Molecular Mechanisms Underlying the Association between Endometriosis and Ectopic Pregnancy
Abstract
1. Introduction
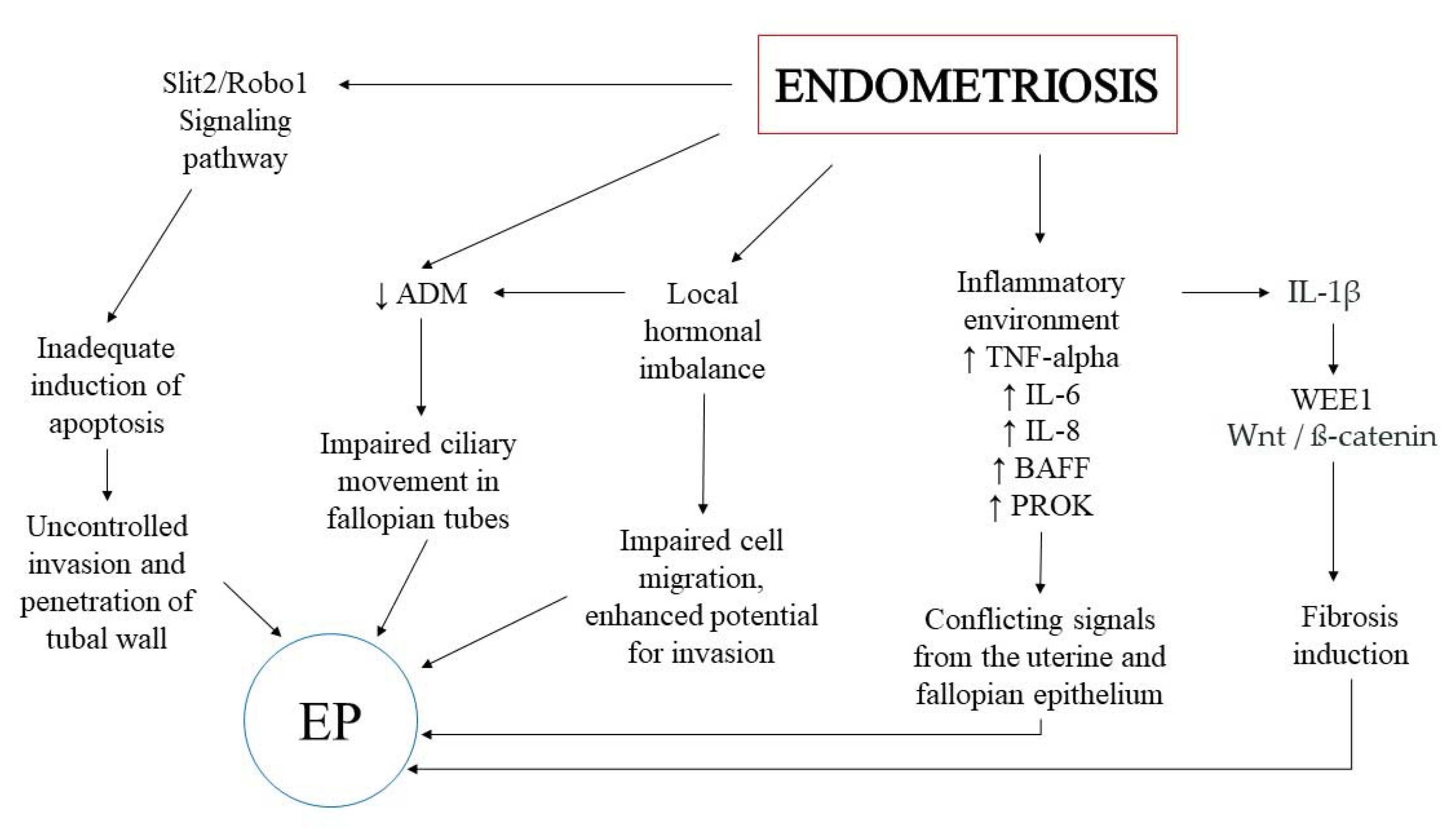
2. The Potential Keypoints in the Association between EP and Endometriosis
3. Inflammatory Environment
4. The Wnt/ß-Catenin Signaling Pathway
5. The Role of Hormones
6. The Endocannabinoid System
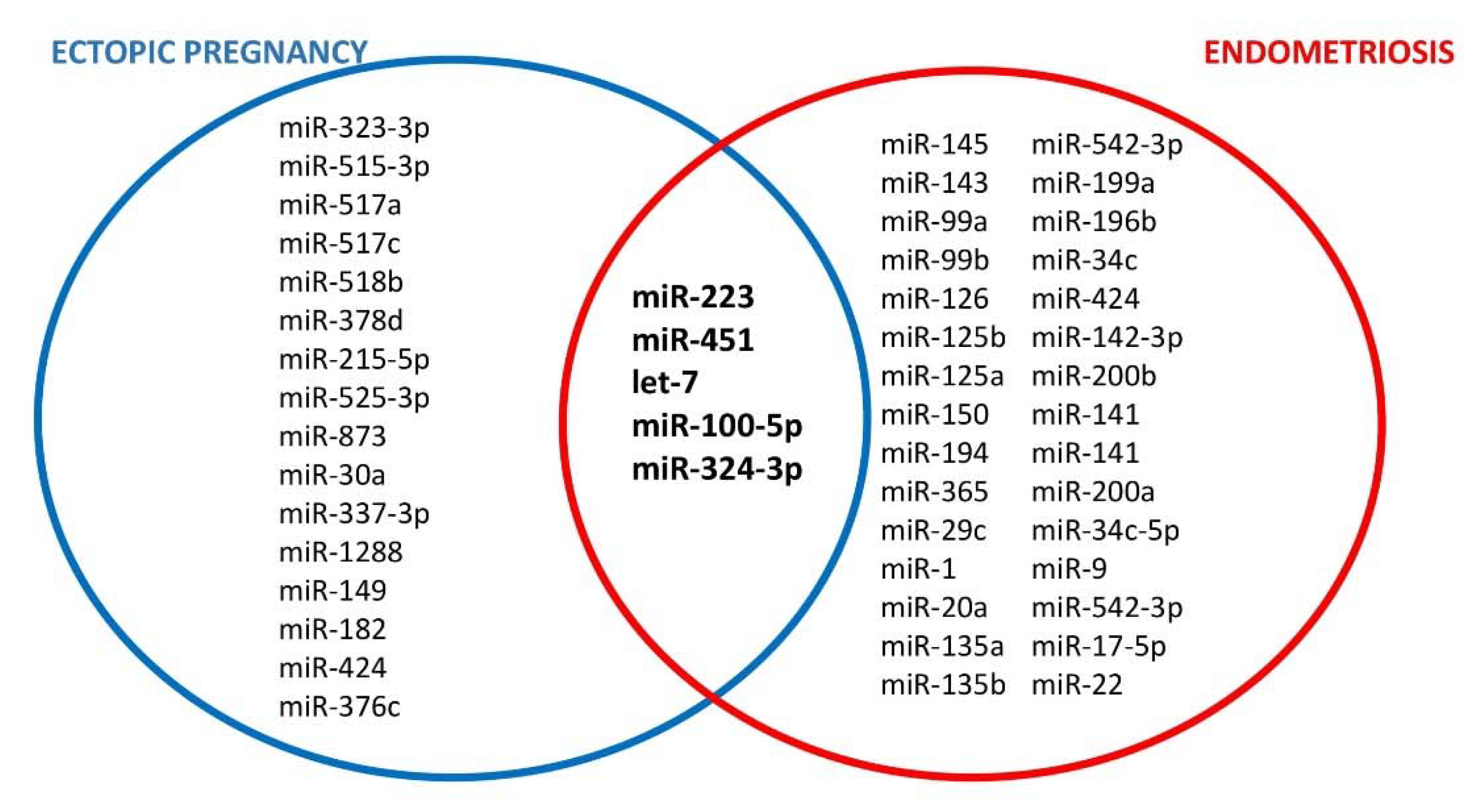
7. The Role of microRNA
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and Treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or Embolic Endometriosis, Due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93. [Google Scholar] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and Pathophysiology of Endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, M.; Zervou, M.I.; Matalliotaki, C.; Rahmioglu, N.; Koumantakis, G.; Kalogiannidis, I.; Prapas, I.; Zondervan, K.; Spandidos, D.A.; Matalliotakis, I.; et al. The Role of Gene Polymorphisms in Endometriosis. Mol. Med. Rep. 2017, 16, 5881–5886. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Fujishita, A.; Hiraki, K.; Matsumoto, A.; Nakashima, M.; Masuzaki, H. Pelvic Pain in Women with Ovarian Endometrioma Is Mostly Associated with Coexisting Peritoneal Lesions. Hum. Reprod. 2013, 28, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Coste, J.; Bouyer, J.; Job-Spira, N. Epidemiology of Ectopic Pregnancy: Incidence and Risk Factors. Contracept. Fertil. Sex. 1992 1996, 24, 135–139. [Google Scholar]
- Weström, L.; Joesoef, R.; Reynolds, G.; Hagdu, A.; Thompson, S.E. Pelvic Inflammatory Disease and Fertility. A Cohort Study of 1,844 Women with Laparoscopically Verified Disease and 657 Control Women with Normal Laparoscopic Results. Sex. Transm. Dis. 1992, 19, 185–192. [Google Scholar] [CrossRef]
- Corpa, J.M. Ectopic Pregnancy in Animals and Humans. Reproduction 2006, 131, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Shaw, J.L.; Murdoch, A.; McDonald, S.E.; Williams, A.R.; Jabbour, H.N.; Duncan, W.C.; Critchley, H.O. Placental Growth Factor: A Promising Diagnostic Biomarker for Tubal Ectopic Pregnancy. J. Clin. Endocrinol. Metab. 2011, 96, E104–E108. [Google Scholar] [CrossRef]
- Yong, P.J.; Matwani, S.; Brace, C.; Quaiattini, A.; Bedaiwy, M.A.; Albert, A.; Allaire, C. Endometriosis and Ectopic Pregnancy: A Meta-Analysis. J. Minim. Invasive Gynecol. 2020, 27, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Farland, L.V.; Prescott, J.; Sasamoto, N.; Tobias, D.K.; Gaskins, A.J.; Stuart, J.J.; Carusi, D.A.; Chavarro, J.E.; Horne, A.W.; Rich-Edwards, J.W.; et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet. Gynecol. 2019, 134, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Poder, L.; Sun, D.; Jha, P. Endometriosis in Pregnancy. Abdom. Radiol. 2020, 45, 1741–1753. [Google Scholar] [CrossRef]
- Ezzati, M.; Djahanbakhch, O.; Arian, S.; Carr, B.R. Tubal Transport of Gametes and Embryos: A Review of Physiology and Pathophysiology. J. Assist. Reprod. Genet. 2014, 31, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The Reproductive Significance of Human Fallopian Tube Cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, C.-L.; Vijayan, M.; Yeung, W.S.; Ng, E.H.; Wang, X.; Wai-Sum, O.; Li, R.H.; Zhang, Y.; Chiu, P.C. Adrenomedullin Insufficiency Alters Macrophage Activities in Fallopian Tube: A Pathophysiologic Explanation of Tubal Ectopic Pregnancy. Mucosal Immunol. 2020, 13, 743–752. [Google Scholar] [CrossRef]
- Shaw, J.L.; Horne, A.W. The Paracrinology of Tubal Ectopic Pregnancy. Mol. Cell. Endocrinol. 2012, 358, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.M.; Briton-Jones, C.; Cheung, C.K.; Leung, S.W.; Cheung, L.P.; Haines, C. Increased Messenger RNA Expression of Vascular Endothelial Growth Factor and Its Receptors in the Implantation Site of the Human Oviduct with Ectopic Gestation. Fertil. Steril. 2004, 82, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-W.; Zheng, Y.; Lu, Y.; Liu, X.; Geng, J.-G. Slit2 Overexpression Results in Increased Microvessel Density and Lesion Size in Mice with Induced Endometriosis. Reprod. Sci. 2013, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Peng, H.; Lu, W.-H.; Shuai, H.-L.; Zha, Q.-B.; Yeung, C.-K.; Li, H.; Wang, L.-J.; Ho Lee, K.K.; Zhu, W.-J.; et al. Role of Slit2/Robo1 in Trophoblast Invasion and Vascular Remodeling during Ectopic Tubal Pregnancy. Placenta 2015, 36, 1087–1094. [Google Scholar] [CrossRef]
- Evans, J.; Catalano, R.D.; Brown, P.; Sherwin, R.; Critchley, H.O.; Fazleabas, A.T.; Jabbour, H.N. Prokineticin 1 Mediates Fetal-Maternal Dialogue Regulating Endometrial Leukemia Inhibitory Factor. FASEB J. 2009, 23, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Feng, Y.; Zou, S.; Weijdegård, B.; Wu, G.; Brännström, M.; Billig, H. The Role of Estrogen in the Pathophysiology of Tubal Ectopic Pregnancy. Am. J. Transl. Res. 2012, 4, 269. [Google Scholar] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The Endometrial Immune Environment of Women with Endometriosis. Hum. Reprod. Update 2019, 25, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Beste, M.T.; Pfäffle-Doyle, N.; Prentice, E.A.; Morris, S.N.; Lauffenburger, D.A.; Isaacson, K.B.; Griffith, L.G. Molecular Network Analysis of Endometriosis Reveals a Role for C-Jun–Regulated Macrophage Activation. Sci. Transl. Med. 2014, 6, 222ra16. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Chang, K.-K.; Sun, H.-X. Immunosuppressive Macrophages Induced by IDO1 Promote the Growth of Endometrial Stromal Cells in Endometriosis. Mol. Med. Rep. 2017, 15, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Edwards, A.K.; Singh, S.S.; Young, S.L.; Lessey, B.A.; Tayade, C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering pro-Inflammatory Cytokines and Angiogenic Growth Factors. J. Immunol. 2015, 195, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Ksiazek, K.; Warnecke, C.; Kuźlan, M.; Korybalska, K.; Tayama, H.; Wiśniewska-Elnur, J.; Pawlaczyk, K.; Trómińska, J.; Breborowicz, A.; et al. Role of Mesothelial Cell-Derived Granulocyte Colony-Stimulating Factor in Interleukin-17-Induced Neutrophil Accumulation in the Peritoneum. Kidney Int. 2007, 71, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Da Gama Coelho Riccio, L.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Rovere Querini, P. Endometriosis, a Disease of the Macrophage. Front. Immunol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Schulke, L.; Berbic, M.; Manconi, F.; Tokushige, N.; Markham, R.; Fraser, I.S. Dendritic Cell Populations in the Eutopic and Ectopic Endometrium of Women with Endometriosis. Hum. Reprod. 2009, 24, 1695–1703. [Google Scholar] [CrossRef]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of Immune Cells in the Pathogenesis of Endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Mariuzzi, L.; Domenis, R.; Orsaria, M.; Marzinotto, S.; Londero, A.P.; Bulfoni, M.; Candotti, V.; Zanello, A.; Ballico, M.; Mimmi, M.C.; et al. Functional Expression of Aryl Hydrocarbon Receptor on Mast Cells Populating Human Endometriotic Tissues. Lab. Investig. 2016, 96, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Marí-Alexandre, J.; Barceló-Molina, M.; Belmonte-López, E.; García-Oms, J.; Estellés, A.; Braza-Boïls, A.; Gilabert-Estellés, J. Micro-RNA Profile and Proteins in Peritoneal Fluid from Women with Endometriosis: Their Relationship with Sterility. Fertil. Steril. 2018, 109, 675–684. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, H.; Zhang, D.; Li, J.; Huang, Z.; Zhao, X.; Zhang, J. Reassessment of Prevalence of Tubal Endometriosis, and Its Associated Clinicopathologic Features and Risk Factors in Premenopausal Women Received Salpingectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100074. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, D.; Ouyang, J.; Liang, Y.; Zhang, H.; Huang, Z.; Liang, G.; Zhu, Q.; Guan, X.; Zhang, J. Effects of Pelvic Endometriosis and Adenomyosis on Ciliary Beat Frequency and Muscular Contractions in the Human Fallopian Tube. Reprod. Biol. Endocrinol. 2018, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, H.; Zhao, X.; Qin, Y.; Liang, G.; He, X.; Zhang, J. Integrated Analysis of MRNA and Protein Expression Profiling in Tubal Endometriosis. Reproduction 2020, 159, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Thomas, S.Y.; Hamilton, K.J.; Young, S.L.; Cook, D.N.; Korach, K.S. Early Endometriosis in Females Is Directed by Immune-Mediated Estrogen Receptor α and IL-6 Cross-Talk. Endocrinology 2018, 159, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.-Y.; Chang, N.; Tsai, J.-L.; Lin, S.-C.; Tsai, S.-J.; Wu, M.-H. Hypoxia-Inhibited DUSP2 Expression Promotes IL-6/STAT3 Signaling in Endometriosis. Am. J. Reprod. Immunol. 2017, 78, e12690. [Google Scholar] [CrossRef]
- Goryszewska-Szczurek, E.; Baryla, M.; Kaczynski, P.; Waclawik, A. Prokineticin 1-Prokineticin Receptor 1 Signaling in Trophoblast Promotes Embryo Implantation and Placenta Development. Sci. Rep. 2021, 11, 13715. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, X. Expression of PROK 1 and Its Receptor PROKR 1 in Endometriosis and Its Clinical Significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 621–627. [Google Scholar] [PubMed]
- Ma, L.; Li, Z.; Xi, S.; Guo, Q.; Zhao, P.; Li, W.; Ai, J.; Chen, X. Tubal Ectopic Pregnancy Occurrence Is Associated with High Expressions of Prokineticin Receptors and Aberrant Secretion of Inflammatory Cytokines. Am. J. Transl. Res. 2020, 12, 5741. [Google Scholar] [PubMed]
- Balasubramaniam, E.S.; Van Noorden, S.; El-Bahrawy, M. The Expression of Interleukin (IL)-6, IL-8, and Their Receptors in Fallopian Tubes with Ectopic Tubal Gestation. Fertil. Steril. 2012, 98, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, X.; Qu, S.; Yang, G.; Shen, N. B Cell Activation Factor (BAFF) Induces Inflammation in the Human Fallopian Tube Leading to Tubal Pregnancy. BMC Pregnancy Childbirth 2019, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Hever, A.; Roth, R.B.; Hevezi, P.; Marin, M.E.; Acosta, J.A.; Acosta, H.; Rojas, J.; Herrera, R.; Grigoriadis, D.; White, E.; et al. Human Endometriosis Is Associated with Plasma Cells and Overexpression of B Lymphocyte Stimulator. Proc. Natl. Acad. Sci. USA 2007, 104, 12451–12456. [Google Scholar] [CrossRef]
- Lekovich, J.; Witkin, S.S.; Doulaveris, G.; Orfanelli, T.; Shulman, B.; Pereira, N.; Rosenwaks, Z.; Spandorfer, S.D. Elevated Serum Interleukin-1β Levels and Interleukin-1β-to-Interleukin-1 Receptor Antagonist Ratio 1 Week after Embryo Transfer Are Associated with Ectopic Pregnancy. Fertil. Steril. 2015, 104, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xue, X.; Tian, H.; Ye, H.; Wang, H.; Wang, R.; Liu, Y.; Zhang, C.; Chen, Q.; Sun, L. WEE1 Promotes Endometriosis via the Wnt/β-Catenin Signaling Pathway. Reprod. Biol. Endocrinol. RBE 2021, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Jia-Rong, Z.; Shuang-Di, L.; Xiao-Ping, W. Eutopic or Ectopic Pregnancy: A Competition between Signals Derived from the Endometrium and the Fallopian Tube for Blastocyst Implantation. Placenta 2009, 30, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Red-Horse, K.; Drake, P.M.; Gunn, M.D.; Fisher, S.J. Chemokine Ligand and Receptor Expression in the Pregnant Uterus: Reciprocal Patterns in Complementary Cell Subsets Suggest Functional Roles. Am. J. Pathol. 2001, 159, 2199–2213. [Google Scholar] [CrossRef]
- Tulac, S.; Nayak, N.R.; Kao, L.C.; Van Waes, M.; Huang, J.; Lobo, S.; Germeyer, A.; Lessey, B.A.; Taylor, R.N.; Suchanek, E.; et al. Identification, Characterization, and Regulation of the Canonical Wnt Signaling Pathway in Human Endometrium. J. Clin. Endocrinol. Metab. 2003, 88, 3860–3866. [Google Scholar] [CrossRef]
- Sonderegger, S.; Pollheimer, J.; Knöfler, M. Wnt Signalling in Implantation, Decidualisation and Placental Differentiation–Review. Placenta 2010, 31, 839–847. [Google Scholar] [CrossRef]
- Nagaraja, A.K.; Andreu-Vieyra, C.; Franco, H.L.; Ma, L.; Chen, R.; Han, D.Y.; Zhu, H.; Agno, J.E.; Gunaratne, P.H.; DeMayo, F.J.; et al. Deletion of Dicer in Somatic Cells of the Female Reproductive Tract Causes Sterility. Mol. Endocrinol. 2008, 22, 2336–2352. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; De Cecco, S.; Trez, D.; Caliari, D.; Aresu, L.; Castagnaro, M. Immunohistochemical Expression of E-Cadherin and β-Catenin in Feline Mammary Tumours. J. Comp. Pathol. 2012, 147, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, W.; Ma, Z.; Wang, G.; Peng, H.; Chen, Y.; Lee, K.K.H.; Yang, X. Enhanced Beta-Catenin Expression and Inflammation Are Associated with Human Ectopic Tubal Pregnancy. Hum. Reprod. 2013, 28, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. In Vitro Effects of a Small-Molecule Antagonist of the Tcf/ß-Catenin Complex on Endometrial and Endometriotic Cells of Patients with Endometriosis. PLoS ONE 2013, 8, e61690. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Involvement of the Wnt/β-Catenin Signaling Pathway in the Cellular and Molecular Mechanisms of Fibrosis in Endometriosis. PLoS ONE 2013, 8, e76808. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Botchorishvili, R.; Pouly, J.L.; Canis, M. Targeting the Wnt/β-Catenin Pathway in Endometriosis: A Potentially Effective Approach for Treatment and Prevention. Mol. Cell. Ther. 2014, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, W.; Xiong, Y.; Liu, H.; Liu, Y. 17 β-Estradiol Promotes Vascular Endothelial Growth Factor Expression via the Wnt/β-Catenin Pathway during the Pathogenesis of Endometriosis. Mol. Hum. Reprod. 2016, 22, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; O’Neill, S.R.; Zhang, Y.; Holtzman, M.J.; Takemaru, K.-I.; Korach, K.S.; Winuthayanon, W. Estrogen Receptor α Is Required for Oviductal Transport of Embryos. FASEB J. 2017, 31, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Liao, S.B.; Chiu, P.C.N.; Tam, W.W.; Ho, J.C.; Ng, E.H.; Ho, P.C.; Yeung, W.S.; Tang, F.; O, W.S. Expression of Adrenomedullin in Human Oviduct, Its Regulation by the Hormonal Cycle and Contact with Spermatozoa, and Its Effect on Ciliary Beat Frequency of the Oviductal Epithelium. J. Clin. Endocrinol. Metab. 2010, 95, E18–E25. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.V.; Dey, S.K.; Critchley, H.O.D.; Horne, A.W. Current Knowledge of the Aetiology of Human Tubal Ectopic Pregnancy. Hum. Reprod. Update 2010, 16, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Liao, S.-B.; Chiu, P.C.N.; Yeung, W.S.B.; Ng, E.H.Y.; Cheung, A.N.Y.; Tang, F.; O, W.S. Effects of Adrenomedullin on the Expression of Inflammatory Cytokines and Chemokines in Oviducts from Women with Tubal Ectopic Pregnancy: An in-Vitro Experimental Study. Reprod. Biol. Endocrinol. RBE 2015, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, G.; Yang, H.; Zhi, D.; Li, L.; Wang, D.; Liu, M.; Su, H. S100A8 Expression in Oviduct Mucosal Epithelial Cells Is Regulated by Estrogen and Affects Mucosal Immune Homeostasis. PLoS ONE 2021, 16, e0260188. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E.; Herrera, G.G.; Anamthathmakula, P.; Rock, J.K.; Willie, A.M.; Harris, E.A.; Takemaru, K.-I.; Winuthayanon, W. Roles of Steroid Hormones in Oviductal Function. Reproduction 2020, 159, R125–R137. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Lee, J.E.; Cho, Y.J.; Park, M.J.; O’Malley, B.W. Genomic Function of Estrogen Receptor β in Endometriosis. Endocrinology 2019, 160, 2495–2516. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.-W. Cellular Changes Consistent with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Formation and Function of the Myofibroblast during Tissue Repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, L.; Yu, Q.; Zhang, Y.; Yan, L.; Chen, Z.-J. The Estrogen-Regulated LncRNA H19/MiR-216a-5p Axis Alters Stromal Cell Invasion and Migration via ACTA2 in Endometriosis. Mol. Hum. Reprod. 2019, 25, 550–561. [Google Scholar] [CrossRef]
- Horne, A.W.; King, A.E.; Shaw, E.; McDonald, S.E.; Williams, A.R.; Saunders, P.T.; Critchley, H.O. Attenuated Sex Steroid Receptor Expression in Fallopian Tube of Women with Ectopic Pregnancy. J. Clin. Endocrinol. Metab. 2009, 94, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Bylander, A.; Lind, K.; Goksör, M.; Billig, H.; Larsson, D.J. The Classical Progesterone Receptor Mediates the Rapid Reduction of Fallopian Tube Ciliary Beat Frequency by Progesterone. Reprod. Biol. Endocrinol. RBE 2013, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.-W. Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Gomel, V.; Martin, D.C. Peritoneal Fluid Progesterone and Progesterone Resistance in Superficial Endometriosis Lesions. Hum. Reprod. 2022, 37, 203–211. [Google Scholar] [CrossRef]
- Peng, B.; Klausen, C.; Campbell, L.; Leung, P.C.K.; Horne, A.W.; Bedaiwy, M.A. Gonadotropin-Releasing Hormone and Gonadotropin-Releasing Hormone Receptor Are Expressed at Tubal Ectopic Pregnancy Implantation Sites. Fertil. Steril. 2016, 105, 1620–1627.e3. [Google Scholar] [CrossRef] [PubMed]
- Dobovišek, L.; Krstanović, F.; Borštnar, S.; Debeljak, N. Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment. Cancers 2020, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Luschnig, P.; Schicho, R. Cannabinoids in Gynecological Diseases. Med. Cannabis Cannabinoids 2019, 2, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Santoro, A.; D’Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The Epigenetics of the Endocannabinoid System. Int. J. Mol. Sci. 2020, 21, 1113. [Google Scholar] [CrossRef]
- Battista, N.; Meccariello, R.; Cobellis, G.; Fasano, S.; Di Tommaso, M.; Pirazzi, V.; Konje, J.C.; Pierantoni, R.; Maccarrone, M. The Role of Endocannabinoids in Gonadal Function and Fertility along the Evolutionary Axis. Mol. Cell. Endocrinol. 2012, 355, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Battista, N.; Pirazzi, V.; Maccarrone, M. The Manifold Actions of Endocannabinoids on Female and Male Reproductive Events. Front. Biosci. 2011, 16, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, H.; Sun, X.; Kingsley, P.J.; Marnett, L.J.; Cravatt, B.F.; Dey, S.K. Differential Regulation of Endocannabinoid Synthesis and Degradation in the Uterus during Embryo Implantation. Prostaglandins Other Lipid Mediat. 2007, 83, 62–74. [Google Scholar] [CrossRef] [PubMed]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. Fluctuation in Anandamide Levels from Ovulation to Early Pregnancy in In-Vitro Fertilization-Embryo Transfer Women, and Its Hormonal Regulation. Hum. Reprod. 2009, 24, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Habayeb, O.M.H.; Taylor, A.H.; Evans, M.D.; Cooke, M.S.; Taylor, D.J.; Bell, S.C.; Konje, J.C. Plasma Levels of the Endocannabinoid Anandamide in Women--a Potential Role in Pregnancy Maintenance and Labor? J. Clin. Endocrinol. Metab. 2004, 89, 5482–5487. [Google Scholar] [CrossRef]
- Almada, M.; Cunha, S.; Fonseca, B.M.; Amaral, C.; Piscitelli, F.; Di Marzo, V.; Correia-da-Silva, G.; Teixeira, N. Anandamide Interferes with Human Endometrial Stromal-Derived Cell Differentiation: An Effect Dependent on Inhibition of Cyclooxygenase-2 Expression and Prostaglandin E2 Release. BioFactors 2016, 42, 277–286. [Google Scholar] [CrossRef]
- Lazzarin, N.; Valensise, H.; Bari, M.; Ubaldi, F.; Battista, N.; Finazzi-Agrò, A.; Maccarrone, M. Fluctuations of Fatty Acid Amide Hydrolase and Anandamide Levels during the Human Ovulatory Cycle. Gynecol. Endocrinol. 2004, 18, 212–218. [Google Scholar] [CrossRef]
- Maccarrone, M.; Valensise, H.; Bari, M.; Lazzarin, N.; Romanini, C.; Finazzi-Agrò, A. Relation between Decreased Anandamide Hydrolase Concentrations in Human Lymphocytes and Miscarriage. Lancet 2000, 355, 1326–1329. [Google Scholar] [CrossRef]
- Schuel, H. Tuning the Oviduct to the Anandamide Tone. J. Clin. Investig. 2006, 116, 2087–2090. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Wang, D.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; DuBois, R.N.; Dey, S.K. Aberrant Cannabinoid Signaling Impairs Oviductal Transport of Embryos. Nat. Med. 2004, 10, 1074–1080. [Google Scholar] [CrossRef]
- Horne, A.W.; Phillips, J.A.; Kane, N.; Lourenco, P.C.; McDonald, S.E.; Williams, A.R.W.; Simon, C.; Dey, S.K.; Critchley, H.O.D. CB1 Expression Is Attenuated in Fallopian Tube and Decidua of Women with Ectopic Pregnancy. PLoS ONE 2008, 3, e3969. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Fonseca, B.M.; Teixeira, N.; Correia-da-Silva, G. The Fundamental Role of the Endocannabinoid System in Endometrium and Placenta: Implications in Pathophysiological Aspects of Uterine and Pregnancy Disorders. Hum. Reprod. Update 2020, 26, 586–602. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Moreira-Pinto, B.; Costa, L.; Felgueira, E.; Oliveira, P.; Rebelo, I. Concentrations of the Endocannabinoid N-Arachidonoylethanolamine in the Follicular Fluid of Women with Endometriosis: The Role of M1 Polarised Macrophages. Reprod. Fertil. Dev. 2021, 33, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Cioffi, R.; Viganò, P.; Candiani, M.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Garavaglia, E.; Panina-Bordignon, P. Elevated Systemic Levels of Endocannabinoids and Related Mediators Across the Menstrual Cycle in Women With Endometriosis. Reprod. Sci. 2016, 23, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Resuehr, D.; Glore, D.R.; Taylor, H.S.; Bruner-Tran, K.L.; Osteen, K.G. Progesterone-Dependent Regulation of Endometrial Cannabinoid Receptor Type 1 (CB1-R) Expression Is Disrupted in Women with Endometriosis and in Isolated Stromal Cells Exposed to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD). Fertil. Steril. 2012, 98, 948–956.e1. [Google Scholar] [CrossRef] [PubMed]
- Fuchs Weizman, N.; Wyse, B.A.; Szaraz, P.; Defer, M.; Jahangiri, S.; Librach, C.L. Cannabis Alters Epigenetic Integrity and Endocannabinoid Signalling in the Human Follicular Niche. Hum. Reprod. 2021, 36, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Lai, E.C. Adult-Specific Functions of Animal MicroRNAs. Nat. Rev. Genet. 2013, 14, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Beermann, J.; Piccoli, M.-T.; Viereck, J.; Thum, T. Non-Coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, K.; Laudański, P.; Issat, T. The Role of Noncoding RNA in the Pathophysiology and Treatment of Premature Ovarian Insufficiency. Int. J. Mol. Sci. 2021, 22, 9336. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The Role of MiRNA in Polycystic Ovary Syndrome (PCOS). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tyc, K.M.; Wong, A.; Scott, R.T.; Tao, X.; Schindler, K.; Xing, J. Analysis of DNA Variants in MiRNAs and MiRNA 3’UTR Binding Sites in Female Infertility Patients. Lab. Investig. 2021, 101, 503–512. [Google Scholar] [CrossRef]
- Pankiewicz, K.; Fijałkowska, A.; Issat, T.; Maciejewski, T.M. Insight into the Key Points of Preeclampsia Pathophysiology: Uterine Artery Remodeling and the Role of MicroRNAs. Int. J. Mol. Sci. 2021, 22, 3132. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, E.; Zajkowska, A.; Bochowicz, A.; Pankiewicz, K.; Szewczyk, G.; Opolski, G.; Maciejewski, T.; Małecki, M.; Fijałkowska, A. Downregulated Expression of MicroRNAs Associated with Cardiac Hypertrophy and Fibrosis in Physiological Pregnancy and the Association with Echocardiographically-Evaluated Myocardial Function. Biomed. Rep. 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, P.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. MicroRNAs Expression Profiling of Eutopic Proliferative Endometrium in Women with Ovarian Endometriosis. Reprod. Biol. Endocrinol. RBE 2013, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, P.; Charkiewicz, R.; Tolwinska, A.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. Profiling of Selected MicroRNAs in Proliferative Eutopic Endometrium of Women with Ovarian Endometriosis. BioMed Res. Int. 2015, 2015, 760698. [Google Scholar] [CrossRef]
- Kolanska, K.; Bendifallah, S.; Canlorbe, G.; Mekinian, A.; Touboul, C.; Aractingi, S.; Chabbert-Buffet, N.; Daraï, E. Role of MiRNAs in Normal Endometrium and in Endometrial Disorders: Comprehensive Review. J. Clin. Med. 2021, 10, 3457. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.M.; Maier, A.C. MiRNAs and LncRNAs: Potential Non-Invasive Biomarkers for Endometriosis. Biomedicines 2021, 9, 1662. [Google Scholar] [CrossRef]
- Tamaru, S.; Kajihara, T.; Mizuno, Y.; Mizuno, Y.; Tochigi, H.; Ishihara, O. Endometrial MicroRNAs and Their Aberrant Expression Patterns. Med. Mol. Morphol. 2020, 53, 131–140. [Google Scholar] [CrossRef]
- Dominguez, F.; Moreno-Moya, J.M.; Lozoya, T.; Romero, A.; Martínez, S.; Monterde, M.; Gurrea, M.; Ferri, B.; Núñez, M.J.; Simón, C.; et al. Embryonic MiRNA Profiles of Normal and Ectopic Pregnancies. PLoS ONE 2014, 9, e102185. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yan, Q.; Xu, F.; Li, Y.; Zhao, W.; Wu, C.; Wang, Y.; Lang, X. MicroRNA-873 Is a Potential Serum Biomarker for the Detection of Ectopic Pregnancy. Cell. Physiol. Biochem. 2017, 41, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, S.; Gao, Z.-M.; Deng, P.; Wang, D.-B. Reduced MicroRNA-451 Expression in Eutopic Endometrium Contributes to the Pathogenesis of Endometriosis. World J. Clin. Cases 2019, 7, 2155–2164. [Google Scholar] [CrossRef]
- Ohlsson Teague, E.M.C.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-Regulated Pathways Associated with Endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Q.; Wang, Z.; Che, Y.; Zheng, S.; Chen, Y.; Zhong, X.; Shi, F. MiR-223: An Immune Regulator in Infectious Disorders. Front. Immunol. 2021, 12, 781815. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.R.; Daley, G.Q. Lin28: A MicroRNA Regulator with a Macro Role. Cell 2010, 140, 445–449. [Google Scholar] [CrossRef]
- Lozoya, T.; Domínguez, F.; Romero-Ruiz, A.; Steffani, L.; Martínez, S.; Monterde, M.; Ferri, B.; Núñez, M.J.; Romero-Espinós, A.; Zamora, O.; et al. The Lin28/Let-7 System in Early Human Embryonic Tissue and Ectopic Pregnancy. PLoS ONE 2014, 9, e87698. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zou, S.; Weijdegård, B.; Chen, J.; Cong, Q.; Fernandez-Rodriguez, J.; Wang, L.; Billig, H.; Shao, R. The Onset of Human Ectopic Pregnancy Demonstrates a Differential Expression of MiRNAs and Their Cognate Targets in the Fallopian Tube. Int. J. Clin. Exp. Pathol. 2014, 7, 64–79. [Google Scholar] [PubMed]
- Cho, S.; Mutlu, L.; Grechukhina, O.; Taylor, H.S. Circulating MicroRNAs as Potential Biomarkers for Endometriosis. Fertil. Steril. 2015, 103, 1252–1260.e1. [Google Scholar] [CrossRef] [PubMed]
- Grechukhina, O.; Petracco, R.; Popkhadze, S.; Massasa, E.; Paranjape, T.; Chan, E.; Flores, I.; Weidhaas, J.B.; Taylor, H.S. A Polymorphism in a Let-7 MicroRNA Binding Site of KRAS in Women with Endometriosis. EMBO Mol. Med. 2012, 4, 206–217. [Google Scholar] [CrossRef]
- Pokrovenko, D.A.; Vozniuk, V.; Medvediev, M.V. MicroRNA Let-7: A Promising Non-Invasive Biomarker for Diagnosing and Treating External Genital Endometriosis. Turk. J. Obstet. Gynecol. 2021, 18, 291–297. [Google Scholar] [CrossRef] [PubMed]
- De Santis, C.; Götte, M. The Role of MicroRNA Let-7d in Female Malignancies and Diseases of the Female Reproductive Tract. Int. J. Mol. Sci. 2021, 22, 7359. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ruiz, A.; Avendaño, M.S.; Dominguez, F.; Lozoya, T.; Molina-Abril, H.; Sangiao-Alvarellos, S.; Gurrea, M.; Lara-Chica, M.; Fernandez-Sanchez, M.; Torres-Jimenez, E.; et al. Deregulation of MiR-324/KISS1/Kisspeptin in Early Ectopic Pregnancy: Mechanistic Findings with Clinical and Diagnostic Implications. Am. J. Obstet. Gynecol. 2019, 220, 480.e1–480.e17. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Z.; Jiang, W.; Ling, Y.; Kuang, H. Reproductive Functions of Kisspeptin/KISS1R Systems in the Periphery. Reprod. Biol. Endocrinol. RBE 2019, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Abdelkareem, A.O.; Alotaibi, F.T.; AlKusayer, G.M.; Ait-Allah, A.S.; Rasheed, S.M.; Helmy, Y.A.; Allaire, C.; Peng, B.; Yong, P.J.; Bedaiwy, M.A. Immunoreactivity of Kisspeptin and Kisspeptin Receptor in Eutopic and Ectopic Endometrial Tissue of Women With and Without Endometriosis. Reprod. Sci. 2020, 27, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-C.; Ma, J.-X. The Role of MiR-324-3p in Polycystic Ovary Syndrome (PCOS) via Targeting WNT2B. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3286–3293. [Google Scholar] [CrossRef]
- Han, Y.; Hu, H.; Zhou, J. Knockdown of LncRNA SNHG7 Inhibited Epithelial-Mesenchymal Transition in Prostate Cancer Though MiR-324-3p/WNT2B Axis in Vitro. Pathol. Res. Pract. 2019, 215, 152537. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Załęcka, J.; Pankiewicz, K.; Issat, T.; Laudański, P. Molecular Mechanisms Underlying the Association between Endometriosis and Ectopic Pregnancy. Int. J. Mol. Sci. 2022, 23, 3490. https://doi.org/10.3390/ijms23073490
Załęcka J, Pankiewicz K, Issat T, Laudański P. Molecular Mechanisms Underlying the Association between Endometriosis and Ectopic Pregnancy. International Journal of Molecular Sciences. 2022; 23(7):3490. https://doi.org/10.3390/ijms23073490
Chicago/Turabian StyleZałęcka, Julia, Katarzyna Pankiewicz, Tadeusz Issat, and Piotr Laudański. 2022. "Molecular Mechanisms Underlying the Association between Endometriosis and Ectopic Pregnancy" International Journal of Molecular Sciences 23, no. 7: 3490. https://doi.org/10.3390/ijms23073490
APA StyleZałęcka, J., Pankiewicz, K., Issat, T., & Laudański, P. (2022). Molecular Mechanisms Underlying the Association between Endometriosis and Ectopic Pregnancy. International Journal of Molecular Sciences, 23(7), 3490. https://doi.org/10.3390/ijms23073490





