Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study
Abstract
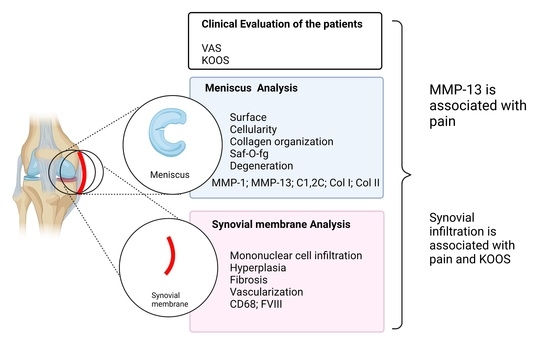
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Synovial Inflammation Features
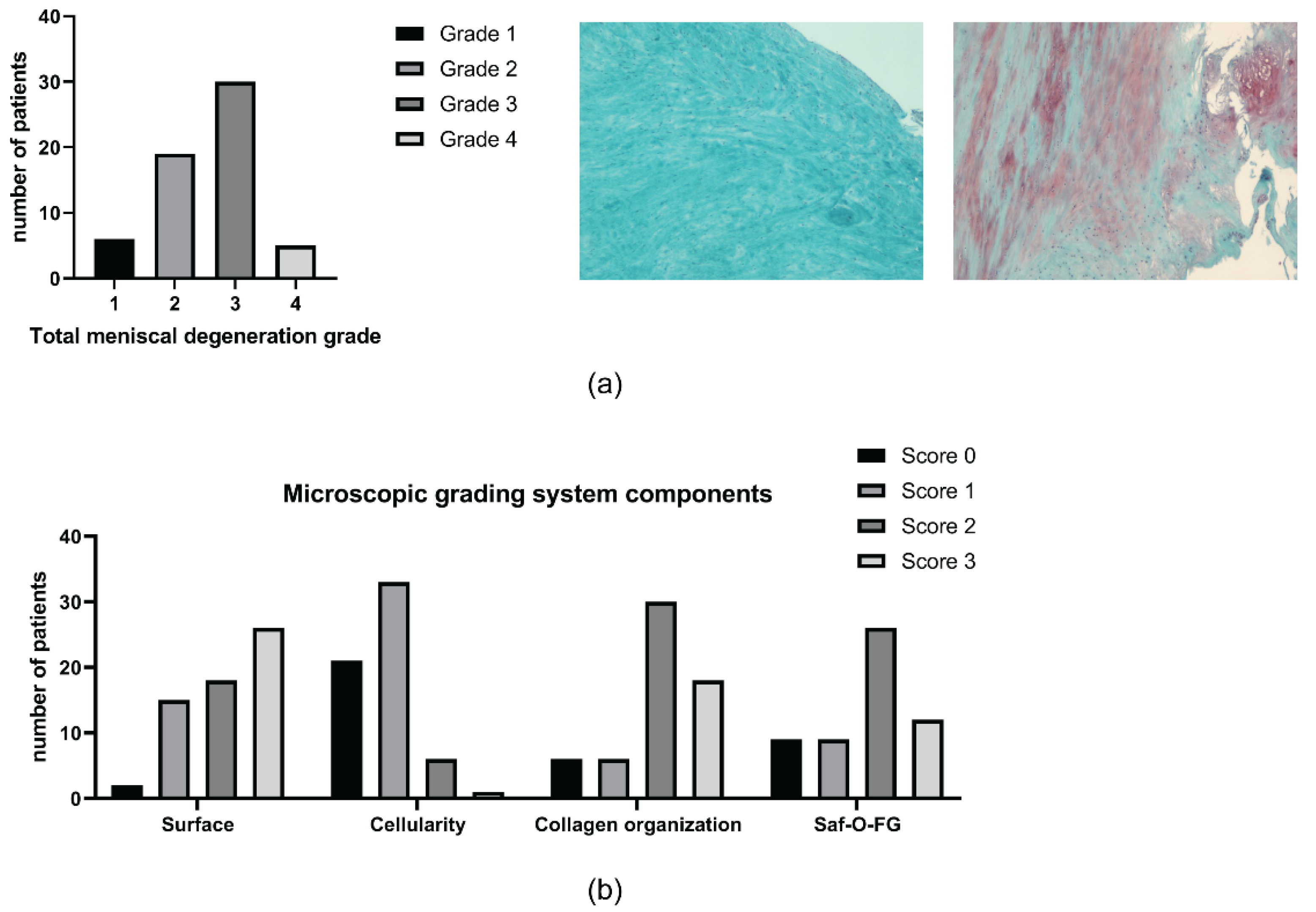
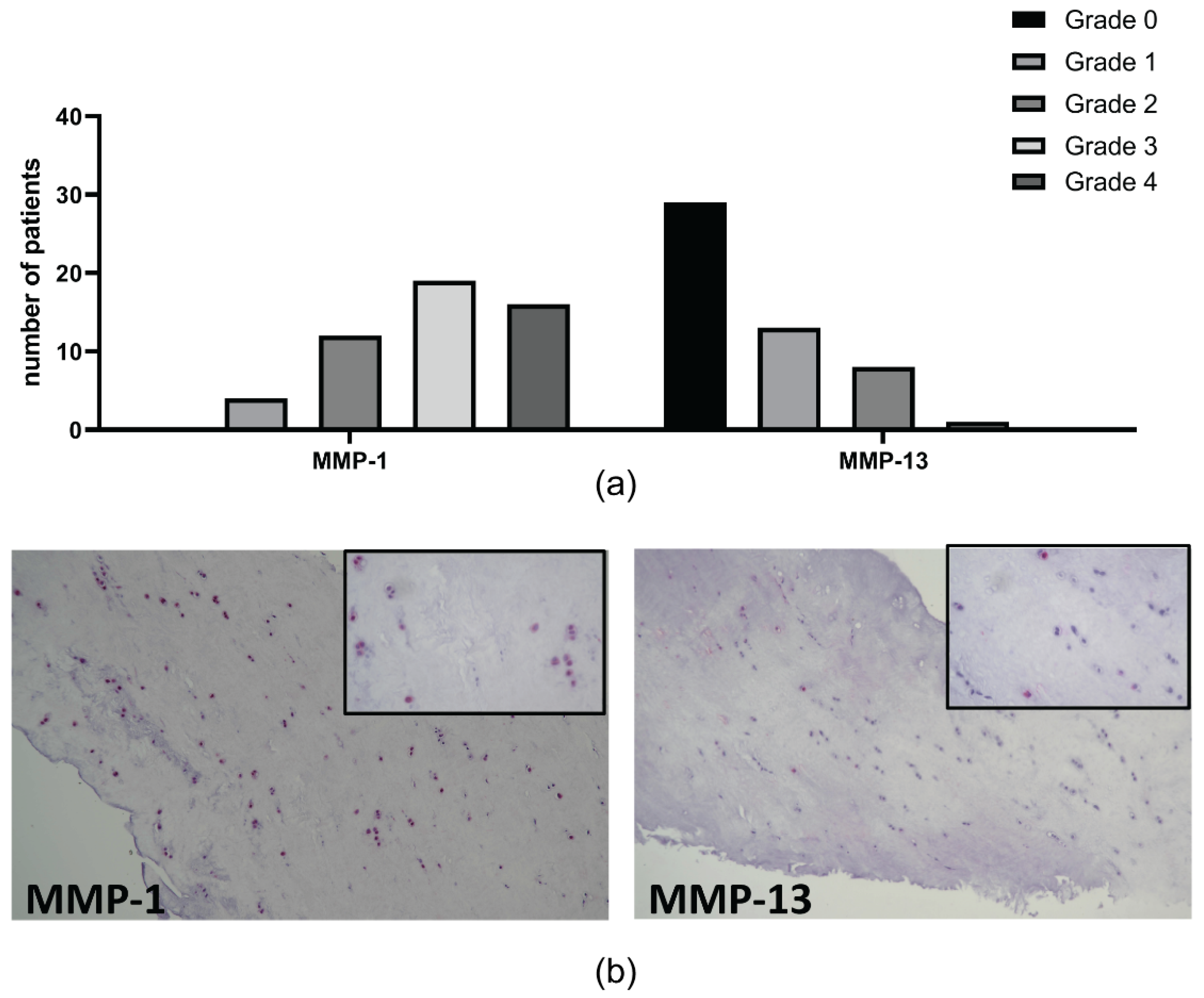
2.3. Meniscal Features
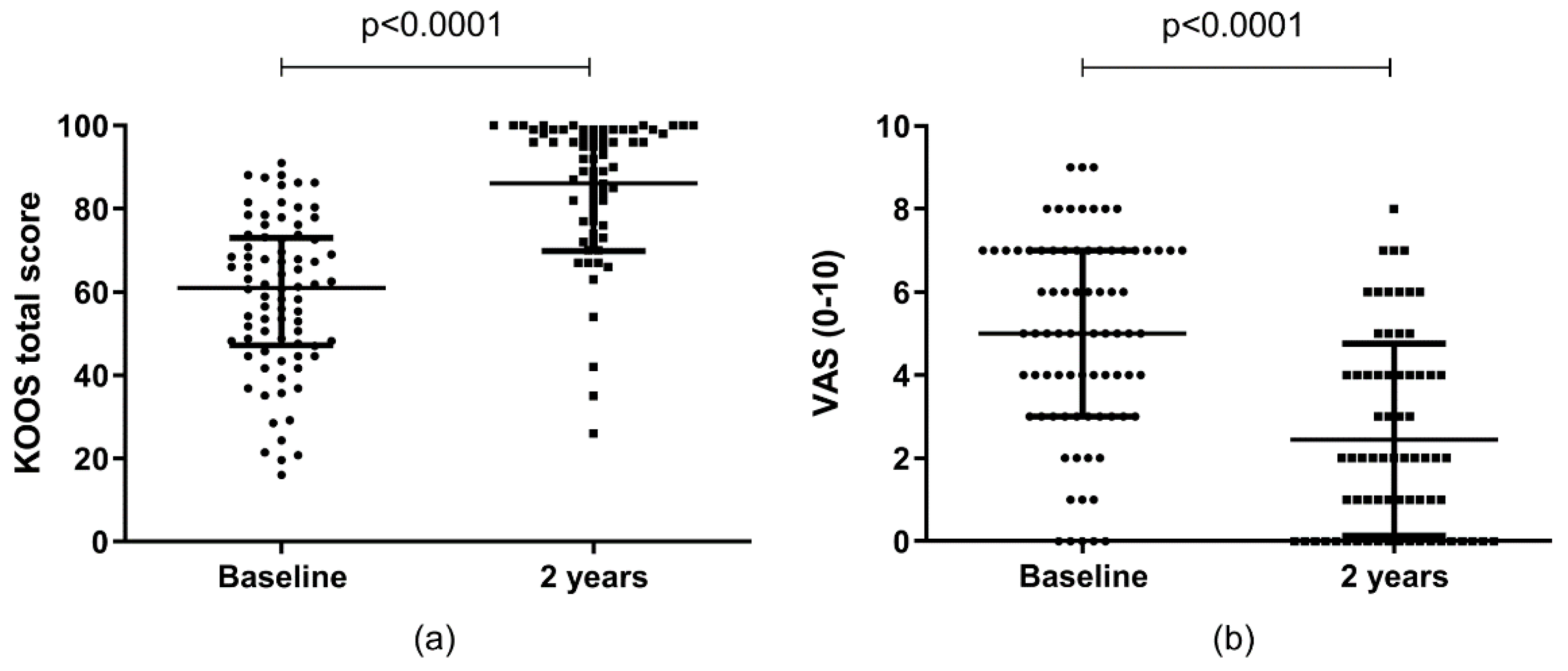
2.4. Clinical Outcomes
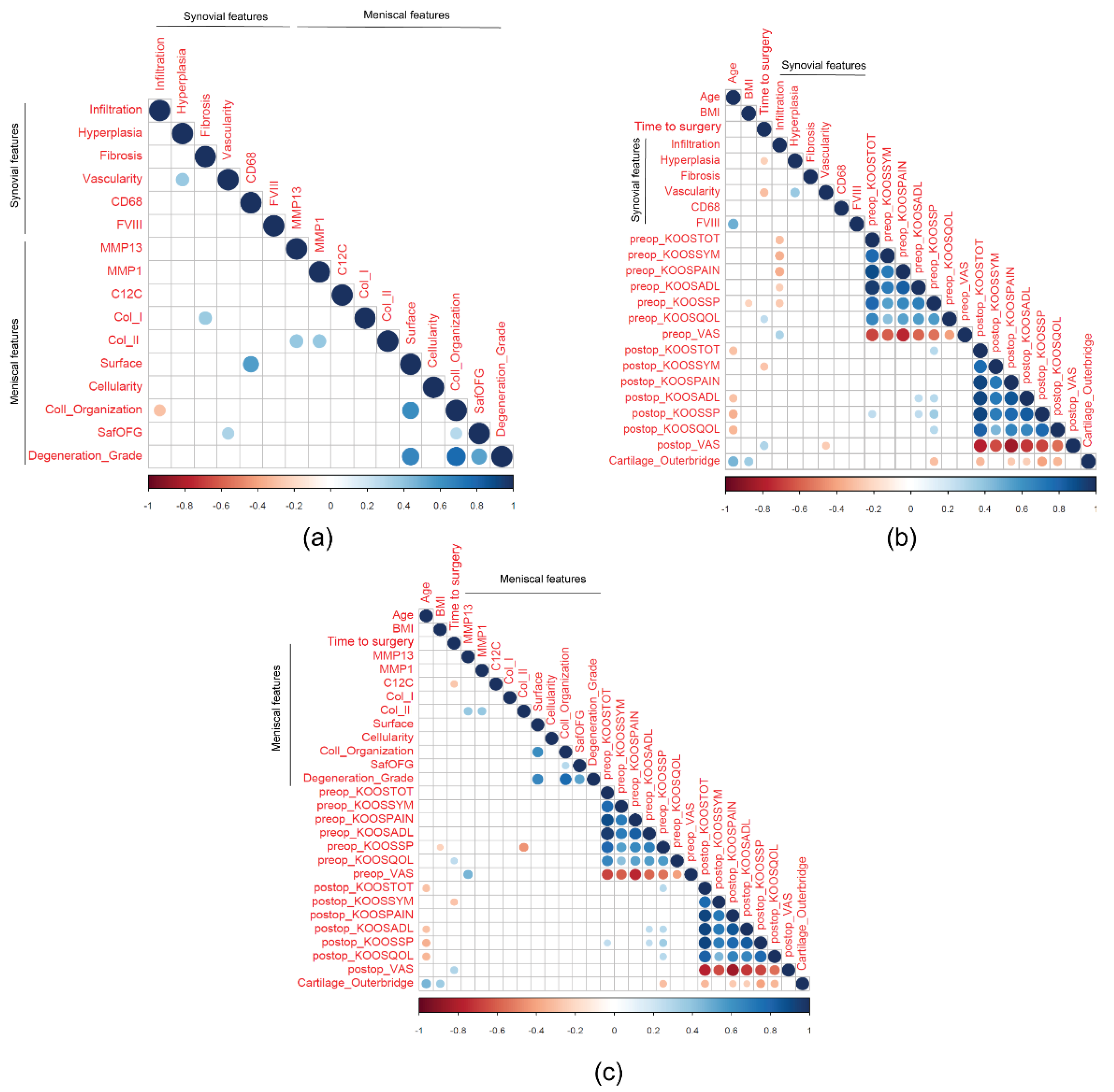
2.5. Correlations
2.6. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment and Clinical Data Collection
4.2. Tissue Sample Collection
4.3. Histological Analyses
4.4. Immunohistochemical Analyses
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. BioMed Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; El Hadi, H.; Granzotto, M.; Rossato, M.; Ramonda, R.; Macchi, V.; De Caro, R.; Vettor, R.; Favero, M. Systemic and Local Adipose Tissue in Knee Osteoarthritis. J. Cell. Physiol. 2017, 232, 1971–1978. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [Green Version]
- Englund, M.; Guermazi, A.; Roemer, F.W.; Aliabadi, P.; Yang, M.; Lewis, C.E.; Torner, J.; Nevitt, M.C.; Sack, B.; Felson, D.T. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009, 60, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Stocco, E.; Porzionato, A.; De Rose, E.; Barbon, S.; De Caro, R.; Macchi, V. Meniscus regeneration by 3D printing technologies: Current advances and future perspectives. J. Tissue Eng. 2022, 13, 20417314211065860. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [Green Version]
- Paxton, E.S.; Stock, M.V.; Brophy, R.H. Meniscal repair versus partial meniscectomy: A systematic review comparing reoperation rates and clinical outcomes. Arthroscopy 2011, 27, 1275–1288. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Hunter, D.J.; Niu, J.; Zhang, Y.; Englund, M.; Javaid, M.K.; Lynch, J.A.; Mohr, A.; Torner, J.; et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: The Framingham and MOST osteoarthritis studies. Osteoarthr. Cartil. 2009, 17, 748–753. [Google Scholar] [CrossRef] [Green Version]
- MacFarlane, L.A.; Yang, H.; Collins, J.E.; Jarraya, M.; Guermazi, A.; Mandl, L.A.; Martin, S.D.; Wright, J.; Losina, E.; Katz, J.N.; et al. Association of Changes in Effusion-Synovitis With Progression of Cartilage Damage Over Eighteen Months in Patients With Osteoarthritis and Meniscal Tear. Arthritis Rheumatol. 2019, 71, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Rossato, M.; Olivotto, E.; Trisolino, G.; Ruggieri, P.; Rubini, A.; Porzionato, A.; Natali, A.; De Caro, R.; et al. Quantitative MRI analysis of infrapatellar and suprapatellar fat pads in normal controls, moderate and end-stage osteoarthritis. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2019, 221, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Hayashi, D.; Roemer, F.W.; Zhu, Y.; Niu, J.; Crema, M.D.; Javaid, M.K.; Marra, M.D.; Lynch, J.A.; El-Khoury, G.Y.; et al. Synovitis in knee osteoarthritis assessed by contrast-enhanced magnetic resonance imaging (MRI) is associated with radiographic tibiofemoral osteoarthritis and MRI-detected widespread cartilage damage: The MOST study. J. Rheumatol. 2014, 41, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.L.; Hunter, D.J.; Niu, J.; Clancy, M.; Guermazi, A.; Genant, H.; Gale, D.; Grainger, A.; Conaghan, P.; Felson, D.T. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1599–1603. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R.; McKeon, B.; Swaim, B.H.; DiCarlo, E.; Asomugha, E.U.; Kanda, V.; Nair, A.; Lee, D.M.; Richmond, J.C.; Katz, J.N.; et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: Molecular characterization and relationship to symptoms. Arthritis Rheum. 2011, 63, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R.; Albert, A.S.; DiCarlo, E.; Rajan, K.B.; Kanda, V.; Asomugha, E.U.; Swaim, B.H.; Katz, J.N.; Goldring, S.R.; Richmond, J.C.; et al. The influence of synovial inflammation and hyperplasia on symptomatic outcomes up to 2 years post-operatively in patients undergoing partial meniscectomy. Osteoarthr. Cartil. 2013, 21, 1392–1399. [Google Scholar] [CrossRef] [Green Version]
- Snoeker, B.; Turkiewicz, A.; Magnusson, K.; Frobell, R.; Yu, D.; Peat, G.; Englund, M. Risk of knee osteoarthritis after different types of knee injuries in young adults: A population-based cohort study. Br. J. Sports Med. 2020, 54, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K.; D’Lima, D.D. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Favero, M.; Belluzzi, E.; Trisolino, G.; Goldring, M.B.; Goldring, S.R.; Cigolotti, A.; Pozzuoli, A.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: A coculture study. J. Cell. Physiol. 2019, 234, 11176–11187. [Google Scholar] [CrossRef]
- Stone, A.V.; Loeser, R.F.; Vanderman, K.S.; Long, D.L.; Clark, S.C.; Ferguson, C.M. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthr. Cartil. 2014, 22, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, E.S.; Smith, M.M.; Little, C.B.; Melrose, J. Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthr. Cartil. 2012, 20, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluzzi, E.; Olivotto, E.; Toso, G.; Cigolotti, A.; Pozzuoli, A.; Biz, C.; Trisolino, G.; Ruggieri, P.; Grigolo, B.; Ramonda, R.; et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect. Tissue Res. 2019, 60, 136–145. [Google Scholar] [CrossRef]
- Driban, J.B.; Harkey, M.S.; Barbe, M.F.; Ward, R.J.; MacKay, J.W.; Davis, J.E.; Lu, B.; Price, L.L.; Eaton, C.B.; Lo, G.H.; et al. Risk factors and the natural history of accelerated knee osteoarthritis: A narrative review. BMC Musculoskelet. Disord 2020, 21, 332. [Google Scholar] [CrossRef]
- Favero, M.; Ramonda, R.; Goldring, M.B.; Goldring, S.R.; Punzi, L. Early knee osteoarthritis. RMD Open 2015, 1, e000062. [Google Scholar] [CrossRef] [PubMed]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Hitchon, C.A.; El-Gabalawy, H.S. The synovium in rheumatoid arthritis. Open Rheumatol. J. 2011, 5, 107–114. [Google Scholar] [CrossRef]
- Trisolino, G.; Favero, M.; Dallari, D.; Tassinari, E.; Traina, F.; Otero, M.; Goldring, S.R.; Goldring, M.B.; Carubbi, C.; Ramonda, R.; et al. Labral calcification plays a key role in hip pain and symptoms in femoroacetabular impingement. J. Orthop. Surg. Res. 2020, 15, 86. [Google Scholar] [CrossRef]
- Abrams, G.D.; Luria, A.; Sampson, J.; Madding, R.A.; Robinson, W.H.; Safran, M.R.; Sokolove, J. Decreased Synovial Inflammation in Atraumatic Hip Microinstability Compared With Femoroacetabular Impingement. Arthroscopy 2017, 33, 553–558. [Google Scholar] [CrossRef]
- Long, Y.; Xie, J.; Zhang, Z.-Q.; Zhang, Z.; Meng, F.; He, A. Substantive molecular and histological changes within the meniscus with tears. BMC Musculoskelet. Disord. 2019, 20, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistelli, M.; Favero, M.; Burini, D.; Trisolino, G.; Dallari, D.; De Franceschi, L.; Goldring, S.R.; Goldring, M.B.; Belluzzi, E.; Filardo, G.; et al. Morphological and ultrastructural analysis of normal, injured and osteoarthritic human knee menisci. Eur. J. Histochem. 2019, 63, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef] [Green Version]
- Brophy, R.H.; Farooq Rai, M.; Zhang, Z.; Torgomyan, A.; Sandell, L.J. Molecular Analysis of Age and Sex-Related Gene Expression in Meniscal Tears with and without a Concomitant Anterior Cruciate Ligament Tear. J. Bone Jt. Surg. 2012, 94, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billinghurst, R.C.; Dahlberg, L.; Ionescu, M.; Reiner, A.; Bourne, R.; Rorabeck, C.; Mitchell, P.; Hambor, J.; Diekmann, O.; Tschesche, H.; et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Investig. 1997, 99, 1534–1545. [Google Scholar] [CrossRef] [Green Version]
- Aurich, M.; Poole, A.R.; Reiner, A.; Mollenhauer, C.; Margulis, A.; Kuettner, K.E.; Cole, A.A. Matrix homeostasis in aging normal human ankle cartilage. Arthritis Rheum. 2002, 46, 2903–2910. [Google Scholar] [CrossRef]
- Cheung, H.S. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect. Tissue Res. 1987, 16, 343–356. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; Dicarlo, E.; Potter, H.G.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef] [Green Version]
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Askari, A.; Naghizadeh, M.M.; Homayounfar, R.; Shahi, A.; Afsarian, M.H.; Paknahad, A.; Kennedy, D.; Ataollahi, M.R. Increased Serum Levels of IL-17A and IL-23 Are Associated with Decreased Vitamin D3 and Increased Pain in Osteoarthritis. PLoS ONE 2016, 11, e0164757. [Google Scholar] [CrossRef]
- Snelling, S.J.; Bas, S.; Puskas, G.J.; Dakin, S.G.; Suva, D.; Finckh, A.; Gabay, C.; Hoffmeyer, P.; Carr, A.J.; Lübbeke, A. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PLoS ONE 2017, 12, e0175109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, D.; Long, L.; Deng, X.; Tao, R.; Huang, G. Correlation between plasma, synovial fluid and articular cartilage Interleukin-18 with radiographic severity in 33 patients with osteoarthritis of the knee. Clin. Exp. Med. 2014, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Song, Y.; Yi, Y.; Qiu, F.; Wang, J.; Li, J.; Jin, Q.; Sacitharan, P.K. Blockade of IL-33 signalling attenuates osteoarthritis. Clin. Transl. Immunol. 2020, 9, e1185. [Google Scholar] [CrossRef] [PubMed]
- Grammens, J.; Van Haver, A.; Danckaers, F.; Booth, B.; Sijbers, J.; Verdonk, P. Small medial femoral condyle morphotype is associated with medial compartment degeneration and distinct morphological characteristics: A comparative pilot study. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2021, 29, 1777–1789. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63 (Suppl. 11), S208–S228. [Google Scholar] [CrossRef] [Green Version]
- Trisolino, G.; Favero, M.; Lazzaro, A.; Martucci, E.; Strazzari, A.; Belluzzi, E.; Goldring, S.R.; Goldring, M.B.; Punzi, L.; Grigolo, B.; et al. Is arthroscopic videotape a reliable tool for describing early joint tissue pathology of the knee? Knee 2017, 24, 1374–1382. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef]
- Monticone, M.; Ferrante, S.; Salvaderi, S.; Rocca, B.; Totti, V.; Foti, C.; Roi, G.S. Development of the Italian version of the knee injury and osteoarthritis outcome score for patients with knee injuries: Cross-cultural adaptation, dimensionality, reliability, and validity. Osteoarthr. Cartil. 2012, 20, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Outerbridge, R.E. The etiology of chondromalacia patellae. J. Bone Jt. Surg. 1961, 43, 752–757. [Google Scholar] [CrossRef]
- Pearle, A.D.; Scanzello, C.R.; George, S.; Mandl, L.A.; DiCarlo, E.F.; Peterson, M.; Sculco, T.P.; Crow, M.K. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr. Cartil. 2007, 15, 516–523. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]


| Absence of Synovial Infiltrate (36 Patients) | Presence of Synovial Infiltrate (38 Patients) | p-Value | |
|---|---|---|---|
| Age | 48.14 (56.66–35.25) | 46.99 (54.08–40.91) | 0.645 |
| BMI | 27.70 (31.80–23.53) | 27.35 (29.92–23.72) | 0.845 |
| Time to surgery | 0.77 (1.69–0.39) | 0.56 (1.49–0.32) | 0.455 |
| Pre-operative | |||
| KOOS | 66.66 (79.91–53.87) | 54.76 (68.45–44.64) | 0.015 |
| KOOS symptoms | 76.78 (85.71–60.71) | 60.71 (85.71–60.71) | 0.006 |
| KOOS pain | 72.22 (82.64–58.33) | 55.55 (69.44–44.44) | 0.002 |
| KOOS ADL | 77.94 (93.75–55.15) | 64.70 (79.78–50.00) | 0.045 |
| KOOS sport | 37.50 (55.00–25.00) | 22.5 (45.00–10.00) | 0.061 |
| KOOS QoL | 43.75 (56.25–31.25) | 40.62 (56.25–25.00) | 0.557 |
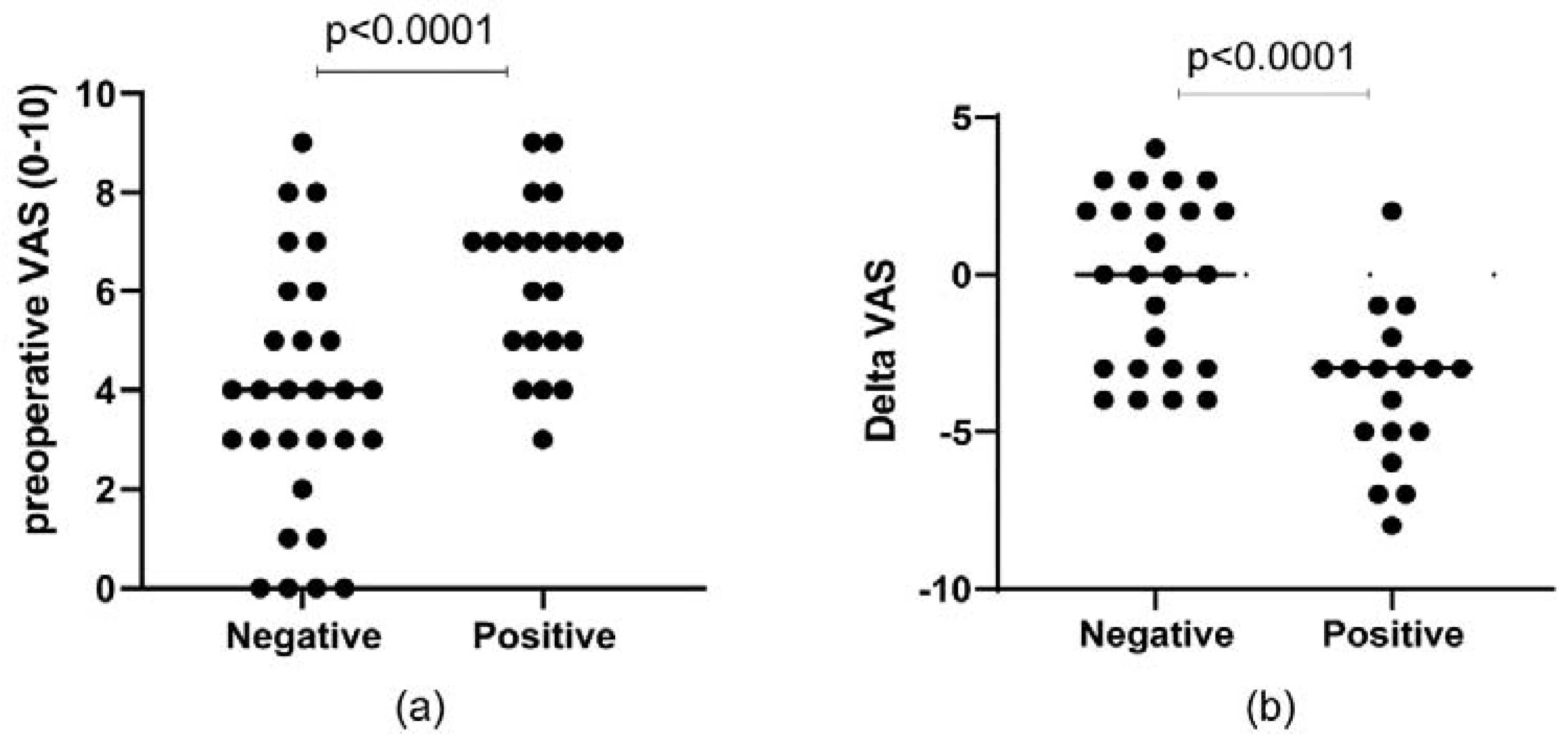
| VAS | 4 (6–2) | 6 (7–4) | 0.005 |
| Post-operative | |||
| KOOS | 94.05 (98.81–76.34) | 91.67 (99.40–80.95) | 0.747 |
| KOOS symptoms | 96.43 (100.00–82.14) | 96.43 (100.00–82.14) | 0.973 |
| KOOS pain | 93.06 (100.00–80.56) | 97.22 (100.00–83.33) | 0.840 |
| KOOS ADL | 99.27 (100.00–75.74) | 97.06 (100.00–83.82) | 0.868 |
| KOOS sport | 90.00 (100.00–57.50) | 90.00 (100.00–65.00) | 0.813 |
| KOOS QoL | 81.25 (100.00–68.75) | 87.50 (100.00–75.00) | 0.433 |
| VAS | 2 (5–0) | 2 (4–0) | 0.370 |
| Δ KOOS | 19.34 (32.89–5.51) | 28.57 (42.86–23.81) | 0.057 |
| Δ KOOS symptoms | 16.07 (25.89–5.35) | 25.00 (35.72–14.28) | 0.081 |
| Δ KOOS pain | 18.05 (31.94–2.08) | 36.11 (41.67–19.44) | 0.032 |
| Δ KOOS ADL | 10.29 (32.72–2.57) | 22.06 (41.18–8.82) | 0.124 |
| Δ KOOS sport | 42.50 (56.25–3.75) | 45.00 (70.00–35.00) | 0.065 |
| Δ KOOS QoL | 31.25 (56.25–18.75) | 37.50 (50.00–25.00) | 0.492 |
| Δ VAS | −1.5 (−4–2) | −3 (−5–−2) | 0.019 |
| Estimate | Std. Error | T Value | p-Value | |
|---|---|---|---|---|
| Preop KOOS | ||||
| KOOS TOTAL | ||||
| (Intercept) | 80.77 | 16.55 | 4.82 | <0.001 |
| Synovial infiltrate | −9.38 | 4.19 | −2.24 | 0.029 |
| Cartilage Outerbridge | 0.48 | 0.56 | −0.85 | 0.399 |
| Duration of symptoms | −0.0007 | 0.96 | −0.001 | 0.999 |
| Age | 0.02 | 0.21 | 0.09 | 0.924 |
| BMI | −0.55 | 0.51 | −1.06 | 0.291 |
| KOOS symptoms | ||||
| (Intercept) | 59.71 | 17.48 | 3.41 | 0.001 |
| Synovial infiltrate | −10.84 | 4.43 | −2.45 | 0.017 |
| Cartilage Outerbridge | −0.53 | 0.59 | −0.89 | 0.377 |
| Duration of symptoms | 0.26 | 1.02 | 0.26 | 0.795 |
| Age | 0.15 | 0.22 | 0.67 | 0.502 |
| BMI | 0.24 | 0.54 | 0.45 | 0.656 |
| KOOS pain | ||||
| (Intercept) | 87.33 | 16.59 | 5.26 | <0.001 |
| Synovial infiltrate | −12.82 | 4.20 | −3.05 | 0.003 |
| Cartilage Outerbridge | −0.01 | 0.56 | −0.02 | 0.983 |
| Duration of symptoms | −0.31 | 0.96 | −0.32 | 0.746 |
| Age | −0.06 | 0.21 | −0.27 | 0.790 |
| BMI | −0.56 | 0.52 | −1.08 | 0.284 |
| Pre-op VAS | ||||
| (Intercept) | 3.06 | 2.22 | 1.38 | 0.172 |
| Synovial infiltrate | 1.61 | 0.56 | 2.87 | 0.005 |
| Cartilage Outerbridge | 0.02 | 0.07 | 0.21 | 0.833 |
| Duration of symptoms | 0.06 | 0.13 | 0.50 | 0.620 |
| Age | 0.01 | 0.03 | 0.52 | 0.607 |
| BMI | 0.005 | 0.07 | 0.07 | 0.945 |
| Estimate | Std. Error | T Value | p-Value | |
|---|---|---|---|---|
| Δ KOOS total score | ||||
| (Intercept) | 13.13 | 20.82 | 0.63 | 0.531 |
| Synovial infiltrate | 10.97 | 5.28 | 2.08 | 0.042 |
| Cartilage Outerbridge | −0.55 | 0.71 | −0.77 | 0.443 |
| Duration of symptoms | −1.072 | 1.17 | −0.91 | 0.365 |
| Age | −0.24 | 0.26 | −0.94 | 0.352 |
| BMI | 0.77 | 0.64 | 1.21 | 0.232 |
| Δ KOOS pain | ||||
| (Intercept) | −7.05 | 21.27 | −0.33 | 0.742 |
| Synovial infiltrate | 13.61 | 5.40 | 2.52 | 0.014 |
| Cartilage Outerbridge | −1.17 | 0.72 | −1.62 | 0.111 |
| Duration of symptoms | −0.89 | 1.20 | −0.75 | 0.459 |
| Age | −0.06 | 0.26 | −0.24 | 0.811 |
| BMI | 1.22 | 0.65 | 1.87 | 0.067 |
| Δ KOOS Sport | ||||
| (Intercept) | 39.07 | 27.88 | 1.40 | 0.167 |
| Synovial infiltrate | 15.15 | 7.08 | 2.14 | 0.037 |
| Cartilage Outerbridge | −0.66 | 0.95 | −0.69 | 0.491 |
| Duration of symptoms | −0.09 | 1.57 | −0.06 | 0.954 |
| Age | −0.92 | 0.34 | −2.67 | 0.01 |
| BMI | 1.52 | 0.86 | 1.78 | 0.081 |
| Δ VAS | ||||
| (Intercept) | 0.17 | 3.11 | 0.05 | 0.957 |
| Synovial infiltrate | −1.91 | 0.79 | −2.42 | 0.019 |
| Cartilage Outerbridge | 0.09 | 0.11 | 0.86 | 0.390 |
| Duration of symptoms | 0.14 | 0.17 | 0.80 | 0.424 |
| Age | −0.002 | 0.04 | −0.06 | 0.955 |
| BMI | −0.07 | 0.09 | −0.72 | 0.473 |
| VAS | Estimate | Std. Error | T Value | p-Value |
|---|---|---|---|---|
| Preop VAS | ||||
| (Intercept) | 3.02 | 2.64 | 1.15 | 0.258 |
| MMP-13 | 1.32 | 0.39 | 3.36 | 0.002 |
| Cartilage Outerbridge | −0.01 | 0.08 | −0.12 | 0.908 |
| Duration of symptoms | −0.06 | 0.19 | −0.32 | 0.749 |
| Age | 0.05 | 0.03 | 1.39 | 0.171 |
| BMI | −0.04 | 0.08 | −0.47 | 0.641 |
| Δ VAS | ||||
| (Intercept) | 2.60 | 3.50 | 0.74 | 0.463 |
| MMP-13 | −1.90 | 0.52 | −3.62 | 0.001 |
| Cartilage Outerbridge | 0.12 | 0.11 | 1.03 | 0.291 |
| Duration of symptoms | 0.42 | 0.24 | 1.70 | 0.097 |
| Age | −0.04 | 0.04 | −1.03 | 0.309 |
| BMI | −0.08 | 0.11 | −0.73 | 0.471 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivotto, E.; Belluzzi, E.; Pozzuoli, A.; Cigolotti, A.; Scioni, M.; Goldring, S.R.; Goldring, M.B.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. Int. J. Mol. Sci. 2022, 23, 3903. https://doi.org/10.3390/ijms23073903
Olivotto E, Belluzzi E, Pozzuoli A, Cigolotti A, Scioni M, Goldring SR, Goldring MB, Ruggieri P, Ramonda R, Grigolo B, et al. Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. International Journal of Molecular Sciences. 2022; 23(7):3903. https://doi.org/10.3390/ijms23073903
Chicago/Turabian StyleOlivotto, Eleonora, Elisa Belluzzi, Assunta Pozzuoli, Augusto Cigolotti, Manuela Scioni, Steven R. Goldring, Mary B. Goldring, Pietro Ruggieri, Roberta Ramonda, Brunella Grigolo, and et al. 2022. "Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study" International Journal of Molecular Sciences 23, no. 7: 3903. https://doi.org/10.3390/ijms23073903
APA StyleOlivotto, E., Belluzzi, E., Pozzuoli, A., Cigolotti, A., Scioni, M., Goldring, S. R., Goldring, M. B., Ruggieri, P., Ramonda, R., Grigolo, B., Trisolino, G., & Favero, M. (2022). Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. International Journal of Molecular Sciences, 23(7), 3903. https://doi.org/10.3390/ijms23073903