Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. CSD Search
3.2. Selenium Derivatives
3.2.1. Oxygen as Electron Donor
3.2.2. Halogen as Electron Donor
3.2.3. Carbon as Electron Donor
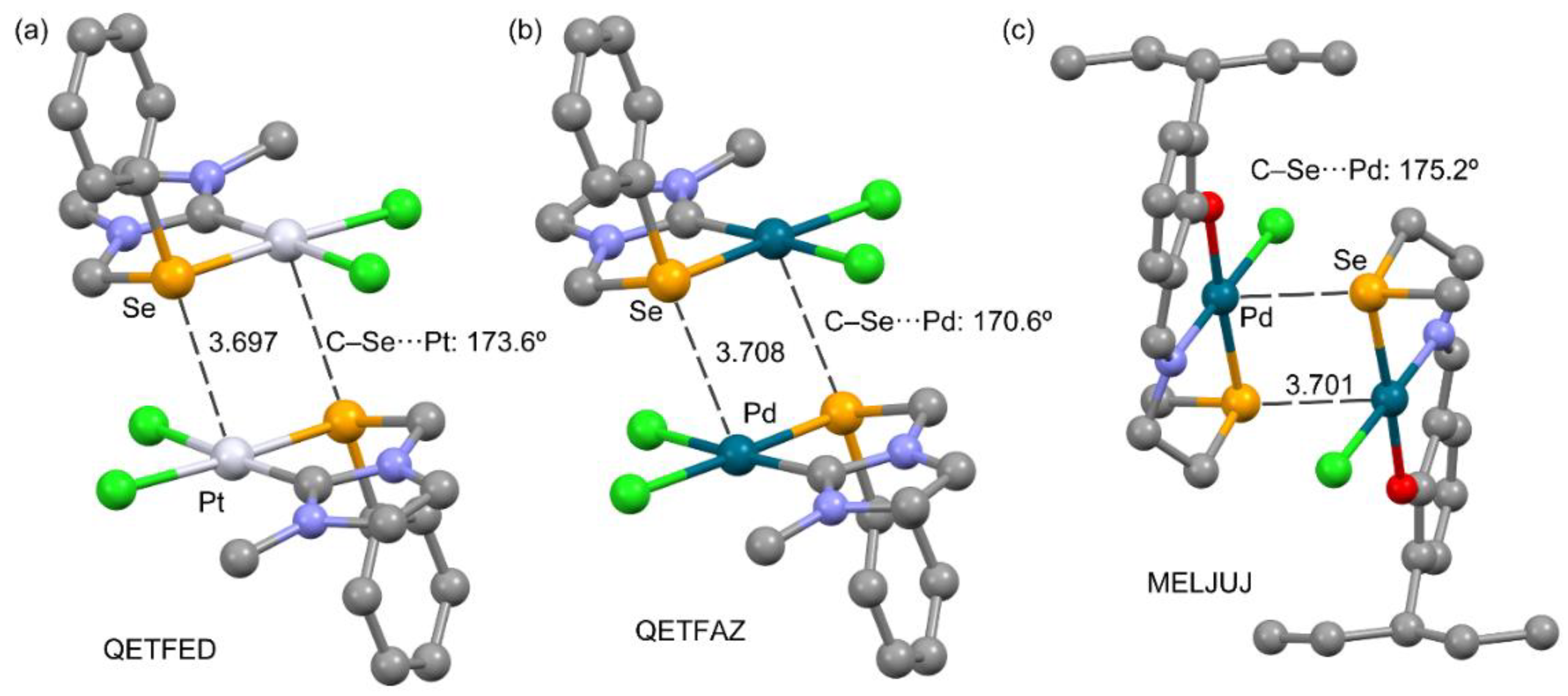
3.2.4. Metal as Electron Donor
3.3. Tellurium Derivatives
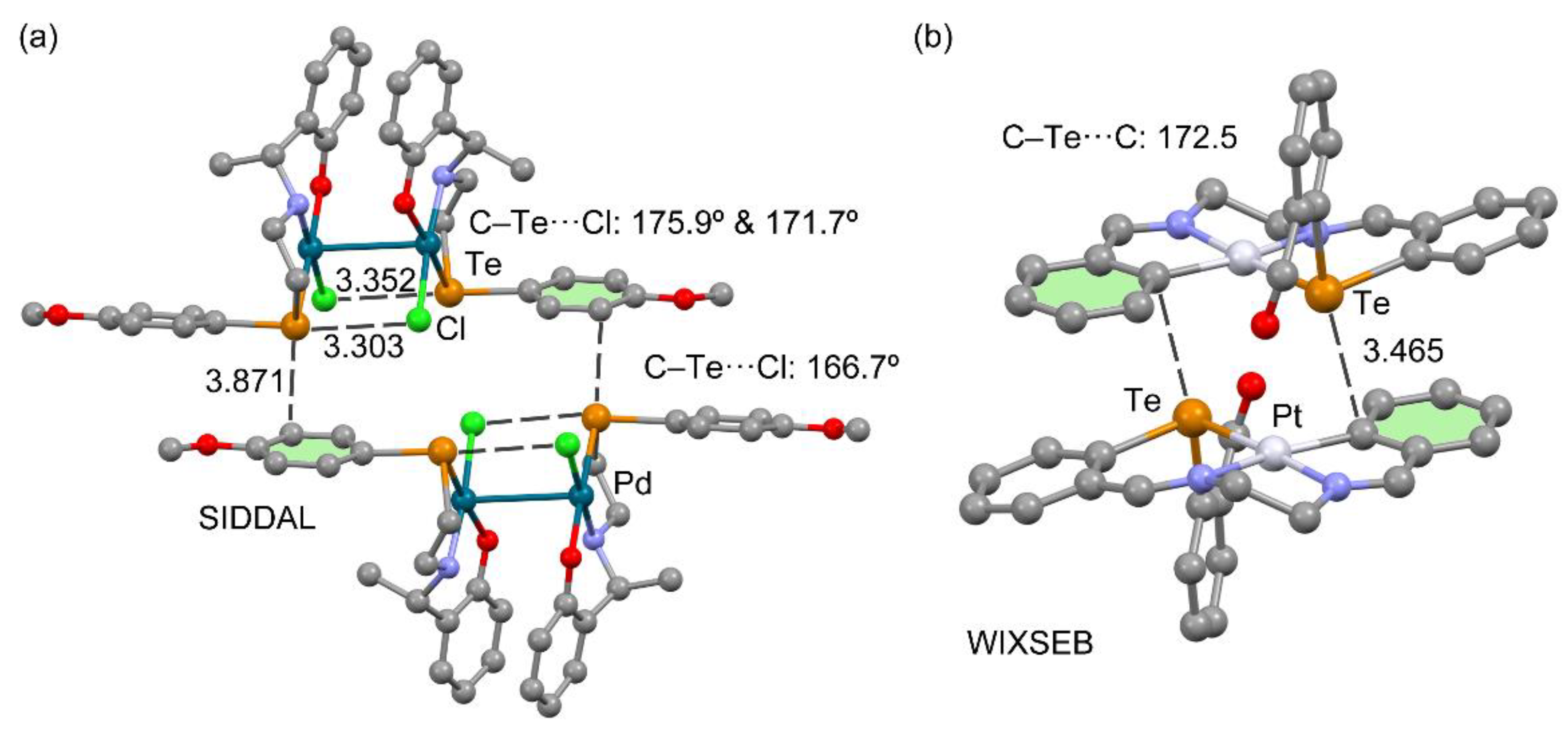
3.3.1. Halogen as Electron Donor
3.3.2. Carbon as Electron Donor
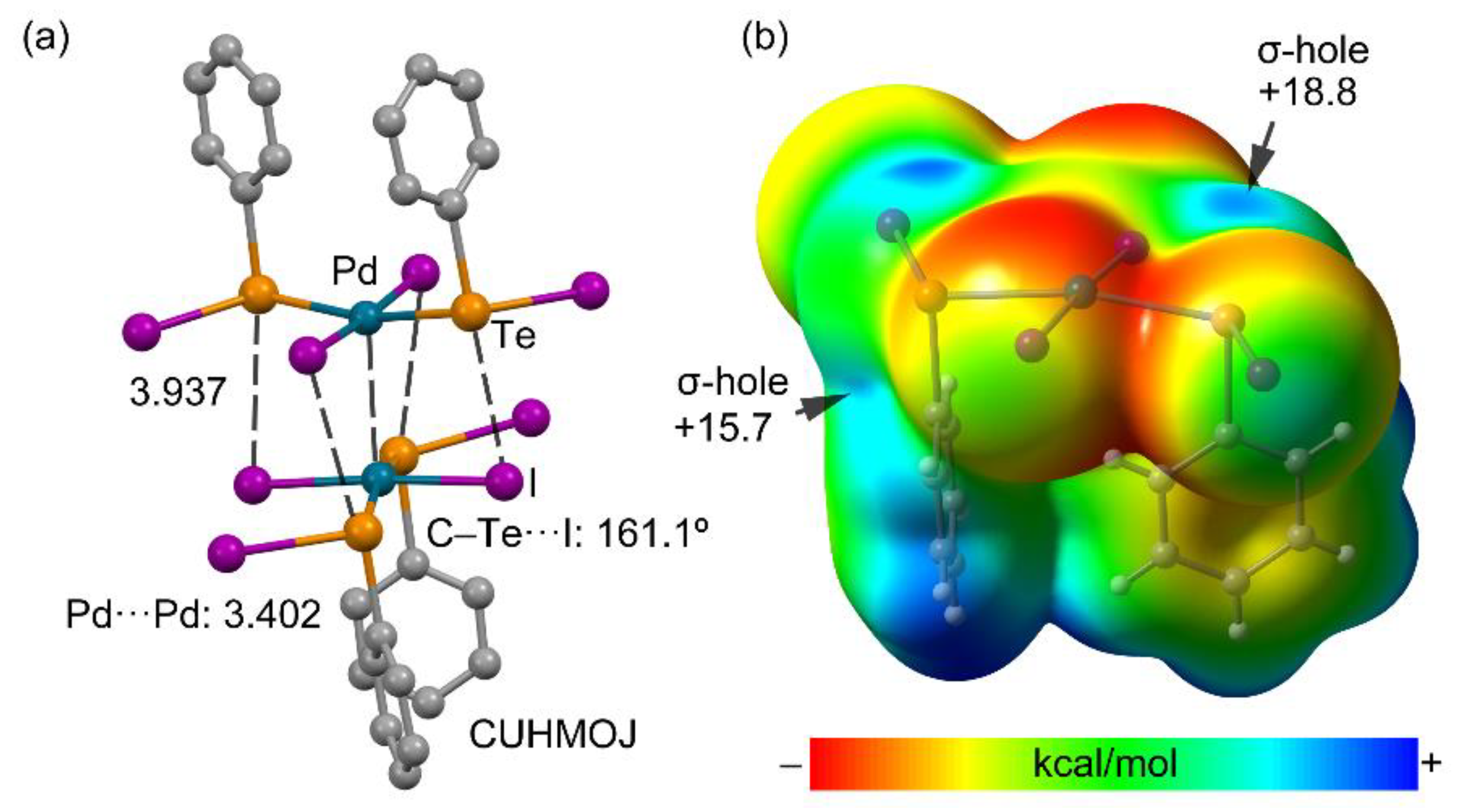
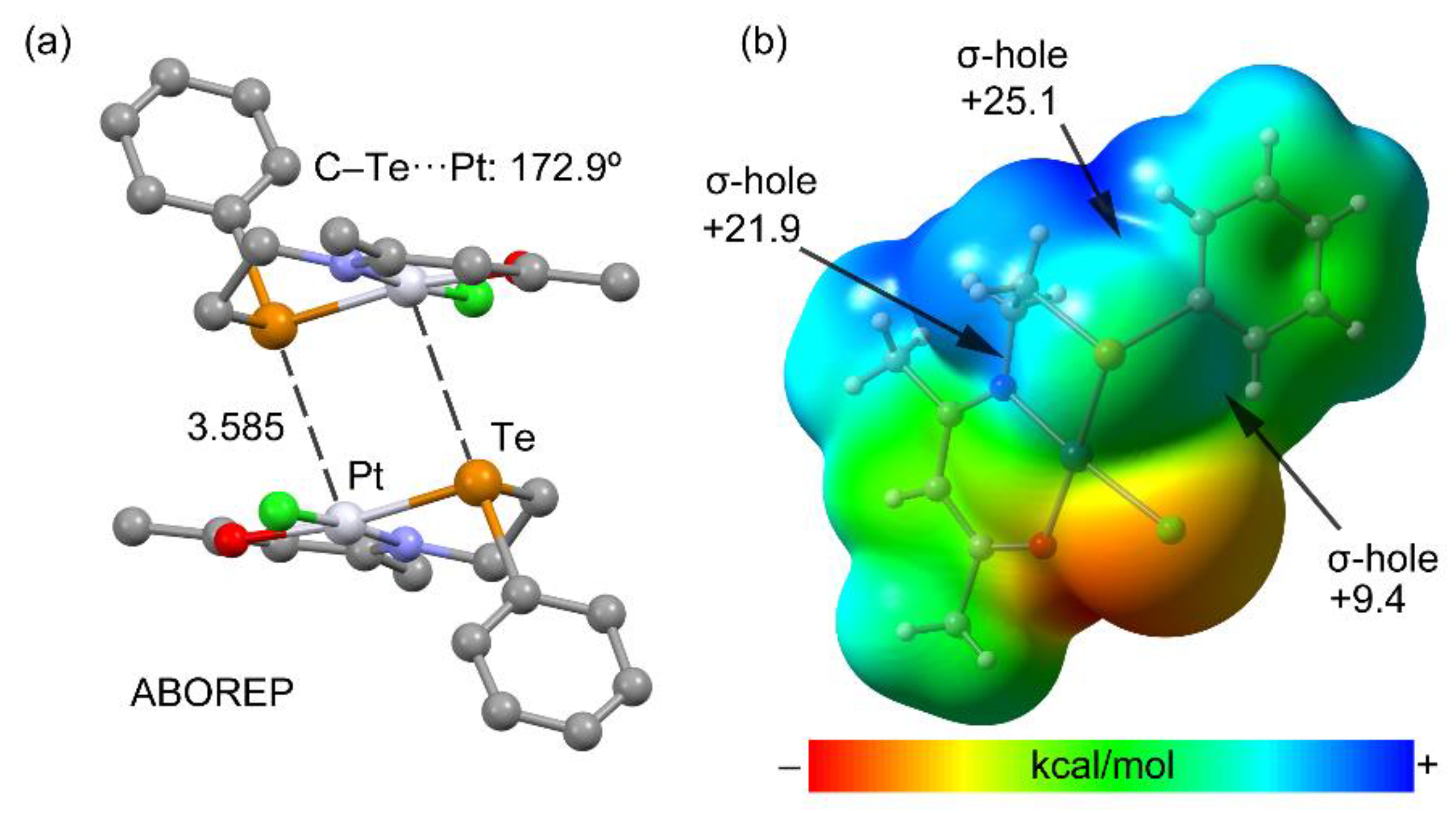
3.3.3. Metal as Electron Donor
4. Theoretical Study
4.1. Energetic Study
4.2. AIM Analysis
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzi, A.; Pigliacelli, C.; Bergamaschi, G.; Gori, A.; Metrangolo, P. Biomimetic engineering of the molecular recognition and self-assembly of peptides and proteins via halogenation. Coord. Chem. Rev. 2020, 411, 213242. [Google Scholar] [CrossRef]
- Daolio, A.; Scilabra, P.; Terraneo, G.; Resnati, G. C(sp3) atoms as tetrel bond donors: A crystallographic survey. Coord. Chem. Rev. 2020, 413, 213265. [Google Scholar] [CrossRef]
- von der Heiden, D.; Vanderkooy, A.; Erdélyi, M. Halogen bonding in solution: NMR spectroscopic approaches. Coord. Chem. Rev. 2020, 407, 213147. [Google Scholar] [CrossRef]
- Gill, H.; Gokel, M.R.; McKeever, M.; Negin, S.; Patel, M.B.; Yin, S.; Gokel, G.W. Supramolecular pore formation as an antimicrobial strategy. Coord. Chem. Rev. 2020, 412, 213264. [Google Scholar] [CrossRef]
- Xu, Y.; Szell, P.M.J.; Kumar, V.; Bryce, D.L. Solid-state NMR spectroscopy for the analysis of element-based non-covalent interactions. Coord. Chem. Rev. 2020, 411, 213237. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jin, W.J. Halogen bonding in room-temperature phosphorescent materials. Coord. Chem. Rev. 2020, 404, 213107. [Google Scholar] [CrossRef]
- Grabowski, S.J. Triel bond and coordination of triel centres–Comparison with hydrogen bond interaction. Coord. Chem. Rev. 2020, 407, 213171. [Google Scholar] [CrossRef]
- Fromm, K.M. Chemistry of alkaline earth metals: It is not all ionic and definitely not boring! Coord. Chem. Rev. 2020, 408, 213192. [Google Scholar] [CrossRef]
- Fourmigué, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084. [Google Scholar] [CrossRef]
- Scheiner, S.; Michalczyk, M.; Zierkiewicz, W. Coordination of anions by noncovalently bonded σ-hole ligands. Coord. Chem. Rev. 2020, 405, 213136. [Google Scholar] [CrossRef]
- Taylor, M.S. Anion recognition based on halogen, chalcogen, pnictogen and tetrel bonding. Coord. Chem. Rev. 2020, 413, 213270. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. σ/π-Hole noble gas bonding interactions: Insights from theory and experiment. Coord. Chem. Rev. 2020, 404, 213112. [Google Scholar] [CrossRef]
- Biot, N.; Bonifazi, D. Chalcogen-bond driven molecular recognition at work. Coord. Chem. Rev. 2020, 413, 213243. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Naming interactions from the electrophilic site. Cryst. Growth. Des. 2014, 14, 2697–2702. [Google Scholar] [CrossRef]
- Terraneo, G.; Resnati, G. Bonding Matters. Cryst. Growth. Des. 2017, 17, 1439–1440. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure. Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure. Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Zahn, S.; Frank, R.; Hey-Hawkins, E.; Kirchner, B. Pnicogen bonds: A new molecular linker? Chem. Eur. J. 2011, 17, 6034–6038. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not only hydrogen bonds: Other noncovalent interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef]
- Daolio, A.; Pizzi, A.; Terraneo, G.; Frontera, A.; Resnati, G. σ-Holes Allow for Attractive Anion· anion Interactions Involving Perrhenate, Permanganate, and Pertechnetate Anions. ChemPhysChem 2021, 22, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Daolio, A.; Pizzi, A.; Calabrese, M.; Terraneo, G.; Bordignon, S.; Frontera, A.; Resnati, G. Molecular Electrostatic Potential and Noncovalent Interactions in Derivatives of Group 8 Elements. Angew. Chem. Int. Ed. 2021, 60, 20723–20727. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Alkorta, I.; Elguero, J.; Mooibroek, T.J.; Frontera, A. Spodium Bonds: Noncovalent Interactions Involving Group 12 Elements. Angew. Chem. Int. Ed. 2020, 59, 17482–17487. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J.H.; Johansson, A.J.; Brinck, T. σ-Holes and σ-lumps direct the Lewis basic and acidic interactions of noble metal nanoparticles: Introducing regium bonds. Phys. Chem. Chem. Phys. 2018, 20, 2676–2692. [Google Scholar] [CrossRef]
- Daolio, A.; Pizzi, A.; Terraneo, G.; Ursini, M.; Frontera, A.; Resnati, G. Anion⋅⋅⋅Anion Coinage Bonds: The Case of Tetrachloridoaurate. Angew. Chem. Int. Ed. 2021, 60, 14385–14389. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. Regium–π bonds: An Unexplored Link between Noble Metal Nanoparticles and Aromatic Surfaces. Chem. Eur. J. 2018, 24, 7228–7234. [Google Scholar] [CrossRef]
- Terrón, A.; Buils, A.; Mooibroek, T.J.; Barceló, M.; García-Raso, A.; Fiol, J.J.; Frontera, A. Synthesis, X-ray characterization and regium bonding interactions of a trichlorido (1-hexylcytosine) gold (III) complex. Chem. Commun. 2020, 56, 3524–3527. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen bonding based recognition processes: A world parallel to hydrogen bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. (Eds.) Halogen Bonding: Fundamentals and Applications; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Erdélyi, M. Halogen bonding in solution. Chem. Soc. Rev. 2012, 41, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Beale, T.M.; Chudzinski, M.G.; Sarwar, M.G.; Taylor, M.S. Halogen bonding in solution: Thermodynamics and applications. Chem. Soc. Rev. 2013, 42, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. (Eds.) Halogen Bonding II Impact on Materials Chemistry and Life Sciences; Springer International Publishing AG: Heidelberg, Germany, 2015. [Google Scholar]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen bonding in supramolecular chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton. Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef]
- Gleiter, R.; Haberhauer, G.; Werz, D.B.; Rominger, F.; Bleiholder, C. From noncovalent chalcogen–chalcogen interactions to supramolecular aggregates: Experiments and calculations. Chem. Rev. 2018, 118, 2010–2041. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen bonding: An overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef]
- Scheiner, S. The pnicogen bond: Its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- del Bene, J.E.; Alkorta, I.; Elguero, J. The Pnicogen Bond in Review: Structures, Binding Energies, Bonding Properties, and Spin-Spin Coupling Constants of Complexes Stabilized by Pnicogen Bonds; Scheiner, S., Ed.; Noncovalent Forces; Springer: Heidelberg, Germany, 2015; pp. 191–264. [Google Scholar]
- Brammer, L. Halogen bonding, chalcogen bonding, pnictogen bonding, tetrel bonding: Origins, current status and discussion. Faraday. Discuss. 2017, 203, 485–507. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Lane, P. σ-Hole bonding and hydrogen bonding: Competitive interactions. Int. J. Quantum. Chem. 2007, 107, 3046–3052. [Google Scholar] [CrossRef]
- Zhu, S.; Xing, C.; Xu, W.; Jin, G.; Li, Z. Halogen bonding and hydrogen bonding coexist in driving self-assembly process. Cryst. Growth. Des. 2004, 4, 53–56. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Forni, A.; Gorynsztejn-Leben, M.; Kaivola, M.; Metrangolo, P.; Milani, R.; Shishido, A.; Pilati, T.; Resnati, G. Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly and Performance of Light-Responsive Supramolecular Polymers. Adv. Funct. Mater. 2012, 22, 2572–2579. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen bonds in crystal engineering: Like hydrogen bonds yet different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Scheiner, S. Forty years of progress in the study of the hydrogen bond. Struct. Chem. 2019, 30, 1119–1128. [Google Scholar] [CrossRef]
- Corradi, E.; Meille, S.V.; Messina, M.T.; Metrangolo, P.; Resnati, G. Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly Processes. Angew. Chem. Int. Ed. 2000, 39, 1782–1786. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The origin of chalcogen-bonding interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef] [PubMed]
- Gurbanov, A.V.; Kuznetsov, M.L.; Mahmudov, K.T.; Pombeiro, A.J.L.; Resnati, G. Resonance Assisted Chalcogen Bonding as a New Synthon in the Design of Dyes. Chem. Eur. J. 2020, 26, 14833–14837. [Google Scholar] [CrossRef] [PubMed]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Murray, J.S.; Fanfrlík, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability I: The effects of aromatic fluorine substitution on the strengths of halogen-bonding interactions involving chlorine, bromine, and iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Scheiner, S. Substituent Effects on Cl···N, S···N, and P···N Noncovalent Bonds. J. Phys. Chem. A 2012, 116, 3487–3497. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Effects of Charge and Substituent on the S···N Chalcogen Bond. J. Phys. Chem. A 2014, 118, 3183–3192. [Google Scholar] [CrossRef]
- Azofra, L.M.; Scheiner, S. Substituent Effects in the Noncovalent Bonding of SO2 to Molecules Containing a Carbonyl Group. The Dominating Role of the Chalcogen Bond. J. Phys. Chem. A 2014, 118, 3835–3845. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadirad, N. Substituent effects incooperativity of chalcogen bonds. Mol. Phys. 2015, 113, 3282–3290. [Google Scholar] [CrossRef]
- Gonzalez-Alvarez, M.; Alzuet, G.; Borras, J.; Agudo, L.C.; Garcia-Granda, S.; Montejo-Bernando, J.M. Comparison of Protective Effects against Reactive Oxygen Species of Mononuclear and Dinuclear Cu(II) Complexes with N-Substituted Benzothiazolesulfonamides. Inorg. Chem. 2005, 44, 9424–9433. [Google Scholar] [CrossRef]
- Gao, L.; Wu, J.-Y.; Li, M.-Y.; Zhang, L.; Zhang, P.; Wu, Q.-F.; Cai, X.-Q.; Chen, F.-T.; Zhang, S.-S. A New Copper(I) Iodide Coordination polymer Incorporating Cu2I2 Double-stranded Stair: Synthesis, Crystal Structure and Luminescent Property. Chin. J. Struct. Chem. 2013, 32, 885–889. [Google Scholar]
- Gomila, R.M.; Mooibroek, T.J.; Frontera, A. In A combined theoretical and CSD perspective on σ-hole interactions with tetrels, pnictogens, chalcogens, halogens, and noble gases. In Hot Topics in Crystal Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Weigend, F.; Häser, M. RI-MP2: First derivatives and global consistency. Theor. Chem. Acc. 1997, 97, 331–340. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic Structure Calculations on Workstation Computers-the Program System turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Kirina, Y.V.; Kukushkin, V.Y. Halogen bonding between metal centers and halocarbons. Chem. Commun. 2016, 52, 5565–5568. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic–Nucleophilic Dualism of Nickel(II) toward Ni···I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef]
- Baykov, S.V.; Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Boyarskiy, V.P. Pt/Pd and I/Br Isostructural Exchange Provides Formation of C–I···Pd, C–Br···Pt, and C–Br···Pd Metal-Involving Halogen Bonding. Cryst. Growth. Des. 2018, 18, 5973–5980. [Google Scholar] [CrossRef]
- Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Matveychuk, Y.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Bifurcated Halogen Bonding C–Br···η2(Cl–Pt). Cryst. Growth. Des. 2019, 19, 1364–1376. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-involving halogen bond Ar–I⋯[dz2PtII] in a platinum acetylacetonate complex. CrystEngComm 2020, 22, 554–563. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Haukka, M.; Levin, O.V.; Frontera, A.; Kukushkin, V.Y. Supramolecular Assembly of Metal Complexes by (Aryl)I⋯dz2 [PtII] Halogen Bonds. Chem. Eur. J. 2020, 26, 7692–7701. [Google Scholar] [CrossRef]
- Nemec, V.; Lisac, K.; Bedeković, N.; Fotović, L.; Stilinović, V.; Cinčić, D. Crystal engineering strategies towards halogen-bonded metal–organic multi-component solids: Salts, cocrystals and salt cocrystals. CrystEngComm 2021, 23, 3063–3083. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. JACS Au 2021, 1, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Rozhkov, A.V.; Katlenok, E.A.; Zhmykhova, M.V.; Ivanov, A.Y.; Kuznetsov, M.L.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Chalcogen Bond. The Case of Platinum(II) Interaction with Se/Te-Based σ-Hole Donors. J. Am. Chem. Soc. 2021, 143, 15701–15710. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Elder, P.J.W.; Dube, P.A.; Greedan, J.E.; Jenkins, H.A.; Britten, J.F.; Vargas-Baca, I. The size of the metal ion controls the structures of the coordination polymers of benzo-2,1,3-selenadiazole. CrystEngComm 2013, 15, 7434–7437. [Google Scholar] [CrossRef]
- Milios, C.J.; Ioannou, P.V.; Raptopoulou, C.P.; Papaefstathiou, G.S. Crystal Engineering with 2,1,3-benzoselenadiazole and Mercury(II) Chloride. Polyhedron 2009, 28, 3199–3202. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta. Cryst. 2016, B72, 171–179. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5050. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem.Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Revision, B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bauzá, A.; Frontera, A. Halogen and Chalcogen Bond Energies Evaluated Using Electron Density Properties. ChemPhysChem 2020, 21, 26–31. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Todd, A.; Keith, T.K. (Eds.) AIMAll (Version 13.05.06); Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Behrens, U.; Berges, P.; Bieganowski, R.; Hinrichs, W.; Schiffling, C.; Klar, G. Cyclic Ligands with Fixed Co-ordination Geometry. Part 5. Complexes of Thianthrene and Selenanthrene with d8 and dl0 Systems. Structures of the 1:1 Complexes of Tetra-alkoxyselenanthrenes with Plaitinum(II) and Mercury(II) Chlorides. J. Chem. Res. 1986, 326, 2801. [Google Scholar]
- Kumar, A.; Agarwal, M.; Singh, A.K.; Butcher, R.J. Palladium(II), platinum(II), ruthenium(II) and mercury(II) complexes of potentially tridentate Schiff base ligands of (E, N, O) type (E=S, Se, Te): Synthesis, crystal structures and applications in Heck and Suzuki coupling reactions. Inorg. Chim. Acta. 2009, 362, 3208–3218. [Google Scholar] [CrossRef]
- Takeda, N.; Isobe, T.; Sasamori, T.; Tokitoh, N. [1,2-Bis (phenyl seleno) benzene] dichlorido palladium(II). Acta. Cryst. E 2007, 63, m2546. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Elsegood, M.R.J.; Kelly, P.F.; Smith, M.B.; Staniland, P.M. Versatile routes to selenoether functionalised tertiary phosphines. Dalton. Trans. 2010, 39, 5216–5218. [Google Scholar] [CrossRef][Green Version]
- Ritch, J.S.; Charette, B.J. An Experimental and Computational Comparison of Phosphorus and Selenium-Based Ligands for Catalysis. Can. J. Chem. 2016, 94, 386. [Google Scholar] [CrossRef]
- Kumar, S.; Saleem, F.; Singh, A.K. ‘Click’ generated 1,2,3-triazole based organosulfur/selenium ligands and their Pd(ii) and Ru(ii) complexes: Their synthesis, structure, and catalytic applications. Dalton. Trans. 2016, 45, 11445–11458. [Google Scholar] [CrossRef]
- Bhaskar, R.; Sharma, A.K.; Yadav, M.K.; Singh, A.K. Sonogashira (Cu and amine free) and Suzuki coupling in air catalyzed via nanoparticles formed in situ from Pd(ii) complexes of chalcogenated Schiff bases of 1-naphthaldehyde and their reduced forms. Dalton. Trans. 2017, 46, 15235–15248. [Google Scholar] [CrossRef]
- Kumar, U.; Dubey, P.; Singh, V.V.; Prakash, O.; Singh, A.K. Sterically hindered selenoether ligands: Palladium(II) complexes as catalytic activators for Suzuki–Miyaura coupling. RSC. Adv. 2014, 4, 41659–41665. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, M.; Singh, A.K. Schiff bases of 1′-hydroxy-2′-acetonaphthone containing chalcogen functionalities and their complexes with and (p-cymene)Ru(II), Pd(II), Pt(II) and Hg(II): Synthesis, structures and applications in C–C coupling reactions. J. Organomet. Chem. 2008, 693, 3533–3545. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Bokach, N.A.; Kukushin, V.Y.; Frontera, A. Metal Centers as Nucleophiles: Oxymoron of Halogen Bond-Involving Crystal Engineering. Chem. Eur. J. 2022, 28, e202103173. [Google Scholar] [PubMed]
- Spokoyny, A.M.; Reuter, M.G.; Stern, C.L.; Ratner, M.A.; Seideman, T.; Mirkin, C.A. Carborane-Based Pincers: Synthesis and Structure of SeBSe and SBS Pd(II) Complexes. J. Am. Chem. Soc. 2009, 131, 9482–9483. [Google Scholar] [CrossRef] [PubMed]
- Klauke, K.; Gruber, I.; Knedel, T.O.; Schmolke, L.; Barthel, J.; Breitzke, H.; Buntkowsky, G.; Janiak, C. Silver, Gold, Palladium, and Platinum N-heterocyclic Carbene Complexes Containing a Selenoether-Functionalized Imidazol-2-ylidene Moiety. Organometallics 2018, 37, 298–308. [Google Scholar] [CrossRef]
- Kostas, I.D.; Steele, B.R.; Terzis, A.; Amosova, S.V.; Martynov, A.V.; Makhaeva, N.A. New Palladium Complexes with S-or Se-Containing Schiff-Base Ligands as Efficient Catalysts for the Suzuki-Miyaura Cross-Coupling Reaction of Aryl Bromides with Phenylboronic Acid under Aerobic Conditions. Eur. J. Inorg. Chem. 2006, 2006, 2642–2646. [Google Scholar] [CrossRef]
- Kirsten, L.; Schwade, V.D.; Selter, L.; Hagenbach, A.; Piquini, P.C.; Lang, E.S.; Abram, U. Pt, Pd and Hg Complexes with Potentially Tridentate Telluroether Ligands. Eur. J. Inorg. Chem. 2015, 2015, 3748–3757. [Google Scholar] [CrossRef]
- Gupta, A.; Deka, R.; Srivastava, K.; Singh, H.B.; Butcher, R.J. Synthesis of Pd(II) complexes of unsymmetrical, hybrid selenoether and telluroether ligands: Isolation of tellura-palladacycles by fine tuning of intramolecular chalcogen bonding in hybrid telluroether ligands. Polyhedron 2019, 172, 95–103. [Google Scholar] [CrossRef]
- Kemmitt, T.; Levason, W.; Webster, M. Chelating ditelluroether complexes of palladium and platinum: Synthesis and multinuclear NMR studies. Structure of dibromo(meso-1,3-bis(phenyltelluro)propane)palladium(II): [Pd{meso-PhTe(CH2)3TePh}Br2]. Inorg. Chem. 1989, 28, 692–696. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, V.; Dhingra, S.K.; Drake, J.E.; Bailey, J.H.E. cis-Dichloro[2-(4-ethoxyphenyltelluro)ethyl methyl sulfide-S,Te]platinum(II) cis-PtCl2[Te(C6H4OC2H5)CH2CH2(CH3)S]. Acta Cryst. C 1992, 48, 655–657. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, P.A.K.; Drake, J.E.; Hursthouse, M.B.; Little, M.E. Tellurium-chlorine secondary interactions in palladium(II) complex of MeOC6H4TeCH2CH2NHCH(CH3)C6H4-2-OH resulting in self-assembled bimolecular aggregates with short palladium-palladium distances. Struct. Chem. 2007, 18, 203–207. [Google Scholar]
- Menon, S.C.; Panda, A.; Singh, H.B.; Butcher, R.J. Synthesis and single crystal X-ray structure of the first cationic Pd(ii) complex of a tellurium-containing polyaza macrocycle: Contrasting reactions of Pd(ii) and Pt(ii) with a 22-membered macrocyclic Schiff base. Chem. Commun. 2000, 143–144. [Google Scholar] [CrossRef]
- Milton, M.D.; Singh, J.D.; Butcher, R.J. Synthesis of β-ketoenamine donors having O, N, Se/Te donor functionalities and their reaction chemistry with Pd(II) and Pt(II) metal ions. Tetrahedron. Lett. 2004, 45, 6745–6747. [Google Scholar] [CrossRef]
- Gomila, R.M.; Bauzá, A.; Frontera, A. Enhancing chalcogen bonding by metal coordination. Dalton. Trans. 2022. [Google Scholar] [CrossRef] [PubMed]
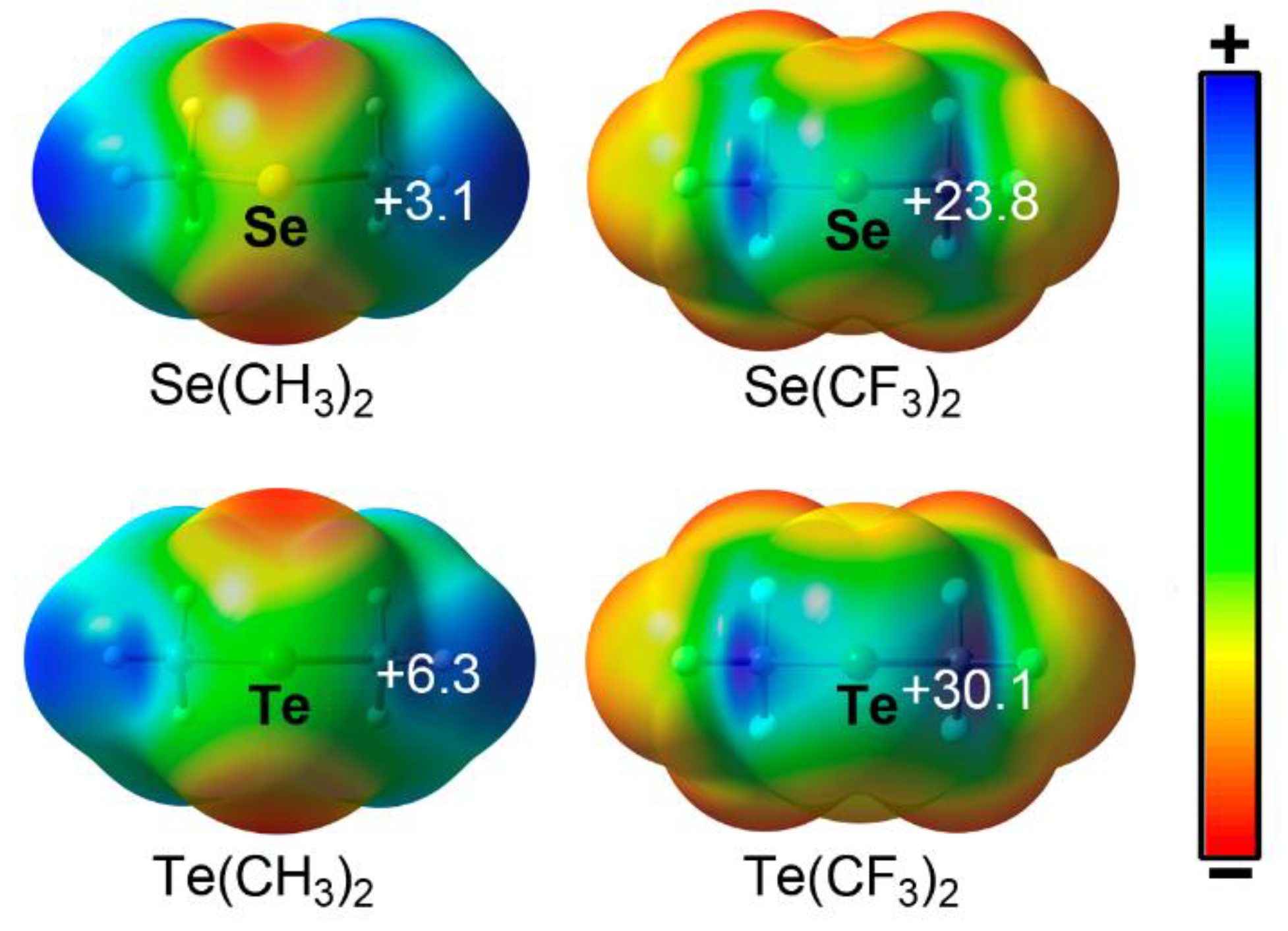
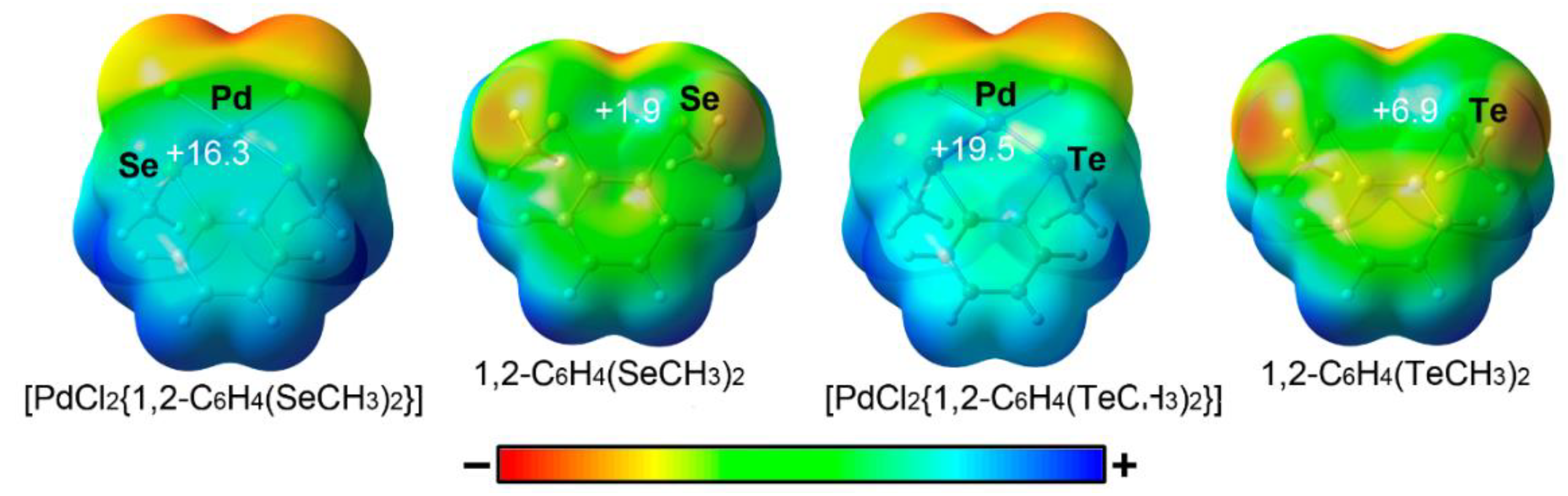
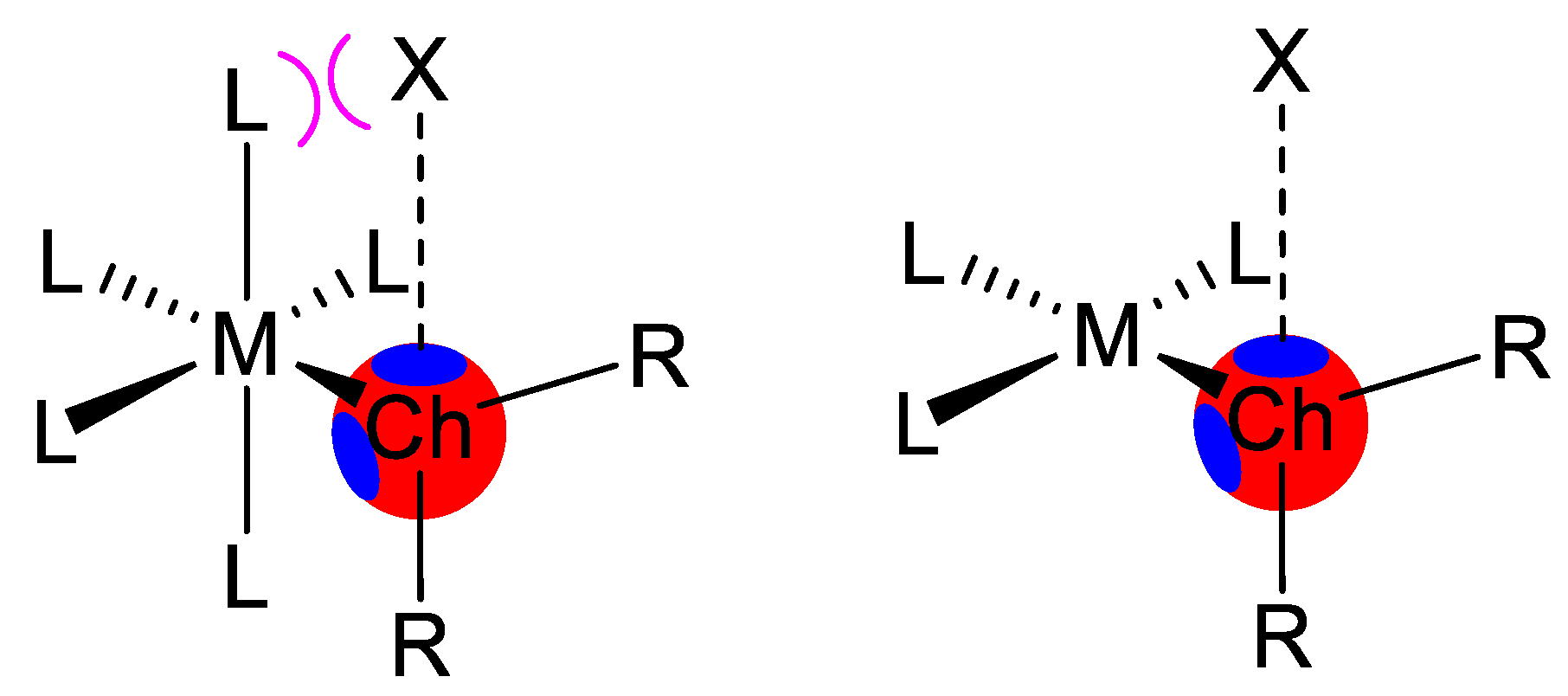
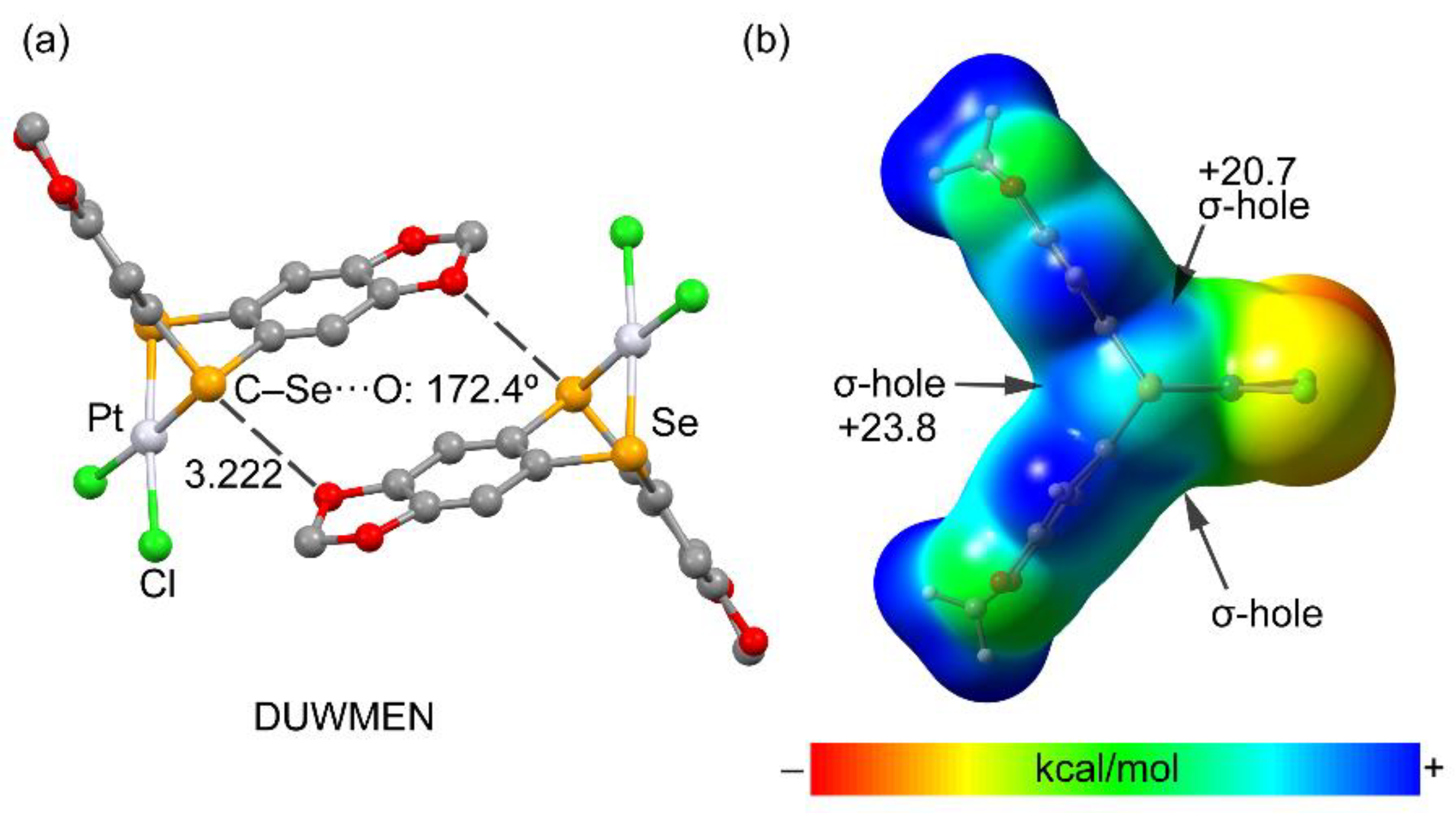
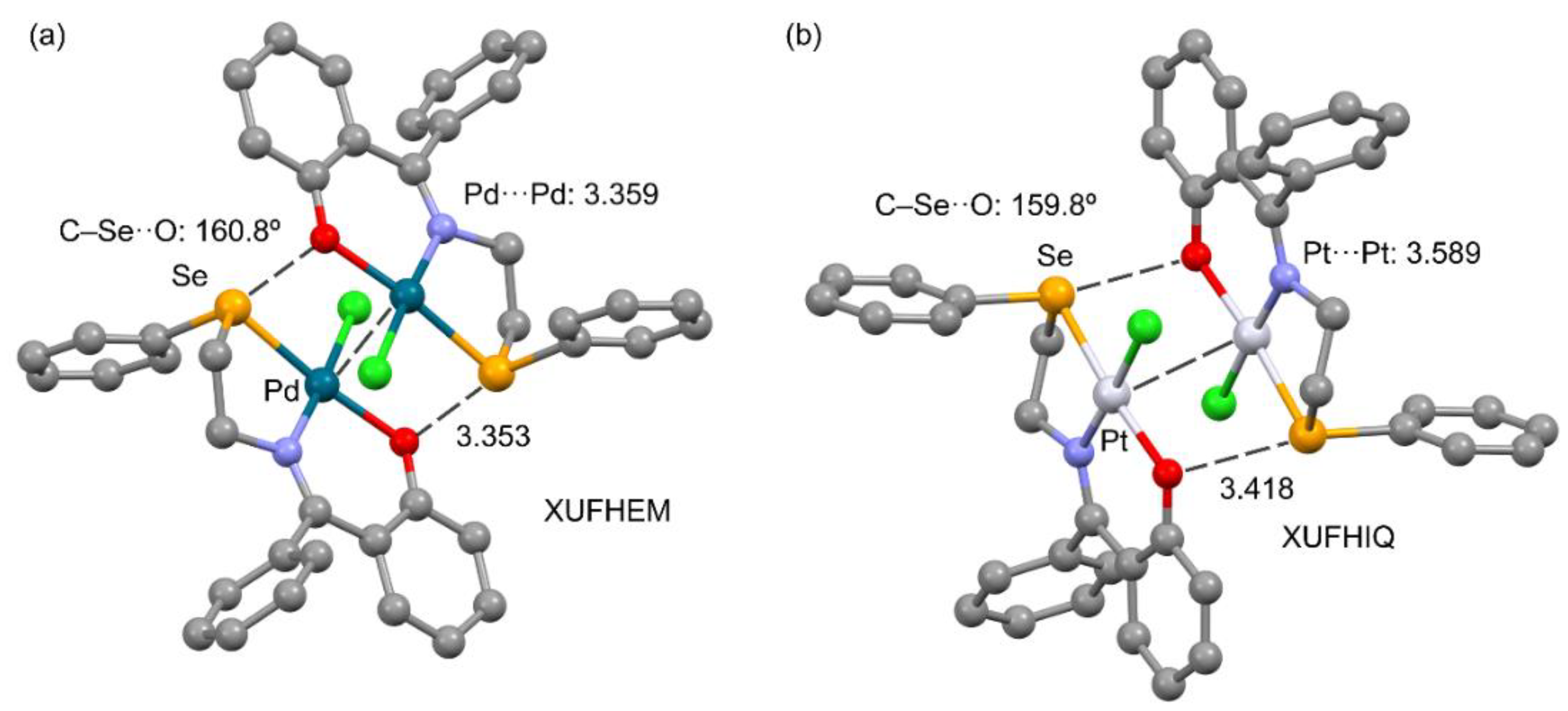
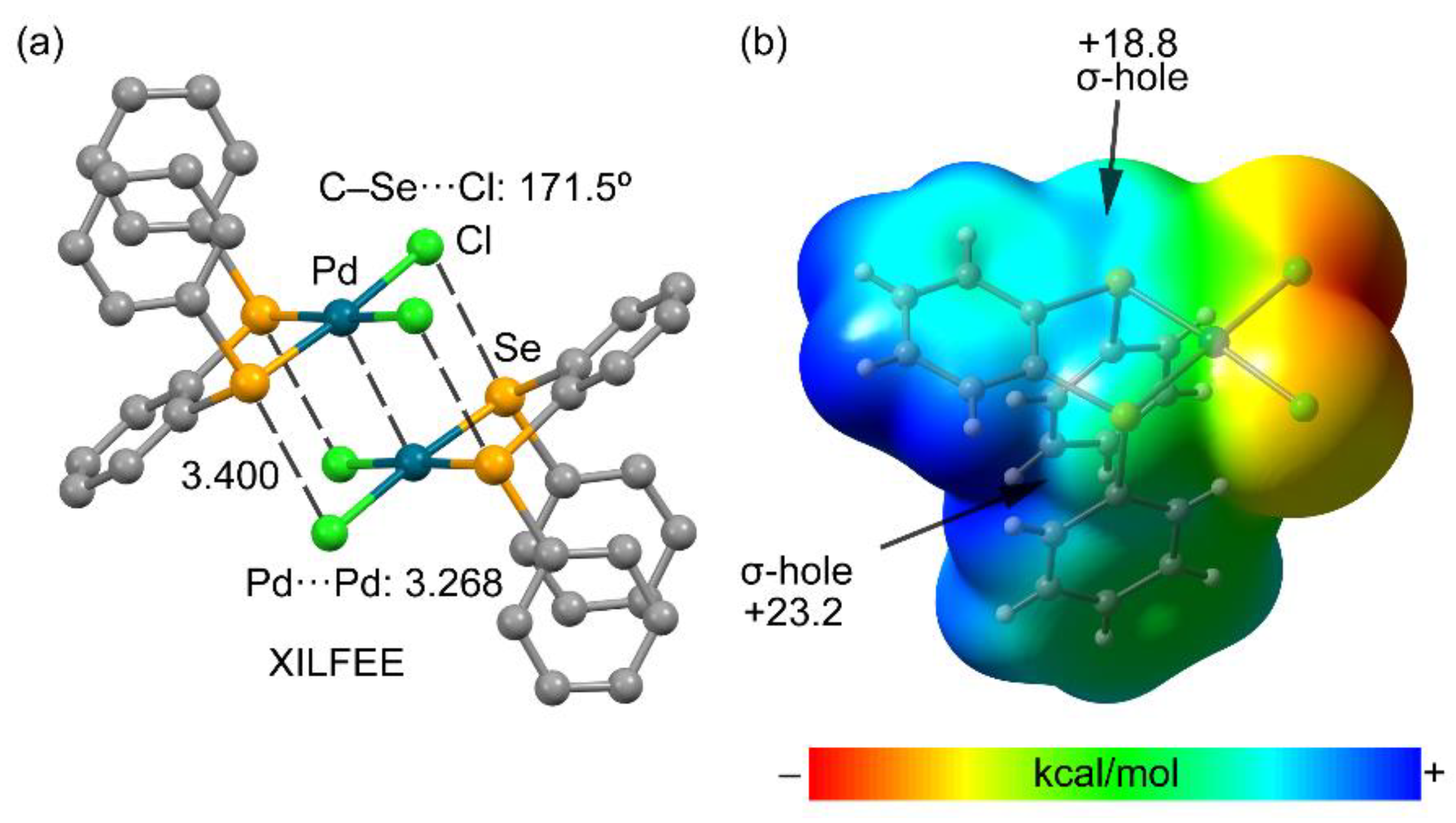


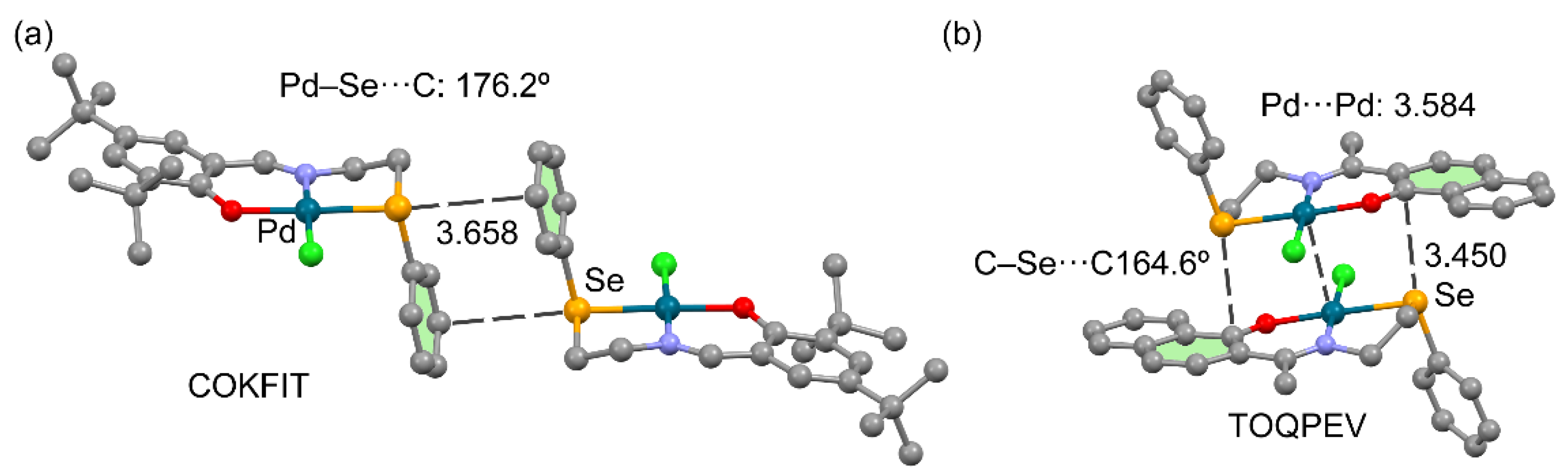
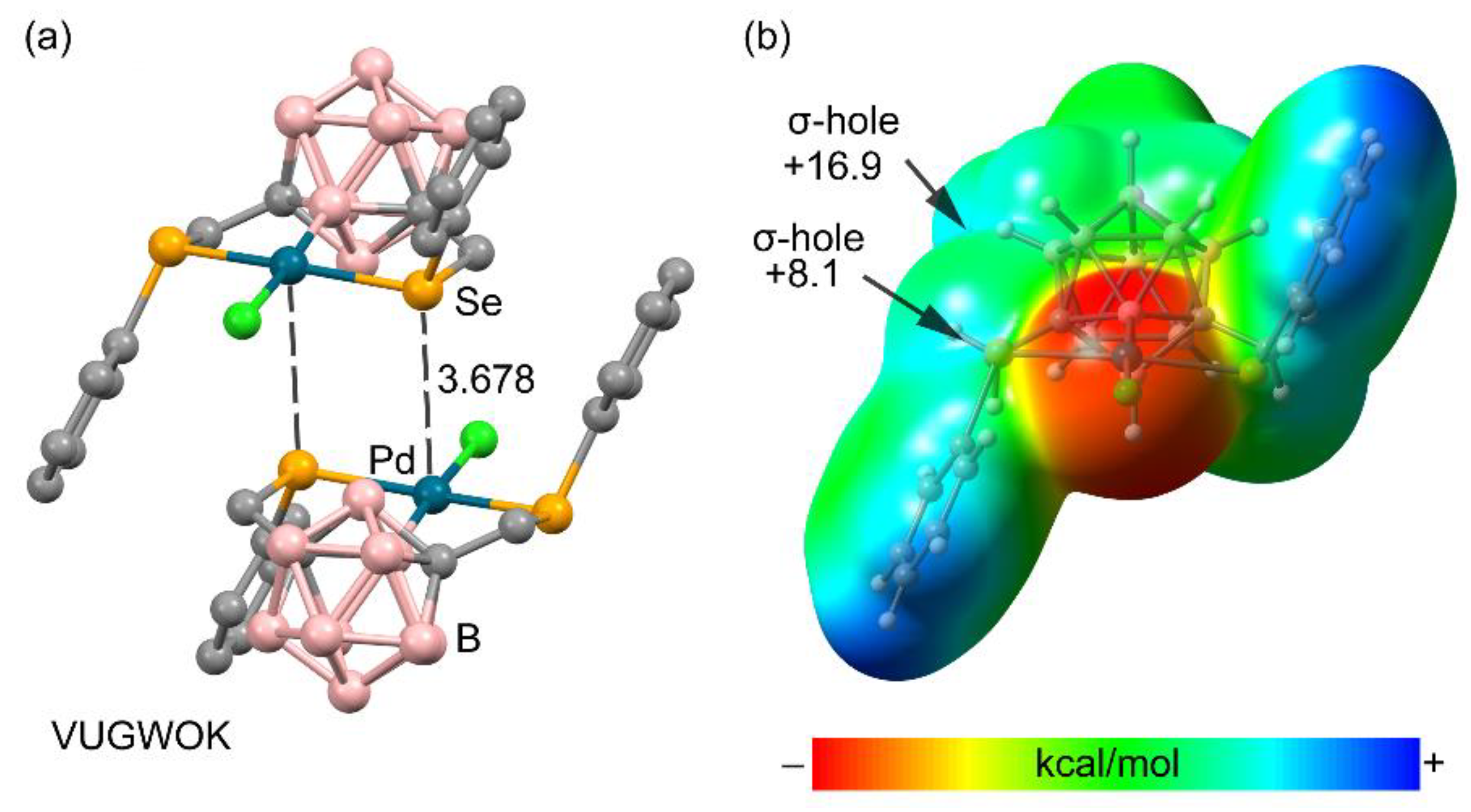

| Ch | α | RvdW |
|---|---|---|
| O | 3.0 | 1.52 |
| S | 11.8 | 1.80 |
| Se | 17.5 | 1.90 |
| Te | 25.9 | 2.06 |
| CSD Code | Name | Formula |
|---|---|---|
| DUWMEN | dichloro-(2,3:7,8-bis(methylenedioxy)-selenanthrene-Se,Se′)-platinum(II) acetone solvate | C14H8Cl2O4PtSe2, C3H6O |
| XUFHEM | chloro-(2-(phenyl((2-(phenylselanyl-kSe)ethyl)imino-kN)methyl)phenolato-kO)-palladium(II) | C21H18ClNOPdSe |
| XUFHIQ | chloro-(2-(phenyl((2-(phenylselanyl-kSe)ethyl)imino-kN)methyl)phenolato-kO)-platinum(II) | C21H18ClNOPtSe |
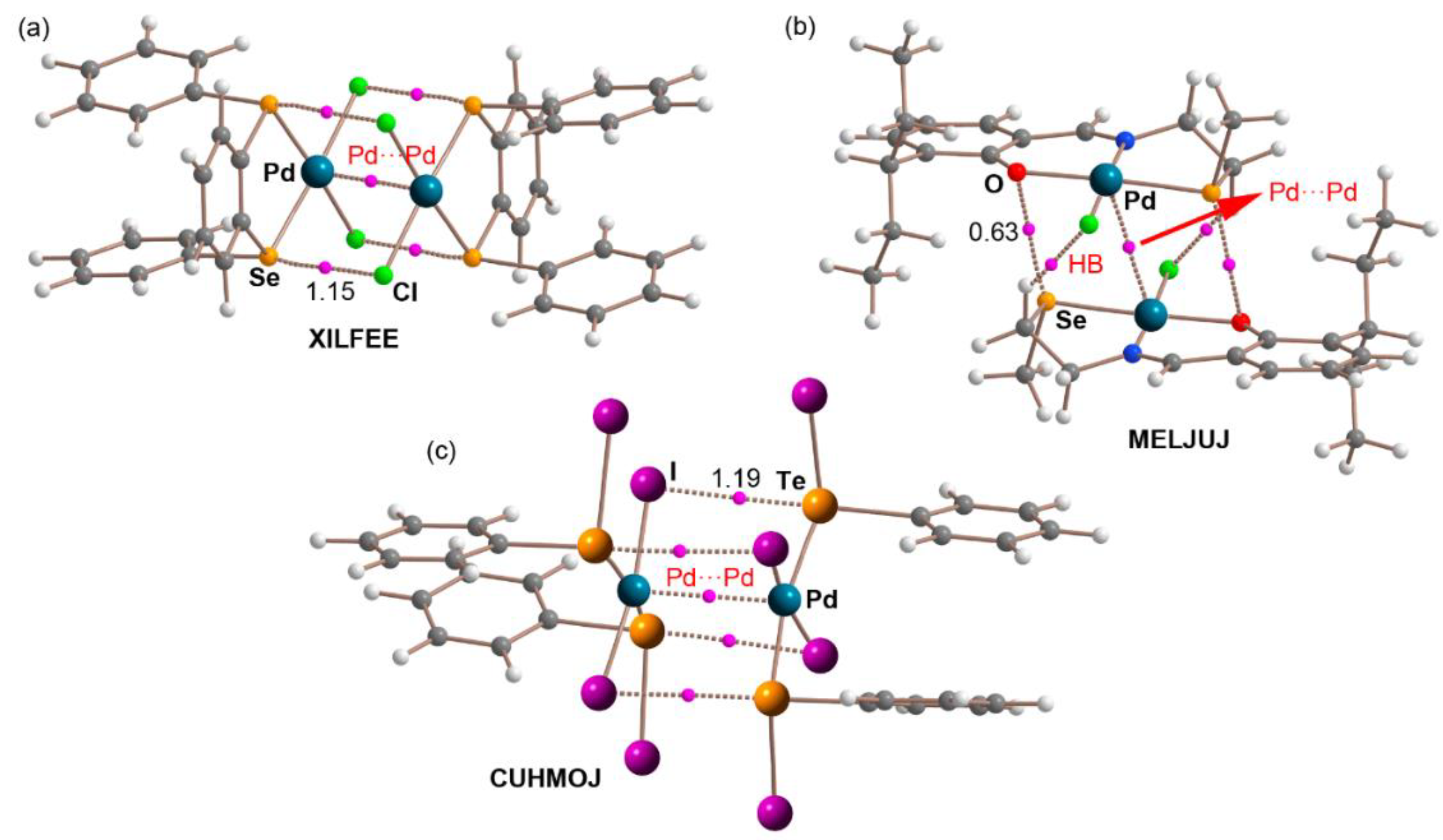
| XILFEE | (1,2-bis(phenylseleno)benzene)-dichlorido-palladium(II) | C18H14Cl2PdSe2 |
| PUYWUC | cichloro-(diphenyl(2-(phenylselanyl)phenyl)phosphine)-platinum | C24H19Cl2PPtSe |
| TAFWIJ | dichloro-(2-(phenylselanyl)aniline)-palladium(II) acetonitrile solvate | C12H11Cl2NPdSe, C2H3N |
| EPULIM | dichloro-(1-(2,6-di-isopropylphenyl)-4-((phenylselanyl)methyl)-1H-1,2,3-triazole)-palladium(II) | C21H25Cl2N3PdSe |
| BEJWIZ | chloro-[8-({[2-(phenylselanyl)ethyl]imino}methyl)naphthalen-1-yl]-palladium(II) | C19H16ClNPdSe |
| COKFIT | chloro-(2,4-di-t-butyl-6-(((2-(phenylselanyl)ethyl)imino)methyl)phenolato)-palladium(II) | C23H30ClNOPdSe |
| TOQPEV | chloro-(2-(1-(2-(phenylselanyl)ethylimino)ethyl)-1-naphtholato-N,O,Se)-palladium(II) | C20H18ClNOPdSe |
| VUGWOK | chloro-(1,7-bis(phenylselenomethyl)-1,7-dicarba-closo-dodecaborate-B,S,S′)-palladium(II) | C16H23B10ClPdSe2 |
| QETFED | dichloro-(1-methyl-3-[(phenylselanyl)methyl]-imidazol-2-ylidene)-platinum(II) acetonitrile solvate | C11H12Cl2N2PtSe, 0.5(C2H3N) |
| QETFAZ | dichloro-(1-methyl-3-[(phenylselanyl)methyl]-imidazol-2-ylidene)-palladium(II) acetonitrile solvate | C11H12Cl2N2PdSe, 0.5(C2H3N) |
| MELJUJ | chloro-((2-((2-methylselanyl)ethyl)iminomethyl)-6-(1-ethylpropyl)phenolato)-palladium(II) | C15H22ClNOPdSe |
| CSD Code | Name | Formula |
|---|---|---|
| CUHMOJ | bis(benzenetellurenyl iodide)-di-iodo-palladium(II) | C12H10I4PdTe2 |
| CODTEX | dichloro-[1-(2-{[2,6-di-isopropylphenyl]tellanyl}phenyl)-N,N-dimethylmethanamine]-palladium | C21H29Cl2NPdTe |
| JAGZIA | dibromo-(meso-1,3-bis(phenyltelluro)propane)-palladium(II) | C15H16Br2PdTe2 |
| TAPYEO | cis-Dichloro-(2-(4-ethoxyphenyl-telluro)ethyl(methyl)sulfido)-platinum(II) | C11H16Cl2OPtSTe |
| SIDDAL | chloro-(2-(1-((2-((4-methoxyphenyl)tellanyl)ethyl)amino)ethyl)phenolato)-palladium(II) dichloromethane solvate | C17H20ClNO2PdTe, 0.5(CH2Cl2) |
| WIXSEB | (μ2-N-(o-phenylene)methyllidene)-N′-((3-formyl-o-phenylene)methylidene)-platinum(II)-tellurium hexafluorophosphate | C23H19N2OPtTe+, F6P− |
| ABOREP | chloro-(4-(2-(phenyltelluro)ethylimino)pentan-2-onato-N,O,Te)-platinum(II) | C13H16ClNOPtTe |
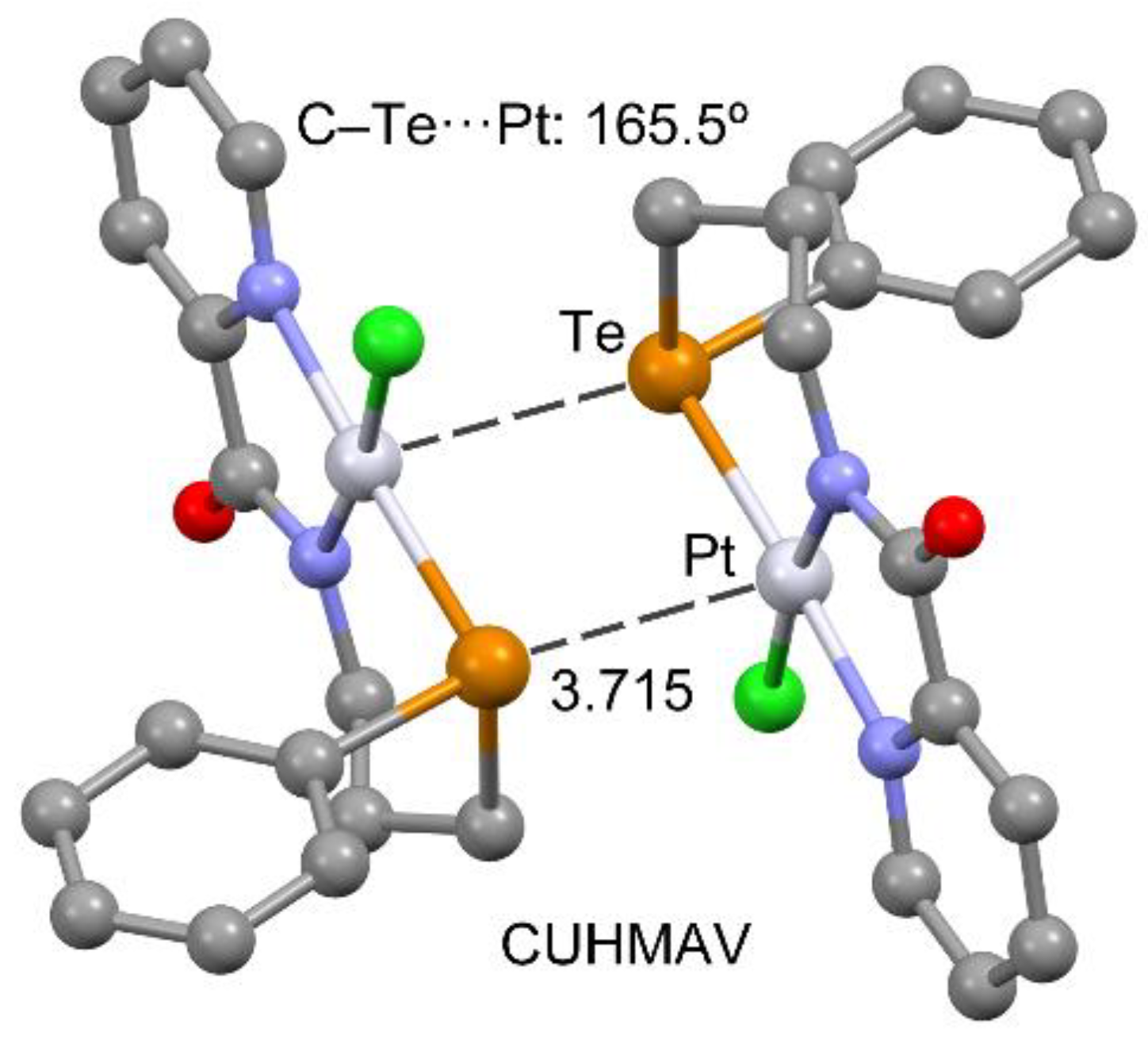
| CUHMAV | chloro-(N-(3-(phenyltellanyl)propyl)pyridine-2-carboxamidato)-platinum(II) | C15H15ClN2OPtTe |
| CSD Code | ΔEBSSE | ΔEChB | d | ∠ | ρ 102 | QD (e) | QA (e) |
|---|---|---|---|---|---|---|---|
| XILFEE | −32.0 | −7.9 a | 3.359 | 165.6 | 1.15 | 0.78 | −0.42 |
| MELJUJ | −31.5 | −2.7 a | 3.445 c | 170.6 c | 0.63 | 0.70 | −0.64 |
| CUHMOJ | −24.5 | −4.8 b | 3.724 | 178.9 | 1.19 | 0.79 | −0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frontera, A.; Bauza, A. Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations. Int. J. Mol. Sci. 2022, 23, 4188. https://doi.org/10.3390/ijms23084188
Frontera A, Bauza A. Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations. International Journal of Molecular Sciences. 2022; 23(8):4188. https://doi.org/10.3390/ijms23084188
Chicago/Turabian StyleFrontera, Antonio, and Antonio Bauza. 2022. "Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations" International Journal of Molecular Sciences 23, no. 8: 4188. https://doi.org/10.3390/ijms23084188
APA StyleFrontera, A., & Bauza, A. (2022). Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations. International Journal of Molecular Sciences, 23(8), 4188. https://doi.org/10.3390/ijms23084188







