Abstract
Searching for adequate and effective compounds displaying antimicrobial activities, especially against Gram-positive bacteria, is an important research area due to the high hospitalization and mortality rates of these bacterial infections in both the human and veterinary fields. In this work, we explored (E)-4-amino-3-((3,5-di-tert-butyl-2-hydroxybenzylidene)amino) benzoic acid (SB-1, harboring an intramolecular hydrogen bond) and (E)-2-((4-nitrobenzilidene)amino)aniline (SB-2), two Schiff bases derivatives. Results demonstrated that SB-1 showed an antibacterial activity determined by the minimal inhibitory concentration (MIC) against Staphylococcus aureus, Enterococcus faecalis, and Bacillus cereus (Gram-positive bacteria involved in human and animal diseases such as skin infections, pneumonia, diarrheal syndrome, and urinary tract infections, among others), which was similar to that shown by the classical antibiotic chloramphenicol. By contrast, this compound showed no effect against Gram-negative bacteria (Klebsiella pneumoniae, Escherichia coli, and Salmonella enterica). Furthermore, we provide a comprehensive physicochemical and theoretical characterization of SB-1 (as well as several analyses for SB-2), including elemental analysis, ESMS, 1H and 13C NMR (assigned by 1D and 2D techniques), DEPT, UV-Vis, FTIR, and cyclic voltammetry. We also performed a computational study through the DFT theory level, including geometry optimization, TD-DFT, NBO, and global and local reactivity analyses.
1. Introduction
At present, it is universally recognized that the emergence of multiresistant bacteria is an increased public health issue [1]. Multiresistant pathogens have been increasingly reported in human and animal diseases [1,2,3,4]. The Centers of Disease Control and Prevention (CDC) presented a threat report listing multiresistant bacteria that included some important Gram-positive pathogens such as Clostridioides difficile (urgent threat), Enterococci, Staphylococcus aureus (MRSA), Streptococcus pneumoniae (serious threats), Group A Streptococcus, and Group B Streptococcus (concerning threat), among others [1,5]. Gram-positive bacteria present a thick peptidoglycan cell wall protecting the cytoplasmic membrane. In contrast, Gram-negative bacteria exhibit a thin peptidoglycan cell wall and an outer phospholipid membrane harboring proteins and lipopolysaccharides [6]. Thus, the study of novel antimicrobial agents is an active area of research aiming to circumvent the increasing emergence of resistance traits in bacteria showing structural differences. In particular, the use of biocide differential compounds (i.e., which affect only a subset of microorganisms), is attracting interest [7].
Schiff bases are compounds harboring a functional group called azomethine (–C=N–), a linker between structures showing the formula R1R2–C=N–R3, where R1, R2, or R3 could be substituted aryl or alkyl groups, among others [8,9,10]. Schiff bases exhibit a broad range of applications, including anticancer drug candidates [11], where their use in antimicrobial chemotherapy is progressively gaining attention [8,12]. However, the precise mechanism of action supporting the antimicrobial activity is not fully understood, although some works provide evidence dealing with the elucidation of the mode of action (e.g., [13]). Many of the Schiff bases exhibiting antimicrobial activity are coordinated with metals (first transition series and other metals), which are thought to exert this effect [14,15,16,17,18]. Nevertheless, the use of Schiff bases with no metal coordination exhibiting antimicrobial activity has been comparatively less explored [19]. Some advantages of using simpler molecules include their straightforward synthesis, which makes them desirable for pharmaceutical applications [20,21].
Our group is particularly interested in non-natural, simple, and small Schiff bases, constituted only by two substituted rings linked by the azomethine group [22]. In particular, we want to understand how to modulate antimicrobial activity by exploring different kinds of substitutions as we previously reported for other Schiff bases [23,24]. In this work, we synthesized and characterized a new Schiff base named SB-1 ((E)-4-amino-3-((3,5-di-tert-butyl-2-hydroxybenzylidene)amino) benzoic acid], harboring an intramolecular hydrogen bond (IHB), which was compared to SB-2 ((E)-2-((4-nitrobenzylidene)amino)aniline] regarding a complete characterization and antimicrobial effects (Figure 1). As stated, SB-1 is a new compound; conversely, SB-2 was already reported as unable to inhibit the bacterial growth of both Gram-positive and Gram-negative bacteria, although its structural characterization was performed only by 1H NMR and its electronical characterization was not disclosed [25]. Thus, comparing SB-1 and SB-2 regarding their structural and antimicrobial properties is a good approach to identify potential substitutions participating in biocide activity.

Figure 1.
Geometry optimizations of SB-1 and SB-2. Blue: N; red: O; white: H; gray: C.
2. Results and Discussion
2.1. Synthesis and Characterizations
SB-1 and SB-2 (Figure 1) were prepared by direct condensation of a primary amine with their corresponding substituent aldehyde (Schemes S1 and S2) in methanol at room temperature, as described in Methods. SB-1 presented a light brown-yellow color, whereas SB-2 exhibited a dark red color; both compounds were obtained with high yield. SB-1 is an amino-benzoic acid linked by the azomethine group with a phenolic ring, presenting an intramolecular hydrogen bond (IHB) between the OH from the phenolic ring and the N from the Schiff base. On the other hand, SB-2 is a phenylenamine ring linked with a nitrobenzene ring, where the IHB is absent. Both SB-1 and SB-2 presented high solubility in DMSO, lower methanol solubility, and apparent water insolubility at room temperature. Since SB-1 is a new compound, we determined its elemental (C, H, N) microanalysis, which agreed with the formula (C22H28N2O3). Furthermore, we performed electrospray mass spectrometry, obtaining the peak at 369.1, in agreement with the proposed molecular formula (Figure S1a). Regarding the melting point, we observed that SB-1 melted at 225–226 °C before decomposition. For SB-2, we also performed elemental analysis (C13H11N3O2) and ESMS (Figure S1b), obtaining similar results to those previously reported [25].
In order to analyze the structure of SB-1 and SB-2, we performed NMR experiments in DMSO-d6 as solvent (the arbitrary numbering of protons used in the 1H NMR studies is shown in (Figure S2).
SB-1 exhibited two narrow signals at 5.78 ppm and 13.36 ppm, assigned to the proton of the amino group (–NH2) and the hydroxyl group (–OH), respectively. A less intense and broader signal appeared at 12.24 ppm, assigned to the carboxylic group’s proton (–COOH). All these assignations were confirmed by D2O exchange (compare Figures S3 and S4). On the other hand, the azomethine (–CH=N–) proton (H4) appeared as a singlet at 8.93 ppm (Figure S3). The other protons corresponding to the amino-benzoic acid ring appeared at 6.80 ppm (d) (H-2) and 7.68 ppm (s) (H-6). Regarding H1, it appeared as a multiplet overlapped with H-5, at about 7.66–7.52 ppm. Finally, H-6 (found in the phenol ring) appeared at 7.38 ppm (d) (Figure S3). All these assignments were confirmed by HHCOSY (Figure S5). To complement these analyses, the 13C NMR spectrum was obtained. We found 14 aromatic carbons between 170 and 113 ppm, whereas tert-butyl carbons were found between 36 and 28 ppm. The azomethine carbon was found at 165 ppm (Figure S6). We also performed a DEPT and CHCOSY analysis to corroborate the SB-1 structure (Figures S7 and S8).
Regarding SB-2, the 1H NMR was already reported [25], which was corroborated by our data (Figure S9). In this context, we complemented this analysis with a D2O exchange, HHCOSY, 13C NMR, and DEPT. These analyses were also helpful for validating our procedure of synthesis and in confirming the SB-2 structure. The 1H NMR and HHCOSY showed the azomethine proton as a singlet at 8.84 ppm. The four protons in the phenylenamine ring (H1 to H4) appeared at 7.02, 7.24, 6.76, and 6.57 ppm, respectively (Figures S9 and S10). On the other hand, the protons corresponding to the nitrobenzene ring (H6, H6’, H7, and H7’) appeared as two intense signals at 8.32 ppm and 8.27 ppm, indicating that they are homotopic (Figures S9 and S10). The D2O exchange corroborated the assignment of the amino group’s protons (–NH2) (5.40 ppm; bs; Figure S11). To complement these results and confirm the proposed structure for SB-2, 13C NMR, DEPT, and CHCOSY (all in DMSO-d6) analyses were performed. In this case, the azomethine carbon was assigned at 154 ppm (see details in Figures S12 and S13a,b).
In order to continue the characterization of both SB-1 and SB-2, the FTIR spectrum (in KBr pellet) was carried out. For SB-1, the FTIR spectrum showed bands that are generally considered symmetric and asymmetric stretching νOH vibrations at 3475.73 cm−1 assigned to the IHB (Figure S14). This assignment is consistent with previously reported signals assigned to similar Schiff bases [26]. Two additional bands were observed at 3379.29 and 3059.10 cm−1, assigned to the νNH stretching vibrations modes (asymmetric and symmetric) of a primary amino group, in accordance with previously reported results for comparable Schiff bases [26]. We assigned the bands at 2962.66 cm−1, 2866.22 cm−1, and 2530.61 cm−1 as typical νC–H bands. Other absorptions were observed at 1666.50 cm−1, 1616.35 cm−1, and 1570.06 cm−1, assigned as νC=O (carbonyl group), νHC=N (azomethine), and νC=C, respectively. Although the azomethine group is usually assigned to absorptions at around 1640 cm−1 [27], the SB-1 azomethine group (1616.35 cm−1) can be explained by the presence of the IHB, as described for other similar molecules [26]. SB-2, for its part, showed a characteristic pattern in line with previous studies [25] (Figure S15).
Finally, for SB-1 and SB-2, the UV-Vis spectrum was recorded in methanol at room temperature (Figure S16). In both cases, the spectrum showed two bands; the first centered approximately at 278 nm, was assigned to a mixed of n → π* (–C=N–) and to π → π* transitions; whereas the band approximately at 377 nm was assigned to π → π* transitions, according to previous reports showing other related compounds [28]. In addition, theoretical studies supported all these assignments (see below).
All these analyses supported both the structure and purity of SB-1 and SB-2, indicating that these compounds were suitable for their electrochemical and biological assays.
2.2. Electrochemical Characterization
Electrochemical characterization of novel molecules helps establish a relationship between a molecule structure and physicochemical properties such as oxidation/reduction potentials. Therefore, cyclic voltammetry analyses for SB-1 and SB-2 were performed to elucidate the oxidation and reduction processes. In addition, cyclic voltammograms for these compounds were compared to a blank solution containing TBAPF6 in anhydrous CH3CN (Figure 2).
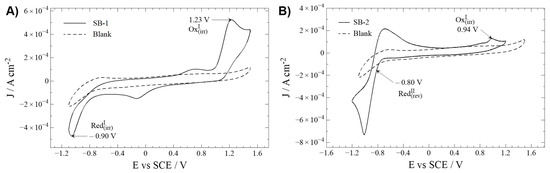
Figure 2.
Cyclic voltammetry profiles of Schiff bases (—) and blank (---). Interface: Pt| 10−3 mol L−1 of compound + 10−1 mol L−1 of TBAPF6 in anhydrous CH3CN under an argon atmosphere. Scan rate: 200 mV s−1. (A) SB-1; (B) SB-2.
Oxidation and reduction processes are studied by direct analysis of the cyclic voltammograms. To distinguish compound-related REDOX processes from secondary reactions with the solvent (e.g., acetonitrile), a working window study is mandatory (Figures S17 and S18) [22]. Similar profiles have been described for similar Schiff bases with an IHB [22,26].
Regarding oxidation, SB-1 and SB-2 display an irreversible oxidation at 1.23 and 0.94 V, respectively. The nature of this irreversible oxidation is typically ascribed to oxidation, taking place somewhere at the aminobenzoic ring, most likely at the –NH2 group [24]. The considerable potential difference is probably due to the electron withdrawal influence of the carboxylic acid group on SB-1, which increases the energy (potential) required to oxidize the amine group.
Concerning reduction processes, SB-1 and SB-2 showed peaks at −0.90 (irreversible) and −0.80 V (quasi-reversible). Typically, the reduction processes in this kind of compound are ascribed to an intramolecular reductive coupling of the azomethine group, which involves a self-protonation reaction, as described for other related Schiff bases. This phenomenon can explain the results for SB-1 [22,23,29]. Without dismissing the possibility that the intramolecular reductive coupling of the azomethine group could occur at SB-2, it should be minded that SB-2 possesses a –NO2 moiety. Organic nitro-aromatic compounds are known for their intense reversible reduction [30,31], the most likely process observed on this molecule.
To complement these assignations, molecular models and the energy of the frontier orbitals HOMO-LUMO is discussed below. For SB-1, the HOMO distribution analysis, which defines the energy of the highest occupied molecular orbital, mainly comprises the amino in the aminobenzoic ring and the hydroxyl in the phenolic ring (for a qualitative molecular orbital diagram, see Figure 3). On the other hand, in SB-2, the HOMO is mainly located at the phenylenamine ring and, to a lesser extent, the azomethine group, supporting the participation of these groups in oxidation processes. In addition, calculations showed that, for SB-1, the LUMO (lowest unoccupied molecular orbital) is mainly distributed around the azomethine group, which is the region where the electron might be withdrawn during the reduction processes. On the other hand, for SB-2, the LUMO is intensively located over the nitrobenzene ring, which supports the idea that the quasi-reversible reduction of SB-2 takes place at the –NO2 moiety. Therefore, the calculation results fairly complement the electrochemical REDOX processes designations.
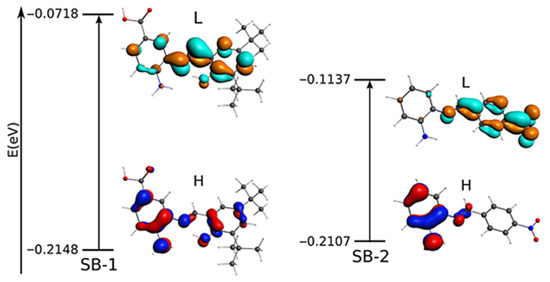
Figure 3.
Qualitative molecular orbital diagram for SB-1 and SB-2 with the most important electronic transitions. Occupied orbitals: red and blue; unoccupied orbitals: orange and cyan. L: LUMO; H: HOMO.
2.3. Geometry Optimizations, TD-DFT, and NBO Studies
In order to complement the experimental data, we performed a geometry optimization. The selected geometry parameter data of SB-1 and SB-2 are listed in Tables S1 and S2, and the optimized geometries are shown in Figures S19 and S20. The optimized structures and geometry parameters agree with available experimental data from a similar Schiff base [29,32]. The azomethine group showed a computed distance and angle degree of 1.286 Å and 121.75° for SB-1; 1.273 Å and 121.88° for SB-2, respectively, in accordance with other Schiff bases [32]. On the other hand, the asymmetric and symmetric vibration mode of –NH2 computed appeared at 3732 cm−1 and 3626 cm−1 for SB-1, and 3696 cm−1 and 3582 cm−1 for SB-2. The νOH appeared at 3787 cm−1 and 3187 cm−1 for the –COOH group and IHB observed in SB-1, respectively. For their part, calculations of frequencies showed that the azomethine appeared at 1680 cm−1 and 1696 cm−1 for SB-1 and SB-2, respectively (Table S3, Figures S21 and S22), corroborating the experimental FTIR assignment (compare with Figures S14 and S15). The calculated energy of the frontier orbital HOMO-LUMO (Figure 3) is reported in eV (E HOMO−LUMO = 0.143 eV for SB-1, and 0.097 eV for SB-2).
To further elucidate the UV-Vis transitions, we conducted time-dependent density functional theory (TD-DFT) calculations for SB-1 and SB-2 in methanol as solvent (Figure S23). The most important absorption bands and other parameters, such as the oscillator strengths and the corresponding transition, are shown in Table S4. The calculated results are in good agreement with experimental data.
To study the IHB of SB-1 in more detail, we evaluated the second-order interaction energy through Natural Bond Orbitals (NBOs) calculations [33]. The interaction energy is related to stabilizing donor-acceptor interactions due to electron delocalization concerning the zeroth-order natural Lewis structure [34]. In addition, NBO analysis provides an efficient method for studying the participation of the IHB in the stability of N-harboring compounds [35]. Thus, from the NBO analysis, the IHB results in stabilization at 26.9 kcal/mol (between the nitrogen lone pairs and an anti-bonding orbital in the –OH group). On the other hand, the oxygen lone pairs can delocalize to the aromatic ring, resulting in stabilization at 39.4 kcal/mol. By contrast, delocalization from the azomethine group to the benzoic acid ring and vice versa contributes to stabilization at 11.1 and 10.9 kcal/mol, respectively. The delocalization from the phenolic ring to the azomethine contributes to stabilizing at 25.2 kcal/mol. These results show that the delocalization among the azomethine group and both aromatic rings provide stability to SB-1. Other interactions involving the –NH2 and –COOH substituents are shown in Table S5. For the numbering of atoms for NBO, see Figure S24.
2.4. Analysis of Global Reactivity
The chemical reactivity and site selectivity were studied using several reactivity descriptors (more details concerning these descriptors are included in the Supplementary Material). According to Table 1, chemical potential (m) indicates that SB-1 presents a higher escaping tendency of electrons from equilibrium than SB-2. Molecular hardness (h) indicates that SB-1 offers a higher resistance to charge transfer than SB-2; the same is concluded through global softness (S), which reveals that SB-2 presents a more effortless charge transfer than SB-1. These two descriptors are not very clear by themselves; thus, it is necessary to resort to electrophilicity (w), indicating that the energy stabilization due to a maximum electron flow from a donor environment favors SB-2, meaning that this compound would be favored energetically from a nucleophilic attack rather than SB-1. Electron-donating power (w−) reveals that SB-1 is more likely to donate charge than SB-2. On the contrary, electron-accepting power (w+) reveals that SB-2 tends to accept a charge more favorably than SB-1. Summing up, these two global descriptors joined together in net electrophilicity indicate that SB-1 is a better electron-donating species than SB-2. Conversely, SB-2 acts better as an electron acceptor than SB-1. For more details regarding these descriptors, please see the Supplementary Material and Figures S25 and S26.

Table 1.
Some global reactivity descriptors and SB-1, SB-2, and precursors for SB-1 were obtained from the B3LYP/6-311++G(d,p) level of theory.
2.5. Analysis of Local Reactivity
Density functional theory (DFT) has successfully explained the theoretical background of molecular properties and chemical concepts [36,37]. In this context, we determined descriptors to analyze chemical reactivity and site selectivity of SB-1 and SB-2. To this aim, we obtained the molecular electrostatic potential (MEP) and local hyper softness (LHS) (Figure 4 and Figure 5). Integrated values of LHS are given in . Please see the Supplementary Material (Tables S6–S8) to check the values of condensed LHS atom by atom.
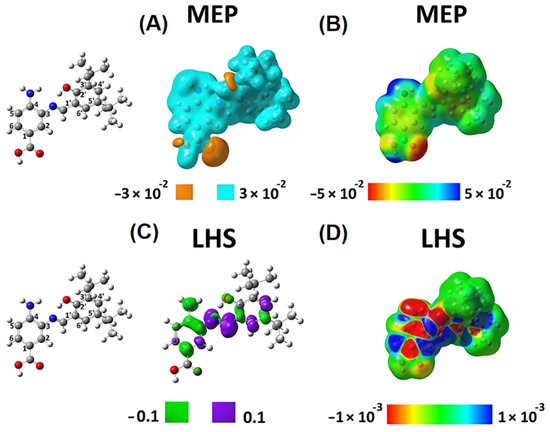
Figure 4.
Local reactivity descriptors of SB-1. Molecular electrostatic potential (MEP) and local hyper softness (LHS) values as 3D pictures. Isovalues of MEP are displayed at ±0.03 (or ±0.03 a.u.) (A), and isovalues of LHS are displayed at ±0.1 (or ±0.1 a.u.) for LHS (C). Values of MEP and LHS projected onto at an electron density isosurface of 0.001 ranging from −0.05 to 0.05 (B) and from −0.001 to 0.001 (D) are depicted, respectively. The corresponding atom numbering is shown on the left.
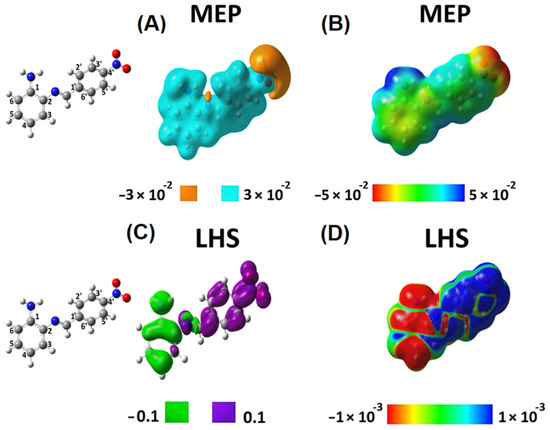
Figure 5.
Local reactivity descriptors of SB-2. Molecular electrostatic potential (MEP) and local hyper softness (LHS) as 3D pictures. Isovalues of MEP are displayed at ±0.03 (or 0.03 a.u.) (A), and isovalues of LHS are displayed at ±0.1 (or 0.1 a.u.) for LHS (C). Values of MEP and LHS projected onto at an electron density isosurface of 0.001 ranging from −0.05 to 0.05 (B) and from −0.001 to 0.001 (D), respectively. The corresponding atom numbering is shown on the left.
These local reactivity descriptors (MEP and LHS) were projected onto an electron density isosurface of 0.001 (or 0.001 a.u.) as previously proposed [38], to produce maps that allow for analyzing of how the values of these descriptors evolve along with the entire molecular structure (Figure 4B,D and Figure 5B,D). For SB-1, Figure 4A shows a predominance of electrons on the hydroxyl groups located at the carboxylic function (amino-benzoic acid ring) and the hydroxyl groups of the phenolic ring. The MEP map in Figure 4B confirms the acidic behavior of the hydrogen of the carboxylic function and the subtle acidic behavior of the hydrogens of the amino group. According to this qualitative information, interactions with a cationic species should occur on the hydroxylic groups’ oxygens. By contrast, a species showing an anionic character should interact with the amino group’s hydrogens and more favorably with the carboxylic function’s hydrogens.
The global softness, S, is 7.123 . According to the LHS descriptor, any possible electrophilic attack will occur on the amino-benzoic acid ring, preferably on the atoms numbered 1, 3, and 4, and the nitrogen of the amino group (Figure 4C). Furthermore, the amino-benzoic ring is the most susceptible region of this molecule to undergo electrophilic attacks. The atoms 1, 3, 4, and 5, along with the nitrogen atom of the amino group, present a total condensed local hyper-softness of -23.79 , while atoms 2 and 6 quantify an amount of 3.17 . These results indicate that this ring is prone to undergo an electrophilic instead of a nucleophilic attack Figure 4D).
On the other hand, the nitrogen atom in the azomethine group and its neighbor carbon sp2 atom are prone to nucleophilic attacks. This characteristic behavior is similar to atoms 4′ and 6′ reactivity on the phenolic ring (Figure 4C). On the phenolic ring, the atoms 2′, 3′, 4′, and 6′ give a total condensed local-hyper softness value of 9.79 and 1′ and 5′ present a total condensed local-hyper softness value of −3.92 . The nitrogen and carbon atoms of the azomethine group present a condensed local-hyper softness value of 18.38 (Figure 4D). Hence, the amino-benzoic ring is the most reactive molecular fragment of SB-1, and electrophilic attacks should predominate.
The azomethine is the second most reactive site of SB-1, where nucleophilic attacks would be oriented to occur. The tert-butyl groups in the phenolic ring show neither electrostatic nor covalent possible interactions, indicating that they could only exert a steric effect, protecting the azomethine group from nucleophilic attacks, facilitating any possible electrophilic attack on the amino-benzoic acid ring.
Concerning SB-2, MEP analysis confirmed that electrons predominate on the nitro group (Figure 5A) and that the hydrogen atoms in the amino group present an acidic behavior (Figure 5B) (as well as in SB-1, Figure 4B). Furthermore, in SB-2, electrophilic attacks will only be oriented towards the phenylenamine ring. Thus, unlike SB-1, SB-2 exhibits a well-separated local reactivity regarding targets for nucleophilic and electrophilic attacks (Figure 5C,D).
For SB-2, the global softness is 9.753 . The LHS map shows that atoms 1, 2, 4, 5, 6, and nitrogen of the amino group are the most susceptible to electrophilic attacks. In contrast, atoms 1′, 2′, 3′, 4′, 5′, 6′ and the nitro group are susceptible to nucleophilic attacks (Figure 5C,D). After adding the negative integrated local hyper-softness values on the phenylenamine ring (atoms, 1, 2, 4, 5, 6, NH2), the resulting value is −75.17 . Conversely, after adding the integrated values of local hyper-softness of the nitrobenzene ring (atoms 1′, 2′, 3′, 4′, 5′, 6′, nitro), we obtained 66.57 . These results show that local reactivities that SB-2 are more favorable than those exhibited by SB-1. Therefore, SB-1 and SB-2 exhibit different local reactivity properties, suggesting that these compounds present, in turn, different biological activities. To end this theoretical analysis, we confirmed the presence of an IHB in SB-1 through Wiberg and Mayer bond order analyses. The same analysis confirmed no IHB in SB-2 (Figures S27 and S28).
2.6. Analysis of Antimicrobial Activity
Previously, it has been described that SB-2 exerted no antimicrobial effect against both Gram-positive and Gram-negative bacteria, including Staphylococcus aureus, Escherichia coli, and Klebsiella sp. [25]. This information was corroborated by determining the minimal inhibitory concentration (MIC) of Gram-positive and Gram-negative bacteria (Table 2; see a resistance/sensitivity profile of tested bacteria in Tables S9 and S10). However, when SB-1 was tested, we found that this compound exerted a potent antimicrobial activity (comparable to that shown by the antibiotic chloramphenicol, which affects protein synthesis [39]) only against Gram-positive pathogens (Table 2). Gram-positive and Gram-negative differ essentially in the configuration of their cell envelopes. Gram-positive bacteria present a cytoplasmic membrane surrounded by a thick cell wall, whereas Gram-negative bacteria have an inner membrane, a thin cell wall, and a second outer membrane over the wall. In both cases, the cell wall is constituted of peptidoglycan [40,41]. The intrinsic resistance of Gram-negative bacteria could explain this differential antimicrobial activity of SB-1 over Gram-positive bacteria due to the exclusion of the compound by the presence of the outer membrane. In this sense, Gram-negative bacteria exhibit intrinsic resistance to vancomycin because of the limit of diffusible molecules through the outer membrane [42]. Previously, it has been reported that Salmonella Typhi ΔompA mutant (which lacks the porin OmpA) and Salmonella Typhi ΔyibP (ΔenvC) mutant (which affect the peptidoglycan hydrolysis) present an increased envelop permeability, which leads to a susceptibility to vancomycin [43]. In this context, we also tested the antimicrobial activity of SB-1 against the Salmonella Typhi ΔompA and ΔyibP (ΔenvC) mutants; nevertheless, we found no effect (data not shown). However, it is necessary to underline that, despite mutations affecting permeability, these mutants still present the outer membrane. That structure could hamper the uptake of the SB-1, impairing the contact with its target. Thereby, we postulate that the presence of the outer membrane, present only in Gram-negative bacteria, could explain why SB-1 cannot efficiently kill this kind of bacteria. Thus, more experimentation is needed to unequivocally determine the molecular bases of the differential antimicrobial activity of SB-1 against Gram-positive bacteria.

Table 2.
Minimal inhibitory concentration (MIC) (µM) of SB-1, SB-2, and precursors for SB-1 (called A and B). Bacteria were cultured for 24 h before MIC determination (n = 3).
As described above, SB-1 is constituted by two rings (amino-benzoic acid ring and the phenolic ring) connected by the azomethine. To assess whether the antimicrobial effect can be attributed to one of these moieties, we tested the precursors for SB-1 synthesis, i.e., A: 3,4-diaminobenzoic acid, and B: 3,5-di-tert-butyl-2-hydroxybenzaldehyde (Scheme S1) against two Gram-positive and one Gram-negative bacteria. Interestingly, we found that none of these precursors presented antimicrobial activity, even against Gram-positive bacteria that showed to be highly susceptible to SB-1. However, since SB-2 also presents an azomethine group, the antimicrobial effect is not plausibly attributed to this part of the compound. Thus, all these results support that the whole structure of SB-1, and not the rings separately, are responsible for the antimicrobial activity, reinforcing the importance of the relationship between structure and activity in this kind of compound [23,24]. In this context, the IHB, which contributes to the SB-1 structure, might influence this compound’s antimicrobial activity.
Thus, SB-1 showed a potent antimicrobial activity only against Gram-positive bacteria (which exhibit resistance to other antibiotics, e.g., penicillin or tetracycline (Table S9), including Staphylococcus aureus, Bacillus cereus, and Enterococcus faecalis (Table 2). Staphylococcus aureus is a non-sporulated pathogen frequently isolated in hospital facilities, which is the etiologic agent of skin, soft tissue, and bone infections. This pathogen is also involved in endocarditis, bacteremia, and toxic shock syndrome, all deadly conditions [44]. Bacillus cereus is a spore-forming ubiquitous pathogen mainly involved in food poisoning [45]. Enterococcus faecalis is a non-sporulated Gram-positive pathogen that colonizes the gastrointestinal tract of humans and animals. This pathogen is considered an important agent for hospital-acquired infections, mainly affecting susceptible patients (i.e., immunocompromised patients) [46]. In addition, Staphylococcus aureus and Enterococcus faecalis are considered a serious threat by the Centers of Disease Control and Prevention regarding the emergence of multiresistant strains [1,5]. Thus, SB-1 could be considered a promising compound to be explored as a potential antimicrobial agent against these pathogens.
3. Materials and Methods
3.1. Materials and Instruments
The reagents used for the synthesis were commercially available by Aldrich, and they were used without further purification. FTIR spectra were obtained on a Tracer-100 FT-IR spectrophotometer (Shimadzu, Kyoto, Japan) with a KBr pellet. 1H NMR, HHCOSY, 13C NMR, CHCOSY, and DEPT 45 for SB-1 and/or SB-2 were recorded on a Bruker AVANCE 400 spectrometer (Bruker, Billerica, MA, USA) (400 MHz for 1H and 100 MHz for 13C) at 25 °C and are reported in ppm downfield from TMS. Unless otherwise stated, samples were dissolved in deuterated DMSO containing tetramethyl silane as an internal reference. Melting points were determined on a Stuart Scientific melting point apparatus SMP3 (Stuart, Staffordshire, UK) in open capillary tubes and were uncorrected. Elemental analyses (CHNS) were performed in a Thermo Flash 2000 Series (Thermo Scientific, Waltham, MA, USA), with a thermal conductivity detector (TCD). In addition, ESMS (electrospray mass spectrometry) of SB-1 was performed with a MS 2020 instrument (Shimadzu, Kyoto, Japan). For the electrochemical experiments, a working solution contained 10−3 mol L−1 of the respective compound (SB-1 and SB-2) with 10−1 mol L−1 tetrabutylammonium hexafluorophosphate (TBAPF6, supporting electrolyte) in anhydrous CH3CN. Before each experiment, the working solution was purged with high purity argon, and an argon atmosphere was maintained during the experiment, as previously reported [24,47]. A polycrystalline non-annealed platinum disc (2 mm diameter) was used as the working electrode. A platinum gauze of a large geometrical area, separated from the cell’s main compartment by a fine sintered glass, was used as the counter electrode. All potentials quoted in this paper refer to an Ag/AgCl electrode in tetramethylammonium chloride to match the potential of a saturated calomel electrode (SCE) at room temperature. All electrochemical experiments were performed at room temperature on a CHI900B bipotentiostat (CH instruments, Austin, TX, USA) interfaced to a PC running the CHI 9.12 software (CH instruments, Austin, TX, USA) that allowed experimental control and data acquisition [48,49].
3.2. Procedure for Preparing SB-1 and SB-2
SB-1 and SB-2 were prepared by direct interaction between the corresponding amino and aldehyde compounds at room temperature and stirring. As a result, the synthesis yield was more than 75% in both cases.
3.2.1. Synthesis of (E)-4-Amino-3-((3,5-di-tert-butyl-2-hydroxybenzylidene)amino) Benzoic Acid (SB-1)
The synthesis was performed by condensation between 3,4-diaminobenzoic acid (precursor A) and 3,5-di-tert-bytyl-2-hydroxy-benzaldehyde (precursor B) (1:1) in 20 mL of methanol as previously described for similar synthesis [22,29]. The reaction was stirred for 24 h at room temperature, without the need of heating or an inert atmosphere. The precipitate was filtered, washed with ethanol and diethyl ether (50/50 v/v), and vacuum dried. A light brown-yellow amorphous compound was obtained (yield: 79%. m.p. = 225–226 °C, dec.). FTIR (KBr, cm−1): 3475 (νOH), 3379 and 3059 (νNH2), 2962 (νC=C), 1666 (νC=O), 1616 (νN=C), 1570 (νC=C). 1H NMR δ = 5.78 (bs; 2H; NH2), 6.80 (d; J = 8.4; 1 H, H2), 7.38 (s; 1H; H6), 7.66–7.52 (m; 2H; H1 and H5), 7.68 (s; 1H; H3), 8.93 (s; 1H; H4), 12.24 (s, 1H; -COOH), 13.36 (s, 1H; -OH).13C NMR δ = 167.72, 164.71, 157.64, 147.32, 140.92, 136.25, 133.61, 129.93, 128.56, 127.58, 120.59, 119.23, 118.85, 114.87, 35.28, 34.29, 31.68, and 29.93; DEPT: δ = 164.72, 129.82, 128.30, 127.55, 120.67, 114.65, 31.60, and 29.80. UV/Vis: (methanol, room temperature) λ nm (ε mol−1 dm3 cm−1): 278 (93.643 × 104), 377 (12.695 × 103). Anal. Calcd. (%) for C22H28N2O3: C, 71.71; H, 7.66; N, 7.60; C/N = 9.43. Found (%): C, 70.46; H, 7.65; N, 7.55, C/N = 9.33. EI-MS (ESI+): m/z calculated for C22H28N2O3 = 368.4, found (M+H)1+ = 369.1.
3.2.2. Synthesis of (E)-2-((4-Nitrobenzylidene)amino)aniline (SB-2)
The synthesis was performed as previously reported [24], with slight modification. Briefly, a condensation between 4-nitrobenzaldehyde and 1,2-phenylenediamine (1:1) was carried out as described 2.2.1. A dark red amorphous compound was obtained (yield: 76%). FTIR (KBr, cm−1): 3464 and 3367 (νNH2), 1608 (νN=C), 1597 (νC=C), 1454 (νC=C), 1342 (νC-N), 1315 (νNO2). 1H NMR: δ = 5.40 (bs; 2H; -NH2), 6.67–6.43 (m; 1 H), 6.75 (d; 1H), 7.02 (t; 1H), 7.24 (d; 1H), 8.30 (dd; 4H), 8.84 (s, 1H). 13C NMR: δ = 153.98, 148.83, 145.41, 142.93, 134.47, 129.88, 129.28, 124.34, 117.51, 116.45, and 115.59; DEPT: δ = 153.96, 129.67, 129.28, 124.33, 117.51, 116.45, and 115.52. UV/Vis: (methanol, room temperature) λ nm (ε mol−1 dm3 cm−1): 281 (31.120 × 103), 416 (11.447 × 103). Anal. Calcd. (%) for C13H11N3O2: C, 64.66; H, 4.55; N, 17.40; C/N = 3.71. Found (%): C, 62.12; H, 4.22; N, 16.56, C/N = 3.75. EI-MS (ESI+): m/z calculated for C13H11N3O2 = 241.2, found (M-H) = 240.0; (M+K+)1+ = 281.2.
3.3. Computational Details
The analyses of local reactivity, geometrical optimizations, frequency, and TD-DFT (in methanol) were performed by the B3LYP functional with no symmetry restrictions and using the 6-311G(d,p) basis set [50,51,52,53]. In addition, frequency calculations were performed to identify the stationary points as minima, where we did not find imaginary frequencies. All calculations were carried out using the Gaussian 09 software package (Gaussian, Wallingford, CT, USA).
We computed 3D pictures of molecular electrostatic potentials (MEP) [54,55,56] and local hyper-softness (LHS) at the B3LYP/6-311++G(d,p) level of theory [54,55,56,57]; however, MEP is routinely computed by using Gaussian 09. To calculate LHS, Cubegen and Cubman programs (Gaussian, Wallingford, CT, USA) were used. Nevertheless, the integrated values of LHS were obtained using the AOMix 6.94 software (SG-Chem, Ottawa, ON, Canada) based on single-point calculations obtained at the B3LYP/6-311G(d,p) level of theory, thus meaning that they do not include diffuse functions as a demanded constrain by AOMix. After applying this constrain, the integration of Fukui functions was performed as follows (Equations (1) and (2)):
where are the squared coefficients defining the linear combination of atomic orbitals that produces the ith molecular occupied orbital and are the squared coefficients defining the linear combination of atomic orbitals that produces the jth molecular virtual orbital. While is an overlap integral between two atomic functions, it has nothing to do with the global softness , and when and that leads to (Equations (3) and (4)):
The given values of and correspond to the Fukui indexes ranging from 0 to 1 each. The dual descriptor index is (Equation (5)):
It ranges from −1 to 1, and after integrating all over the atomic domain , the resulting value will be defined in the interval (−1–1). A positive value within the (0–1) interval indicates that the molecular region is prone to undergo nucleophilic attacks; on the contrary, a negative value within the (−1–0) interval reveals that the zone is prone to undergo electrophilic attacks. The condensed LHS on the kth atom is obtained through the following operational formula (Equation (6)):
Then, ranges from negative to positive values under the following restriction (Equation (7)):
The latter is a consequence of the “normalization” to zero of LHS when integrated on all space and not only on an atomic domain , ; but this is a consequence of its approximated operational formula . The complete operational formula does not obey the restriction mentioned above.
More details concerning these descriptors are exposed in the Supplementary Material.
3.4. Antimicrobial Activity
SB-1 and SB-2, as well as the precursors for SB-1 (A: 3,4-diaminobenzoic acid, and B: 3,5-di-tert-butyl-2-hydroxybenzaldehyde) were evaluated for their in-vitro growth inhibitory activity against the Gram-positive, clinical isolates Staphylococcus aureus, Bacillus cereus, Enterococcus faecalis, from the Hospital Clínico de la Universidad de Chile (Santiago de Chile, Chile) (Table S9). In addition, we also tested the Gram-negative pathogens Salmonella enterica subsp. enterica serovar Typhimurium (ATCC14028s) [58,59], Salmonella enterica subsp. enterica serovar Typhi STH2370 [43,60,61] and clinical isolates from the Hospital Clínico de la Universidad de Chile (Escherichia coli and Klebsiella pneumoniae) (Table S2). Minimum inhibitory concentration (MIC) was obtained by broth dilution essentially as described [62]. MIC is defined as the lowest concentration of the tested compounds at which no growth of the strain was observed after the incubation [62]. Microorganisms were previously cultured in Luria-Bertani broth (LB, Bacto peptone, 10 g/L; Bacto yeast extract, 5 g/L; NaCl, 5 g/L) at 37 °C with shaking to OD600 = 1.4 (stationary phase). Further dilutions of microorganisms (0.5 McFarland) were performed with Bacto Tryptic Soy broth (pancreatic digest casein 17.0 g/L, papaic digest of soybean 3.0 g/L, dextrose 2.5 g/L, sodium chloride 5.0 g/L, dipotassium phosphate 2.5 g/L). Stock solutions of the tested compounds were prepared in dimethyl sulfoxide (DMSO) or ethanol 95% for chloramphenicol (Cam, antibiotic used as positive control). The inoculated wells were incubated at 37 °C for 24 h. As control, DMSO alone was used. Only when the tested compounds’ inhibition effect was higher than the effect of DMSO alone was the compound considered to exhibit detectable antimicrobial activity. The MIC values of the tested compounds were obtained as µM. All the experiments were performed in biological and technical triplicate in each assay. For details of dilutions used for MIC calculation, see Table S11.
4. Conclusions
In this work, we synthesized and characterized SB-1, and compared it with SB-2. We found that only SB-1 exhibited an intramolecular hydrogen bond (IHB), corroborated by 1H NMR, D2O exchange, and FTIR. Furthermore, we found that SB-1 exerted a potent antimicrobial activity only against Gram-positive pathogens. Nevertheless, this antimicrobial activity cannot be attributed to their precursor rings separately. Thus, this result is promising regarding the synthesis of new potential antimicrobial agents against bacteria considered to be a serious threat due to the emergence of multiresistant strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23052553/s1.
Author Contributions
Conceptualization, A.C. and J.A.F.; methodology, M.G., A.C., D.P.-H., J.I.M.-A., E.A., M.P., C.O. and J.A.F.; software, D.P.-H. and J.I.M.-A.; validation, M.G., A.C., R.M.-G., D.P.-H., J.I.M.-A., M.P., C.O. and J.A.F.; formal analysis, A.C. and J.A.F.; investigation, M.G., A.C., R.M.-G., J.I.M.-A., E.A., M.P., M.M.R.-Z., A.S. and J.A.F.; resources, M.G., A.C., D.P.-H., J.I.M.-A., E.A., M.P., C.O. and J.A.F.; data curation, M.G., A.C. and J.A.F.; writing—original draft preparation, A.C. and J.A.F.; writing—review and editing, A.C. and J.A.F.; visualization, A.C. and J.A.F.; supervision, A.C. and J.A.F.; project administration, A.C. and J.A.F.; funding acquisition, M.G., A.C. and J.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT INICIO 11170637 (ANID) and Núcleo UNAB DI-02-19/N. M. Gacitúa thanks to FONDECYT 1190011 (ANID), Juan A. Fuentes thanks to FONDECYT 1181638 (ANID), and R. Morales-Guevara thanks to fellowship ANID 21210811.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We especially thank to María Angélica del Valle for instrumental facilities. We take this opportunity to dedicate this manuscript to María Angélica del Valle, for her long career dedicated to the field of electrodeposition kinetics of heterocycle-derived polymers, and electro-polymerization, and for the occasion of her retirement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dodds, D.R. Antibiotic resistance: A current epilogue. Biochem. Pharmacol. 2017, 134, 139–146. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Balaban, N.Q.; Baquero, F.; Courvalin, P.; Glaser, P.; Gophna, U.; Kishony, R.; Molin, S.; Tonjum, T. Antibiotic resistance: Turning evolutionary principles into clinical reality. FEMS Microbiol. Rev. 2020, 44, 171–188. [Google Scholar] [CrossRef]
- Kwon, J.H.; Powderly, W.G. The post-antibiotic era is here. Science 2021, 373, 471. [Google Scholar] [CrossRef]
- CDC Biggest Threat Data. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fbiggest_threats.html (accessed on 23 February 2022).
- Madigan, M. Brock. In Biology of Microorganisms; Pearson: London, UK, 2017. [Google Scholar]
- Pérez-Cobas, A.E.; Artacho, A.; Knecht, H.; Ferrús, M.L.; Friedrichs, A.; Ott, S.J.; Moya, A.; Latorre, A.; Gosalbes, M.J. Differential Effects of Antibiotic Therapy on the Structure and Function of Human Gut Microbiota. PLoS ONE 2013, 8, e80201. [Google Scholar] [CrossRef]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Medchemcomm 2018, 9, 409–436. [Google Scholar] [CrossRef]
- Weng, Q.; Yi, J.; Chen, X.; Luo, D.; Wang, Y.; Sun, W.; Kang, J.; Han, Z. Controllable Synthesis and Biological Application of Schiff Bases from d-Glucosamine and Terephthalaldehyde. ACS Omega 2020, 5, 24864–24870. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Sun, S.; Xu, Y. Cyanide Boosting Copper Catalysis: A Mild Approach to Fluorescent Benzazole Derivatives from Nonemissive Schiff Bases in Biological Media. Org. Lett. 2020, 22, 3361–3366. [Google Scholar] [CrossRef]
- El Deeb, S.; Ma, B.N.; Baecker, D.; Gust, R. Studies on the stability of the anticancer-active [N,N’-bis(salicylidene)-1,2-phenylenediamine]chloridoiron(III) complex under pharmacological-like conditions. Inorg. Chim. Acta 2019, 487, 76–80. [Google Scholar] [CrossRef]
- Murtaza, G.; Mumtaz, A.; Khan, F.A.; Ahmad, S.; Azhar, S.; Khan, S.A.; Najam-Ul-Haq, M.; Atif, M.; Khan, S.A.; Maalik, A.; et al. Recent pharmacological advancements in schiff bases: A review. Acta Pol. Pharm. 2014, 71, 531–535. [Google Scholar]
- Teran, R.; Guevara, R.; Mora, J.; Dobronski, L.; Barreiro-Costa, O.; Beske, T.; Perez-Barrera, J.; Araya-Maturana, R.; Rojas-Silva, P.; Poveda, A.; et al. Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules 2019, 24, 2696. [Google Scholar] [CrossRef] [Green Version]
- Sangwan, V.; Singh, D.P. Macrocyclic Schiff base complexes as potent antimicrobial agents: Synthesis, characterization and biological studies. Mater. Sci. Eng. C Mater Biol. Appl. 2019, 105, 110119. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Pervaiz, M.; Ahmad, I.; Yousaf, M.; Kirn, S.; Munawar, A.; Saeed, Z.; Adnan, A.; Gulzar, T.; Kamal, T.; Ahmad, A.; et al. Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 642–649. [Google Scholar] [CrossRef]
- Rambabu, A.; Pradeep Kumar, M.; Ganji, N.; Daravath, S.; Shivaraj. DNA binding and cleavage, cytotoxicity and antimicrobial studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of 1-((E)-(4-(trifluoromethoxy)phenylimino)methyl)naphthalen-2-ol Schiff base. J. Biomol. Struct. Dyn. 2020, 38, 307–316. [Google Scholar] [CrossRef]
- Bajema, E.A.; Roberts, K.F.; Meade, T.J. Cobalt-Schiff Base Complexes: Preclinical Research and Potential Therapeutic Uses. Met Ions Life Sci. 2019, 19, 269–274. [Google Scholar] [CrossRef]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Makurvet, F.D. Biologics vs. small molecules: Drug costs and patient access. Med. Drug Discov. 2021, 9, 100075. [Google Scholar] [CrossRef]
- Petrus, M.L.; Bouwer, R.K.M.; Lafont, U.; Athanasopoulos, S.; Greenham, N.C.; Dingemans, T.J. Small-molecule azomethines: Organic photovoltaics via Schiff base condensation chemistry. J. Mater. Chem. A 2014, 2, 9474–9477. [Google Scholar] [CrossRef] [Green Version]
- Carreno, A.; Gacitua, M.; Paez-Hernandez, D.; Polanco, R.; Preite, M.; Fuentes, J.A.; Mora, G.C.; Chavez, I.; Arratia-Perez, R. Spectral, theoretical characterization and antifungal properties of two phenol derivative Schiff bases with an intramolecular hydrogen bond. New J. Chem. 2015, 39, 7822–7831. [Google Scholar] [CrossRef]
- Carreño, A.; Zúñiga, C.; Páez-Hernández, D.; Gacitúa, M.; Polanco, R.; Otero, C.; Arratia-Pérez, R.; Fuentes, J.A. Study of the structure–bioactivity relationship of three new pyridine Schiff bases: Synthesis, spectral characterization, DFT calculations and biological assays. New J. Chem. 2018, 42, 8851–8863. [Google Scholar] [CrossRef]
- Carreno, A.; Rodriguez, L.; Paez-Hernandez, D.; Martin-Trasanco, R.; Zuniga, C.; Oyarzun, D.P.; Gacitua, M.; Schott, E.; Arratia-Perez, R.; Fuentes, J.A. Two New Fluorinated Phenol Derivatives Pyridine Schiff Bases: Synthesis, Spectral, Theoretical Characterization, Inclusion in Epichlorohydrin-beta-Cyclodextrin Polymer, and Antifungal Effect. Front. Chem. 2018, 6, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.N.; Sharif, S.A.I.; El-Tajory, A.N.; Elamari, A.A. Synthesis and Antibacterial Activities of Some Schiff Bases. E-J. Chem. 2011, 8, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Carreno, A.; Paez-Hernandez, D.; Cantero-Lopez, P.; Zuniga, C.; Nevermann, J.; Ramirez-Osorio, A.; Gacitua, M.; Oyarzun, P.; Saez-Cortez, F.; Polanco, R.; et al. Structural Characterization, DFT Calculation, NCI, Scan-Rate Analysis and Antifungal Activity against Botrytis cinerea of (E)-2-{[(2-Aminopyridin-2-yl)imino]-methyl}-4,6-di-tert-butylphenol (Pyridine Schiff Base). Molecules 2020, 25, 2741. [Google Scholar] [CrossRef]
- Issa, Y.M.; Hassib, H.B.; Abdelaal, H.E.; Kenawi, I.M. Spectral investigation of the intramolecular charge-transfer in some aminotriazole Schiff bases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1364–1374. [Google Scholar] [CrossRef]
- Czaplinska, B.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Slodek, A.; Korzec, M.; Musiol, R. Theoretical and Experimental Investigations of Large Stokes Shift Fluorophores Based on a Quinoline Scaffold. Molecules 2020, 25, 2488. [Google Scholar] [CrossRef]
- Carreno, A.; Vega, A.; Zarate, X.; Schott, E.; Gacitua, M.; Valenzuela, N.; Preite, M.; Manriquez, J.M.; Chavez, I. Synthesis, Characterization and Computational Studies of (E)-2-{[(2-Aminopyridin-3-Yl)Imino]-Methyl}-4,6-Di-Tert-Butylphenol. Quim. Nova 2014, 37, 584–588. [Google Scholar] [CrossRef]
- Ohba, T.; Ishida, H.; Yamaguchi, T.; Horiuchi, T.; Ohkubo, K. Carbon dioxide-promoted electrochemical reduction of aromatic nitro compounds to azoxy compounds in acetonitrile. J. Chem. Soc. Chem. Commun. 1994, 263–264. [Google Scholar] [CrossRef]
- Huang, Y.; Lessard, J. Electrochemical Behaviour of Nitrobenzene, Nitrosobenzene, Azobenzene, and Azoxybenzene on Hg, Pt, Cu, and Ni Electrodes in Aprotic Medium. Electroanalysis 2016, 28, 2716–2727. [Google Scholar] [CrossRef]
- Carreno, A.; Ladeira, S.; Castel, A.; Vega, A.; Chavez, I. (E)-2-{[(2-Amino-pyridin-3-yl)imino]-meth-yl}-4,6-di-tert-butyl-phenol. Acta Cryst. Sect E Struct Rep. Online 2012, 68, o2507–o2508. [Google Scholar] [CrossRef]
- Deshmukh, M.M.; Gadre, S.R.; Bartolotti, L.J. Estimation of intramolecular hydrogen bond energy via molecular tailoring approach. J. Phys. Chem. A 2006, 110, 12519–12523. [Google Scholar] [CrossRef]
- Purser, G.H. Lewis Structures Are Models for Predicting Molecular Structure, Not Electronic Structure. J. Chem. Educ. 1999, 76, 1013–1018. [Google Scholar] [CrossRef]
- Aviles-Moreno, J.R.; Berden, G.; Oomens, J.; Martinez-Haya, B. Isolated complexes of the amino acid arginine with polyether and polyamine macrocycles, the role of proton transfer. Phys. Chem. Chem. Phys. 2017, 19, 31345–31351. [Google Scholar] [CrossRef]
- Barrales-Martinez, C.; Martinez-Araya, J.I.; Jaque, P. 1,3-Dipolar Cycloadditions by a Unified Perspective Based on Conceptual and Thermodynamics Models of Chemical Reactivity. J. Phys. Chem. A 2021, 125, 801–815. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. The importance of diffuse functions in basis sets to produce reliable 3D pictures of dual descriptor. Chem. Phys. Lett. 2019, 724, 29–34. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 2002, 109, 7968–7979. [Google Scholar] [CrossRef]
- Oong, G.C.; Tadi, P. Chloramphenicol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shrivastava, R.; Chng, S.S. Lipid trafficking across the Gram-negative cell envelope. J. Biol. Chem. 2019, 294, 14175–14184. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar]
- Pimenta, A.L.; Young, J.; Holland, I.B.; Blight, M.A. Antibody analysis of the localisation, expression and stability of HlyD, the MFP component of the E. coli haemolysin translocator. Mol. Gen. Genet. 1999, 261, 122–132. [Google Scholar] [CrossRef]
- Nevermann, J.; Silva, A.; Otero, C.; Oyarzun, D.P.; Barrera, B.; Gil, F.; Calderon, I.L.; Fuentes, J.A. Identification of Genes Involved in Biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front. Microbiol. 2019, 10, 104. [Google Scholar] [CrossRef]
- Hernandez-Aristizabal, I.; Ocampo-Ibanez, I.D. Antimicrobial Peptides with Antibacterial Activity against Vancomycin-Resistant Staphylococcus aureus Strains: Classification, Structures, and Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 7927. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Martlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins (Basel) 2021, 13, 98. [Google Scholar] [CrossRef]
- Sadowy, E. Mobile genetic elements beyond the VanB-resistance dissemination among hospital-associated enterococci and other Gram-positive bacteria. Plasmid 2021, 114, 102558. [Google Scholar] [CrossRef]
- del Valle, M.A.; Gacitúa, M.; Díaz, F.R.; Armijo, F.; Río, R.D. Electrosynthesis of polythiophene nanowires via mesoporous silica thin film templates. Electrochem. Commun. 2009, 11, 2117–2120. [Google Scholar] [CrossRef]
- Diaz, F.R.; Pardo, M.A.; Gacitua, M.A.; del Valle, M.A. Electrode Modified with a Polymer of Aniline and 3-Hexylthiophene to Be Assayed in the Selective Determination of Nitrate. J. Chil. Chem. Soc. 2020, 65, 5023–5026. [Google Scholar] [CrossRef]
- de Valle, M.A. dsDNA Sensing Capabilities of Metallopolymers Electrochemically Deposited from Ruthenium-Pyrrole and—Thiophene Complexes. Int. J. Electrochem. Sci. 2019, 14, 8131–8140. [Google Scholar] [CrossRef]
- Hay, P.J. Gaussian basis sets for molecular calculations. The representation of 3d orbitals in transition-metal atoms. J. Chem. Phys. 1977, 66, 4377–4384. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. Theory Comput. Modeling (Theor. Chim. Acta) 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Politzer, P. Atomic and molecular energies as functionals of the electrostatic potential. Theor. Chem. Acc. 2004, 111, 395–399. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li-F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Jarvik, T.; Smillie, C.; Groisman, E.A.; Ochman, H. Short-term signatures of evolutionary change in the Salmonella enterica serovar Typhimurium 14028 genome. J. Bacteriol. 2010, 192, 560–567. [Google Scholar] [CrossRef] [Green Version]
- Berrocal, L.; Fuentes, J.A.; Trombert, A.N.; Jofre, M.R.; Villagra, N.A.; Valenzuela, L.M.; Mora, G.C. stg fimbrial operon from S. Typhi STH2370 contributes to association and cell disruption of epithelial and macrophage-like cells. Biol. Res. 2015, 48, 34. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, C.; Ugalde, J.A.; Mora, G.C.; Alvarez, S.; Contreras, I.; Santiviago, C.A. Draft Genome Sequence of Salmonella enterica Serovar Typhi Strain STH2370. Genome Announc. 2014, 2, e00104-14. [Google Scholar] [CrossRef] [Green Version]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderon, I.L.; Fuentes, J.A. “One for all”: Functional transfer of OMV-mediated polymyxin B resistance from Salmonella enterica sv. Typhi ΔtolR and ΔdegS to susceptible bacteria. Front. Microbiol. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Moore, C.B.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Denning, D.W.; Donnelly, J.P.; Dromer, F.; Dupont, B.; Rex, J.H.; et al. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 2003, 9, 467–474. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).