Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia
Abstract
:1. Introduction
2. Results
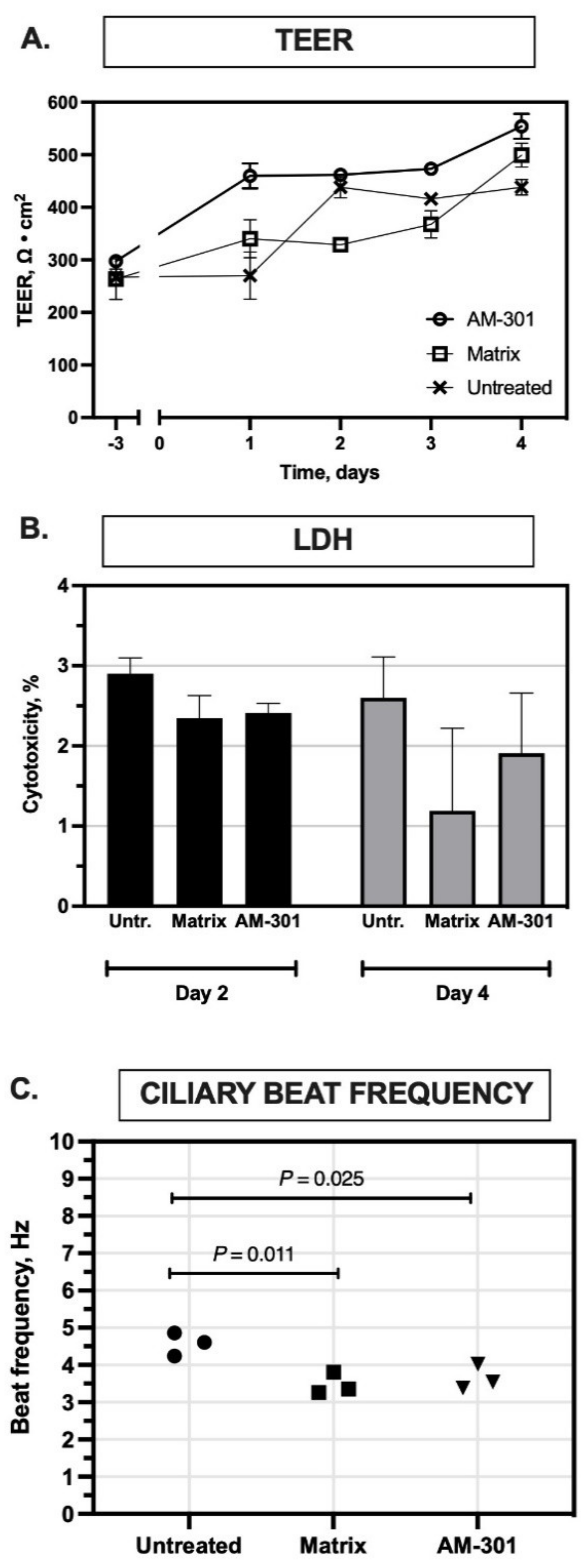
2.1. In Vitro Safety
2.2. In Vitro Efficacy
2.2.1. Pre-Viral Load (Prophylactic) Efficacy
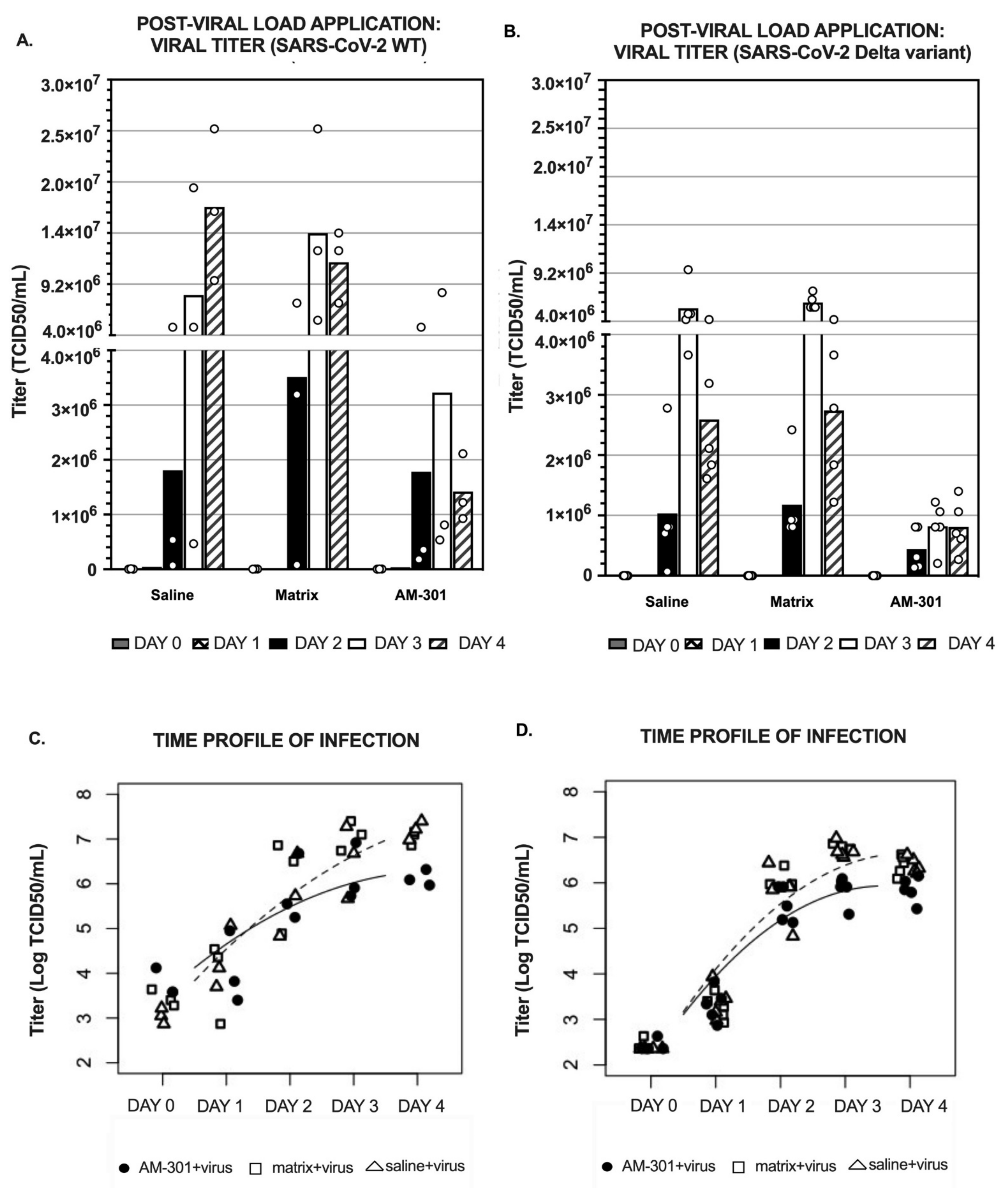
2.2.2. Post-Viral Load (Mitigation) Efficacy
3. Discussion
4. Materials and Methods
4.1. Virus and Cell Cultures
4.2. Nasal Spray Formulation
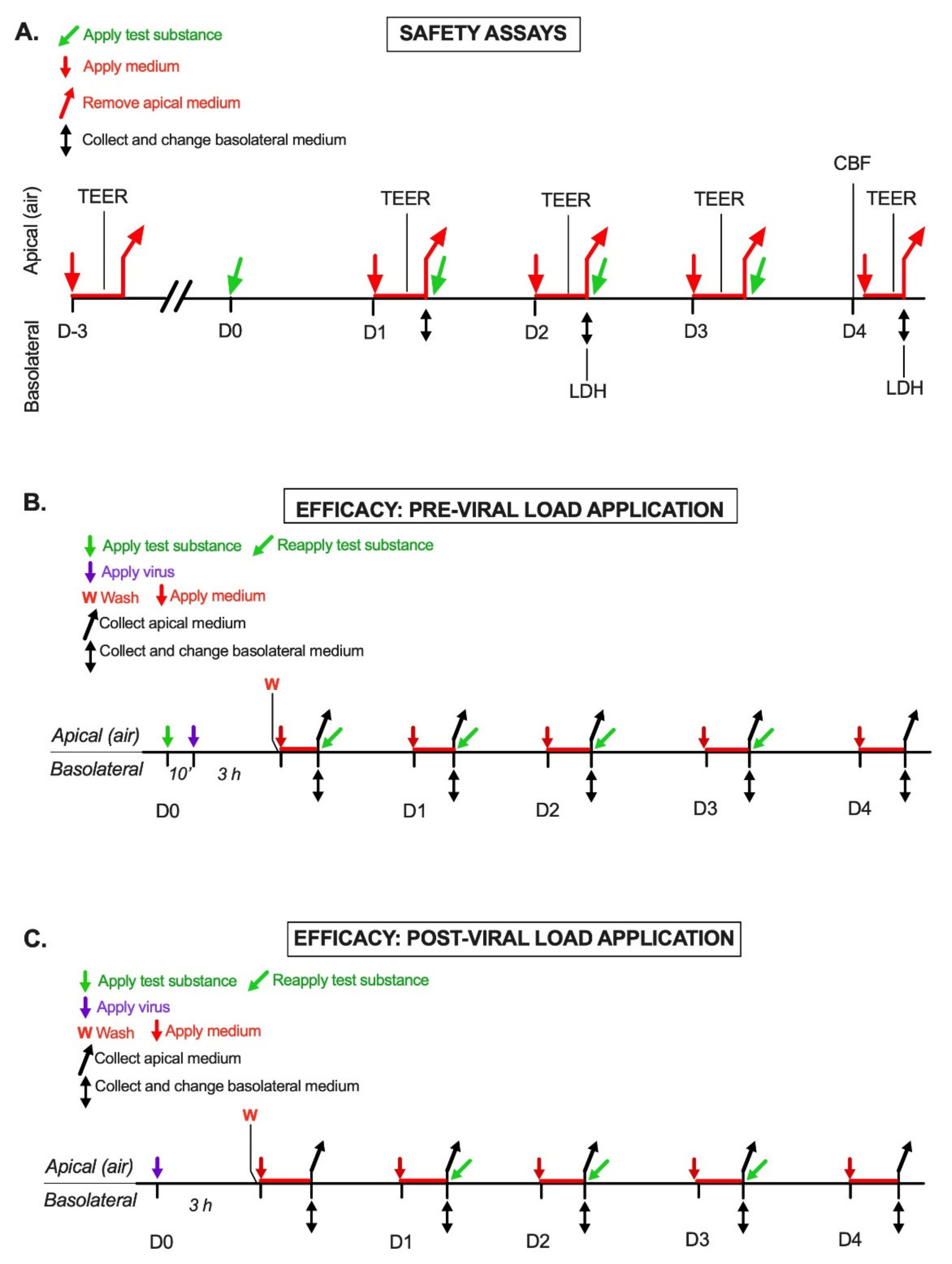
4.3. Safety Assay Design
4.3.1. Transepithelial Electric Resistance (TEER) Assay
4.3.2. Lactate Dehydrogenase (LDH) Assay
4.3.3. Cilia Beating Frequency (CBF) Assay
4.4. Efficacy Assays Design
4.4.1. Pre-Viral Load Protocol
- (1)
- Viral sampling: 300 μL of the medium was applied to the apical surface. After 20 min at 34 °C, the conditioned apical medium was collected and stored at −70 °C until analysis.
- (2)
- Medium change: Inserts were transferred to a new 24-well plate with 500 μL/well fresh medium. The conditioned basolateral medium was collected and stored at −70 °C for possible future analyses.
- (3)
- Repeat treatment: The apical surface was treated with a product or left untreated, as described above, and the inserts were returned to the 34 °C incubator.
4.4.2. Post-Viral Load Protocol
4.5. Viral Titer Assay
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pifarre, I.A.H.; Acosta, E.; Lopez-Casasnovas, G.; Lo, A.; Nicodemo, C.; Riffe, T.; Myrskyla, M. Years of life lost to COVID-19 in 81 countries. Sci. Rep. 2021, 11, 3504. [Google Scholar] [CrossRef] [PubMed]
- Aristodemou, K.; Buchhass, L.; Claringbould, D. The COVID-19 crisis in the EU: The resilience of healthcare systems, government responses and their socio-economic effects. Eurasian Econ. Rev. 2021, 11, 251–281. [Google Scholar] [CrossRef]
- Kerlin, M.P.; Costa, D.K.; Davis, B.S.; Admon, A.J.; Vranas, K.C.; Kahn, J.M. Actions Taken by US Hospitals to Prepare for Increased Demand for Intensive Care During the First Wave of COVID-19: A National Survey. Chest 2021, 160, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Verelst, F.; Kuylen, E.; Beutels, P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID-19) cases, March 2020. Eur. Surveill. 2020, 25, 2000323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeri, N.C.; Shrestha, N.; Rahman, M.S.; Zaki, R.; Tan, Z.; Bibi, S.; Baghbanzadeh, M.; Aghamohammadi, N.; Zhang, W.; Haque, U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020, 49, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Morgenstern, C.; Kelly, J.; Lowe, R.; CMMID COVID-19 Working Group; Jit, M. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021, 19, 40. [Google Scholar] [CrossRef]
- Bo, Y.; Guo, C.; Lin, C.; Zeng, Y.; Li, H.B.; Zhang, Y.; Hossain, M.S.; Chan, J.W.M.; Yeung, D.W.; Kwok, K.O.; et al. Effectiveness of non-pharmaceutical interventions on COVID-19 transmission in 190 countries from 23 January to 13 April 2020. Int. J. Infect. Dis. 2021, 102, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Mak, T.M.; Cui, L.; Toh, M.; Lim, Y.D.; Lee, P.H.; Lee, T.H.; Chia, P.Y.; et al. Clinical and virological features of SARS-CoV-2 variants of concern: A retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin. Infect. Dis. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Tang, J.W.; Bahnfleth, W.P.; Bluyssen, P.M.; Buonanno, G.; Jimenez, J.L.; Kurnitski, J.; Li, Y.; Miller, S.; Sekhar, C.; Morawska, L.; et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Hosp. Infect. 2021, 110, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Sikkema, L.; Waghray, A.; Heimberg, G.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smillie, C.; et al. Human Cell Atlas Lung Biological, N. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 2021, 27, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, J.; Hong, S.P.; Choi, S.Y.; Yang, M.J.; Ju, Y.S.; Kim, Y.T.; Kim, H.M.; Rahman, M.D.T.; Chung, M.K.; et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Gallo, O.; Locatello, L.G.; Mazzoni, A.; Novelli, L.; Annunziato, F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021, 14, 305–316. [Google Scholar] [CrossRef]
- Neant, N.; Lingas, G.; Le Hingrat, Q.; Ghosn, J.; Engelmann, I.; Lepiller, Q.; Gaymard, A.; Ferre, V.; Hartard, C.; Plantier, J.C.; et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc. Natl. Acad. Sci. USA 2021, 118, e2017962118. [Google Scholar] [CrossRef]
- Killingley, B.; Mann, A.; Kalinova, M.; Boyers, A.; Goonawardane, N.; Zhou, J.; Lindsell, K.; Hare, S.S.; Brown, J.; Frise, R.; et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge. Nat. Portf. J. 2022. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, A.L.; Popescu, S.V. SARS-CoV-2 transmission without symptoms. Science 2021, 371, 1206–1207. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Suman, J.D. Current understanding of nasal morphology and physiology as a drug delivery target. Drug Deliv. Transl. Res. 2013, 3, 4–15. [Google Scholar] [CrossRef]
- Froba, M.; Grosse, M.; Setz, C.; Rauch, P.; Auth, J.; Spanaus, L.; Munch, J.; Ruetalo, N.; Schindler, M.; Morokutti-Kurz, M.; et al. Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta. Int. J. Mol. Sci. 2021, 22, 13202. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.J.; Sarr, A.B.; Grant, P.G.; Phillips, T.D.; Woode, G.N. In vitro studies on the use of clay, clay minerals and charcoal to adsorb bovine rotavirus and bovine coronavirus. Vet. Microbiol. 1998, 63, 137–146. [Google Scholar] [CrossRef]
- Bellou, M.I.; Syngouna, V.I.; Tselepi, M.A.; Kokkinos, P.A.; Paparrodopoulos, S.C.; Vantarakis, A.; Chrysikopoulos, C.V. Interaction of human adenoviruses and coliphages with kaolinite and bentonite. Sci. Total Environ. 2015, 517, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Cromeans, T. Effects of bentonite clay solids on poliovirus concentration from water by microporous filter methods. Appl. Environ. Microbiol. 1985, 49, 795–798. [Google Scholar] [CrossRef] [Green Version]
- Kelessidis, V.C. Investigations on the thixotropy of bentonite suspensions. Energy Sources Part A Recovery Util. Environ. Effects. 2008, 30, 1729–1746. [Google Scholar] [CrossRef]
- Farsani, S.M.; Deijs, M.; Dijkman, R.; Molenkamp, R.; Jeeninga, R.E.; Ieven, M.; Goossens, H.; van der Hoek, L. Culturing of respiratory viruses in well-differentiated pseudostratified human airway epithelium as a tool to detect unknown viruses. Influenza Other Respir. Viruses 2015, 9, 51–57. [Google Scholar] [CrossRef]
- Juozapaitis, M.; Aguiar Moreira, E.; Mena, I.; Giese, S.; Riegger, D.; Pohlmann, A.; Hoper, D.; Zimmer, G.; Beer, M.; Garcia-Sastre, A.; et al. An infectious bat-derived chimeric influenza virus harbouring the entry machinery of an influenza A virus. Nat. Commun. 2014, 5, 4448. [Google Scholar] [CrossRef] [Green Version]
- Tapparel, C.; Sobo, K.; Constant, S.; Huang, S.; Van Belle, S.; Kaiser, L. Growth and characterization of different human rhinovirus C types in three-dimensional human airway epithelia reconstituted in vitro. Virology 2013, 446, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Padey, B.; Julien, T.; Trouillet-Assant, S.; Traversier, A.; Errazuriz-Cerda, E.; Fouret, J.; Dubois, J.; Gaymard, A.; Lescure, F.X.; et al. Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia. Cell Rep. Med. 2020, 1, 100059. [Google Scholar] [CrossRef] [PubMed]
- Nehrig, J.; Grosse, N.; Hohenfeld, I.P.; Fais, F.; Meyer, T.; Hohlfeld, J.M.; Badorrek, P. Efficacy and safety of a drug-free, barrier-forming nasal spray for allergic rhinitis: Randomized, open-label, crossover noninferiority trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Gallagher, C. Extending the Linear Model With R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. J. Am. Stat. Assoc. 2007, 102, 1477. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Froba, M.; Graf, P.; Grosse, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Eccles, R. Iota-Carrageenan as an Antiviral Treatment for the Common Cold. Open Virol. J. 2020, 14, 9–15. [Google Scholar] [CrossRef]
- Witten, J.; Ribbeck, K. The particle in the spider’s web: Transport through biological hydrogels. Nanoscale 2017, 9, 8080–8095. [Google Scholar] [CrossRef] [Green Version]
- Khediri, F.; Mrad, A.I.; Azzouz, M.; Doughi, H.; Najjar, T.; Mathiex-Fortunet, H.; Garnier, P.; Cortot, A. Efficacy of diosmectite (smecta) in the treatment of acute watery diarrhoea in adults: A multicentre, randomized, double-blind, placebo-controlled, parallel group study. Gastroenterol. Res. Pract. 2011, 2011, 783196. [Google Scholar] [CrossRef] [Green Version]
- Syngouna, V.I.; Chrysikopoulos, C.V. Interaction between viruses and clays in static and dynamic batch systems. Environ. Sci. Technol. 2010, 44, 4539–4544. [Google Scholar] [CrossRef]
- Dunn, D.B.; Hitchborn, J.H. The Use of Bentonite in the Purification of Plant Viruses. Virology 1965, 25, 171–192. [Google Scholar] [CrossRef]
- Lipson, S.M.; Stotzky, G. Specificity of virus adsorption to clay minerals. Can. J. Microbiol. 1985, 31, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Maxim, L.D.; Niebo, R.; McConnell, E.E. Bentonite toxicology and epidemiology—A review. Inhal. Toxicol. 2016, 28, 591–617. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, M.; Vennemann, A.; Wohlleben, W. Lung Toxicity Analysis of Nano-Sized Kaolin and Bentonite: Missing Indications for a Common Grouping. Nanomaterials 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.S. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef]
- Dimitri-Pinheiro, S.; Soares, R.; Barata, P. The Microbiome of the Nose-Friend or Foe? Allergy Rhinol. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, N.S.; Pinski, A.N.; Monsibais, A.N.; Jankeel, A.; Doratt, B.M.; Cinco, I.R.; Ibraim, I.; Messaoudi, I. Acute SARS-CoV-2 infection is associated with an increased abundance of bacterial pathogens, including Pseudomonas aeruginosa in the nose. Cell Rep. 2021, 36, 109637. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Herrera, D.; Serrano, J.; Roldan, S.; Sanz, M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin. Oral. Investig. 2020, 24, 2925–2930. [Google Scholar] [CrossRef]
- Huang, N.; Perez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef]
- Papineni, R.S.; Rosenthal, F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol. Med. 1997, 10, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fevre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife-Livestock-Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, B.; Benaoudia, S.; Huang, S.; Bonfante, R.; Wiszniewski, L.; Tseligka, E.D.; Tapparel, C.; Constant, S. Antiviral drug screening by assessing epithelial functions and innate immune responses in human 3D airway epithelium model. Antiviral. Res. 2018, 156, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Capobianchi, M.R.; Rueca, M.; Messina, F.; Giombini, E.; Carletti, F.; Colavita, F.; Castilletti, C.; Lalle, E.; Bordi, L.; Vairo, F.; et al. Molecular characterization of SARS-CoV-2 from the first case of COVID-19 in Italy. Clin. Microbiol. Infect. 2020, 26, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wiszniewski, L.; Constant, S.; Roggen, E. Potential of in vitro reconstituted 3D human airway epithelia (MucilAir) to assess respiratory sensitizers. Toxicol. In Vitro 2013, 27, 1151–1156. [Google Scholar] [CrossRef]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Constant, S.; Perek, N.; Boudard, D.; Delavenne, X. Pharmacological characterization of the 3D MucilAir nasal model. Eur. J. Pharm. Biopharm. 2019, 139, 186–196. [Google Scholar] [CrossRef]
- Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. The R Project for Statistical Computing. 2018. Available online: https://www.R-project.org (accessed on 31 January 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fais, F.; Juskeviciene, R.; Francardo, V.; Mateos, S.; Guyard, M.; Viollet, C.; Constant, S.; Borelli, M.; Hohenfeld, I.P. Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia. Int. J. Mol. Sci. 2022, 23, 4062. https://doi.org/10.3390/ijms23074062
Fais F, Juskeviciene R, Francardo V, Mateos S, Guyard M, Viollet C, Constant S, Borelli M, Hohenfeld IP. Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia. International Journal of Molecular Sciences. 2022; 23(7):4062. https://doi.org/10.3390/ijms23074062
Chicago/Turabian StyleFais, Fabio, Reda Juskeviciene, Veronica Francardo, Stéphanie Mateos, Manuela Guyard, Cécile Viollet, Samuel Constant, Massimo Borelli, and Ilja P. Hohenfeld. 2022. "Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia" International Journal of Molecular Sciences 23, no. 7: 4062. https://doi.org/10.3390/ijms23074062
APA StyleFais, F., Juskeviciene, R., Francardo, V., Mateos, S., Guyard, M., Viollet, C., Constant, S., Borelli, M., & Hohenfeld, I. P. (2022). Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia. International Journal of Molecular Sciences, 23(7), 4062. https://doi.org/10.3390/ijms23074062







