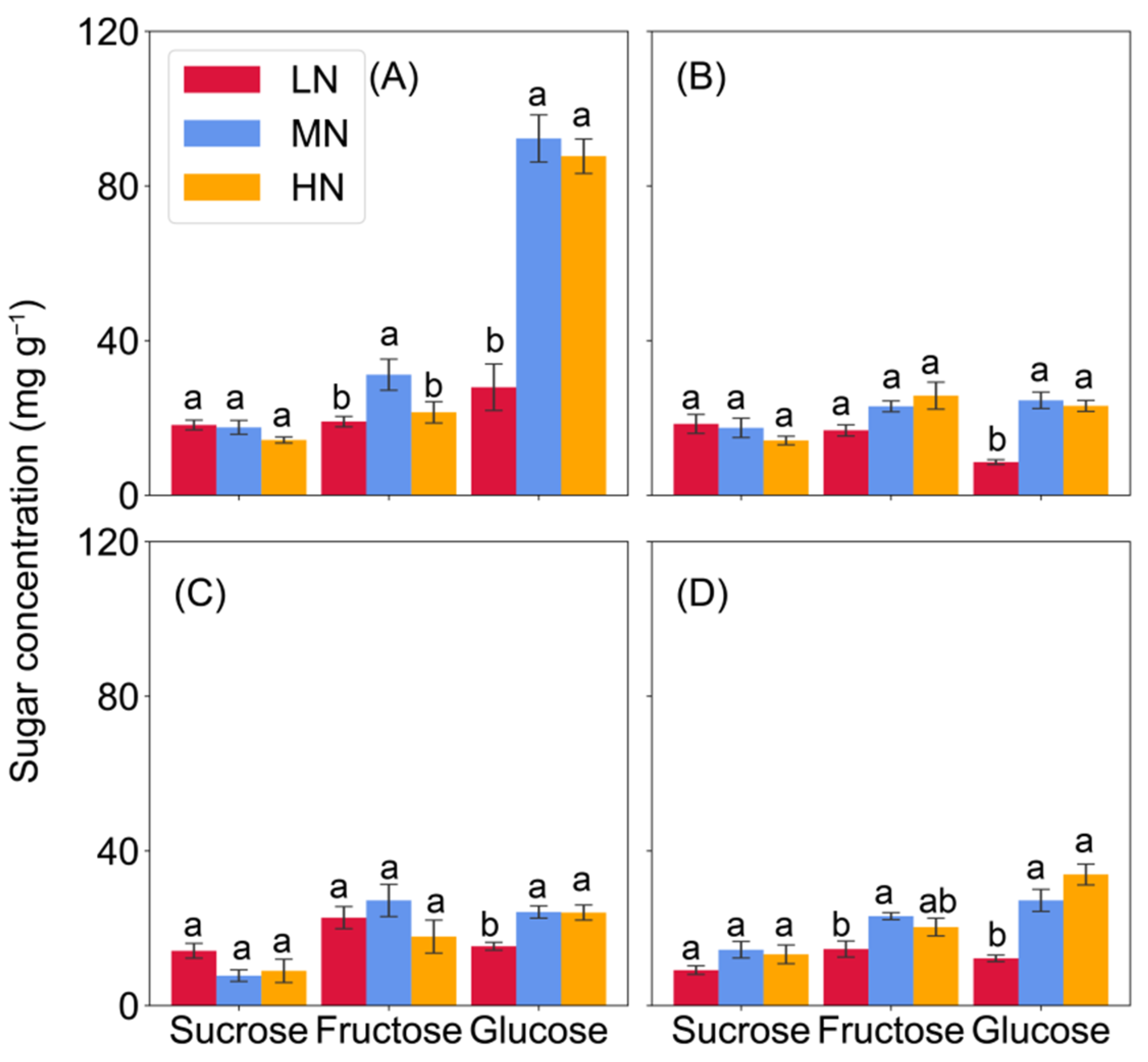
2.2. Soluble Sugar Concentration of Leaf and Nodes
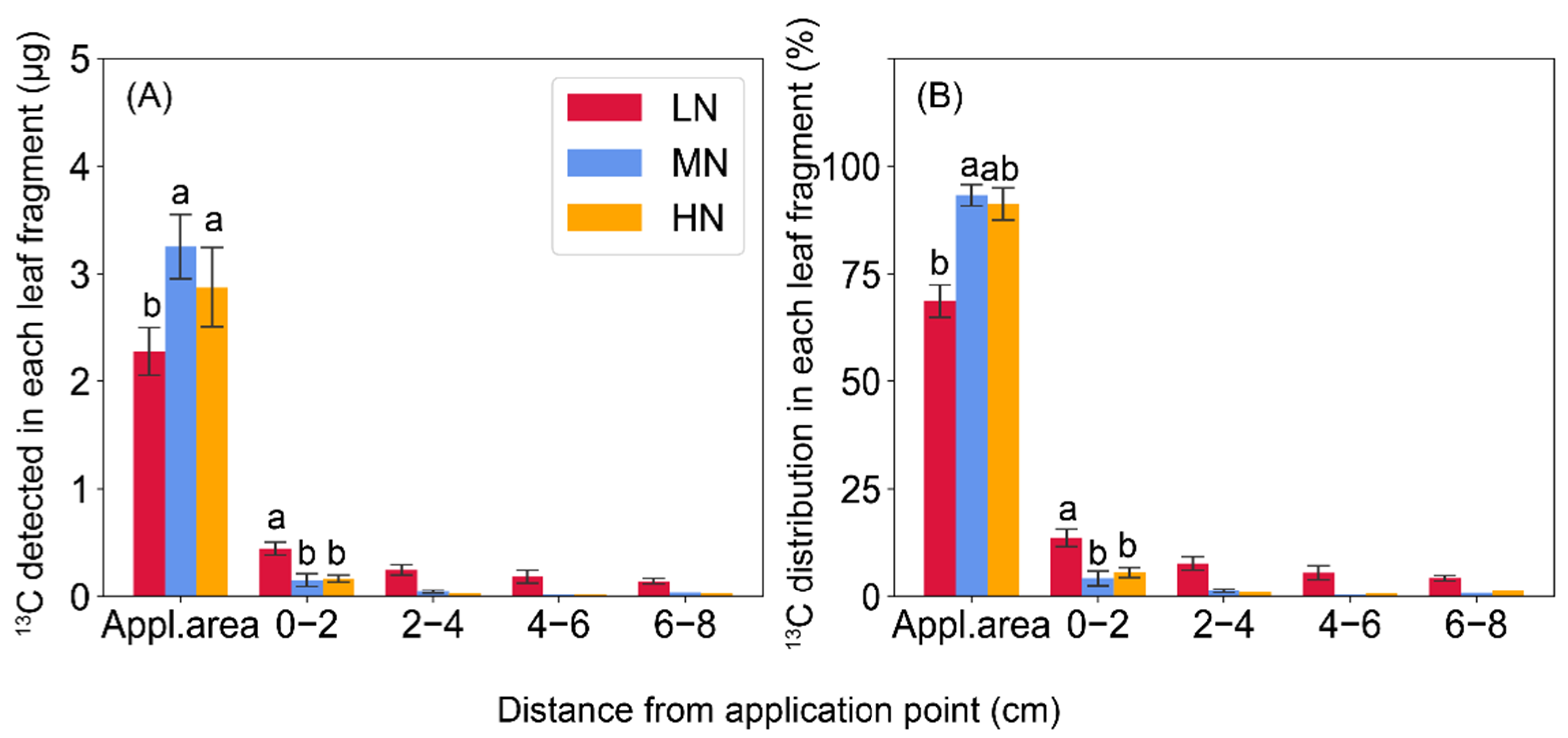
2.3. 13C-Sucrose Export in Leaves
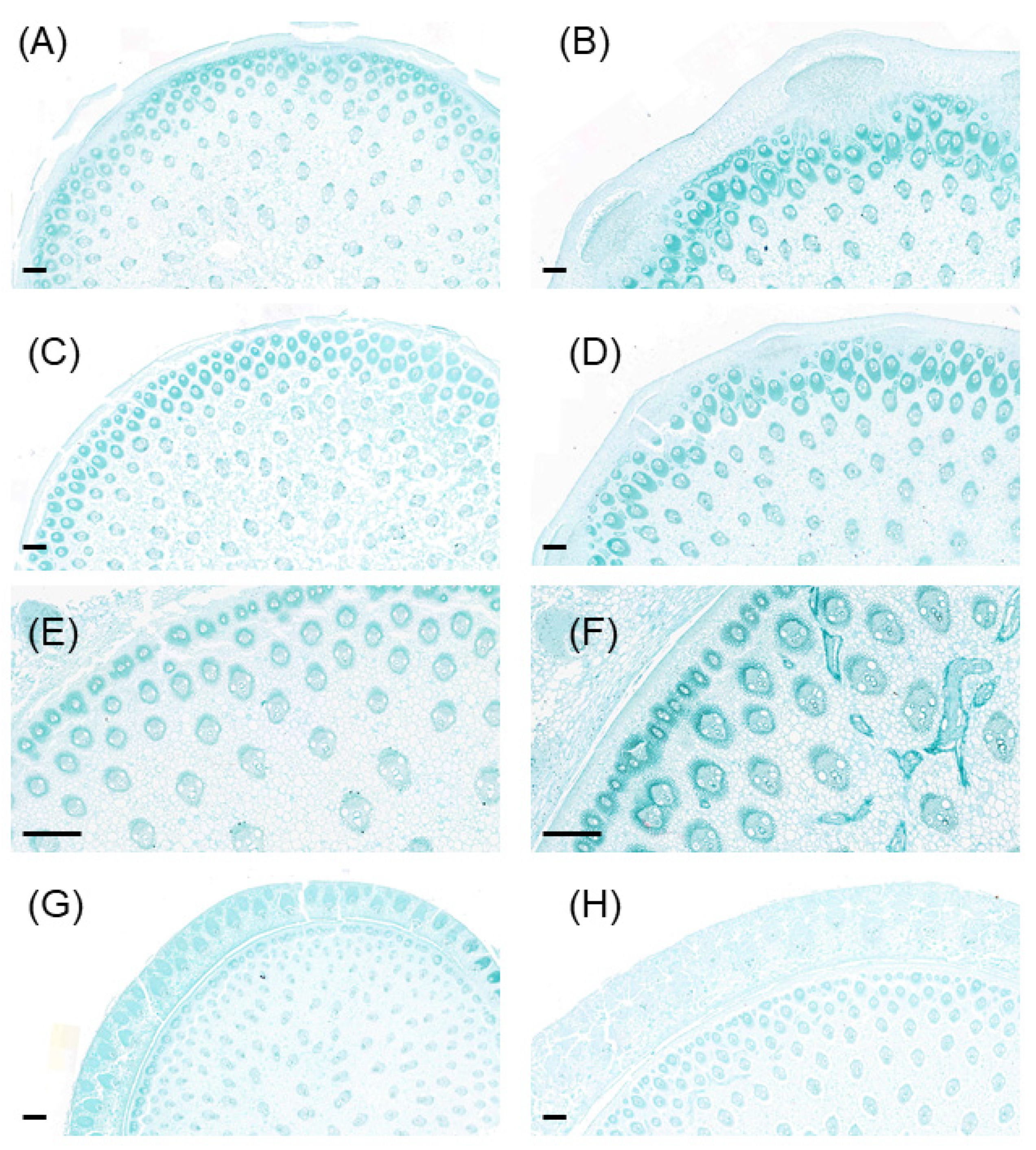
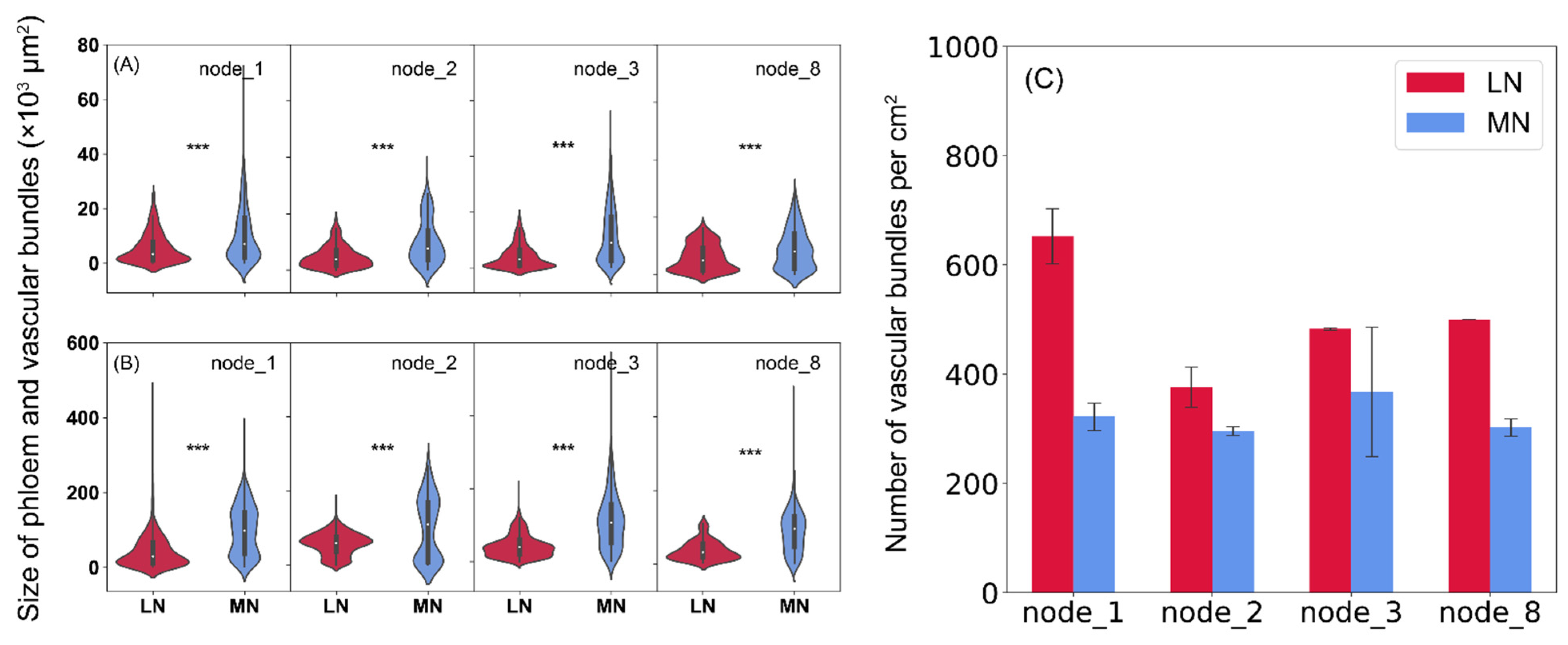
2.4. Anatomic Analysis of Stem Nodes
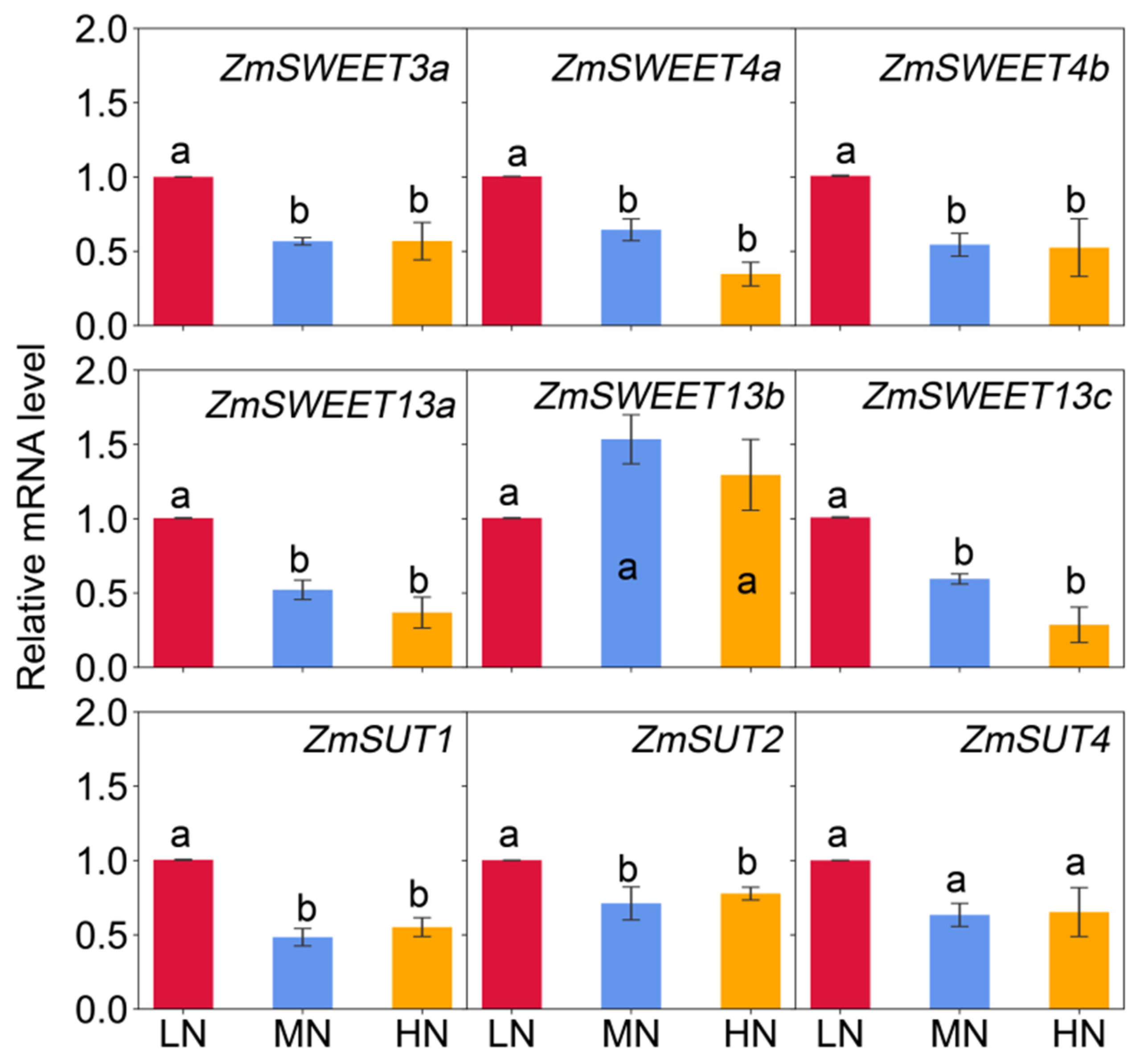
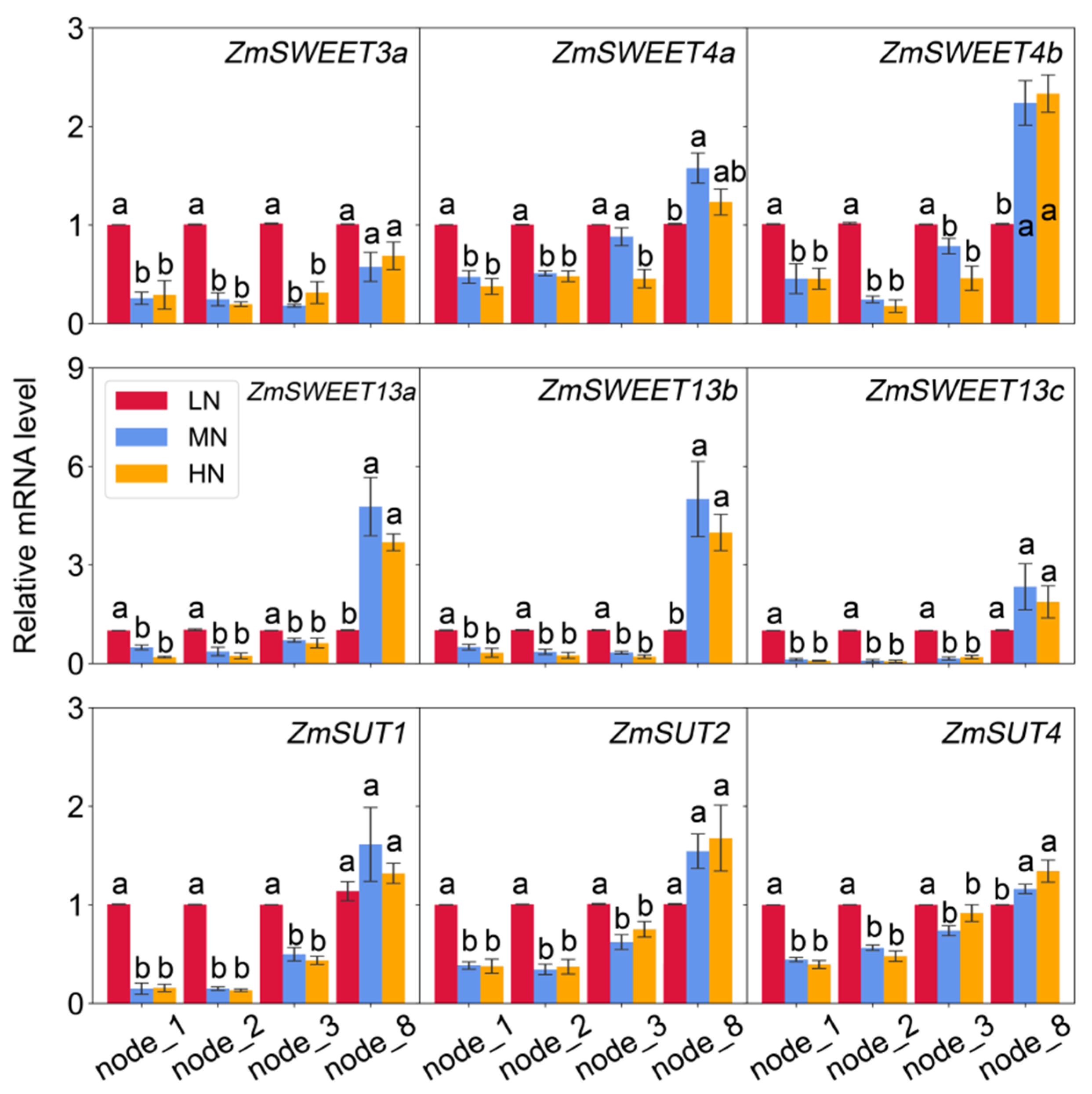
2.5. Transcript Abundance of Genes Involving in Sugar Transport in Leaves and Nodes
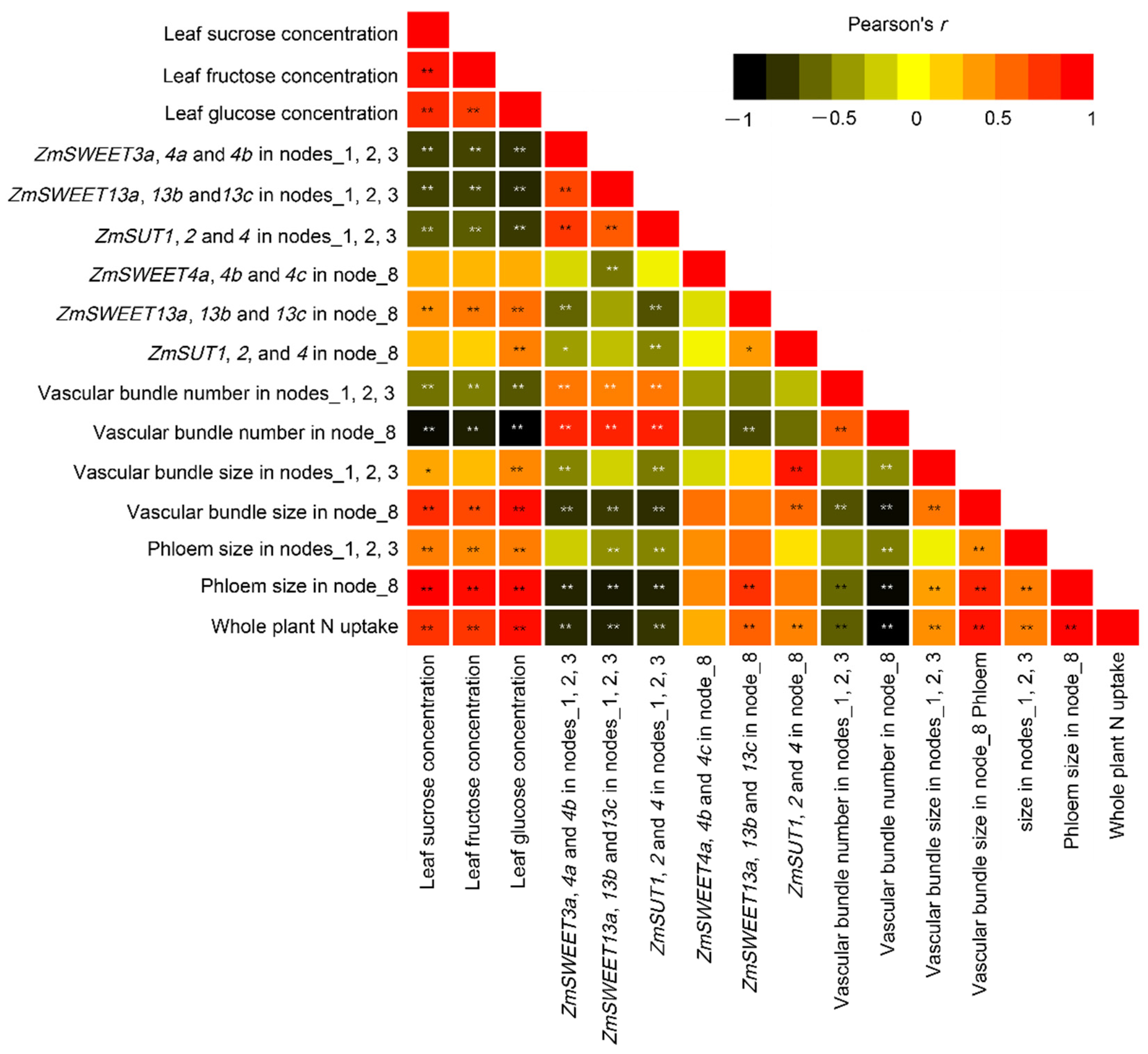
2.6. Correlation Analysis
3. Discussion
3.1. Structural Characteristics of Stem Nodes in Response to N Availability
3.2. Differential Responses of Sugar Partitioning to N Availability in the Lower and Upper Nodes
3.3. Developmental Impacts on the Whole-Plant Carbon Partitioning as Affected by N Availability
4. Materials and Methods
4.1. Plant Growth Conditions and Experimental Design
4.2. Growth Measurements and Plant Sampling
4.3. Paraffin Section and Observation
4.4. Soluble Sugar Quantification
4.5. RNA Isolation, Reverse Transcription, and Real Time PCR (qPCR)
4.6. 13C-Sucrose Export Assay
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signaling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L.; Braun, D.M. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci. 2010, 178, 341–349. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Carbon flow in the plant-soil-microbe continuum at different growth stages of maize grown in a Mollisol. Arch. Agron. Soil. Sci. 2017, 63, 362–374. [Google Scholar] [CrossRef]
- Braun, D.M.; Slewinski, T.L. Genetic control of carbon partitioning in grasses: Roles of Sucrose Transporters and Tie-dyed loci in phloem loading. Plant Physiol. 2009, 149, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F.B. Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Lo, S.; Ho, T.D. Source-Sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Bihmidine, S.; Hunter, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Engels, C.; Kirkby, E.; White, P. Mineral Nutrition, Yield and Source-Sink Relationships. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 85–133. [Google Scholar]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Node-controlled allocation of mineral elements in Poaceae. Curr. Opin. Plant Biol. 2017, 39, 18–24. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.F. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Oshida, K.T.Y.; Ma, J.F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 2017, 541, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Slewinski, T.L.; Meeley, R.; Braun, D.M. Sucrose transporter1 functions in phloem loading in maize leaves. J. Exp. Bot. 2009, 60, 881–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, R.F.; Leach, K.A.; Boyer, N.R.; Swyers, M.J.; Benitez-Alfonso, Y.; Skopelitis, T.; Luo, A.; Sylvester, A.; Jackson, D.; Braun, D.M. Sucrose transporter ZmSut1 expression and localization uncover new insights into sucrose phloem loading. Plant Physiol. 2016, 172, 1876–1898. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qu, X.; Hou, B.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Eom, J.; Chen, L.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L.; Garg, A.; Johal, G.S.; Braun, D.M. Maize SUT1 functions in phloem loading. Plant Signal. Behav. 2010, 5, 687–690. [Google Scholar] [CrossRef] [Green Version]
- Stelpflug, S.C.; Sekhon, R.S.; Vaillancourt, B.; Hirsch, C.N.; Buell, C.R.; de Leon, N.; Kaeppler, S.M. An expanded maize gene expression atlas based on RNA sequencing and its use to explore root development. Plant Genome 2016, 9, e2014–e2015. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar transporters in plants: New insights and discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [Green Version]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.; Yang, J.; Yang, B.; Frommer, W.; Sosso, D. Impaired phloem loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef]
- Paul, M.J.; Watson, A.; Griffiths, C.A. Linking fundamental science to crop improvement through understanding source and sink traits and their integration for yield enhancement. J. Exp. Bot. 2020, 71, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Jiang, Y.; Zhao, M.; Qi, H.; Li, C. Nitrogen supply regulates vascular bundle structure and matter transport characteristics of spring maize under high plant density. Front. Plant Sci. 2021, 11, 602739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, H.; Zhang, B.; Huang, S.; Wang, P. Timing of water deficit limits maize kernel setting in association with changes in the source-flow-sink relationship. Front. Plant Sci. 2018, 9, 1326. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Botha, F.C. Distribution patterns of neutral invertase and sugar content in sugarcane internodal tissues. Plant Physiol. Biochem. 2000, 38, 819–824. [Google Scholar] [CrossRef]
- Bihmidine, S.; Baker, R.F.; Hoffner, C.; Braun, D.M. Sucrose accumulation in sweet sorghum stems occurs by apoplasmic phloem unloading and does not involve differential Sucrose transporter expression. BMC Plant Biol. 2015, 15, 186. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Gupta, A.; Laxmi, A. Glucose control of root growth direction in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 2981–2993. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Gupta, A.; Laxmi, A. Glucose and phytohormone interplay in controlling root directional growth in Arabidopsis. Plant Signal. Behav. 2014, 9, e29219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence, lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [PubMed]
- Cliquet, J.; Deléens, E.; Mariotti, A. C and N Mobilization from stalk and leaves during kernel filling by 13C and 15N tracing in Zea mays L. Plant Physiol. 1990, 94, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Himelblau, E.; Amasino, R.M. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J. Plant Physiol. 2001, 158, 1317–1323. [Google Scholar] [CrossRef]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Zhao, Z.; Ge, T.; Gunina, A.; Li, Y.; Zhu, Z.; Peng, P.; Wu, J.; Kuzyakov, Y. Carbon and nitrogen availability in paddy soil affects rice photosynthate allocation, microbial community composition, and priming, combining continuous 13C labeling with PLFA analysis. Plant Soil 2019, 445, 137–152. [Google Scholar] [CrossRef]


| Items | LN | MN | HN |
|---|---|---|---|
| Shoot DM (g plant−1) | 16.23 ± 1.17 b | 43.41 ± 2.48 a | 45.99 ± 1.96 a |
| Root DM (g plant−1) | 5.58 ± 0.64 b | 15.82 ± 1.20 a | 16.18 ± 1.25 a |
| R/S ratio | 0.35 ± 0.04 a | 0.36 ± 0.02 a | 0.36 ± 0.04 a |
| Total N uptake (g plant−1) | 0.20 ± 0.02 c | 1.05 ± 0.05 b | 1.24 ± 0.03 a |
| Total leaf area (cm2) | 2553.6 ± 134.0 b | 4394.8 ± 132.1 a | 4346.3 ± 64.9 a |
| SPAD | 22.30 ± 0.69 b | 48.05 ± 0.55 a | 49.05 ± 1.23 a |
| Pn (μmol CO2 m−2 s−1) | 8.00 ± 1.08 b | 23.24 ± 1.14 a | 21.80 ± 2.43 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Ning, P.; Feng, X.; Ren, H.; Cui, M.; Yang, L. Characterization of Stem Nodes Associated with Carbon Partitioning in Maize in Response to Nitrogen Availability. Int. J. Mol. Sci. 2022, 23, 4389. https://doi.org/10.3390/ijms23084389
Zhao Y, Ning P, Feng X, Ren H, Cui M, Yang L. Characterization of Stem Nodes Associated with Carbon Partitioning in Maize in Response to Nitrogen Availability. International Journal of Molecular Sciences. 2022; 23(8):4389. https://doi.org/10.3390/ijms23084389
Chicago/Turabian StyleZhao, Yujing, Peng Ning, Xiaojie Feng, Hanshuo Ren, Mingtang Cui, and Lu Yang. 2022. "Characterization of Stem Nodes Associated with Carbon Partitioning in Maize in Response to Nitrogen Availability" International Journal of Molecular Sciences 23, no. 8: 4389. https://doi.org/10.3390/ijms23084389
APA StyleZhao, Y., Ning, P., Feng, X., Ren, H., Cui, M., & Yang, L. (2022). Characterization of Stem Nodes Associated with Carbon Partitioning in Maize in Response to Nitrogen Availability. International Journal of Molecular Sciences, 23(8), 4389. https://doi.org/10.3390/ijms23084389






