Pep19 Has a Positive Effect on Insulin Sensitivity and Ameliorates Both Hepatic and Adipose Tissue Phenotype of Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Groups
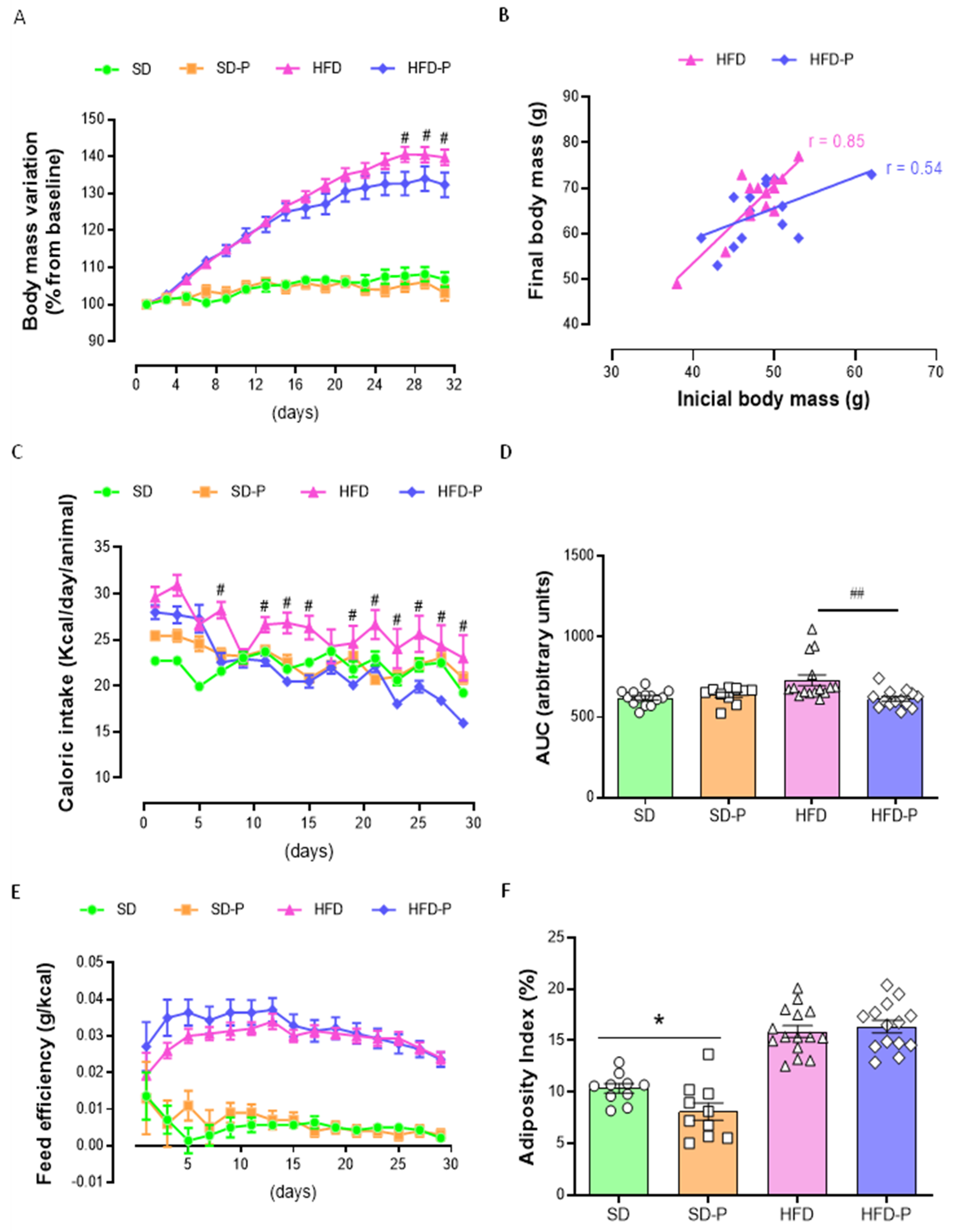
2.3. Body Mass and Food Intake
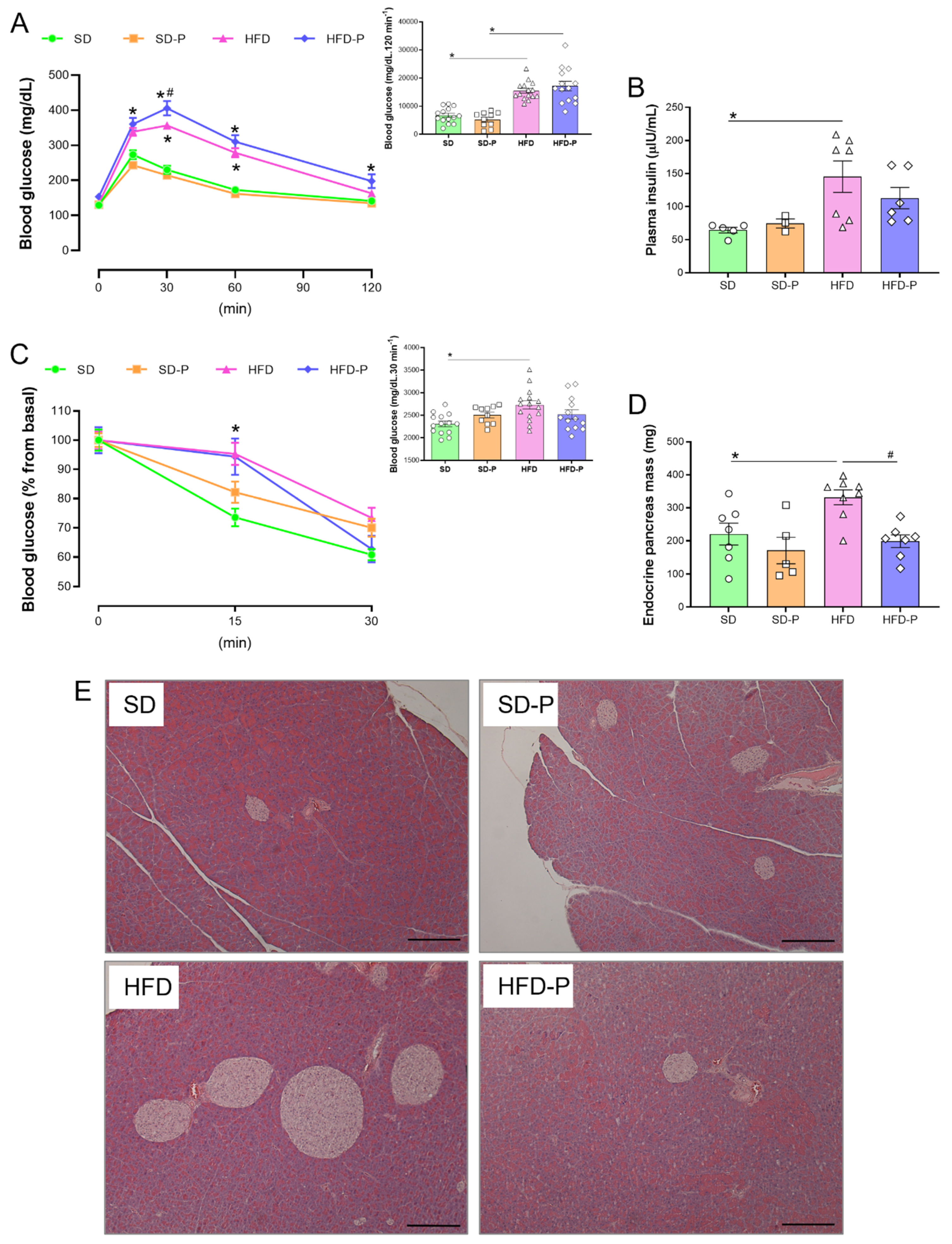
2.4. Glucose Tolerance and Insulin Sensitivity Assessment
2.5. Euthanasia and Tissue Harvesting
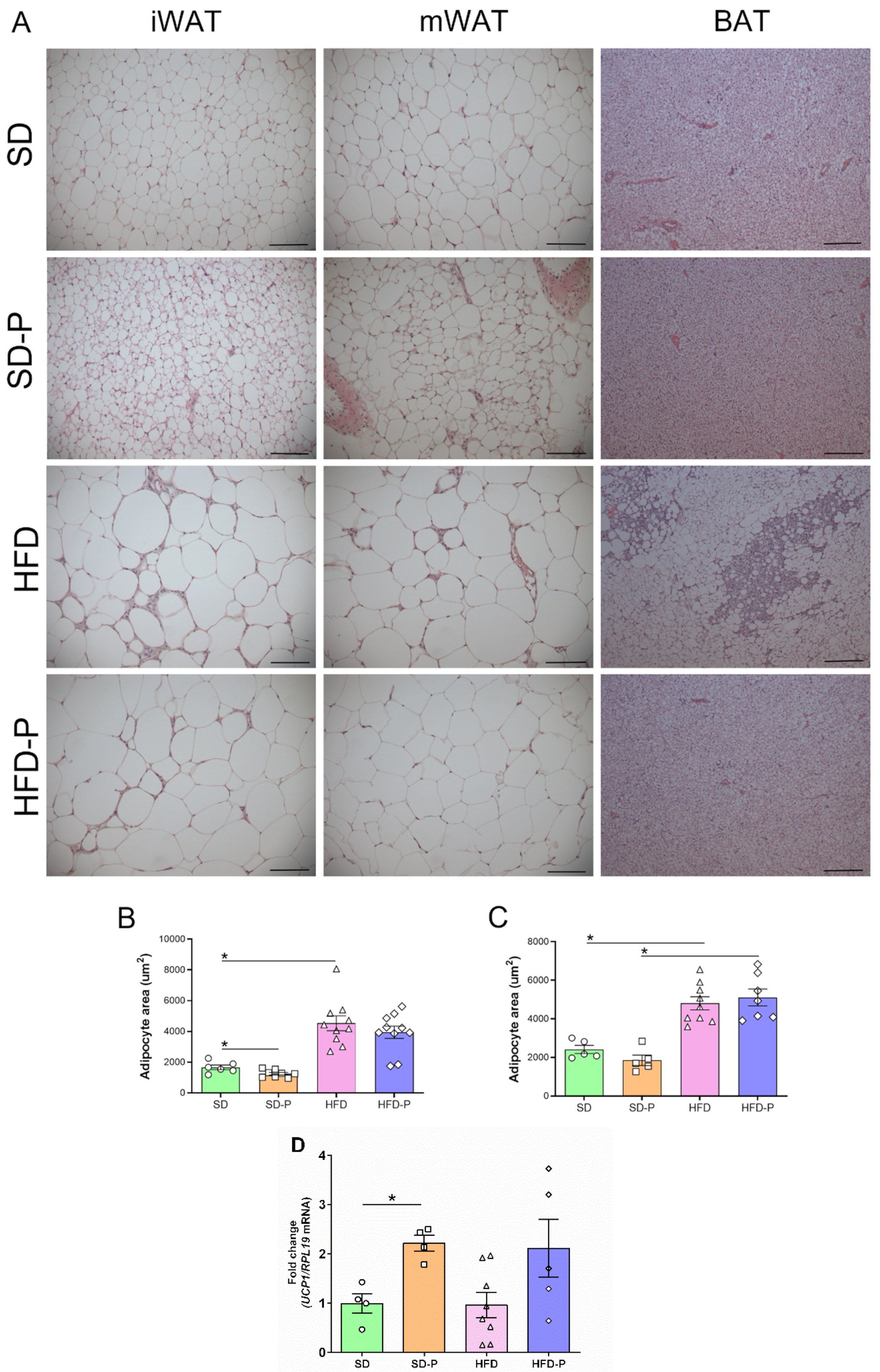
2.6. Histological Analyzes
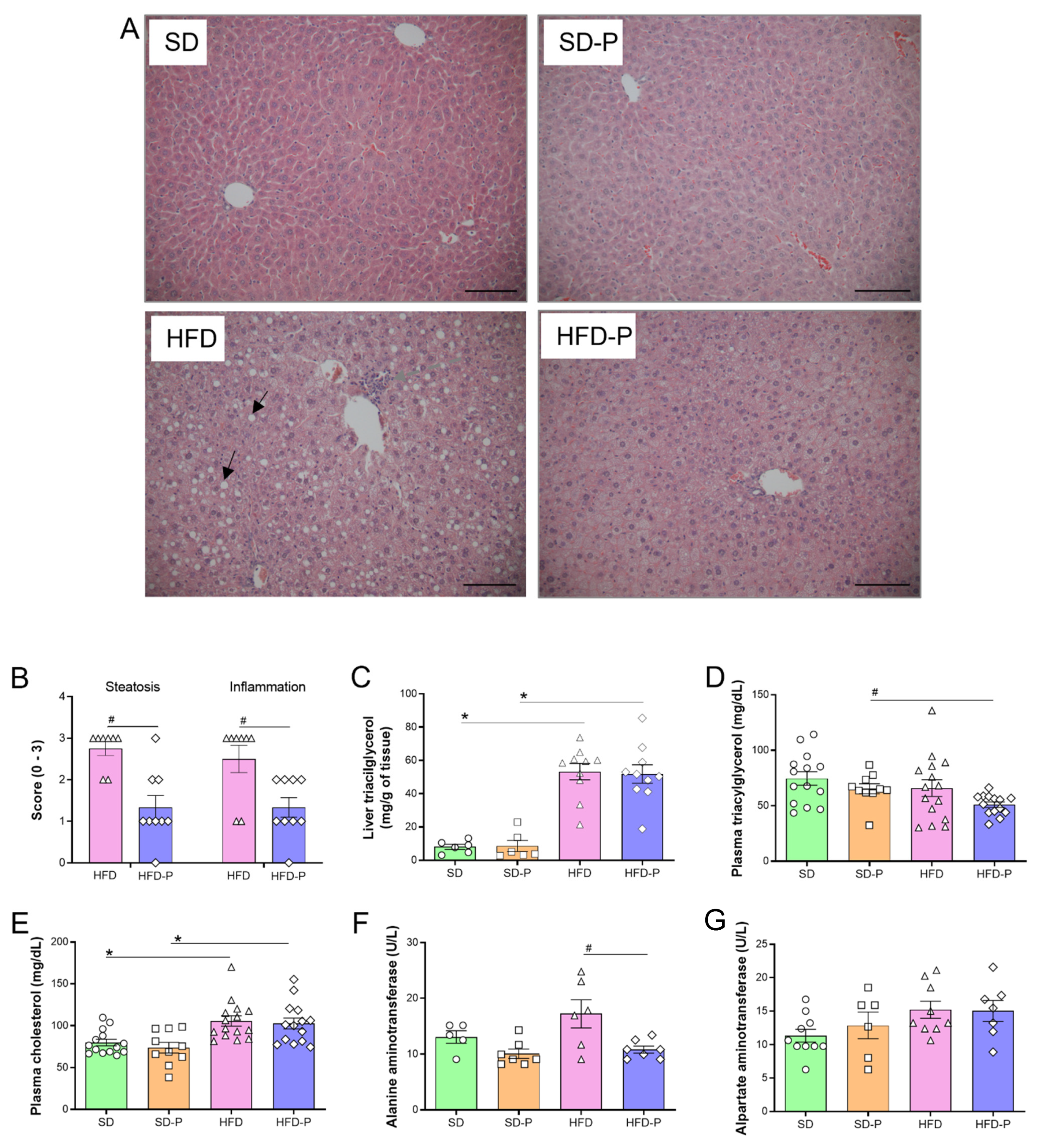
2.7. Histopathological and Biochemical Liver Analyses
2.8. Real-Time PCR
2.9. Behavioral Tests
2.10. Statistical Analysis
3. Results
3.1. Pep19 Attenuates Body-Weight Gain in Mice Fed a High-Fat Diet (HFD)
3.2. Pep19 Modulates Insulin Sensitivity and Mass of Endocrine Pancreas in Obese Mice
3.3. Pep 19 Ameliorates Hepatic Steatosis and Inflammation
3.4. Pep 19 Effects on Adipose Tissue Morphology and UCP1 mRNA Expression
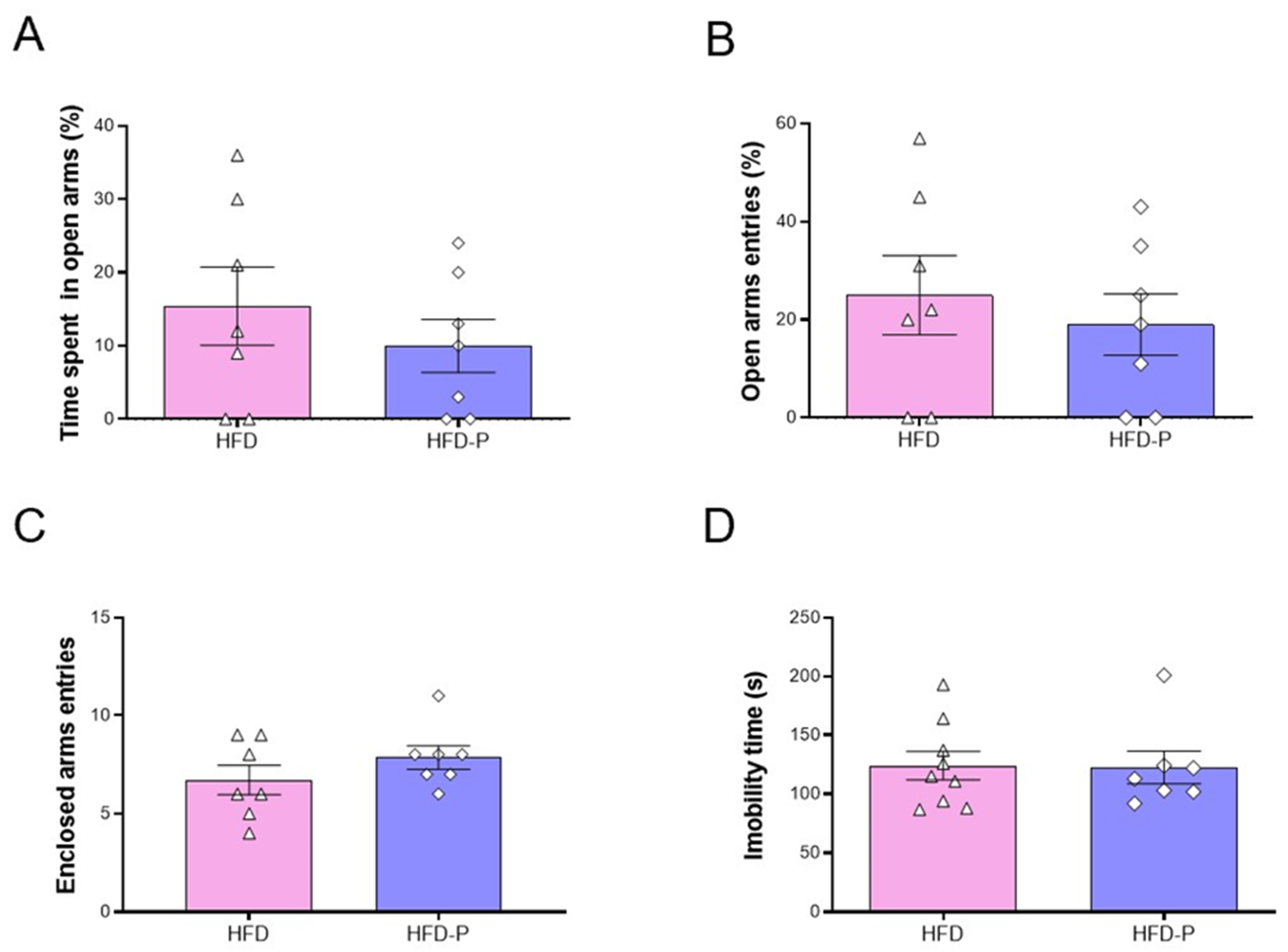
3.5. Pep19 Treatment Does Not Affect Behavior
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.; Kim, K.; Wilding, J.; Federation, W.O. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Keaver, L.; Webber, L.; Dee, A.; Shiely, F.; Marsh, T.; Balanda, K.; Perry, I. Application of the UK foresight obesity model in Ireland: The health and economic consequences of projected obesity trends in Ireland. PLoS ONE 2013, 8, e79827. [Google Scholar] [CrossRef]
- Richey, J.M.; Woolcott, O. Re-visiting the endocannabinoid system and its therapeutic potential in obesity and associated diseases. Curr. Diabetes Rep. 2017, 17, 99. [Google Scholar] [CrossRef]
- Pagotto, U.; Vicennati, V.; Pasquali, R. The endocannabinoid system and the treatment of obesity. Ann. Med. 2005, 37, 270–275. [Google Scholar] [CrossRef]
- Cote, M.; Matias, I.; Lemieux, I.; Petrosino, S.; Almeras, N.; Despres, J.; Di Marzo, V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007, 31, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [Green Version]
- De Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.d.O.; Pereira, S.S.; de Oliveira, L.L.; Gouveia Peluzio, M.d.C.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef]
- Duarte, C.; Alonso, R.; Bichet, N.; Cohen, C.; Soubrié, P.; Thiébot, M.-H. Blockade by the Cannabinoid CB1 Receptor Antagonist, Rimonabant (SR141716), of the Potentiation by Quinelorane of Food-Primed Reinstatement of Food-Seeking Behavior. Neuropsychopharmacology 2004, 29, 911–920. [Google Scholar] [CrossRef]
- Ravinet Trillou, C.; Delgorge, C.; Menet, C.; Arnone, M.; Soubrié, P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. 2004, 28, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P.; Golay, A.; Sjöström, L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 2005, 353, 2121–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi-Sunyer, F.X.; Aronne, L.J.; Heshmati, H.M.; Devin, J.; Rosenstock, J.; RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: A randomized controlled trial. Jama 2006, 295, 761–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheen, A.J.; Finer, N.; Hollander, P.; Jensen, M.D.; Van Gaal, L.F.; Group, R.-D.S. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: A randomised controlled study. Lancet 2006, 368, 1660–1672. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rössner, S.; Group, R.-E.S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Perwitz, N.; Fasshauer, M.; Klein, J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm. Metab. Res. 2006, 38, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Moreira, F.A.; Crippa, J.A.S. The psychiatric side-effects of rimonabant. Braz. J. Psychiatry 2009, 31, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Bermudez-Silva, F.; Viveros, M.; McPartland, J.; De Fonseca, F.R. The endocannabinoid system, eating behavior and energy homeostasis: The end or a new beginning? Pharmacol. Biochem. Behav. 2010, 95, 375–382. [Google Scholar] [CrossRef]
- Reckziegel, P.; Festuccia, W.T.; Britto, L.R.G.; Jang, K.L.L.; Romao, C.M.; Heimann, J.C.; Fogaca, M.V.; Rodrigues, N.S.; Silva, N.R.; Guimaraes, F.S.; et al. A novel peptide that improves metabolic parameters without adverse central nervous system effects. Sci. Rep. 2017, 7, 14781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Thomas, B.F.; Zhang, Y. Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: Current approaches for therapeutics development. Curr. Top. Med. Chem. 2019, 19, 1418–1435. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.R.; Kooijman, S.; van Dam, A.D.; Pelgrom, L.R.; Berbée, J.F.; Visseren, C.A.; van Aggele, R.C.; van den Hoek, A.M.; Sips, H.C.; Lombès, M. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 2014, 28, 5361–5375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Räder, A.F.B.; Weinmüller, M.; Reichart, F.; Schumacher-Klinger, A.; Merzbach, S.; Gilon, C.; Hoffman, A.; Kessler, H. Orally Active Peptides: Is There a Magic Bullet? Angew. Chem. Int. Ed. 2018, 57, 14414–14438. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.T.; Pliatsika, D.; Gall, F.M.; Fischer, T.; Riedl, R. New perspectives in oral peptide delivery. Drug Discov. Today 2021, 26, 1097–1105. [Google Scholar] [CrossRef]
- Dale, C.S.; Pagano Rde, L.; Rioli, V.; Hyslop, S.; Giorgi, R.; Ferro, E.S. Antinociceptive action of hemopressin in experimental hyperalgesia. Peptides 2005, 26, 431–436. [Google Scholar] [CrossRef]
- Heimann, A.S.; Gomes, I.; Dale, C.S.; Pagano, R.L.; Gupta, A.; de Souza, L.L.; Luchessi, A.D.; Castro, L.M.; Giorgi, R.; Rioli, V.; et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 20588–20593. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Matsui, T. Intestinal absorption of small peptides: A review. Int. J. Food Sci. Technol. 2019, 54, 1942–1948. [Google Scholar] [CrossRef] [Green Version]
- Heimann, A.S.; Dale, C.S.; Guimarães, F.S.; Reis, R.A.M.; Navon, A.; Shmuelov, M.A.; Rioli, V.; Gomes, I.; Devi, L.L.; Ferro, E.S. Hemopressin as a breakthrough for the cannabinoid field. Neuropharmacology 2021, 183, 108406. [Google Scholar] [CrossRef]
- Heimann, A.S.; Giardini, A.C.; Sant’Anna, M.B.; Dos Santos, N.B.; Gewehr, M.C.F.; Munhoz, C.D.; Castro, L.M.; Picolo, G.; Remer, R.A.; Ferro, E.S. NFKF is a synthetic fragment derived from rat hemopressin that protects mice from neurodegeneration. Neurosci. Lett. 2020, 721, 134765. [Google Scholar] [CrossRef]
- Camargo, L.L.; Denadai-Souza, A.; Yshii, L.M.; Lima, C.; Teixeira, S.A.; Cerqueira, A.R.A.; Gewehr, M.C.F.; Fernandes, E.S.; Schenka, A.A.; Muscará, M.N.; et al. The potential anti-inflammatory and anti-nociceptive effects of rat hemopressin (PVNFKFLSH) in experimental arthritis. Eur. J. Pharmacol. 2020, 890, 173636. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, C.B.; Heimann, A.S.; Remer, R.A.; Russo, L.C.; Colquhoun, A.; Forti, F.L.; Ferro, E.S. Intracellular Peptides in Cell Biology and Pharmacology. Biomolecules 2019, 9, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogaça, M.V.; Sonego, A.B.; Rioli, V.; Gozzo, F.C.; Dale, C.S.; Ferro, E.S.; Guimarães, F.S. Anxiogenic-like effects induced by hemopressin in rats. Pharmacol. Biochem. Behav. 2015, 129, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, Z.; Kovacs, G.; Tömböly, C.; Benedek, G.; Horvath, G. The effects of peptide and lipid endocannabinoids on arthritic pain at the spinal level. Anesth. Analg. 2012, 114, 1346–1352. [Google Scholar] [CrossRef] [Green Version]
- Dale, C.S.; Pagano Rde, L.; Rioli, V. Hemopressin: A novel bioactive peptide derived from the alpha1-chain of hemoglobin. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. S1), 105–106. [Google Scholar] [CrossRef] [Green Version]
- Dodd, G.T.; Worth, A.A.; Hodkinson, D.J.; Srivastava, R.K.; Lutz, B.; Williams, S.R.; Luckman, S.M. Central functional response to the novel peptide cannabinoid, hemopressin. Neuropharmacology 2013, 71, 27–36. [Google Scholar] [CrossRef]
- Dodd, G.T.; Mancini, G.; Lutz, B.; Luckman, S.M. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J. Neurosci. 2010, 30, 7369–7376. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.N.O.; Llanos, R.P.; Eichler, R.A.S.; Oliveira, T.B.; Gozzo, F.C.; Festuccia, W.T.; Ferro, E.S. New Intracellular Peptide Derived from Hemoglobin Alpha Chain Induces Glucose Uptake and Reduces Blood Glycemia. Pharmaceutics 2021, 13, 2175. [Google Scholar] [CrossRef]
- Hideaki, K.; Masayoshi, K.; Shigeru, S.; Nobuyasu, M.; Toshio, S. Oral administration of peptides derived from bonito bowels decreases blood pressure in spontaneously hypertensive rats by inhibiting angiotensin converting enzyme. Comp. Biochem. Physiol. Part C: Comp. Pharmacol. 1993, 104, 351–353. [Google Scholar] [CrossRef]
- Nakao, R.; Shen, W.; Shimajiri, Y.; Kainou, K.; Sato, Y.; Ulla, A.; Ohnishi, K.; Ninomiya, M.; Ohno, A.; Uchida, T.; et al. Oral intake of rice overexpressing ubiquitin ligase inhibitory pentapeptide prevents atrophy in denervated skeletal muscle. NPJ Sci. Food 2021, 5, 25. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins: An update. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Beverly, R.L.; Underwood, M.A.; Dallas, D.C. Peptidomics analysis of milk protein-derived peptides released over time in the preterm infant stomach. J. Proteome Res. 2019, 18, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Underwood, M.A.; Dallas, D.C. Release of functional peptides from mother’s milk and fortifier proteins in the premature infant stomach. PLoS ONE 2018, 13, e0208204. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Dallas, D.C. Peptides Released from Foremilk and Hindmilk Proteins by Breast Milk Proteases Are Highly Similar. Front. Nutr. 2017, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins—Mechanisms of action. J. Nutr. Biochem. 2014, 25, 503–514. [Google Scholar] [CrossRef]
- Brunetta, H.S.; de Paula, G.C.; de Oliveira, J.; Martins, E.L.; Dos Santos, G.J.; Galina, A.; Rafacho, A.; de Bem, A.F.; Nunes, E.A. Decrement in resting and insulin-stimulated soleus muscle mitochondrial respiration is an early event in diet-induced obesity in mice. Exp. Physiol. 2019, 104, 306–321. [Google Scholar] [CrossRef]
- Brunetta, H.S.; de Paula, G.C.; Fritzen, M.; Cecchini, M.S.; Santos, G.J.d.; Nazari, E.M.; Rafacho, A.; de Bem, A.F.; Nunes, E.A. Leucine increases muscle mitochondrial respiration and attenuates glucose intolerance in diet-induced obesity in Swiss mice. J. Funct. Foods 2019, 62, 103544. [Google Scholar] [CrossRef]
- Parada, C.A.; de Oliveira, I.P.; Gewehr, M.C.F.; Machado-Neto, J.A.; Lima, K.; Eichler, R.A.S.; Lopes, L.R.; Bechara, L.R.G.; Ferreira, J.C.B.; Festuccia, W.T.; et al. Effect of FKBP12-Derived Intracellular Peptides on Rapamycin-Induced FKBP-FRB Interaction and Autophagy. Cells 2022, 11, 385. [Google Scholar] [CrossRef]
- Giozzet, V.A.; Rafacho, A.; Boschero, A.C.; Carneiro, E.M.; Bosqueiro, J.R. Dexamethasone treatment in vivo counteracts the functional pancreatic islet alterations caused by malnourishment in rats. Metabolism 2008, 57, 617–624. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves-Neto, L.M.; Ferreira, F.B.; Souza, L.; Santos, C.d.; Boschero, A.C.; Facundo, V.A.; Santos, A.R.; Nunes, E.A.; Rafacho, A. Disruption of glucose tolerance caused by glucocorticoid excess in rats is partially prevented, but not attenuated, by arjunolic acid. Indian J. Exp. Biol. 2014, 52, 972–982. [Google Scholar] [PubMed]
- Santos, N.B.D.; Franco, R.D.; Camarini, R.; Munhoz, C.D.; Eichler, R.A.S.; Gewehr, M.C.F.; Reckziegel, P.; Llanos, R.P.; Dale, C.S.; Silva, V.; et al. Thimet Oligopeptidase (EC 3.4.24.15) Key Functions Suggested by Knockout Mice Phenotype Characterization. Biomolecules 2019, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, K., II. Mathematical contributions to the theory of evolution. II. Skew variation in homogeneous material. Proc. R. Soc. Lond. 1985, 57, 257–260. [Google Scholar]
- Brown, G.T.; Kleiner, D.E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016, 65, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Kalinovich, A.V.; de Jong, J.M.; Cannon, B.; Nedergaard, J. UCP1 in adipose tissues: Two steps to full browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef]
- Kim, S.H.; Plutzky, J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab. J. 2016, 40, 12–21. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. The therapeutic potential of brown adipose tissue. Hepatobiliary Surg. Nutr. 2013, 2, 286. [Google Scholar]
- Loyd, C.; Obici, S. Brown fat fuel use and regulation of energy homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 368–372. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Luong, Q.; Huang, J.; Lee, K.Y. Deciphering white adipose tissue heterogeneity. Biology 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, C. Quantification of UCP1 function in human brown adipose tissue. Adipocyte 2017, 6, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedoe, P.P.; Hoeke, G.; Kooijman, S.; Dijk, W.; Buijs, J.T.; Kersten, S.; Havekes, L.M.; Hiemstra, P.S.; Berbée, J.F.; Boon, M.R. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J. Lipid Res. 2015, 56, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 919–933. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, T.; Nicoloro, S.M.; Zhou, Z.; Shen, Y.; Liu, J.; Coffey, N.J.; Cinar, R.; Godlewski, G.; Gao, B.; Aouadi, M. Decreasing CB1 receptor signaling in Kupffer cells improves insulin sensitivity in obese mice. Mol. Metab. 2017, 6, 1517–1528. [Google Scholar] [CrossRef]
- Dinh, C.H.; Szabo, A.; Yu, Y.; Camer, D.; Wang, H.; Huang, X.F. Bardoxolone Methyl Prevents Mesenteric Fat Deposition and Inflammation in High-Fat Diet Mice. Sci. World J. 2015, 2015, 549352. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Du, Z.; Shu, X.; Zhu, L.; Wu, J.; Gao, Q.; Wang, L.; Chen, N.; Li, Y.; Luo, M.; et al. Role of RAGE in obesity-induced adipose tissue inflammation and insulin resistance. Cell Death Discov. 2021, 7, 305. [Google Scholar] [CrossRef]
- Miao, X.; Wang, Y.; Wang, W.; Lv, X.; Wang, M.; Yin, H. The mAb against adipocyte fatty acid-binding protein 2E4 attenuates the inflammation in the mouse model of high-fat diet-induced obesity via toll-like receptor 4 pathway. Mol. Cell. Endocrinol. 2015, 403, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Shin, H.; Park, J.Y.; Rho, J.G.; Son, D.H.; Kim, K.W.; Seong, J.K.; Yoon, S.H.; Kim, W. A novel peripheral cannabinoid 1 receptor antagonist, AJ5012, improves metabolic outcomes and suppresses adipose tissue inflammation in obese mice. FASEB J. 2019, 33, 4314–4326. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kim, W. Peripheral CB1R as a modulator of metabolic inflammation. FASEB J. 2021, 35, e21232. [Google Scholar] [CrossRef] [PubMed]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Nhieu, J.T.-V.; Belot, M.P.; Zimmer, A.; Even, P. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, S.B.; Hernandez-Ono, A.; Rask-Madsen, C.; Haas, J.T.; Alemán, J.O.; Suzuki, R.; Scapa, E.F.; Agarwal, C.; Carey, M.C.; Stephanopoulos, G. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008, 7, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L. Endocannabinoid activation at hepatic CB 1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Osei-Hyiaman, D.; Liu, J.; Zhou, L.; Godlewski, G.; Harvey-White, J.; Jeong, W.-i.; Bátkai, S.; Marsicano, G.; Lutz, B.; Buettner, C. Hepatic CB 1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008, 118, 3160–3169. [Google Scholar] [CrossRef]
- Azar, F.; Courtet, K.; Dekky, B.; Bonnier, D.; Dameron, O.; Colige, A.; Legagneux, V.; Theret, N. Integration of miRNA-regulatory networks in hepatic stellate cells identifies TIMP3 as a key factor in chronic liver disease. Liver Int. 2020, 40, 2021–2033. [Google Scholar] [CrossRef]
- Jourdan, T.; Djaouti, L.; Demizieux, L.; Gresti, J.; Vergès, B.; Degrace, P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 2010, 59, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, D.; Zhang, F.; Tu, Y.; Xia, Y.; Wang, H.; Zhou, B.; Zhang, Y.; Wu, J.; Gao, X. Liver Patt1 deficiency protects male mice from age-associated but not high-fat diet-induced hepatic steatosis. J. Lipid Res. 2012, 53, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Després, J.-P.; Ross, R.; Boka, G.; Alméras, N.; Lemieux, I. Effect of rimonabant on the high-triglyceride/low–HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: The ADAGIO-Lipids trial. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdan, T.; Demizieux, L.; Gresti, J.; Djaouti, L.; Gaba, L.; Vergès, B.; Degrace, P. Antagonism of peripheral hepatic cannabinoid receptor-1 improves liver lipid metabolism in mice: Evidence from cultured explants. Hepatology 2012, 55, 790–799. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.G.; Kanda, S.; Petersen, M.C.; Jornayvaz, F.R.; Samuel, V.T.; Bhanot, S.; Petersen, K.F.; Jurczak, M.J.; Shulman, G.I. ApoA5 knockdown improves whole-body insulin sensitivity in high-fat-fed mice by reducing ectopic lipid content. J. Lipid Res. 2015, 56, 526–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Schnabl, K.; Gabler, S.-M.; Willershäuser, M.; Reber, J.; Karlas, A.; Laurila, S.; Lahesmaa, M.; u Din, M.; Bast-Habersbrunner, A.; et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell 2018, 175, 1561–1574.e12. [Google Scholar] [CrossRef] [Green Version]
- Laurila, S.; Sun, L.; Lahesmaa, M.; Schnabl, K.; Laitinen, K.; Klén, R.; Li, Y.; Balaz, M.; Wolfrum, C.; Steiger, K.; et al. Secretin activates brown fat and induces satiation. Nat. Metab. 2021, 3, 798–809. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Daniel, H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol. Sci. 2002, 23, 434–440. [Google Scholar] [CrossRef]
- Herrera-Ruiz, D.; Knipp, G.T. Current perspectives on established and putative mammalian oligopeptide transporters. J. Pharm. Sci. 2003, 92, 691–714. [Google Scholar] [CrossRef]
- Hu, Y.; Smith, D.E.; Ma, K.; Jappar, D.; Thomas, W.; Hillgren, K.M. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol. Pharm. 2008, 5, 1122–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandsch, M. Drug transport via the intestinal peptide transporter PepT1. Curr. Opin. Pharmacol. 2013, 13, 881–887. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, B.G.; Albericio, F. Peptide Therapeutics 2.0; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- De la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2020, 25, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ingredients | SD | HFD | ||
|---|---|---|---|---|
| g/Kg | kcal | g/Kg | Kcal | |
| Maize starch | 415 | 1660 | 14.3 | 57.2 |
| Soybean meal | 305 | 1281 | 410 | 1722 |
| Sucrose | 80 | 320 | 80 | 320 |
| Maltodextrin | 70 | 280 | 70 | 280 |
| Lard | 0 | 0 | 302 | 2718 |
| Soy oil | 0 | 0 | 0 | 0 |
| Soy fatty acid | 50 | 350 | 50 | 350 |
| Cellulose | 31.7 | 0 | 25.4 | 0 |
| L-cysteine | 1.8 | 7.2 | 1.8 | 7.2 |
| Choline chloride | 1.5 | 0 | 1.5 | 0 |
| Butylhydroxytoluene | 0.014 | 0 | 0.028 | 0 |
| Vitamin’s mix | 10.0 | 40 | 10 | 40 |
| Total | - | 3938 | - | 5494 |
| SD | SD-P | HFD | HFD-P | |
|---|---|---|---|---|
| Absolute Mass (g) | ||||
| Inguinal adipose tissue | 0.93 ± 0.07 | 0.71 ± 0.10 | 1.99 ± 0.15 a | 2.13 ± 0.16 b |
| Epididymal adipose tissue | 1.66 ± 0.11 | 1.32 ± 0.15 | 3.48 ± 0.19 a | 3.14 ± 0.18 b |
| Mesenteric adipose tissue | 1.18 ± 0.05 | 0.75 ± 0.07 * | 2.71 ± 0.18 a | 2.77 ± 0.25 b |
| Retroperitoneal adipose tissue | 1.29 ± 0.07 | 0.89 ± 0.14 * | 2.10 ± 0.16 a | 2.13 ± 0.13 b |
| Brown adipose tissue | 0.29 ± 0.01 | 0.20 ± 0.02 * | 0.40 ± 0.02 a | 0.45 ± 0.03 b |
| Liver | 1.76 ± 0.05 | 1.64 ± 0.06 | 2.46 ± 0.14 a | 2.46 ± 0.16 b |
| Pancreas | 0.31 ± 0.01 | 0.29 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.02 |
| Heart | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| Muscle | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.23 ± 0.01 |
| Relative Mass (%) | ||||
| Inguinal adipose tissue | 1.79 ± 0.14 | 1.44 ± 0.20 * | 2.93 ± 0.16 | 3.22 ± 0.18 ##, b |
| Epididymal adipose tissue | 3.17 ± 0.21 | 2.68 ± 0.28 * | 5.19 ± 0.23 | 4.84 ± 0.26 ##, b |
| Mesenteric adipose tissue | 2.26 ± 0.09 | 1.54 ± 0.12 ** | 4.00 ± 0.19 | 4.18 ± 0.30 b |
| Retroperitoneal adipose tissue | 2.46 ± 0.12 | 1.80 ± 0.27 ** | 3.10 ± 0.19 | 3.24 ± 0.16 #, b |
| Brown adipose tissue | 0.56 ± 0.03 | 0.42 ± 0.05 * | 0.59 ± 0.03 | 0.69 ± 0.04 ##, b |
| Liver | 3.37 ± 0.06 | 3.39 ± 0.10 | 3.66 ± 0.16 | 3.76 ± 0.18 |
| Pancreas | 0.59 ± 0.03 | 0.61 ± 0.03 a | 0.40 ± 0.02 a | 0.41 ± 0.03 b |
| Heart | 0.31 ± 0.01 | 0.33 ± 0.01 * | 0.26 ± 0.01 a | 0.26 ± 0.01 b |
| Muscle | 0.48 ± 0.01 | 0.52 ± 0.01 * | 0.36 ± 0.02 a | 0.35 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvério, R.; Barth, R.; Heimann, A.S.; Reckziegel, P.; dos Santos, G.J.; Romero-Zerbo, S.Y.; Bermúdez-Silva, F.J.; Rafacho, A.; Ferro, E.S. Pep19 Has a Positive Effect on Insulin Sensitivity and Ameliorates Both Hepatic and Adipose Tissue Phenotype of Diet-Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 4082. https://doi.org/10.3390/ijms23084082
Silvério R, Barth R, Heimann AS, Reckziegel P, dos Santos GJ, Romero-Zerbo SY, Bermúdez-Silva FJ, Rafacho A, Ferro ES. Pep19 Has a Positive Effect on Insulin Sensitivity and Ameliorates Both Hepatic and Adipose Tissue Phenotype of Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2022; 23(8):4082. https://doi.org/10.3390/ijms23084082
Chicago/Turabian StyleSilvério, Renata, Robson Barth, Andrea S. Heimann, Patrícia Reckziegel, Gustavo J. dos Santos, Silvana Y. Romero-Zerbo, Francisco J. Bermúdez-Silva, Alex Rafacho, and Emer S. Ferro. 2022. "Pep19 Has a Positive Effect on Insulin Sensitivity and Ameliorates Both Hepatic and Adipose Tissue Phenotype of Diet-Induced Obese Mice" International Journal of Molecular Sciences 23, no. 8: 4082. https://doi.org/10.3390/ijms23084082
APA StyleSilvério, R., Barth, R., Heimann, A. S., Reckziegel, P., dos Santos, G. J., Romero-Zerbo, S. Y., Bermúdez-Silva, F. J., Rafacho, A., & Ferro, E. S. (2022). Pep19 Has a Positive Effect on Insulin Sensitivity and Ameliorates Both Hepatic and Adipose Tissue Phenotype of Diet-Induced Obese Mice. International Journal of Molecular Sciences, 23(8), 4082. https://doi.org/10.3390/ijms23084082







