The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment
Abstract
:1. Introduction
2. Results
2.1. Benzene Measurement on Interdigitated 200 µm Thin Functionalized Chip
2.2. Benzene Measurement (in Gas) on Interdigitated 75 µm Thin
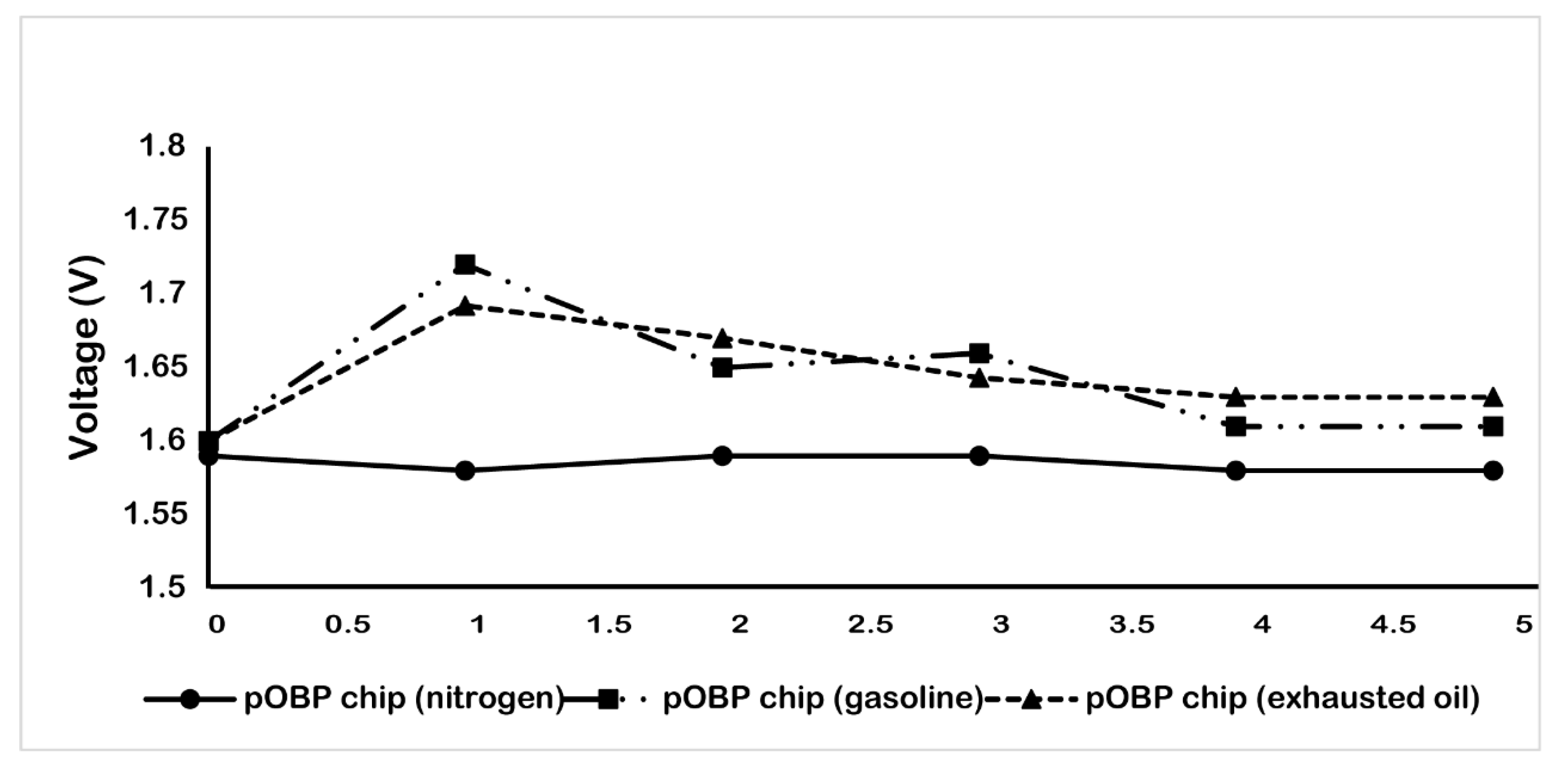
2.3. Real Matrix
3. Materials and Methods
3.1. Materials
3.2. Expression and Purification of the pOBP
3.3. Electrode Preparation and Impedance Measurements
3.4. Gas Chamber Design and Setup
3.5. Surface Derivatization and Functionalization
3.6. Benzene Measurements
- Drying the air inside the gas chamber;
- Exposition of each sample in the gas chamber under a constant flow of humid air in the absence and in the presence of an increasing concentration of benzene;
- Impedance data were collected stepwise in discrete intervals following the evolution of the system up to a steady state.
3.7. Real Matrix Measurements
3.8. Firmware and Software Configuration
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem. J. 1996, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P. Perireceptor events in olfaction. J. Neurobiol. 1996, 30, 3–19. [Google Scholar] [CrossRef]
- Spinelli, S.; Ramoni, R.; Grolli, S.; Bonicel, J.; Cambillau, C.; Tegoni, M. The Structure of the Monomeric Porcine Odorant Binding Protein Sheds Light on the Domain Swapping Mechanism. Biochemistry 1998, 37, 7913–7918. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Mastrogiacomo, R.; Iovinella, I.; Tuccori, E.; Persaud, K.C. Structure and biotechnological applications of odorant-binding proteins. Appl. Microbiol. Biotechnol. 2014, 98, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Capo, A.; Pennacchio, A.; Varriale, A.; D’Auria, S.; Staiano, M. The porcine odorant-binding protein as molecular probe for benzene detection. PLoS ONE 2018, 13, e0202630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance Biosensors: Applications to Sustainability and Remaining Technical Challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance biosensor for peanut protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Jahne, M.; Rogers, S.; Suni, I.I. Detection of Listeria Monocytogenes by Electrochemical Impedance Spectroscopy. Electroanalysis 2013, 25, 2231–2237. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Lassiter, K.; Li, Y.; Hargis, B.; Tung, S.; Berghman, L.; Bottje, W. Interdigitated array microelectrode based impedance immunosensor for detection of avian influenza virus H5N1. Talanta 2009, 79, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Johansson, G. Capacitance measurements of antibody-antigen interactions in a flow system. Anal. Chem. 1997, 69, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, V.M.; Riepl, M.; Wolfbeis, O.S. Capacitive monitoring of protein immobilization and antigen-antibody reactions on monomolecular alkylthiol films on gold electrodes. Biosens. Bioelectron. 1997, 12, 977–989. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Di Giovanni, S.; Varriale, A.; Staiano, M.; Di Pietrantonio, F.; Notargiacomo, A.; Zeni, L.; D’Auria, S. Easy to use plastic optical fiber-based biosensor for detection of butanal. PLoS ONE 2015, 10, e0116770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capone, S.; Pascali, C.D.; Francioso, L.; Siciliano, P.; Persaud, K.C.; Pisanelli, A.M. Electrical characterization of a pig odorant binding protein by Impedance Spectroscopy. In Proceedings of the SENSORS, 2009 IEEE, Christchurch, New Zealand, 25–28 October 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 1758–1762. [Google Scholar]






| 200 µm Sensor Chip | |||||||
|---|---|---|---|---|---|---|---|
| A | W | W+E_1.2 µM | W+B_64 pM | W+B_640 pM | W+B_1.2 nM | W+B_1.2 µM | |
| Empty chip | 2.159 ± (0.010) | 2.322 ± (0.013) | 2.280 ± (0.010) | 2.295 ± (0.002) | 2.286 ± (0.001) | 2.304 ± (0.003) | 2.282 ± (0.009) |
| pOBP chip | 2.159 ± (0.010) | 2.408 ± (0.011) | 2.320 ± (0.012) | 2.390 ± (0.001) | 2.430 ± (0.001) | 2.512 ± (0.002) | 2.546 ± (0.010) |
| GlnBP chip | 2.159 ± (0.010) | 2.401 ± (0.016) | 2.300 ± (0.013) | 2.287 ± (0.001) | 2.290 ± (0.002) | 2.292 ± (0.010) | 2.301 ± (0.008) |
| pOBP—GlnBP | 0.000 ± (0.020) | 0.007 ± (0.027) | 0.020 ± (0.025) | 0.103 ± (0.002) | 0.140 ± (0.003) | 0.220 ± (0.012) | 0.245 ± (0.018) |
| 200 µm Sensor Chip | ||||||
|---|---|---|---|---|---|---|
| N | N+E_1.2 µM | N+B_64 pM | N+B_640 pM | N+B_1.2 nM | N+B_1.2 µM | |
| Empty chip | 2.322 ± (0.001) | 2.325 ± (0.002) | 2.324 ± (0.000) | 2.325 ± (0.003) | 2.324 ± (0.001) | 2.325 ± (0.001) |
| pOBP chip | 2.340 ± (0.002) | 2.327 ± (0.003) | 2.338 ± (0.001) | 2.336 ± (0.004) | 2.336 ± (0.001) | 2.334 ± (0.003) |
| GlnBP chip | 2.333 ± (0.001) | 2.331 ± (0.009) | 2.330 ± (0.006) | 2.330 ± (0.003) | 2.330 ± (0.006) | 2.330 ± (0.009) |
| 75 µm Sensor Chip | ||||||
|---|---|---|---|---|---|---|
| N | N+E_1.2 µM | N+B_64 pM | N+B_640 pM | N+B_1.2 nM | N+B_1.2 µM | |
| Empty chip | 1.622 ± (0.000) | 1.625 ± (0.001) | 1.624 ± (0.001) | 1.625 ± (0.001) | 1.624 ± (0.001) | 1.625 ± (0.001) |
| pOBP chip | 1.640 ± (0.000) | 1.627 ± (0.001) | 1.820 ± (0.001) | 1.710 ± (0.001) | 1.660 ± (0.001) | 1.610 ± (0.001) |
| GlnBP chip | 1.633 ± (0.001) | 1.631 ± (0.003) | 1.630 ± (0.002) | 1.620 ± (0.001) | 1.624 ± (0.002) | 1.628 ± (0.002) |
| pOBP—GlnBP | 0.007 ± (0.001) | -0.004 ± (0.004) | 0.190 ± (0.003) | 0.090 ± (0.002) | 0.036 ± (0.003) | −0.018 ± (0.003) |
| Interdigitated Electrodes | Power Consumption | Analog Output |
|---|---|---|
| 200 and 75 μm | I < 1.2 mA | Ov: 0.5–2.5 Volt |
| Gold-plated PCB | NsV: 3.3 Volt | LPF: 3 Hz |
| Waterproof | OsV: 3~6 Volt | RefV: 1.80 Volt |
| Dust proof | VCO: 11 kHz (5 to 50 kHz) | |
| Particle proof | OpT: 0~30 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capo, A.; Cozzolino, S.; Cavallari, A.; Bruno, U.; Calabrese, A.; Pennacchio, A.; Camarca, A.; Staiano, M.; D’Auria, S.; Varriale, A. The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment. Int. J. Mol. Sci. 2022, 23, 4039. https://doi.org/10.3390/ijms23074039
Capo A, Cozzolino S, Cavallari A, Bruno U, Calabrese A, Pennacchio A, Camarca A, Staiano M, D’Auria S, Varriale A. The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment. International Journal of Molecular Sciences. 2022; 23(7):4039. https://doi.org/10.3390/ijms23074039
Chicago/Turabian StyleCapo, Alessandro, Serena Cozzolino, Adolfo Cavallari, Ugo Bruno, Alessia Calabrese, Angela Pennacchio, Alessandra Camarca, Maria Staiano, Sabato D’Auria, and Antonio Varriale. 2022. "The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment" International Journal of Molecular Sciences 23, no. 7: 4039. https://doi.org/10.3390/ijms23074039
APA StyleCapo, A., Cozzolino, S., Cavallari, A., Bruno, U., Calabrese, A., Pennacchio, A., Camarca, A., Staiano, M., D’Auria, S., & Varriale, A. (2022). The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment. International Journal of Molecular Sciences, 23(7), 4039. https://doi.org/10.3390/ijms23074039









