Salivary and Lacrimal Gland Alterations of the Epidermal Fatty Acid-Binding Protein (E-FABP) in Non-Obese Diabetic Mice †
Abstract
1. Introduction
2. Results
2.1. Aqueous Tear Production Alterations in the NOD and Wild Type Mice
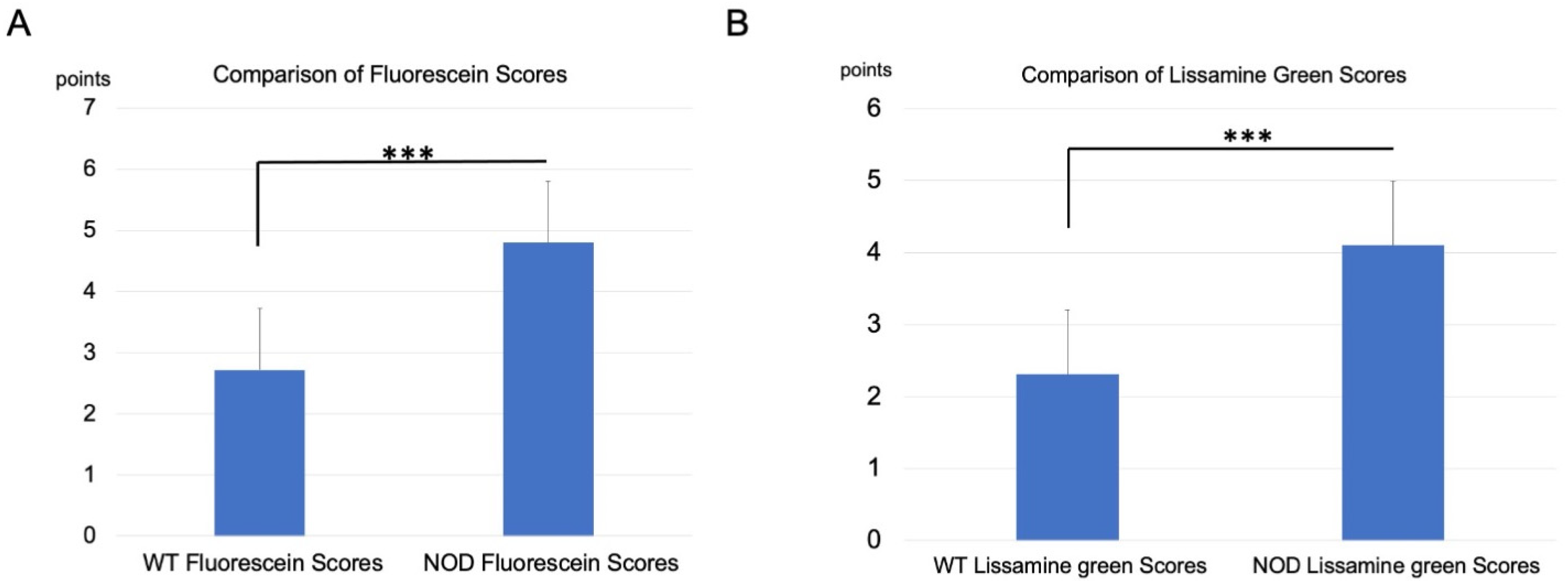
2.2. Corneal Vital Staining and Tear Film Stability Changes
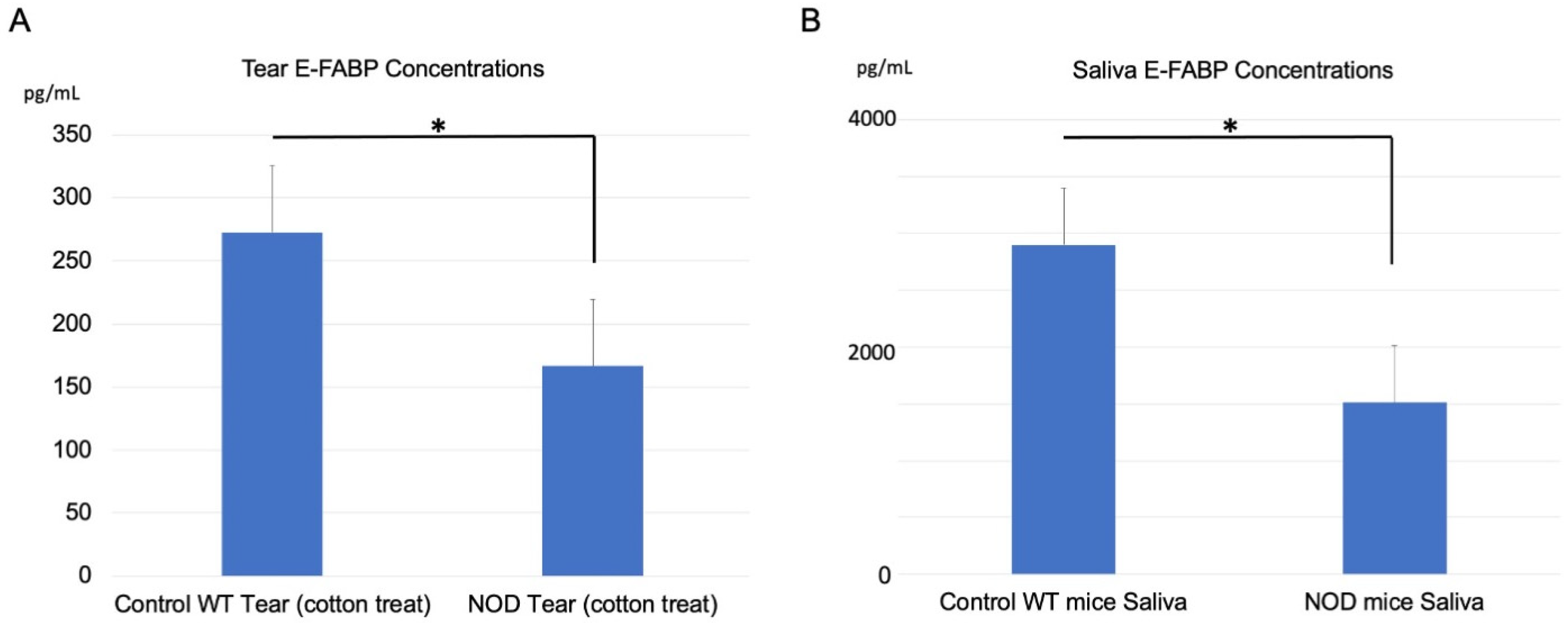
2.3. Comparison of E-FABP Concentration in the Tears and Saliva
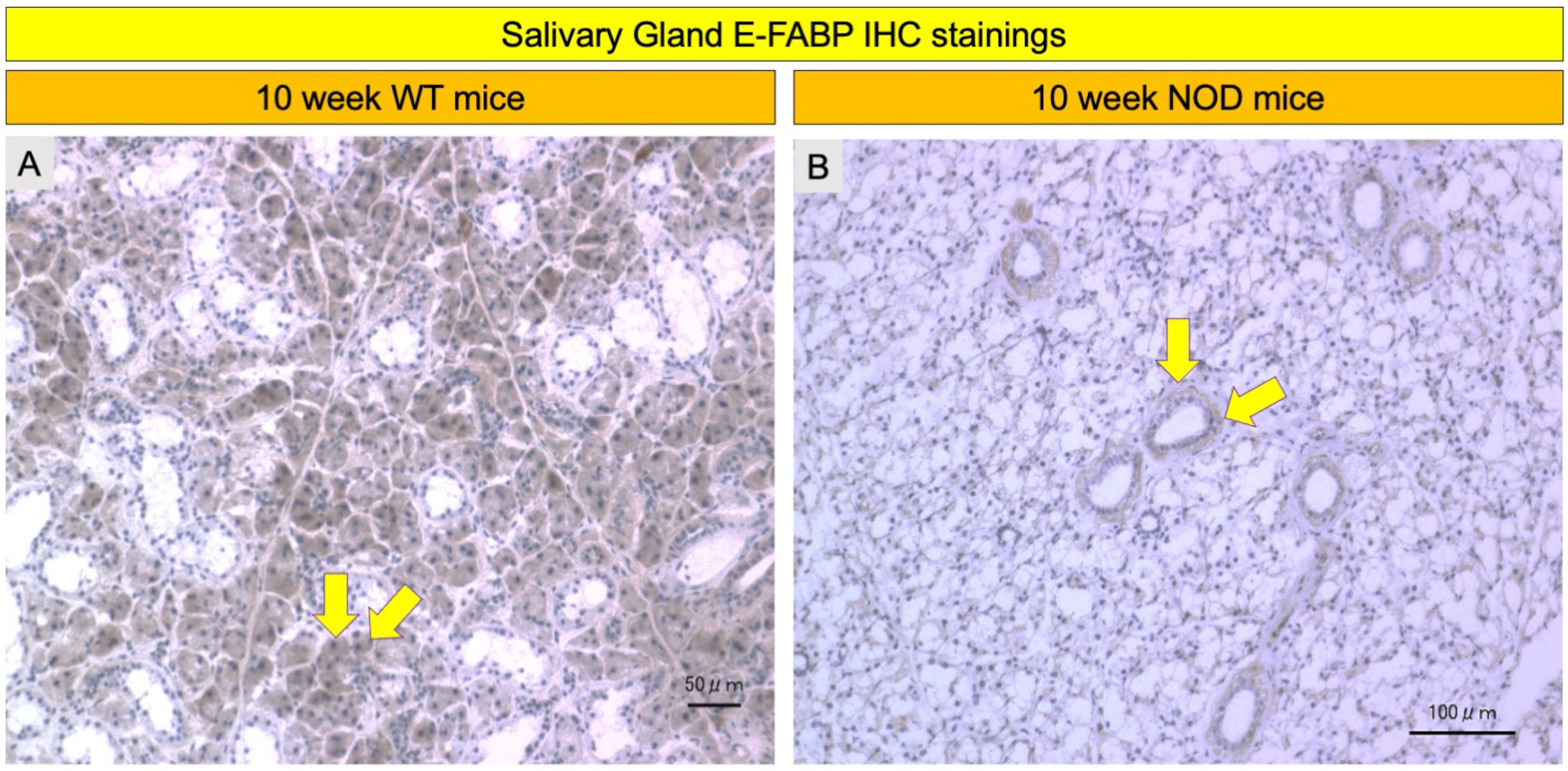
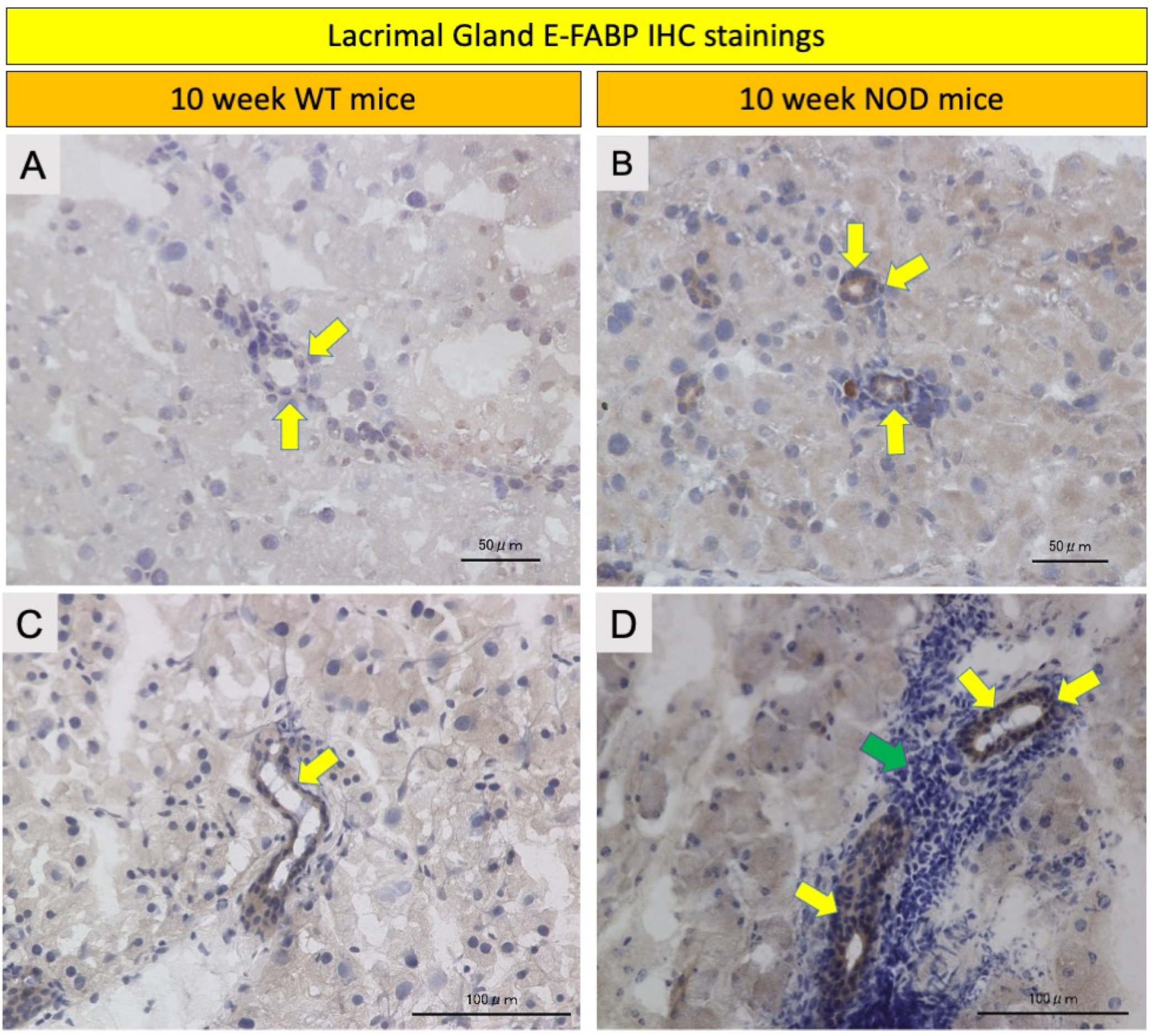
2.4. Comparison of E-FABP Marker Immunohistochemistry Stainings
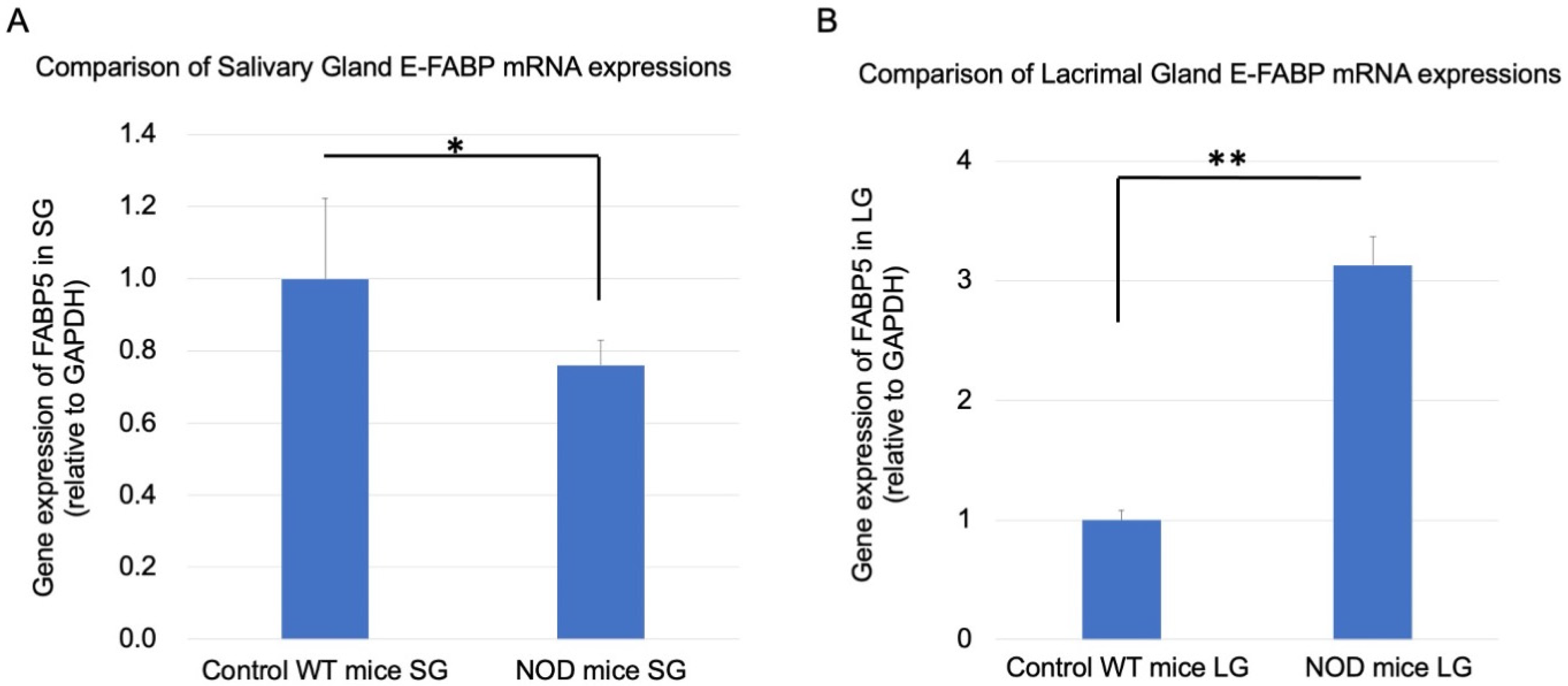
2.5. Comparison of Real-Time RT-PCR Lacrimal and Salivary Gland E-FABP mRNA Expressions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Aqueous Tear Production Measurements
4.3. Collection of Tears, Saliva, and Serum
4.4. Enzyme-Linked Immunosorbent Assay for E-FABP
4.5. Corneal Epithelial Cell Damage Evaluation
4.6. Immunohistochemistry Staining for E-FABP
4.7. Real-Time PCR for Lacrimal and Salivary Gland E-FABP mRNA Expression
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Carrasco, M.; Fuentes-Alexandro, S.; Escarcega, R.O.; Salgado, G.; Riebeling, C.; Cervera, R. Pathophysiology of Sjogren’s syndrome. Arch. Med. Res. 2006, 37, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, R.; Vogelsang, P.; Volchenkov, R.; Espinosa, A.; Wahren-Herlenius, M.; Appel, S. The complexity of Sjogren’s syndrome: Novel aspects on pathogenesis. Immunol. Lett. 2011, 141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; Singh, T.P. Sjogren’s syndrome: Review of the aetiology, Pathophysiology & Potential therapeutic interventions. J. Clin. Exp. Dent. 2017, 9, e584–e589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baldini, C.; Giusti, L.; Ciregia, F.; Da Valle, Y.; Giacomelli, C.; Donadio, E.; Sernissi, F.; Bazzichi, L.; Giannaccini, G.; Bombardieri, S.; et al. Proteomic analysis of saliva: A unique tool to distinguish primary Sjogren’s syndrome from secondary Sjogren’s syndrome and other sicca syndromes. Arthritis Res. Ther. 2011, 13, R194. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Vupputuri, A.; Sekhar, S.; Krishnan, S.; Venugopal, K.; Natarajan, K.U. Heart-type fatty acid-binding protein (H-FABP) as an early diagnostic biomarker in patients with acute chest pain. Indian Heart J. 2015, 67, 538–542. [Google Scholar] [CrossRef]
- Ohrfelt, A.; Andreasson, U.; Simon, A.; Zetterberg, H.; Edman, A.; Potter, W.; Holder, D.; Devanarayan, V.; Seeburger, J.; Smith, A.D.; et al. Screening for new biomarkers for subcortical vascular dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Dis. Extra 2011, 1, 31–42. [Google Scholar] [CrossRef]
- Smathers, R.L.; Petersen, D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 170–191. [Google Scholar] [CrossRef]
- Siegenthaler, G.; Hotz, R.; Chatellard-Gruaz, D.; Jaconi, S.; Saurat, J.H. Characterization and expression of a novel human fatty acid-binding protein: The epidermal type (E-FABP). Biochem. Biophys. Res. Commun. 1993, 190, 482–487. [Google Scholar] [CrossRef]
- Owada, Y.; Yoshimoto, T.; Kondo, H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J. Chem. Neuroanat. 1996, 12, 113–122. [Google Scholar] [CrossRef]
- O’Shaughnessy, R.F.; Seery, J.P.; Celis, J.E.; Frischauf, A.; Watt, F.M. PA-FABP, a novel marker of human epidermal transit amplifying cells revealed by 2D protein gel electrophoresis and cDNA array hybridisation. FEBS Lett. 2000, 486, 149–154. [Google Scholar] [CrossRef]
- Watanabe, R.; Fujii, H.; Odani, S.; Sakakibara, J.; Yamamoto, A.; Ito, M.; Ono, T. Molecular cloning of a cDNA encoding a novel fatty acid-binding protein from rat skin. Biochem. Biophys. Res. Commun. 1994, 200, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Beesley, C.; Foster, C.S.; Chen, H.; Rudland, P.S.; West, D.C.; Fujii, H.; Smith, P.H.; Ke, Y. Human cutaneous fatty acid-binding protein induces metastasis by up-regulating the expression of vascular endothelial growth factor gene in rat Rama 37 model cells. Cancer Res. 2001, 61, 4357–4364. [Google Scholar] [PubMed]
- Adamson, J.; Morgan, E.A.; Beesley, C.; Mei, Y.; Foster, C.S.; Fujii, H.; Rudland, P.S.; Smith, P.H.; Ke, Y. High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene 2003, 22, 2739–2749. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; Zimmerman, A.W. Fatty acid-binding proteins of nervous tissue. J. Mol. Neurosci. 2001, 16, 133–142; discussion 137–151. [Google Scholar] [CrossRef]
- Suojalehto, H.; Kinaret, P.; Kilpelainen, M.; Toskala, E.; Ahonen, N.; Wolff, H.; Alenius, H.; Puustinen, A. Level of Fatty Acid Binding Protein 5 (FABP5) Is Increased in Sputum of Allergic Asthmatics and Links to Airway Remodeling and Inflammation. PLoS ONE 2015, 10, e0127003. [Google Scholar] [CrossRef]
- Moore, S.M.; Holt, V.V.; Malpass, L.R.; Hines, I.N.; Wheeler, M.D. Fatty acid-binding protein 5 limits the anti-inflammatory response in murine macrophages. Mol. Immunol. 2015, 67, 265–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Rao, E.; Yan, F.; Li, Q.; Zhang, Y.; Silverstein, K.A.; Liu, S.; Sauter, E.; Cleary, M.P.; et al. Fatty acid-binding protein E-FABP restricts tumor growth by promoting IFN-beta responses in tumor-associated macrophages. Cancer Res. 2014, 74, 2986–2998. [Google Scholar] [CrossRef]
- Wakamatsu, T.H.; Dogru, M.; Matsumoto, Y.; Kojima, T.; Kaido, M.; Ibrahim, O.M.; Sato, E.A.; Igarashi, A.; Ichihashi, Y.; Satake, Y.; et al. Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 201–210. [Google Scholar] [CrossRef]
- Shinzawa, M.; Dogru, M.; Den, S.; Ichijima, T.; Higa, K.; Kojima, T.; Seta, N.; Nomura, T.; Tsubota, K.; Shimazaki, J. Epidermal Fatty Acid-Binding Protein: A Novel Marker in the Diagnosis of Dry Eye Disease in Sjogren Syndrome. Int. J. Mol. Sci. 2018, 19, 3463. [Google Scholar] [CrossRef]
- Masli, S.; Dartt, D.A. Mouse Models of Sjogren’s Syndrome with Ocular Surface Disease. Int. J. Mol. Sci. 2020, 21, 9112. [Google Scholar] [CrossRef]
- Xu, K.P.; Katagiri, S.; Takeuchi, T.; Tsubota, K. Biopsy of labial salivary glands and lacrimal glands in the diagnosis of Sjogren’s syndrome. J. Rheumatol. 1996, 23, 76–82. [Google Scholar] [PubMed]
- Delaleu, N.; Nguyen, C.Q.; Peck, A.B.; Jonsson, R. Sjogren’s syndrome: Studying the disease in mice. Arthritis Res. Ther. 2011, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model. Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef]
- Gonzales, J.A.; Shiboski, S.C.; Bunya, V.Y.; Akpek, E.K.; Rose-Nussbaumer, J.; Seitzman, G.D.; Criswell, L.A.; Shiboski, C.H.; Lietman, T.M. Ocular Clinical Signs and Diagnostic Tests Most Compatible With Keratoconjunctivitis Sicca: A Latent Class Approach. Cornea 2020, 39, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Owada, Y.; Suzuki, I.; Noda, T.; Kondo, H. Analysis on the phenotype of E-FABP-gene knockout mice. Mol. Cell. Biochem. 2002, 239, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Liu, Q.; Brittingham, K.C.; Liu, Y.; Gruenthal, M.; Gorgun, C.Z.; Hotamisligil, G.S.; Stout, R.D.; Suttles, J. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J. Immunol. 2007, 179, 313–321. [Google Scholar] [CrossRef]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- da Costa, G.; Lamy, E.; Capela e Silva, F.; Andersen, J.; Sales Baptista, E.; Coelho, A.V. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogru, M.; Kojima, T.; Simsek, C.; Nagata, T.; Tsubota, K. Salivary and Lacrimal Gland Alterations of the Epidermal Fatty Acid-Binding Protein (E-FABP) in Non-Obese Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 3491. https://doi.org/10.3390/ijms23073491
Dogru M, Kojima T, Simsek C, Nagata T, Tsubota K. Salivary and Lacrimal Gland Alterations of the Epidermal Fatty Acid-Binding Protein (E-FABP) in Non-Obese Diabetic Mice. International Journal of Molecular Sciences. 2022; 23(7):3491. https://doi.org/10.3390/ijms23073491
Chicago/Turabian StyleDogru, Murat, Takashi Kojima, Cem Simsek, Taeko Nagata, and Kazuo Tsubota. 2022. "Salivary and Lacrimal Gland Alterations of the Epidermal Fatty Acid-Binding Protein (E-FABP) in Non-Obese Diabetic Mice" International Journal of Molecular Sciences 23, no. 7: 3491. https://doi.org/10.3390/ijms23073491
APA StyleDogru, M., Kojima, T., Simsek, C., Nagata, T., & Tsubota, K. (2022). Salivary and Lacrimal Gland Alterations of the Epidermal Fatty Acid-Binding Protein (E-FABP) in Non-Obese Diabetic Mice. International Journal of Molecular Sciences, 23(7), 3491. https://doi.org/10.3390/ijms23073491







