The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review
Abstract
1. Introduction
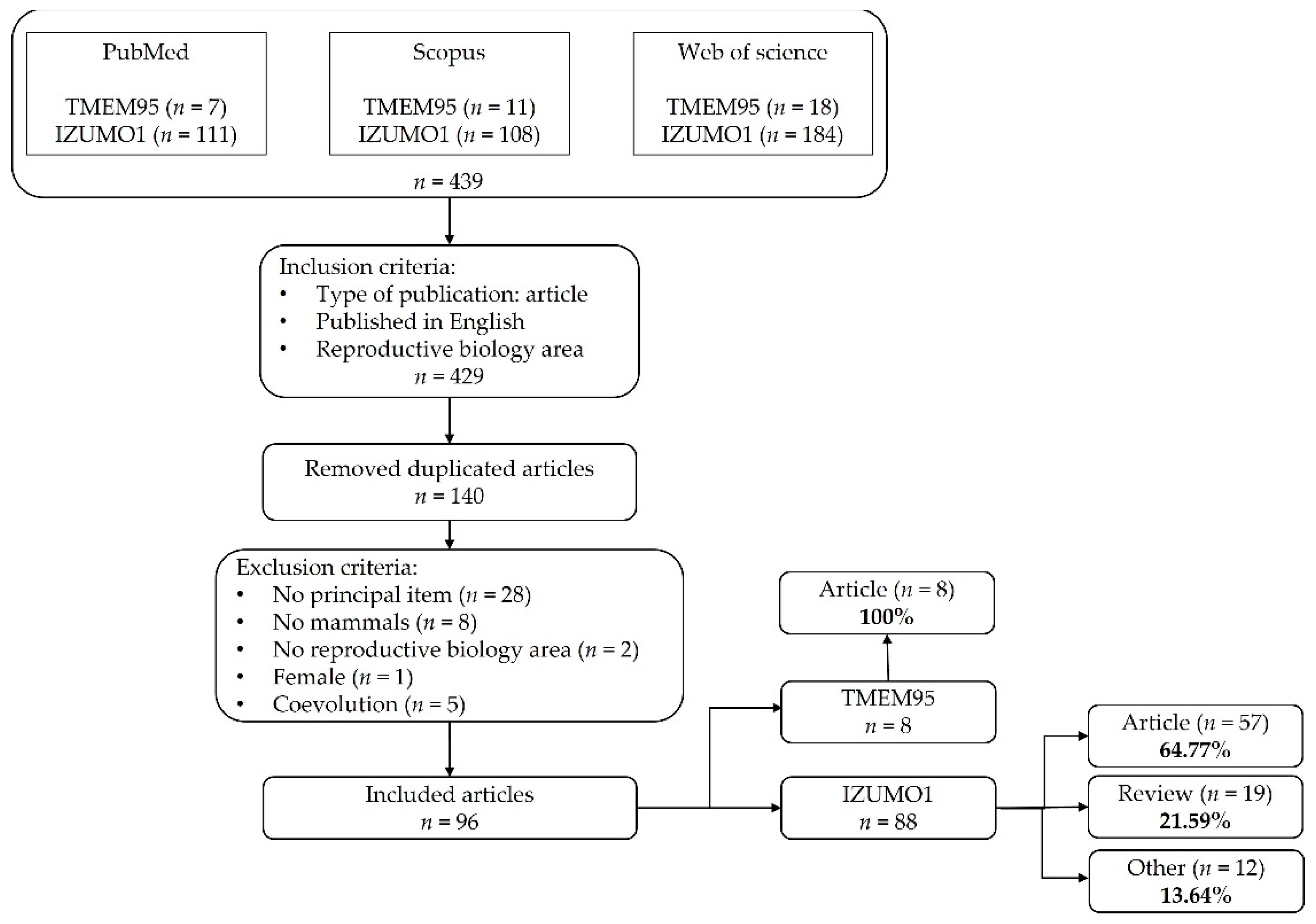
2. Materials and Methods
2.1. Search Strategy and Information Processing
2.2. Selection of Relevant Studies and Data Analysis
3. Results and Discussion
3.1. Compilation of Relevant Bibliographic Sources
3.2. Bibliometric Analysis
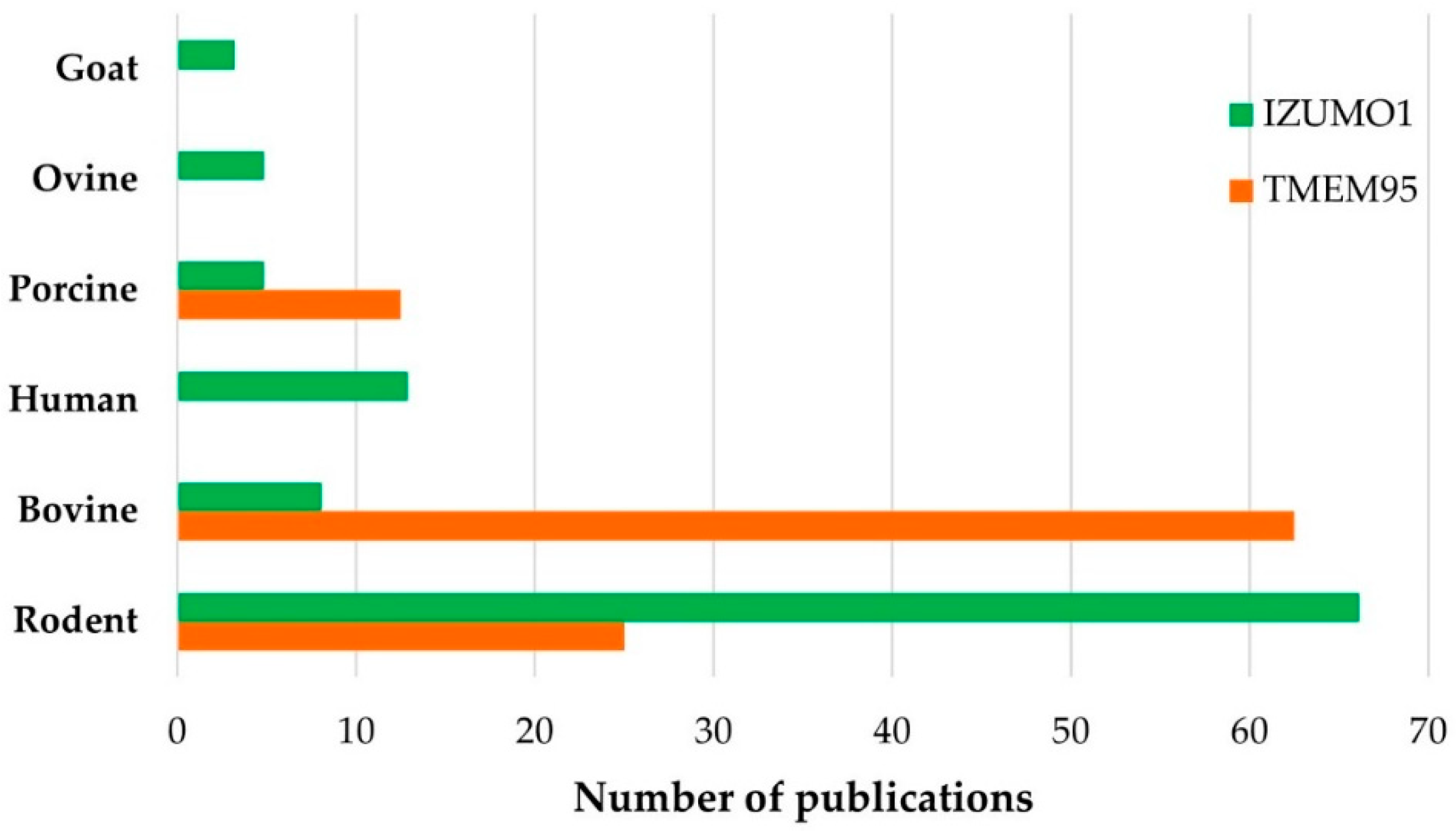
3.3. Bibliographical Analysis
3.4. Analysis of TMEM95
3.5. Analysis of IZUMO1
3.5.1. Characterization and Localization of IZUMO1
| Ref. | Specie | Age | Sample | Main Findings |
|---|---|---|---|---|
| [16] | Human | n.d. | PS |
|
| [39] | Mouse Rat Hamster | Adult | CE |
|
| [40] | Bovine a (n = 15) | >1 year | E EF |
|
| [41] | Mouse b | >12 weeks | CE VD |
|
| [43] | Mouse c | 10–12 weeks | Distal regions of CE |
|
| [47] | Wistar rat | 8–10 weeks | E |
|
| [48] | Mouse | 8 weeks | WT sperm ΔCyt/ΔCyt sperm |
|
| [49] | Datong yak (n = 16) | 6, 18, 30 and 72 months | Multitissues d |
|
| [50] | Sheep e (n = 760) | 3 years | DNA |
|
| [42] | Piétrain boar (n = 12) | Different sexually mature | EF |
|
| [51] | Ram | n.d. | T E EF |
|
| [52] | Mouse f | Retired male breeders | CE |
|
| [53] | Bovine | n.d. | Multitissues g |
|
| [54] | Sheep (n = 5) Cashmere goat (n = 6) | 12 months | T |
|
| [55] | Boar h | n.d. | Sperm Multitissues i |
|
| [56] | Mouse | >8 weeks | CE |
|
| [57] | Mouse | n.d. | Sperm j |
|
| [58] | Cashmere goat | n.d. | EF |
|
| [59] | Mouse k | >8 weeks | Sperm from transgenic N204Q-IZUMO |
|
| [60] | Mouse | 9–16 weeks | Sperm |
|
| [61] | Human (n = 20) | n.d. | Sperm from healthy men |
|
3.5.2. Role of IZUMO1 in Mammalian Fertilization
| Ref. | Specie | Age (Weeks) | Sample | Main Findings |
|---|---|---|---|---|
| [8] | Human Mouse Boar | n.d. | PS |
|
| [18] | Mouse | n.d. | E |
|
| [33] | Human | n.d. | Sperm a |
|
| [34] | Mouse | 8 | CE |
|
| [78] | Mouse | 8–10 | CE VD |
|
| [80] | Mouse b | 12–24 | CE |
|
| [81] | Mouse | >8 | Sperm c |
|
| [82] | Mouse b | 12–14 | CE |
|
| [83] | Human | n.d. | PS |
|
| [84] | Boar d | n.d. | E |
|
| [85] | Mouse | >10–12 | CE VD |
|
4. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austin, C.R. Scientific and Clinical Aspects of Fertilization and Implantation. Proc. R. Soc. Med. 1974, 67, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Bernecic, N.C.; Gadella, B.M.; Leahy, T.; de Graaf, S.P. Novel methods to detect capacitation-related changes in spermatozoa. Theriogenology 2019, 137, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Florman, H.M.; Fissore, R.A. Fertilization in Mammals. In Knobil and Neill’s Physiology of Reproduction: Two-Volume Set; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 1, pp. 149–196. ISBN 9780123977694. [Google Scholar]
- Kay, V.; Robertson, L. Hyperactivated motility of human spermatozoa: A review of physiological function and application in assisted reproduction. Hum. Reprod. Update 1998, 4, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Bhakta, H.H.; Refai, F.H.; Avella, M.A. The molecular mechanisms mediating mammalian fertilization. Development 2019, 146, dev176966. [Google Scholar] [CrossRef]
- Bianchi, E.; Wright, G.J. Find and fuse: Unsolved mysteries in sperm-egg recognition. PLoS Biol. 2020, 18, e3000953. [Google Scholar] [CrossRef]
- Bianchi, E.; Wright, G.J. Cross-species fertilization: The hamster egg receptor, Juno, binds the human sperm ligand, Izumo1. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140101. [Google Scholar] [CrossRef]
- Yanagimachi, R. Zona-free hamster eggs: Their use in assessing fertilizing capacity and examining chromosomes of human spermatozoa. Gamete Res. 1984, 10, 187–232. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef]
- Inoue, N. Novel insights into the molecular mechanism of sperm–egg fusion via IZUMO1. J. Plant Res. 2017, 130, 475–478. [Google Scholar] [CrossRef]
- Lorenzetti, D.; Poirier, C.; Zhao, M.; Overbeek, P.A.; Harrison, W.; Bishop, C.E. A transgenic insertion on mouse chromosome 17 inactivates a novel immunoglobulin superfamily gene potentially involved in sperm-egg fusion. Mamm. Genome 2014, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Oda, S.; Shikano, T.; Ohnuki, T.; Uematsu, Y.; Sakagami, J.; Tada, N.; Miyazaki, S.; Kudo, A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 2000, 24, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Ziyyat, A.; Prenant, M.; Wrobel, E.; Wolf, J.P.; Levy, S.; Le Naour, F.; Boucheix, C. Reduced fertility of female mice lacking CD81. Dev. Biol. 2006, 290, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef]
- Aydin, H.; Sultana, A.; Li, S.; Thavalingam, A.; Lee, J.E. Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 2016, 534, 562–565. [Google Scholar] [CrossRef]
- Ohto, U.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Inoue, N.; Shimizu, T. Structure of IZUMO1–JUNO reveals sperm–oocyte recognition during mammalian fertilization. Nature 2016, 534, 566–569. [Google Scholar] [CrossRef]
- Satouh, Y.; Inoue, N.; Ikawa, M.; Okabe, M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012, 125, 4985–4990. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Okabe, M. The mechanism of sperm-egg interaction and the involvement of IZUMO1 in fusion. Asian J. Androl. 2011, 13, 81–87. [Google Scholar] [CrossRef]
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef]
- Fujihara, Y.; Lu, Y.; Noda, T.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Yu, Z.; Matzuk, R.M.; Matzuk, M.M.; Ikawa, M. Spermatozoa lacking Fertilization Influencing Membrane Protein (FIMP) fail to fuse with oocytes in mice. Dev. Biol. 2020, 117, 9393–9400. [Google Scholar] [CrossRef]
- Lamas-Toranzo, I.; Hamze, J.G.; Bianchi, E.; Fernández-Fuertes, B.; Pérez-Cerezales, S.; Laguna-Barraza, R.; Fernández-González, R.; Lonergan, P.; Gutiérrez-Adán, A.; Wright, G.J.; et al. TMEM95 is a sperm membrane protein essential for mammalian fertilization. eLife 2020, 9, e53913. [Google Scholar] [CrossRef] [PubMed]
- Pausch, H.; Kölle, S.; Wurmser, C.; Schwarzenbacher, H.; Emmerling, R.; Jansen, S.; Trottmann, M.; Fuerst, C.; Götz, K.U.; Fries, R. A Nonsense Mutation in TMEM95 Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle. PLoS Genet. 2014, 10, e1004044. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fuertes, B.; Laguna-Barraza, R.; Fernandez-Gonzalez, R.; Gutierrez-Adan, A.; Blanco-Fernandez, A.; O’Doherty, A.M.; Di Fenza, M.; Kelly, A.K.; Kölle, S.; Lonergan, P. Subfertility in bulls carrying a nonsense mutation in transmembrane protein 95 is due to failure to interact with the oocyte vestments. Biol. Reprod. 2017, 97, 50–60. [Google Scholar] [CrossRef]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of heavy metals on human male fertility—An overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Riquelme, N.; Huerta-Retamal, N.; Gómez-Torres, M.J.; Martínez-Espinosa, R.M. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants 2020, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: Anupdated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar]
- Georgadaki, K.; Khoury, N.; Spandidos, D.A.; Zoumpourlis, V. The molecular basis of fertilization (Review). Int. J. Mol. Med. 2016, 38, 979–986. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, Z.; Li, R.; Wu, H.; Xu, S.; Kang, Y.; Cao, Y.; Chen, X.; Zhu, Y.; Xu, S.; et al. Consultation and treatment behaviour of infertile couples in China: A population-based study. Reprod. Biomed. Online 2019, 38, 917–925. [Google Scholar] [CrossRef]
- Yumura, Y.; Tsujimura, A.; Imamoto, T.; Umemoto, Y.; Kobayashi, H.; Shiraishi, K.; Shin, T.; Taniguchi, H.; Chiba, K.; Miyagawa, Y.; et al. Nationwide survey of urological specialists regarding male infertility: Results from a 2015 questionnaire in Japan. Reprod. Med. Biol. 2018, 17, 44–51. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, H.; Yang, Q.; Shi, T.; Pan, C.; Lei, C.; Dang, R.; Chen, H.; Lan, X. Identification of novel alternative splicing transcript and expression analysis of bovine TMEM95 gene. Gene 2016, 575, 531–536. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S.; Chen, R.; Lv, X.; Pan, C. A novel synonymous SNP (A47A) of the TMEM95 gene is significantly associated with the reproductive traits related to testis in male piglets. Arch. Anim. Breed 2017, 60, 235–241. [Google Scholar] [CrossRef]
- Granados-Gonzalez, V.; Aknin-Seifer, I.; Touraine, R.L.; Chouteau, J.; Wolf, J.P.; Levy, R. Preliminary study on the role of the human IZUMO gene in oocyte–spermatozoa fusion failure. Fertil. Steril. 2008, 90, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hagihara, Y.; Wright, D.; Suzuki, T.; Wada, I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat. Commun. 2015, 6, 8858. [Google Scholar] [CrossRef] [PubMed]
- Shireesha, S.; Sudhakar, K.; Vinoo, R.; Seshaiah, C.V.; Metta, M. Bioinformatic characterization of the Transmembrane protein95 gene (TMEM95) in Murrah buffalo (Bubalus bubalis). Vet. Arh. 2021, 91, 11–18. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, K.; Zhang, G.; Cao, Y.; Zhang, M.; Chen, H.; Lei, C.; Lan, X.; Zhao, Y. Detection of bovine TMEM95 p.Cys161X mutation in 13 Chinese indigenous cattle breeds. Animals 2019, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Kobe, B.; Deisenhofer, J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 1995, 374, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Barbaux, S.; Ialy-Radio, C.; Chalbi, M.; Dybal, E.; Homps-Legrand, M.; Do Cruzeiro, M.; Vaiman, D.; Wolf, J.P.; Ziyyat, A. Sperm SPACA6 protein is required for mammalian Sperm-Egg Adhesion/Fusion. Sci. Rep. 2020, 10, 5535. [Google Scholar] [CrossRef]
- Ellerman, D.; Pei, J.; Gupta, S.; Snell, J.; Myles, D.; Primakoff, P. Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol. Reprod. Dev. 2009, 76, 1188–1199. [Google Scholar] [CrossRef]
- Fukuda, M.; Sakase, M.; Fukushima, M.; Harayama, H. Changes of IZUMO1 in bull spermatozoa during the maturation, acrosome reaction, and cryopreservation. Theriogenology 2016, 86, 2179–2188.e3. [Google Scholar] [CrossRef]
- Saito, T.; Wada, I.; Inoue, N. Alternative splicing of the Izumo1 gene ensures triggering gamete fusion in mice. Sci. Rep. 2019, 9, 3151. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Fernandez-Fuertes, B.; Recuero, S.; Mateo, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Sebkova, N.; Ded, L.; Vesela, K.; Dvorakova-Hortova, K. Progress of sperm IZUMO1 relocation during spontaneous acrosome reaction. Reproduction 2014, 147, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, J.; Miranda, P.V.; Spiridonov, N.A.; Yoon, S.Y.; Fissore, R.A.; Johnson, G.R.; Visconti, P.E. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J. Cell Sci. 2009, 122, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, M.J.; Digilio, L.; Klotz, K.; Shetty, J.; Flickinger, C.J.; Herr, J.C. Equatorial Segment Protein (ESP) Is a Human Alloantigen Involved in Sperm-Egg Binding and Fusion. J. Androl. 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Gaikwad, A.S.; Anderson, A.L.; Merriner, D.J.; O’Connor, A.E.; Houston, B.J.; Aitken, R.J.; O’Bryan, M.K.; Nixon, B. GLIPR1L1 is an IZUMO-binding protein required for optimal fertilization in the mouse. BMC Biol. 2019, 17, 86. [Google Scholar] [CrossRef]
- Baker, M.A.; Hetherington, L.; Weinberg, A.; Naumovski, N.; Velkov, T.; Pelzing, M.; Dolman, S.; Condina, M.R.; Aitken, R.J. Analysis of Phosphopeptide Changes as Spermatozoa Acquire Functional Competence in the Epididymis Demonstrates Changes in the Post-translational Modification of Izumo1. J. Proteome Res. 2012, 11, 5252–5264. [Google Scholar] [CrossRef]
- Young, S.A.M.; Miyata, H.; Satouh, Y.; Muto, M.; Larsen, M.R.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CRISPR/Cas9-mediated mutation revealed cytoplasmic tail is dispensable for IZUMO1 function and male fertility. Reproduction 2016, 152, 665–672. [Google Scholar] [CrossRef]
- Kalwar, Q.; Ding, X.; Ahmad, A.A.; Chu, M.; Wu, X.; Bao, P.; Yan, P. Expression Analysis of IZUMO1 Gene during Testicular Development of Datong Yak (Bos Grunniens). Animals 2019, 9, 292. [Google Scholar] [CrossRef]
- Hu, W.; Dong, X.; Tian, Z.; Zhang, Z.; Tang, J.; Liang, B.; Liu, Q.; Chu, M. Expression, structure and function analysis of the sperm-oocyte fusion genes Juno and Izumo1 in sheep (Ovis aries). J. Anim. Sci. Biotechnol. 2021, 12, 37. [Google Scholar] [CrossRef]
- Gundogan, G.I.; Aktas, A. Immunolocalization of Fertilin β, IZUMO1, and P34H in Ram Spermatozoa. Biopreserv. Biobank. 2021, 19, 470–482. [Google Scholar] [CrossRef]
- Miranda, P.V.; Allaire, A.; Sosnik, J.; Visconti, P.E. Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol. Reprod. 2009, 80, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, E. Molecular cloning and characterization of Izumo1 gene from bovine testis. J. Anim. Sci. Technol. 2015, 57, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xing, W.J.; Han, B.D.; Wu, Q.; Zhao, L.; Bao, X.H.; Bou, S. Molecular cloning and characterization of Izumo1 gene from sheep and cashmere goat reveal alternative splicing. Mol. Biol. Rep. 2011, 38, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, J.S.; Lee, Y.; Song, B.S.; Sim, B.W.; Kim, S.U.; Saitoh, T.; Yazawa, H.; Nunoya, T.; Chang, K.T. Molecular Cloning, Characterization of Porcine IZUMO1, an IgSF Family Member. Reprod. Domest. Anim. 2013, 48, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hamada, D.; Kamikubo, H.; Hirata, K.; Kataoka, M.; Yamamoto, M.; Ikawa, M.; Okabe, M.; Hagihara, Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013, 140, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Wada, I. Monitoring dimeric status of IZUMO1 during the acrosome reaction in living spermatozoon. Cell Cycle 2018, 17, 1279–1285. [Google Scholar] [CrossRef]
- Liu, Z.-D.; Xing, W.-J.; Wang, L.-Q.; Lü, L.-X. Prokaryotic Expression, Ascitic Polyclonal Antibody Preparation and Identification of Cashmere Goat Izumo1. Agric. Sci. China 2010, 9, 605–613. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Okabe, M. Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem. Biophys. Res. Commun. 2008, 377, 910–914. [Google Scholar] [CrossRef]
- Kato, K.; Satouh, Y.; Nishimasu, H.; Kurabayashi, A.; Morita, J.; Fujihara, Y.; Oji, A.; Ishitani, R.; Ikawa, M.; Nureki, O. Structural and functional insights into IZUMO1 recognition by JUNO in mammalian fertilization. Nat. Commun. 2016, 7, 12198. [Google Scholar] [CrossRef]
- Kumar, P.; Wang, M.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Wang, W.; von Brandenstein, M.; Isachenko, V. Unraveling Subcellular and Ultrastructural Changes During Vitrification of Human Spermatozoa: Effect of a Mitochondria-Targeted Antioxidant and a Permeable Cryoprotectant. Front. Cell Dev. Biol. 2021, 9, 672862. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Yamagata, K.; Ikawa, M.; Moss, S.B.; Okabe, M. Aberrant Distribution of ADAM3 in Sperm from Both Angiotensin-Converting Enzyme (Ace)-and Calmegin (Clgn)-Deficient Mice 1. Biol. Reprod. 2006, 75, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Kasahara, T.; Ikawa, M.; Okabe, M. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS ONE 2010, 5, e10301. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.R.; Evans, J.P. Multivariate analysis of male reproductive function in Inpp5b−/− mice reveals heterogeneity in defects in fertility, sperm-egg membrane interaction and proteolytic cleavage of sperm ADAMs. Mol. Hum. Reprod. 2010, 16, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, J.; Buffone, M.G.; Visconti, P.E. Analysis of CAPZA3 localization reveals temporally discrete events during the acrosome reaction. J. Cell. Physiol. 2010, 224, 575–580. [Google Scholar] [CrossRef]
- Nishimura, H.; Gupta, S.; Myles, D.G.; Primakoff, P. Characterization of mouse sperm TMEM190, a small transmembrane protein with the trefoil domain: Evidence for co-localization with IZUMO1 and complex formation with other sperm proteins. Reproduction 2011, 141, 437–451. [Google Scholar] [CrossRef][Green Version]
- Marcello, M.R.; Jia, W.; Leary, J.A.; Moore, K.L.; Evans, J.P. Lack of tyrosylprotein sulfotransferase-2 activity results in altered sperm-egg interactions and loss of ADAM3 and ADAM6 in epididymal sperm. J. Biol. Chem. 2011, 286, 13060–13070. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, L.; Shi, D.S.; Jiang, J.R. Effects of latrunculin A on the relocation of sperm IZUMO1 during gamete interaction in mouse. Mol. Reprod. Dev. 2017, 84, 1183–1190. [Google Scholar] [CrossRef]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Fujimura, L.; Sugiyama, H.; Suganami, A.; Tamura, Y.; Hatano, M.; Miyado, K.; Toshimori, K. Deletion of Eqtn in mice reduces male fertility and sperm-egg adhesion. Reproduction 2018, 156, 579–590. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, J.; Zhang, H.; Wen, Y.; Zhang, H.; Cui, Y.; Tian, J.; Jiang, M.; Liu, X.; Wang, G.; et al. Proteomic Analysis of Dpy19l2-Deficient Human Globozoospermia Reveals Multiple Molecular Defects. Proteom. Clin. Appl. 2019, 13, e1900007. [Google Scholar] [CrossRef]
- Inoue, N.; Hagihara, Y.; Wada, I. Evolutionarily conserved sperm factors, DCST1 and DCST2, are required for gamete fusion. eLife 2021, 10, e66313. [Google Scholar] [CrossRef]
- Rival, C.M.; Xu, W.; Shankman, L.S.; Morioka, S.; Arandjelovic, S.; Lee, C.S.; Wheeler, K.M.; Smith, R.P.; Haney, L.B.; Isakson, B.E.; et al. Phosphatidylserine on viable sperm and phagocytic machinery in oocytes regulate mammalian fertilization. Nat. Commun. 2019, 10, 4456. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Saito, T.; Wada, I. Unveiling a novel function of CD9 in surface compartmentalization of oocytes. Development 2020, 147, dev189985. [Google Scholar] [CrossRef] [PubMed]
- Nagdas, S.K.; Smith, L.; Medina-Ortiz, I.; Hernandez-Encarnacion, L.; Raychoudhury, S. Identification of Bovine Sperm Acrosomal Proteins that Interact with a 32kDa Acrosomal Matrix Protein. Mol. Cell. Biochem. 2016, 414, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Yamatoya, K.; Ito, C.; Araki, M.; Furuse, R.; Toshimori, K. One-step collagenase method for zona pellucida removal in unfertilized eggs: Easy and gentle method for large-scale preparation. Reprod. Med. Biol. 2011, 10, 97–103. [Google Scholar] [CrossRef]
- Miyado, K.; Yamada, G.; Yamada, S.; Hasuwa, H.; Nakamura, Y.; Ryu, F.; Suzuki, K.; Kosai, K.; Inoues, K.; Ogura, A.; et al. Requirement of CD9 on the Egg Plasma Membrane for Fertilization. Science 2000, 287, 321–324. [Google Scholar] [CrossRef]
- Le Naour, F.; Rubinstein, E.; Jasmin, C.; Prenant, M.; Boucheix, C. Severely Reduced Female Fertility in CD9-Deficient Mice. Science 2000, 287, 319–321. [Google Scholar] [CrossRef]
- Chalbi, M.; Barraud-Lange, V.; Ravaux, B.; Howan, K.; Rodriguez, N.; Soule, P.; Ndzoudi, A.; Boucheix, C.; Rubinstein, E.; Wolf, J.P.; et al. Binding of sperm protein Izumo1 and its egg receptor juno drives cd9 accumulation in the intercellular contact area prior to fusion during mammalian fertilization. Development 2014, 141, 3732–3739. [Google Scholar] [CrossRef]
- Huang, T.T.F.; Yanagimachi, R. Inner acrosomal membrane of mammalian spermatozoa: Its properties and possible functions in fertilization. Am. J. Anat. 1985, 174, 249–268. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamagata, K.; Tsumura, K.; Nakanishi, T.; Baba, T. Acrosome reaction of mouse epididymal sperm on oocyte zona pellucida. J. Reprod. Dev. 2007, 53, 255–262. [Google Scholar] [CrossRef][Green Version]
- Inoue, N.; Satouh, Y.; Ikawa, M.; Okabe, M.; Yanagimachi, R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 2011, 108, 20008–20011. [Google Scholar] [CrossRef]
- Yamatoya, K.; Kousaka, M.; Ito, C.; Nakata, K.; Hatano, M.; Araki, Y.; Toshimori, K. Cleavage of SPACA1 regulates assembly of sperm–egg membrane fusion machinery in mature spermatozoa. Biol. Reprod. 2020, 102, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Fard Jahromi, S.S.; Shamsir, M.S. Construction and Analysis of the Cell Surface’s Protein Network for Human Sperm-Egg Interaction. ISRN Bioinform. 2013, 2013, 962760. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, F.; Nakai, M.; Men, N.T.; Kato, N.; Kaneko, H.; Noguchi, J.; Otoi, T.; Kikuchi, K. Roles of the zona pellucida and functional exposure of the sperm-egg fusion factor ‘IZUMO’ during in vitro fertilization in pigs. Anim. Sci. J. 2014, 85, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Wada, I.; Inoue, N. Sperm IZUMO1-dependent gamete fusion influences male fertility in mice. Int. J. Mol. Sci. 2019, 20, 4809. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Noda, T.; Satouh, Y.; Morohoshi, A.; Yuri, S.; Ogawa, M.; Lu, Y.; Isotani, A.; Ikawa, M. Sperm IZUMO1 Is Required for Binding Preceding Fusion With Oolemma in Mice and Rats. Front. Cell Dev. Biol. 2022, 9, 810118. [Google Scholar] [CrossRef]
- Gómez-Torres, M.J.; Huerta-Retamal, N.; Robles-Gómez, L.; Sáez-Espinosa, P.; Aizpurua, J.; Avilés, M.; Romero, A. Arylsulfatase a remodeling during human sperm in vitro capacitation using field emission scanning electron microscopy (FE-SEM). Cells 2021, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, M.J.; Robles-Gómez, L.; Huerta-Retamal, N.; Sáez-Espinosa, P.; Avilés, M.; Aizpurua, J.; Romero, A. FE-SEM Characterization of α-Mannose Density and Surface Mapping Changes in Human Sperm Head During In Vitro Capacitation. Microsc. Microanal. 2020, 26, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Naz, R.K. Presence and Incidence of Izumo Antibodies in Sera of Immunoinfertile Women and Men. Am. J. Reprod. Immunol. 2013, 69, 256–263. [Google Scholar] [CrossRef]
- Mortazavi, B.; Fard, N.; Karkhane, A.; Shokrpoor, S.; Heidari, F. Evaluation of multi-epitope recombinant protein as a candidate for a contraceptive vaccine. J. Reprod. Immunol. 2021, 145, 103325. [Google Scholar] [CrossRef]
- Xue, F.; Wang, L.; Liu, Y.; Tang, H.; Xu, W.; Xu, C. Vaccination with an Epitope Peptide of IZUMO1 to Induce Contraception in Female Mice. Am. J. Reprod. Immunol. 2016, 75, 474–485. [Google Scholar] [CrossRef]
- Naz, R.K. Immunocontraceptive effect of Izumo and enhancement by combination vaccination. Mol. Reprod. Dev. 2008, 75, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lv, Z.; Shi, J.; Hu, Y.; Xu, C. Immunocontraceptive potential of the Ig-like domain of Izumo. Mol. Reprod. Dev. 2009, 76, 794–801. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Huang, T.H.; Wang, D.G.; Xie, Q.D.; Ma, L.; Chen, D.Y. In Vitro and In Vivo Studies Evaluating Recombinant Plasmid pCXN2-mIzumo as a Potential Immunocontraceptive Antigen. Am. J. Reprod. Immunol. 2009, 61, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Huang, T.H.; Xie, Q.D.; An, G. Investigation of Recombinant Mouse Sperm Protein Izumo as a Potential Immunocontraceptive Antigen. Am. J. Reprod. Immunol. 2008, 59, 225–234. [Google Scholar] [CrossRef]
- Naz, R.K. Vaccine for human contraception targeting sperm Izumo protein and YLP12 dodecamer peptide. Protein Sci. 2014, 23, 857–868. [Google Scholar] [CrossRef]

| Ref. | Specie | Study Groups | Immuno- Localization | Main Findings |
|---|---|---|---|---|
| [20] | Mouse | Multitissue a | Confocal laser scanning microscopy |
|
| [22] | Mouse | Fresh sperm (n = 20) Cryopreserved sperm (n = 20) | Epifluorescence inverted microscopy |
|
| [23] | Bovine | Cryopreserved sperm WT (n = 10) Hz (n = 10) Mt (n = 13) | Confocal laser scanning microscopy |
|
| [24] | Bovine | WT (n = 2) Hz (n = 3) Mt (n = 5) | Upright microscopy |
|
| [31] | Bovine | Multitissue b (n = 3) | n.a. |
|
| [32] | Boar c | Testis (n = 289) | n.a. |
|
| [35] | Buffalo | Herford cattle genome sequence | n.a. |
|
| [36] | Bovine d | DNA (n = 765) | n.a. |
|
| Ref. | Specie | Protein | Main Findings |
|---|---|---|---|
| [21] | Mouse | FIMP |
|
| [38] | Mouse | SPACA6 |
|
| [44] | Mouse | TSSK6 |
|
| [46] | Mouse | GLIPR1L1 |
|
| [62] | Mouse | ADAM3 |
|
| [63] | Mouse | ACE3 |
|
| [64] | Mouse | INPP5B |
|
| [65] | Mouse | CAPZA3 |
|
| [66] | Mouse | TMEM190 |
|
| [67] | Mouse | TPST2 |
|
| [68] | Mouse | LatA |
|
| [69] | Mouse | EQTN |
|
| [70] | Human | Dpy19I2 |
|
| [71] | Mouse | DCST1/2 SPACA6 |
|
| [72] | Mouse | PtdSer |
|
| [73] | Mouse | CD9 |
|
| [74] | Bovine | OMC32 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Falcó, M.; Sáez-Espinosa, P.; López-Botella, A.; Aizpurua, J.; Gómez-Torres, M.J. The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 3929. https://doi.org/10.3390/ijms23073929
Hernández-Falcó M, Sáez-Espinosa P, López-Botella A, Aizpurua J, Gómez-Torres MJ. The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(7):3929. https://doi.org/10.3390/ijms23073929
Chicago/Turabian StyleHernández-Falcó, Miranda, Paula Sáez-Espinosa, Andrea López-Botella, Jon Aizpurua, and María José Gómez-Torres. 2022. "The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review" International Journal of Molecular Sciences 23, no. 7: 3929. https://doi.org/10.3390/ijms23073929
APA StyleHernández-Falcó, M., Sáez-Espinosa, P., López-Botella, A., Aizpurua, J., & Gómez-Torres, M. J. (2022). The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review. International Journal of Molecular Sciences, 23(7), 3929. https://doi.org/10.3390/ijms23073929







