Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New?
Abstract
1. Introduction
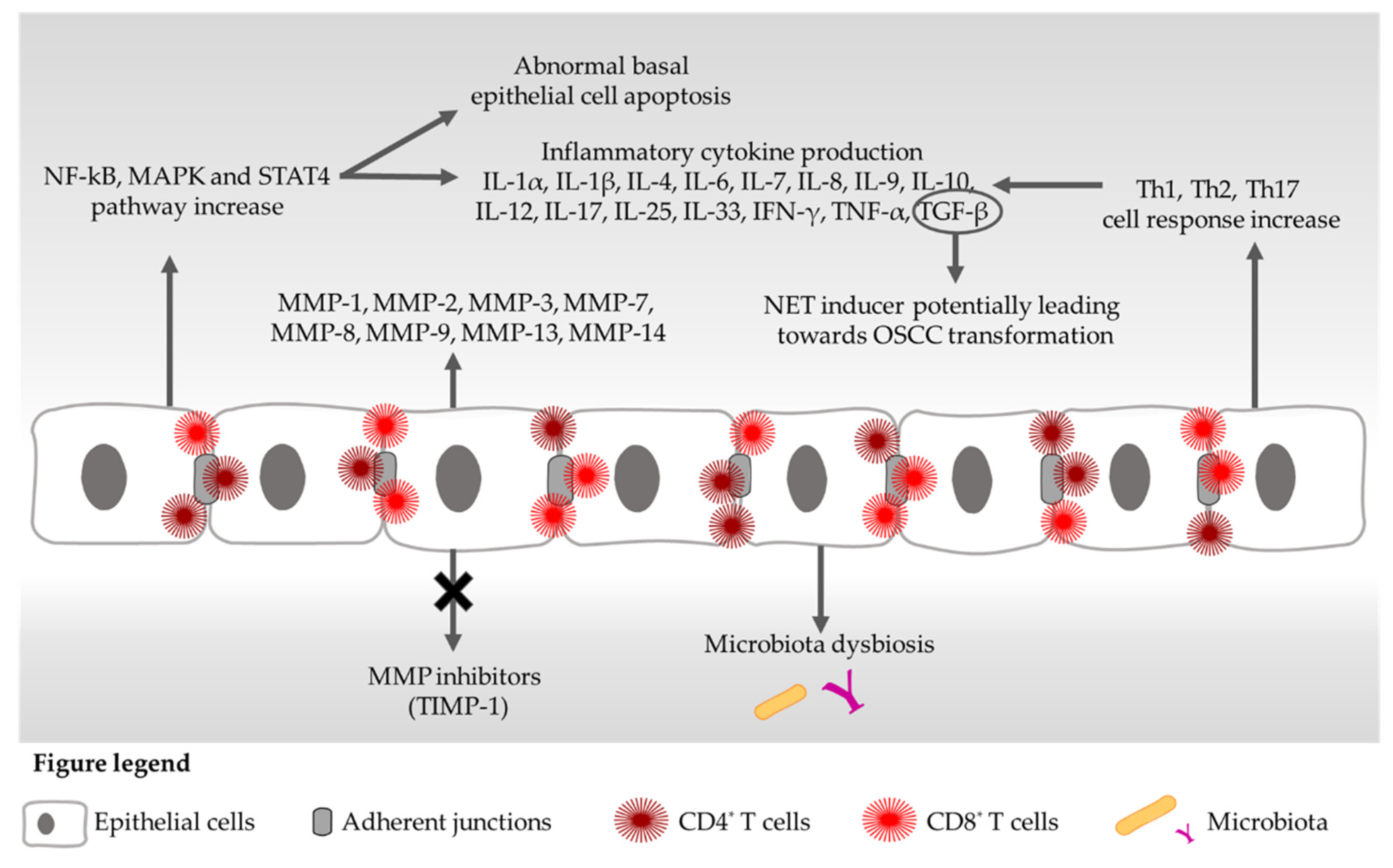
2. Cytokines and OLP
3. Matrix Metalloproteinases and OLP
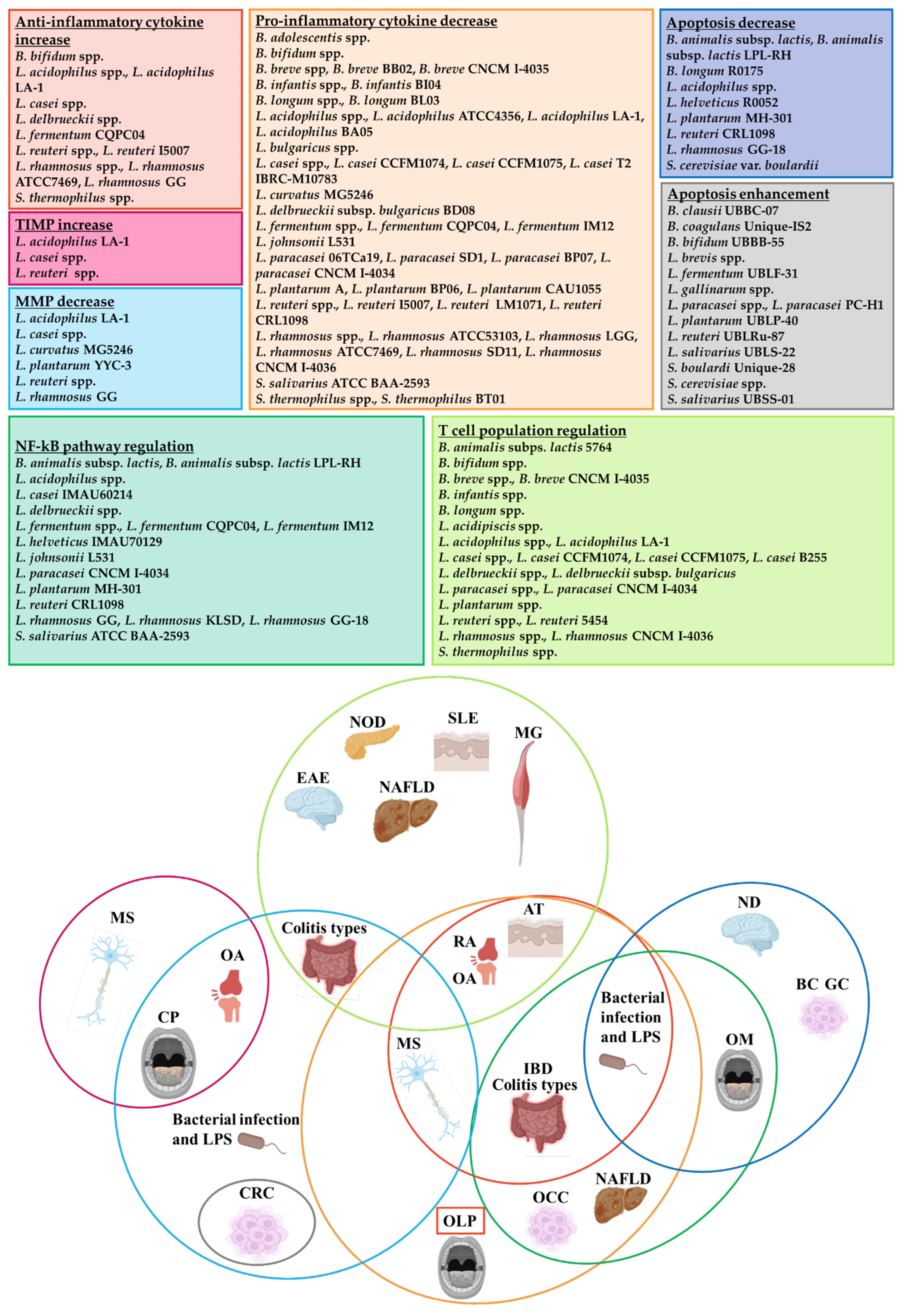
4. Probiotic Effects on Cytokine/MMP-Mediated Signalling Pathways
5. Probiotics and NF-kB Pathway: Evidence from Autoimmune Diseases and OLP
6. OLP Apoptotic Pathways and Probiotics
7. Probiotics, T Cells and Autoimmunity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Castells, E.; Figueiredo, R.; Berini-Aytes, L.; Gay-Escoda, C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e685–e690. [Google Scholar] [CrossRef] [PubMed]
- Bombeccari, G.P.; Guzzi, G.; Tettamanti, M.; Giannì, A.B.; Baj, A.; Pallotti, F.; Spadari, F. Oral lichen planus and malignant transformation: A longitudinal cohort study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 328–334. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Daume, L.; Kreis, C.; Bohner, L.; Kleinheinz, J.; Jung, S. Does the Clinical Form of Oral Lichen Planus (OLP) Influence the Oral Health–Related Quality of Life (OHRQoL)? Int. J. Environ. Res. Public Health 2020, 17, 6633. [Google Scholar] [CrossRef]
- Cheng, Y.-S.L.; Gould, A.; Kurago, Z.; Fantasia, J.; Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 332–354. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Villa, T.G.; Sánchez-Pérez, Á.; Sieiro, C. Oral lichen planus: A microbiologist point of view. Int. Microbiol. 2021, 24, 275–289. [Google Scholar] [CrossRef]
- Hallopeau, H. Sur un cas de lichen de Wilson gingival avec neoplastic voisine dans la region maxillaire. Bull. Soc. Fr. Dermatol. Syphiligr. 1910, 17, 32. [Google Scholar]
- Barnes, L.; Eveson, J.; Reichart, P.; Sidransky, D. Pathology and Genetics of Head and Neck Tumours, International Agency for Research on Cancer (IARC), 3rd ed.; IARC Press: Lyon, France, 2005. [Google Scholar]
- Liu, Y.; Messadi, D.V.; Wu, H.; Hu, S. Oral lichen planus is a unique disease model for studying chronic inflammation and oral cancer. Med. Hypotheses 2010, 75, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2021, 50, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; González-Moles, M.Á.; Warnakulasuriya, S. Oral cancer development in lichen planus and related conditions-3.0 evidence level: A systematic review of systematic reviews. Oral Dis. 2021, 27, 1919–1935. [Google Scholar] [CrossRef]
- Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Zhao, Z.Z.; Zhou, X.J.; Khan, A.; Seymour, G.J.; Bigby, M. The pathogenesis of oral lichen planus. Crit. Rev. Oral Biol. Med. 2002, 13, 350–365. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Tan, Y.; Zhou, G. Probiotics: A non-conventional therapy for oral lichen planus. Arch. Oral Biol. 2017, 81, 90–96. [Google Scholar] [CrossRef]
- Roopashree, M.R.; Gondhalekar, R.V.; Shashikanth, M.C.; George, J.; Thippeswamy, S.H.; Shukla, A. Pathogenesis of oral lichen planus-a review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef]
- Liu, J.; Geng, F.; Sun, H.; Wang, X.; Zhang, H.; Yang, Q.; Zhang, J. Candida albicans induces TLR2/MyD88/NF-κB signaling and inflammation in oral lichen planus-derived keratinocytes. J. Infect. Dev. Ctries. 2018, 12, 780–786. [Google Scholar] [CrossRef]
- Lodi, G.; Scully, C.; Carrozzo, M.; Griffiths, M.; Sugerman, P.B.; Thongprasom, K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 100, 40–51. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Sun, W.; Du, G.; Zhou, G. Inflammation-related cytokines in oral lichen planus: An overview. J. Oral Pathol. Med. 2015, 44, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Shao, F.; Zheng, S.; Tan, Z.; He, Y. Alteration of Streptococcus salivarius in Buccal Mucosa of Oral Lichen Planus and Controlled Clinical Trial in OLP Treatment. Probiotics Antimicrob. Proteins 2020, 12, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, M.; Lucchese, A.; Lajolo, C.; Campus, G.; Lauritano, D.; Serpico, R. Topical Retinoids in Oral Lichen Planus Treatment: An Overview. Dermatology 2013, 226, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Malik, U.; Gupta, S.; Malik, S.D.; Vashishth, S.; Zaheeruddin; Raju, M.S. Treatment of symptomatic oral lichen planus (OLP) with 0.1% tacrolimus powder in Oraguard-B–A pilot prospective study. Saudi Dent. J. 2012, 24, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune System Stimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef]
- Jung, J.-I.; Baek, S.-M.; Nguyen, T.H.; Kim, J.W.; Kang, C.-H.; Kim, S.; Imm, J.-Y. Effects of Probiotic Culture Supernatant on Cariogenic Biofilm Formation and RANKL-Induced Osteoclastogenesis in RAW 264.7 Macrophages. Molecules 2021, 26, 733. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Cámara, M.; Verma, C.; Eremin, O.; Kulkarni, A.D.; Lobo, D.N. Modulation of T Regulatory and Dendritic Cell Phenotypes Following Ingestion of Bifidobacterium longum, AHCC® and Azithromycin in Healthy Individuals. Nutrients 2019, 11, 2470. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, F.; Ma, H.; Chen, S. Exopolysaccharide-Producing Bifidobacterium adolescentis Strains with Similar Adhesion Property Induce Differential Regulation of Inflammatory Immune Response in Treg/Th17 Axis of DSS-Colitis Mice. Nutrients 2019, 11, 782. [Google Scholar] [CrossRef]
- Fan, Z.; Ross, R.P.; Stanton, C.; Hou, B.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus casei CCFM1074 Alleviates Collagen-Induced Arthritis in Rats via Balancing Treg/Th17 and Modulating the Metabolites and Gut Microbiota. Front. Immunol. 2021, 12, 680073. [Google Scholar] [CrossRef]
- Wang, Y.; Du, G.; Shi, L.; Shen, X.; Shen, Z.; Liu, W. Altered expression of CCN1 in oral lichen planus associated with keratinocyte activation and IL-1β, ICAM1, and CCL5 up-regulation. J. Oral Pathol. Med. 2020, 49, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Du, R.; Hong, Y.; Jia, L.; Zeng, Q.; Cheng, B. IL-1 Alpha Regulates CXCL1, CXCL10 and ICAM1 in Network Form in Oral Keratinocytes. Clin. Lab. 2013, 59, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Javvadi, L.R.; Parachuru, V.P.B.; Milne, T.J.; Seymour, G.J.; Rich, A.M. Expression of IL33 and IL35 in oral lichen planus. Arch. Dermatol. Res. 2018, 310, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Cheng, B.; Myers, S.; Bowles, W.; Ho, V.; Ondrey, F. A comparison of the pro-inflammatory, NF-κB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clin. Immunol. 2005, 114, 278–283. [Google Scholar] [CrossRef]
- Abboud, C.S.; da Brandão, E.H.S.; Cunha, K.R.L.; Sousa Brito, K.; de Gallo, C.B.; Molon, A.C.; Horliana, A.C.R.T.; Franco, A.S.L.; Thongprasom, K.; Rodrigues, M.F.S.D. Serum and salivary cytokines in patients with oral lichen planus treated with Photobiomodulation. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Chiamulera, M.M.A.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef]
- Dikova, V.; Principe, S.; Bagan, J. Salivary inflammatory proteins in patients with oral potentially malignant disorders. J. Clin. Exp. Dent. 2019, 11, e659–e664. [Google Scholar] [CrossRef]
- Mehrbani, S.; Motahari, P.; Azar, F.; Ahari, M. Role of interleukin-4 in pathogenesis of oral lichen planus: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2020, e410–e415. [Google Scholar] [CrossRef]
- Mozaffari, H.R.; Zavattaro, E.; Saeedi, M.; Lopez-Jornet, P.; Sadeghi, M.; Safaei, M.; Imani, M.M.; Nourbakhsh, R.; Moradpoor, H.; Golshah, A.; et al. Serum and salivary interleukin-4 levels in patients with oral lichen planus: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 123–131. [Google Scholar] [CrossRef]
- Humberto, J.S.M.; Pavanin, J.V.; da Rocha, M.J.A.; Motta, A.C.F. Cytokines, cortisol, and nitric oxide as salivary biomarkers in oral lichen planus: A systematic review. Braz. Oral Res. 2018, 32, e82. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Wang, H.; Luo, Z.; Wang, Y.; Guan, X. IL-25 promotes Th2-type reactions and correlates with disease severity in the pathogenesis of oral lichen planus. Arch. Oral Biol. 2019, 98, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zeng, J.; Sroussi, H.; Yu, J.; Xu, J.; Cheng, X.; Fan, Y. Interactions between Golli-MBP and Th1/Th2 cytokines in patients with oral lichen planus. Oral Dis. 2014, 20, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Z.; He, M.-J.; Long, L.; Mu, D.-L.; Xu, M.-S.; Xing, X.; Zeng, X.; Liao, G.; Dan, H.-X.; Chen, Q.-M. Interferon-γ and interleukin-4 detected in serum and saliva from patients with oral lichen planus. Int. J. Oral Sci. 2014, 6, 22–26. [Google Scholar] [CrossRef]
- Mozaffari, H.R.; Sharifi, R.; Hayati, M.; Imani, M.M.; Lopez-Jornet, P.; Golshah, A.; Moradpoor, H.; Rezaei, R.; Sadeghi, M. Evaluation of serum and salivary interferon-γ levels in patients with oral lichen planus: A systematic review and meta-analysis of case-control studies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Maehara, T.; Moriyama, M.; Kawano, S.; Hayashida, J.-N.; Furukawa, S.; Ohta, M.; Tanaka, A.; Yamauchi, M.; Ohyama, Y.; Kiyoshima, T.; et al. Cytokine Profiles Contribute to Understanding the Pathogenic Difference Between Good Syndrome and Oral Lichen Planus. Medicine 2015, 94, e704. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, Q.; Deng, Y.; Wang, Y.; Du, G.; Song, C.; Li, C.; Zhu, M.; Chen, G.; Tang, G. Mixed and inhomogeneous expression profile of Th1/Th2 related cytokines detected by cytometric bead array in the saliva of patients with oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 142–151. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Liu, Q.; Tan, J.; Hu, X.; Wang, J.; Wang, Q.; Wang, X. The cellular character of liquefaction degeneration in oral lichen planus and the role of interferon gamma. J. Oral Pathol. Med. 2017, 46, 1015–1022. [Google Scholar] [CrossRef]
- Malekzadeh, H.; Robati, M.; Yousefimanesh, H.; Ghafourian Boroujerdnia, M.; Nadripour, R. Salivary Interferon Gamma and Interleukin-4 Levels in Patients Suffering from Oral Lichen Planus. Cell J. 2015, 17, 554–558. [Google Scholar] [CrossRef]
- Liu, W.; Dan, H.; Wang, Z.; Jiang, L.; Zhou, Y.; Zhao, M.; Chen, Q.; Zeng, X. IFN-Gamma and IL-4 in Saliva of Patients with Oral Lichen Planus: A Study in an Ethnic Chinese Population. Inflammation 2009, 32, 176–181. [Google Scholar] [CrossRef]
- Mozaffari, H.R.; Molavi, M.; Lopez-Jornet, P.; Sadeghi, M.; Safaei, M.; Imani, M.M.; Sharifi, R.; Moradpoor, H.; Golshah, A.; Jamshidy, L. Salivary and Serum Interferon-Gamma/Interleukin-4 Ratio in Oral Lichen Planus Patients: A Systematic Review and Meta-Analysis. Medicina 2019, 55, 257. [Google Scholar] [CrossRef]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, M.; Zhang, S.; Wang, Z.; Jiang, L.; Shen, J.; Bai, J.; Gao, F.; Zhou, M.; Chen, Q. NF-κB-dependent cytokines in saliva and serum from patients with oral lichen planus: A study in an ethnic Chinese population. Cytokine 2008, 41, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Cheng, B.; Myers, S.; Miller, L.; Ho, V.; Ondrey, F. The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol. Carcinog. 2005, 44, 77–82. [Google Scholar] [CrossRef]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171. [Google Scholar] [CrossRef]
- Abdel-Haq, A.; Kusnierz-Cabala, B.; Darczuk, D.; Sobuta, E.; Dumnicka, P.; Wojas-Pelc, A.; Chomyszyn-Gajewska, M. Interleukin-6 and neopterin levels in the serum and saliva of patients with Lichen Planus and oral Lichen Planus. J. Oral Pathol. Med. 2014, 43, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Q.; Yang, S.; Wang, Q.; Xu, J.; Guo, B. The relationship between levels of salivary and serum interleukin-6 and oral lichen planus: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 743–749.e9. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.R.; Sharifi, R.; Sadeghi, M. Interleukin-6 levels in the serum and saliva of patients with oral lichen planus compared with healthy controls: A meta-analysis study. Cent. Eur. J. Immunol. 2018, 43, 103–108. [Google Scholar] [CrossRef]
- Xu, X.-H.; Liu, Y.; Feng, L.; Yang, Y.-S.; Liu, S.-G.; Guo, W.; Zhou, H.-X.; Li, Z.-Q.; Zhang, L.; Meng, W.-X. Interleukin-6 released by oral lichen planus myofibroblasts promotes angiogenesis. Exp. Ther. Med. 2021, 21, 291. [Google Scholar] [CrossRef]
- Yin, M.; Li, G.; Song, H.; Lin, S. Identifying the association between interleukin-6 and lichen planus: A meta-analysis. Biomed. Rep. 2017, 6, 571–575. [Google Scholar] [CrossRef][Green Version]
- Shahidi, M.; Jafari, S.; Barati, M.; Mahdipour, M.; Gholami, M.S. Predictive value of salivary microRNA-320a, vascular endothelial growth factor receptor 2, CRP and IL-6 in Oral lichen planus progression. Inflammopharmacology 2017, 25, 577–583. [Google Scholar] [CrossRef]
- Mozaffari, H.R.; Ramezani, M.; Mahmoudiahmadabadi, M.; Omidpanah, N.; Sadeghi, M. Salivary and serum levels of tumor necrosis factor-alpha in oral lichen planus: A systematic review and meta-analysis study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, e183–e189. [Google Scholar] [CrossRef]
- Malarkodi, T.; Sathasivasubramanian, S. Quantitative Analysis of Salivary TNF- α in Oral Lichen Planus Patients. Int. J. Dent. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Wang, J.T.; Chia, J.S.; Chiang, C.P. Serum interleukin-8 level is a more sensitive marker than serum interleukin-6 level in monitoring the disease activity of oral lichen planus. Br. J. Dermatol. 2005, 152, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Toader, M.P.; Taranu, T.; Constantin, M.M.; Olinici, D.; Mocanu, M.; Costan, V.V.; Toader, S. High serum level of interleukin-6 is linked with dyslipidemia in oral lichen planus. Exp. Ther. Med. 2021, 22, 987. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Y.; Sun, Q.; Jiang, C.; Zhu, M.; Song, C.; Li, C.; Du, G.; Deng, Y.; Nie, H.; et al. Enhanced T-cell proliferation and IL-6 secretion mediated by overexpression of TRIM21 in oral lesions of patients with oral lichen planus. J. Oral Pathol. Med. 2020, 49, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wei, W.; Wang, Y.; Song, C.; Pan, L.; Sun, K.; Du, G.; Deng, Y.; Tang, G. TRIM21 causes abnormal expression of IL-6 in oral lichen planus via the TRIB2-MAPK signal axis. Am. J. Transl. Res. 2020, 12, 4648–4658. [Google Scholar]
- Tavangar, A.; Khozeimeh, F.; Ghoreishian, F.; Boroujeni, M. Serum level of Interleukin-8 in subjects with diabetes, diabetes plus oral lichen planus, and oral lichen planus: A biochemical study. Dent. Res. J. 2016, 13, 413. [Google Scholar] [CrossRef]
- Tavangar, A.; Ghalayani, P.; Boroujeni, M.; Ghoreishian, F. Salivary levels of interleukin-8 in oral lichen planus and diabetic patients: A biochemical study. Dent. Res. J. 2017, 14, 209. [Google Scholar] [CrossRef]
- Pekiner, F.N.; Demirel, G.Y.; Borahan, M.O.; Özbayrak, S. Cytokine profiles in serum of patients with oral lichen planus. Cytokine 2012, 60, 701–706. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, Y.; Zhang, Z.; Li, W.; Ji, X.; Liu, X.; Jin, J.; Xu, S.; Cui, H.; Cheng, Z.; et al. Total glucosides of paeony improves the immunomodulatory capacity of MSCs partially via the miR-124/STAT3 pathway in oral lichen planus. Biomed. Pharmacother. 2018, 105, 151–158. [Google Scholar] [CrossRef]
- Jablonska, E.; Garley, M.; Surazynski, A.; Grubczak, K.; Iwaniuk, A.; Borys, J.; Moniuszko, M.; Ratajczak-Wrona, W. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus–Possible implications for the development of oral cancer. Immunobiology 2020, 225, 151901. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zeng, X.; Han, Q.; Lin, M.; Long, L.; Dan, H.; Zhou, G.; Chen, Q. Overexpression and Selectively Regulatory Roles of IL-23/IL-17 Axis in the Lesions of Oral Lichen Planus. Mediat. Inflamm. 2014, 2014, 701094. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Gao, X.; Ma, L.; Zhou, Z.; Shen, X.; Liu, W. Expression of Foxp3 and interleukin-17 in lichen planus lesions with emphasis on difference in oral and cutaneous variants. Arch. Dermatol. Res. 2014, 306, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, C.; Zhou, Z.; Liu, W.; Shi, L.; Shen, X. Altered expression of interleukin-17A and its targeting microRNAs in oral lichen planus: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 619–624.e1. [Google Scholar] [CrossRef]
- Ge, X.; Xie, H.; Nguyen, T.; Zhao, B.; Xu, J.; Du, J. Renin Promotes STAT4 Phosphorylation to Induce IL-17 Production in Keratinocytes of Oral Lichen Planus. iScience 2020, 23, 100983. [Google Scholar] [CrossRef]
- Javvadi, L.R.; Parachuru, V.P.B.; Milne, T.J.; Seymour, G.J.; Rich, A.M. Regulatory T-cells and IL17A(+) cells infiltrate oral lichen planus lesions. Pathology 2016, 48, 564–573. [Google Scholar] [CrossRef]
- Yang, J.; Tan, Y.; Zhou, G. T cell-derived exosomes containing cytokines induced keratinocytes apoptosis in oral lichen planus. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Zhang, J.; Ma, J.-Z.; Liang, X.-Y.; Chen, G.-Y.; Lu, R.; Du, G.-F.; Zhou, G. MicroRNA-155-IFN-γ Feedback Loop in CD4+T Cells of Erosive type Oral Lichen Planus. Sci. Rep. 2015, 5, 16935. [Google Scholar] [CrossRef]
- Tao, X.; Li, C.; Rhodus, N.L.; Xia, J.; Yang, X.; Cheng, B. Simultaneous detection of IFN-gamma and IL-4 in lesional tissues and whole unstimulated saliva from patients with oral lichen planus. J. Oral Pathol. Med. 2008, 37, 83–87. [Google Scholar] [CrossRef]
- Sun, A.; Wu, Y.-H.; Chang, J.Y.-F.; Wang, Y.-P.; Chiang, C.-P.; Chia, J.-S. FoxP3+CD4+, IFN-γ+CD4+, and IFN-γ+CD8+ cell levels in erosive and non-erosive types of oral lichen planus patients. J. Dent. Sci. 2021, 16, 751–756. [Google Scholar] [CrossRef]
- Gu, G.M.; Martin, M.D.; Darveau, R.P.; Truelove, E.; Epstein, J. Oral and serum IL-6 levels in oral lichen planus patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 673–678. [Google Scholar] [CrossRef] [PubMed]
- de Monteiro, B.V.B.; dos Pereira, J.S.; Nonaka, C.F.W.; Godoy, G.P.; da Silveira, É.J.D.; da Miguel, M.C.C. Immunoexpression of Th17-related Cytokines in Oral Lichen Planus. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Pouralibaba, F.; Babaloo, Z.; Pakdel, F.; Aghazadeh, M. Serum Level of Interleukin 17 in Patients with Erosive and Non erosive Oral Lichen Planus. J. Dent. Res. Dent. Clin. Dent. Prospects 2013, 7, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.; Cheng, B.; Bowles, W.; Myers, S.; Miller, L.; Ondrey, F. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006, 12, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, N.A.; El-Wakeel, N.; Shaker, O.G. Levels of Salivary IFN-gamma, TNF-Alfa, and TNF Receptor-2 As Prognostic Markers in (Erosive) Oral Lichen Planus. Mediat. Inflamm. 2010, 2010, 847632. [Google Scholar] [CrossRef]
- Ge, X.; Wang, L.; Li, M.; Xu, N.; Yu, F.; Yang, F.; Li, R.; Zhang, F.; Zhao, B.; Du, J. Vitamin D/VDR signaling inhibits LPS-induced IFNγ and IL-1β in Oral epithelia by regulating hypoxia-inducible factor-1α signaling pathway. Cell Commun. Signal. 2019, 17, 18. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; Wang, X.; Liu, M.; Li, L.; Yu, X.; Wang, Y.; Wu, Y.; Wang, W.; Gao, M.; et al. Study on the Role of Salivary Flora and NF-κB Inflammatory Signal Pathway in Oral Lichen Planus. Inflammation 2020, 43, 994–1008. [Google Scholar] [CrossRef]
- de Carvalho, M.F.M.S.; Cavalieri, D.; Do Nascimento, S.; Lourenço, T.G.B.; Ramos, D.V.R.; da Pasqualin, D.C.; Martins, L.A.L.; Rocha, F.A.; Heller, D.; Marti, L. Cytokines Levels and Salivary Microbiome Play A Potential Role in Oral Lichen Planus Diagnosis. Sci. Rep. 2019, 9, 18137. [Google Scholar] [CrossRef]
- Wang, K.; Miao, T.; Lu, W.; He, J.; Cui, B.; Li, J.; Li, Y.; Xiao, L. Analysis of oral microbial community and Th17-associated cytokines in saliva of patients with oral lichen planus. Microbiol. Immunol. 2015, 59, 105–113. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Xiong, X.; Yu, T.; Wang, X.; Meng, W.; Wang, H.; Luo, G.; Ge, L. Oral lichen-planus-associated fibroblasts acquire myofibroblast characteristics and secrete pro-inflammatory cytokines in response to Porphyromonas gingivalis lipopolysaccharide stimulation. BMC Oral Health 2018, 18, 197. [Google Scholar] [CrossRef]
- Zanetta, P.; Squarzanti, D.F.; Sorrentino, R.; Rolla, R.; Aluffi Valletti, P.; Garzaro, M.; Dell’Era, V.; Amoruso, A.; Azzimonti, B. Oral microbiota and vitamin D impact on oropharyngeal squamous cell carcinogenesis: A narrative literature review. Crit. Rev. Microbiol. 2021, 47, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, Z.; Lei, L.; Sun, Z.; Zhou, M.; Dan, H.; Zeng, X.; Chen, Q. Interaction Between Oral Lichen Planus and Chronic Periodontitis with Th17-Associated Cytokines in Serum. Inflammation 2013, 36, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, Q.; Luo, Z.; Xu, C.; Liu, J.; Dan, H.; Xu, Y.; Zeng, X.; Chen, Q. Oral lichen planus may enhance the expression of Th17-associated cytokines in local lesions of chronic periodontitis. Clin. Oral Investig. 2014, 18, 1647–1654. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Geng, N.; Tian, K.; Jack Windsor, L. MMPs, TIMP-2, and TGF-β1 in the cancerization of oral lichen planus. Head Neck 2008, 30, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Arduino, P.G.; Maggiora, M.; Curmei, E.; Manavella, V.; Broccoletti, R.; Aimetti, M. Effect of a structured plaque control on MMP-1 and MMP-9 crevicular levels in patients with desquamative gingivitis associated with oral lichen planus. Clin. Oral Investig. 2019, 23, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, A.S.; Dursun, R.; Dundar, N.; Avunduk, M.C.; Hakki, S.S. MMP-1, MMP-9, and TIMP-1 levels in oral lichen planus patients with gingivitis or periodontitis. Arch. Oral Biol. 2013, 58, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Paulusová, V.; Laco, J.; Dřízhal, I.; Slezák, R. Expression of Matrix Metalloproteinase 9 in Patients with Oral Lichen Planus. Acta Medica 2012, 55, 23–26. [Google Scholar] [CrossRef]
- Venugopal, A.; Uma Maheswari, T. Expression of matrix metalloproteinase-9 in oral potentially malignant disorders: A systematic review. J. Oral Maxillofac. Pathol. 2016, 20, 474. [Google Scholar] [CrossRef]
- Wang, H.; Guan, X.; Luo, Z.; Liu, Y.; Ren, Q.; Zhao, X. The association and potentially destructive role of Th9/IL-9 is synergistic with Th17 cells by elevating MMP9 production in local lesions of oral lichen planus. J. Oral Pathol. Med. 2018, 47, 425–433. [Google Scholar] [CrossRef]
- Hazzaa, H.H.; El Shiekh, M.A.M.; Abdelgawad, N.; Gouda, O.M.; Kamal, N.M. Correlation of VEGF and MMP-2 levels in oral lichen planus: An in vivo immunohistochemical study. J. Oral Biol. Craniofac. Res. 2020, 10, 747–752. [Google Scholar] [CrossRef]
- Agarwal, N.; Carnelio, S.; Rodrigues, G. Immunohistochemical and clinical significance of matrix metalloproteinase-2 and its inhibitor in oral lichen planus. J. Oral Maxillofac. Pathol. 2019, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, H.; Xiao, Y.; Wang, L. miR-125b inhibits keratinocyte proliferation and promotes keratinocyte apoptosis in oral lichen planus by targeting MMP-2 expression through PI3 K/Akt/mTOR pathway. Biomed. Pharmacother. 2016, 80, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Mahboobi, N.; Shirazian, S.; Harirchi, I. Serum and Saliva MMP-3 in Patients with OLP and Oral SCC. J. Contemp. Dent. Pract. 2015, 16, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Farzin, M.; Mardani, M.; Ghabanchi, J.; Fattahi, M.J.; Rezaee, M.; Heydari, S.T.; Andisheh Tadbir, A. Serum level of matrix metalloproteinase-3 in patients with oral lichen planus. Iran. Red Crescent Med. J. 2012, 14, 10–13. [Google Scholar] [PubMed]
- Mazzarella, N.; Femiano, F.; Gombos, F.; De Rosa, A.; Giuliano, M. Matrix metalloproteinase gene expression in oral lichen planus: Erosive vs. reticular forms. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Cui, J. COX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinoma. Asian Pac. J. Trop. Med. 2013, 6, 640–643. [Google Scholar] [CrossRef]
- Totan, A.; Miricescu, D.; Parlatescu, I.; Mohora, M.; Greabu, M. Possible salivary and serum biomarkers for oral lichen planus. Biotech. Histochem. 2015, 90, 552–558. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I. Serum and saliva collagenase-3 (MMP-13) in patients with oral lichen planus and oral squamous cell carcinoma. Med. J. Islam. Repub. Iran 2015, 29, 218. [Google Scholar]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, D.; Xie, Y.; Yao, X.; Li, Y. Bifidobacterium infantis Induces Protective Colonic PD-L1 and Foxp3 Regulatory T Cells in an Acute Murine Experimental Model of Inflammatory Bowel Disease. Gut Liver 2019, 13, 430–439. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Lee, C.-G.; So, J.-S.; Chae, C.-S.; Hwang, J.-S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.-C.; Im, S.-H. Generation of regulatory dendritic cells and CD4 + Foxp3 + T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, G.; Meng, F.; Yang, W.; Hu, J.; Ye, L.; Shi, C.; Wang, C. Immunological mechanisms involved in probiotic-mediated protection against Citrobacter rodentium-induced colitis. Benef. Microbes 2016, 7, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Zhang, J.; Mu, J.; Zalan, Z.; Hegyi, F.; Takács, K.; Zhao, X.; Du, M. Protective effect of Lactobacillus fermentum CQPC04 on dextran sulfate sodium–induced colitis in mice is associated with modulation of the nuclear factor-κB signaling pathway. J. Dairy Sci. 2019, 102, 9570–9585. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Cai, S.; Yu, H.; Wang, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri Ameliorates Intestinal Inflammation and Modulates Gut Microbiota and Metabolic Disorders in Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2020, 12, 2298. [Google Scholar] [CrossRef]
- Hegazy, S.K. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145. [Google Scholar] [CrossRef]
- Silveira, D.S.C.; Veronez, L.C.; Lopes-Júnior, L.C.; Anatriello, E.; Brunaldi, M.O.; Pereira-da-Silva, G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J. Gastroenterol. 2020, 26, 6782–6794. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Paulino, D.S.; Brambilla, S.R.; Camargo, J.A.; Persinoti, G.F.; Carvalheira, J.B.C. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 2018, 24, 1995–2008. [Google Scholar] [CrossRef]
- Yue, Y.-C.; Yang, B.-Y.; Lu, J.; Zhang, S.-W.; Liu, L.; Nassar, K.; Xu, X.-X.; Pang, X.-Y.; Lv, J.-P. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb. Cell Fact. 2020, 19, 213. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus casei and Lactobacillus rhamnosus GG Decrease Colon Cancer Cell Invasion In Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Chen, J.; Liu, Y.; Wen, W.; Li, Y.; Huang, X. Lactobacillus delbrueckii Ameliorates Intestinal Integrity and Antioxidant Ability in Weaned Piglets after a Lipopolysaccharide Challenge. Oxid. Med. Cell. Longev. 2020, 2020, 6028606. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.-Y.; Rod-in, W.; Monmai, C.; Sohn, M.; Kim, T.-R.; Jeon, M.-G.; Park, W.J. Anti-inflammatory potential of Lactobacillus reuteri LM1071 via eicosanoid regulation in LPS-stimulated RAW 264.7 cells. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Hsu, W.-F.; Chang, J.-S.; Shih, C.-K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef]
- Jung, J.-I.; Kim, Y.G.; Kang, C.-H.; Imm, J.-Y. Effects of Lactobacillus curvatus MG5246 on inflammatory markers in Porphyromonas gingivalis lipopolysaccharide-sensitized human gingival fibroblasts and periodontitis rat model. Food Sci. Biotechnol. 2022, 31, 111–120. [Google Scholar] [CrossRef]
- Takeda, S.; Igoshi, K.; Tsend-Ayush, C.; Oyunsuren, T.; Sakata, R.; Koga, Y.; Arima, Y.; Takeshita, M. Lactobacillus paracasei strain 06TCa19 suppresses inflammatory chemokine induced by Helicobacter pylori in human gastric epithelial cells. Hum. Cell 2017, 30, 258–266. [Google Scholar] [CrossRef]
- Tuo, Y.; Song, X.; Song, Y.; Liu, W.; Tang, Y.; Gao, Y.; Jiang, S.; Qian, F.; Mu, G. Screening probiotics from Lactobacillus strains according to their abilities to inhibit pathogen adhesion and induction of pro-inflammatory cytokine IL-8. J. Dairy Sci. 2018, 101, 4822–4829. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Chu, B.; Yuan, L.; Liu, N.; Zhu, Y.; Wang, J. Lactobacillus johnsonii L531 Alleviates the Damage Caused by Salmonella typhimurium via Inhibiting TLR4, NF-κB, and NLRP3 Inflammasome Signaling Pathways. Microorganisms 2021, 9, 1983. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Li, X.-Q.; Zhang, W.; Zhou, D.; Liu, H.-Y.; Wang, J.-F. Dose-Dependent Effects of Lactobacillus rhamnosus on Serum Interleukin-17 Production and Intestinal T-Cell Responses in Pigs Challenged with Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 1787–1798. [Google Scholar] [CrossRef]
- Goyal, N.; Shukla, G. Probiotic Lactobacillus rhamnosus GG Modulates the Mucosal Immune Response in Giardia intestinalis-Infected BALB/c Mice. Dig. Dis. Sci. 2013, 58, 1218–1225. [Google Scholar] [CrossRef]
- Maghsood, F.; Mirshafiey, A.; Farahani, M.M.; Modarressi, M.H.; Jafari, P.; Motevaseli, E. Dual Effects of Cell Free Supernatants from Lactobacillus acidophilus and Lactobacillus rhamnosus GG in Regulation of MMP-9 by Up-Regulating TIMP-1 and Down-Regulating CD147 in PMADifferentiated THP-1 Cells. Cell J. 2018, 19, 559–568. [Google Scholar] [CrossRef]
- Lim, S.-M.; Jang, H.M.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Lactobacillus fermentum IM12 attenuates inflammation in mice by inhibiting NF-κB-STAT3 signalling pathway. Benef. Microbes 2017, 8, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, W.-K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Pahumunto, N.; Basic, A.; Östberg, A.-K.; Teanpaisan, R.; Dahlen, G. Oral Lactobacillus strains reduce cytotoxicity and cytokine release from peripheral blood mononuclear cells exposed to Aggregatibacter actinomycetemcomitans subtypes in vitro. BMC Microbiol. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Griet, M.; Zelaya, H.; Mateos, M.V.; Salva, S.; Juarez, G.E.; de Valdez, G.F.; Villena, J.; Salvador, G.A.; Rodriguez, A.V. Soluble Factors from Lactobacillus reuteri CRL1098 Have Anti-Inflammatory Effects in Acute Lung Injury Induced by Lipopolysaccharide in Mice. PLoS ONE 2014, 9, e110027. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Min, H.-K.; Na, H.S.; ye Kwon, J.; Ryu, J.; Cho, K.-H.; Choi, J.; Jung, K.; Lee, S.-Y.; Kim, S.J.; et al. Combinatmarion treatment with Lactobacillus acidophilus LA-1, vitamin B, and curcumin ameliorates the progression of osteoarthritis by inhibiting the pro-inflammatory mediators. Immunol. Lett. 2020, 228, 112–121. [Google Scholar] [CrossRef]
- So, J.-S.; Song, M.-K.; Kwon, H.-K.; Lee, C.-G.; Chae, C.-S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.-H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef]
- Digehsara, S.G.; Name, N.; Sartipnia, N.; Karim, E.; Taheri, S.; Ebrahimi, M.T.; Arasteh, J. Analysis of inflammasomes and CYP27B1 genes in cuprizone demyelinated C57BL/6 mice and evaluation of Th1 and Th2 patterns after oral administration of Lactobacillus casei strain T2 (IBRC-M10783). Microb. Pathog. 2021, 155, 104931. [Google Scholar] [CrossRef]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef]
- Marlina, E.; Goodman, R.N.; Mercadante, V.; Shephard, M.; McMillan, R.; Hodgson, T.; Leeson, R.; Porter, S.; Barber, J.A.; Fedele, S.; et al. A proof of concept pilot trial of probiotics in symptomatic oral lichen planus (CABRIO). Oral Dis. 2021. [Google Scholar] [CrossRef]
- Vincenzi, A.; Goettert, M.I.; Volken de Souza, C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Pai, S.; Thomas, R. Immune deficiency or hyperactivity-Nf-κb illuminates autoimmunity. J. Autoimmun. 2008, 31, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ku, S.; Lim, T.; Park, S.Y.; Park, M.S.; Ji, G.E.; O’Brien, K.; Hwang, K.T. Antioxidant and Anti-Inflammatory Properties of Recombinant Bifidobacterium bifidum BGN4 Expressing Antioxidant Enzymes. Microorganisms 2021, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.U. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-κB activation. World J. Gastroenterol. 2006, 12, 3729. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuzuki, Y.; Hokari, R.; Komoto, S.; Kurihara, C.; Kawaguchi, A.; Nagao, S.; Miura, S. Anti-inflammatory effects of the genus Bifidobacterium on macrophages by modification of phospho-IκB and SOCS gene expression. Int. J. Exp. Pathol. 2009, 90, 131–140. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, S.-H.; Kim, M.G.; Lee, H.J.; Kim, G.-B. Lactobacillus plantarum CAU1055 ameliorates inflammation in lipopolysaccharide-induced RAW 264.7 cells and a dextran sulfate sodium–induced colitis animal model. J. Dairy Sci. 2019, 102, 6718–6725. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef]
- Preising, J.; Philippe, D.; Gleinser, M.; Wei, H.; Blum, S.; Eikmanns, B.J.; Niess, J.-H.; Riedel, C.U. Selection of Bifidobacteria Based on Adhesion and Anti-Inflammatory Capacity In Vitro for Amelioration of Murine Colitis. Appl. Environ. Microbiol. 2010, 76, 3048–3051. [Google Scholar] [CrossRef]
- Ding, L.; Gong, Y.; Yang, Z.; Zou, B.; Liu, X.; Zhang, B.; Li, J. Lactobacillus rhamnosus GG Ameliorates Liver Injury and Hypoxic Hepatitis in Rat Model of CLP-Induced Sepsis. Dig. Dis. Sci. 2019, 64, 2867–2877. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Lun, J.; Gao, J.; Gao, X.; Gong, Z.; Wan, Y.; He, X.; Cao, H. Inhibitory Effects of the Lactobacillus rhamnosus GG Effector Protein HM0539 on Inflammatory Response Through the TLR4/MyD88/NF-κB Axis. Front. Immunol. 2020, 11, 2232. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Sultana, N.; Hayashi, I.; Fukamachi, M.; Sugiyama, M. Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis. Molecules 2019, 24, 2970. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Danshiitsoodol, N.; Kanno, K.; Uchida, T.; Sugiyama, M. The Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Ameliorates Inflammatory Responses in DSS–Induced Ulcerative Colitis. Microorganisms 2021, 9, 2243. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Plaza-Díaz, J.; Robles-Bolívar, P.; Valente-Godínez, H.; Sáez-Lara, M.J.; Abadía-Molina, F.; Gómez-Llorented, C.; Gil, Á.; Álvarez-Mercado, A.I. Bifidobacterium breve CNCM I-4035, Lactobacillus paracasei CNCM I-4034 and Lactobacillus rhamnosus CNCM I-4036 Modulate Macrophage Gene Expression and Ameliorate Damage Markers in the Liver of Zucker-Lepr fa/fa Rats. Nutrients 2021, 13, 202. [Google Scholar] [CrossRef]
- Sougioultzis, S.; Simeonidis, S.; Bhaskar, K.R.; Chen, X.; Anton, P.M.; Keates, S.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem. Biophys. Res. Commun. 2006, 343, 69–76. [Google Scholar] [CrossRef]
- Yao, X.; Yin, C.; Shen, L.; Xie, S. Expressions of NF--kappaBp65, TRAF2, cyclinD1 and their association with cell apoptosis in oral lichen planus. Nan Fang Yi Ke Da Xue Xue Bao 2007, 27, 1657–1660. [Google Scholar]
- Shi, Y.; Shen, L.; Yin, C. Expression of caspase-8, receptor interacting protein and nuclear factor-kappaBp65 in oral lichen planus. Zhonghua Kou Qiang Yi Xue Za Zhi 2010, 45, 11–15. [Google Scholar]
- Zhou, G.; Xia, K.; Du, G.; Chen, X.; Xu, X.; Lu, R.; Zhou, H. Activation of nuclear factor-kappa B correlates with tumor necrosis factor-alpha in oral lichen planus: A clinicopathologic study in atrophic-erosive and reticular form. J. Oral Pathol. Med. 2009, 38, 559–564. [Google Scholar] [CrossRef]
- Santoro, A.; Majorana, A.; Bardellini, E.; Festa, S.; Sapelli, P.; Facchetti, F. NF-kappaB expression in oral and cutaneous lichen planus. J. Pathol. 2003, 201, 466–472. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Li, Z.; Zhou, M.; Chen, Q.; Zeng, X.; Chen, Y. The Activation of NF-κB in Infiltrated Mononuclear Cells Negatively Correlates with Treg Cell Frequency in Oral Lichen Planus. Inflammation 2015, 38, 1683–1689. [Google Scholar] [CrossRef]
- Lin, X.; Sun, H.; Zhen, Y.; Zhang, H.; Shi, H.; Wang, X. Low expression of glucocorticoid receptor α in oral lichen planus correlates with activation of nuclear factor κB: A preliminary study. J. Oral Pathol. Med. 2014, 43, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Rusanen, P.; Marttila, E.; Uittamo, J.; Hagström, J.; Salo, T.; Rautemaa-Richardson, R. TLR1-10, NF-κB and p53 expression is increased in oral lichenoid disease. PLoS ONE 2017, 12, e0181361. [Google Scholar] [CrossRef] [PubMed]
- Thi Do, T.; Phoomak, C.; Champattanachai, V.; Silsirivanit, A.; Chaiyarit, P. New evidence of connections between increased O-GlcNAcylation and inflammasome in the oral mucosa of patients with oral lichen planus. Clin. Exp. Immunol. 2018, 192, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Burgk, J.L.; Gaidt, M.M.; Schmidt, T.; Ebert, T.S.; Bartok, E.; Hornung, V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015, 45, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-J.; Han, L.-H.; Cong, R.-S.; Liang, J. Caspase Family Proteases and Apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Mattila, R.; Syrjänen, S. Caspase cascade pathways in apoptosis of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 618–623. [Google Scholar] [CrossRef]
- Bascones-Ilundain, C.; Gonzalez-Moles, M.A.; Esparza-Gómez, G.; Gil-Montoya, J.A.; Bascones-Martínez, A. Importance of apoptotic mechanisms in inflammatory infiltrate of oral lichen planus lesions. Anticancer Res. 2006, 26, 357–362. [Google Scholar]
- Shan, J.; Ma, J.-M.; Wang, R.; Liu, Q.-L.; Fan, Y. Proliferation and Apoptosis of Peripheral Blood Mononuclear Cells in Patients with Oral Lichen Planus. Inflammation 2013, 36, 419–425. [Google Scholar] [CrossRef]
- Abdel-Latif, A.M.; Abuel-Ela, H.A.; El-Shourbagy, S.H. Increased caspase-3 and altered expression of apoptosis-associated proteins, Bcl-2 and Bax in lichen planus. Clin. Exp. Dermatol. 2009, 34, 390–395. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Li, X. The expression and changes of apoptosis protein Bcl-2 and Bax in oral lichen planus. Shanghai Kou Qiang Yi Xue 2015, 24, 465–469. [Google Scholar] [PubMed]
- Bascones-Ilundain, C.; González-Moles, M.; Campo-Trapero, J.; Gil-Montoya, J.; Esparza-Gómez, G.; Cano-Sánchez, J.; Bascones-Martínez, A. No differences in caspase-3 and Bax expression in atrophic-erosive vs. reticular oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 2007, 22, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Nafarzadeh, S.; Jafari, S.; Bijani, A. Assessment of bax and bcl-2 immunoexpression in patients with oral lichen planus and oral squamous cell carcinoma. Int. J. Mol. Cell. Med. 2013, 2, 136–142. [Google Scholar] [PubMed]
- Chamorro-Petronacci, C.M.; Lafuente-Ibanez De Mendoza, I.; Suarez-Peñaranda, J.M.; Padin-Iruegas, E.; Blanco-Carrion, A.; Lorenzo-Pouso, A.I.; Ortega, K.L.; Pérez-Sayáns, M. Immunohistochemical Characterization of Bcl-2 in Oral Potentially Malignant Disorders. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 706–712. [Google Scholar] [CrossRef]
- Pigatti, F.M.; de Taveira, L.A.A.; Soares, C.T. Immunohistochemical expression of Bcl-2 and Ki-67 in oral lichen planus and leukoplakia with different degrees of dysplasia. Int. J. Dermatol. 2015, 54, 150–155. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, X.; Mao, X.; Wen, D.; Zhang, H.; Wang, J. Inflammatory and immune-related factor Caspase 1 contributes to the development of oral lichen planus. Arch. Oral Biol. 2021, 131, 105244. [Google Scholar] [CrossRef]
- Sklavounou-Andrikopoulou, A.; Chrysomali, E.; Iakovou, M.; Garinis, G.A.; Karameris, A. Elevated serum levels of the apoptosis related molecules TNF-alpha, Fas/Apo-1 and Bcl-2 in oral lichen planus. J. Oral Pathol. Med. 2004, 33, 386–390. [Google Scholar] [CrossRef]
- Neppelberg, E.; Johannessen, A.C.; Jonsson, R. Apoptosis in oral lichen planus. Eur. J. Oral Sci. 2001, 109, 361–364. [Google Scholar] [CrossRef]
- Brant, J.M.C.; Vasconcelos, A.C.; Rodrigues, L.V. Role of apoptosis in erosive and reticular oral lichen planus exhibiting variable epithelial thickness. Braz. Dent. J. 2008, 19, 179–185. [Google Scholar] [CrossRef][Green Version]
- Rusiecki, R.; Witkowski, J.; Jaszczewska-Adamczak, J. MDM2-p53 Interaction Inhibitors: The Current State-of-Art and Updated Patent Review (2010-Present). Recent Pat. Anticancer Drug Discov. 2019, 14, 324–369. [Google Scholar] [CrossRef]
- Squarzanti, D.F.; Cena, T.; Sorrentino, R.; Migliario, M.; Chiocchetti, A.; Rimondini, L.; Azzimonti, B.; Valente, G. Implications on pathogenesis and risk of oral lichen planus neoplastic transformation: An ex-vivo retrospective immunohistochemical study. Histol. Histopathol. 2019, 34, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Hadzi-Mihailovic, M.; Petrovic, R.; Raybaud, H.; Stanimirovic, D.; Ozar Koray, M. Expression and role of p53 in oral lichen planus patients. J. BUON 2017, 22, 1278–1286. [Google Scholar] [PubMed]
- Shiva, A.; Zamanian, A.; Arab, S.; Boloki, M. Immunohistochemical Study of p53 Expression in Patients with Erosive and Non-Erosive Oral Lichen Planus. J. Dent. 2018, 19, 118–123. [Google Scholar]
- Shailaja, G.; Kumar, J.V.; Baghirath, P.V.; Kumar, U.; Ashalata, G.; Krishna, A.B. Estimation of malignant transformation rate in cases of oral epithelial dysplasia and lichen planus using immunohistochemical expression of Ki-67, p53, BCL-2, and BAX markers. Dent. Res. J. 2015, 12, 235–242. [Google Scholar]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2021. [Google Scholar] [CrossRef]
- Shi, Y.; Meng, L.; Zhang, C.; Zhang, F.; Fang, Y. Extracellular vesicles of Lacticaseibacillus paracasei PC-H1 induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2 signaling pathway. Microbiol. Res. 2021, 255, 126921. [Google Scholar] [CrossRef]
- Li, J.Q.; Li, J.L.; Xie, Y.H.; Wang, Y.; Shen, X.N.; Qian, Y.; Han, J.X.; Chen, Y.X.; Fang, J. Saccharomyces cerevisiae may serve as a probiotic in colorectal cancer by promoting cancer cell apoptosis. J. Dig. Dis. 2020, 21, 571–582. [Google Scholar] [CrossRef]
- Yenuganti, V.R.; Yadala, R.; Azad, R.; Singh, S.; Chiluka, V.; Ahire, J.; Reddanna, P. In vitro evaluation of anticancer effects of different probiotic strains on HCT-116 cell line. J. Appl. Microbiol. 2021, 131, 1958–1969. [Google Scholar] [CrossRef]
- Pakbin, B.; Pishkhan Dibazar, S.; Allahyari, S.; Javadi, M.; Farasat, A.; Darzi, S. Probiotic Saccharomyces cerevisiae var. boulardii supernatant inhibits survivin gene expression and induces apoptosis in human gastric cancer cells. Food Sci. Nutr. 2021, 9, 692–700. [Google Scholar] [CrossRef]
- Pakbin, B.; Dibazar, S.P.; Allahyari, S.; Javadi, M.; Amani, Z.; Farasat, A.; Darzi, S. Anticancer Properties of Probiotic Saccharomyces boulardii Supernatant on Human Breast Cancer Cells. Probiotics Antimicrob. Proteins 2022, 1–9. [Google Scholar] [CrossRef]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chu, B.; Chen, S.; Li, Y.; Liu, N.; Zhu, Y.; Zhou, D. Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms 2021, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Dargahi, L.; Naserpour, T.; Mirzanejad, Y.; Alizadeh, S.A.; Peymani, A.; Nassiri-Asl, M. Probiotic mixture of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 attenuates hippocampal apoptosis induced by lipopolysaccharide in rats. Int. Microbiol. 2019, 22, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Guida, A.; Salerno, C.; Contaldo, M.; Esposito, V.; Laino, L.; Serpico, R.; Lucchese, A. Oral lichen planus: A narrative review. Front. Biosci. (Elite Ed.) 2014, 6, 370–376. [Google Scholar] [CrossRef]
- Zhong, E.F.; Chang, A.; Stucky, A.; Chen, X.; Mundluru, T.; Khalifeh, M.; Sedghizadeh, P.P. Genomic Analysis of Oral Lichen Planus and Related Oral Microbiome Pathogens. Pathogens 2020, 9, 952. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Chen, C.; Shao, B.; Zhao, L.; Zhou, Q.; Liu, J.; Wang, G.; Yuan, W.; Sun, Z. Interaction between intestinal microbiota and tumour immunity in the tumour microenvironment. Immunology 2021, 164, 476–493. [Google Scholar] [CrossRef]
- Khorasani, S.; Mahmoudi, M.; Kalantari, M.R.; Lavi Arab, F.; Esmaeili, S.-A.; Mardani, F.; Tabasi, N.; Rastin, M. Amelioration of regulatory T cells by Lactobacillus delbrueckii and Lactobacillus rhamnosus in pristane-induced lupus mice model. J. Cell. Physiol. 2019, 234, 9778–9786. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, Z.; Sui, L.; Xu, Y.; Wang, L.; Qiao, X.; Cui, W.; Jiang, Y.; Zhou, H.; Tang, L.; et al. Lactobacillus johnsonii activates porcine monocyte derived dendritic cells maturation to modulate Th cellular immune response. Cytokine 2021, 144, 155581. [Google Scholar] [CrossRef]
- Bernardo, D.; Sánchez, B.; Al-Hassi, H.O.; Mann, E.R.; Urdaci, M.C.; Knight, S.C.; Margolles, A. Microbiota/host crosstalk biomarkers: Regulatory response of human intestinal dendritic cells exposed to Lactobacillus extracellular encrypted peptide. PLoS ONE 2012, 7, e36262. [Google Scholar] [CrossRef]
- Al-Hassi, H.O.; Mann, E.R.; Sanchez, B.; English, N.R.; Peake, S.T.C.; Landy, J.; Man, R.; Urdaci, M.; Hart, A.L.; Fernandez-Salazar, L.; et al. Altered human gut dendritic cell properties in ulcerative colitis are reversed by Lactobacillus plantarum extracellular encrypted peptide STp. Mol. Nutr. Food Res. 2014, 58, 1132–1143. [Google Scholar] [CrossRef]
- Bajić, S.S.; Đokić, J.; Dinić, M.; Tomić, S.; Popović, N.; Brdarić, E.; Golić, N.; Tolinački, M. GABA potentiate the immunoregulatory effects of Lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro. Sci. Rep. 2020, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Manirarora, J.N.; Kosiewicz, M.M.; Alard, P. Feeding lactobacilli impacts lupus progression in (NZBxNZW)F1 lupus-prone mice by enhancing immunoregulation. Autoimmunity 2020, 53, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Ekmekciu, I.; von Klitzing, E.; Neumann, C.; Bacher, P.; Scheffold, A.; Bereswill, S.; Heimesaat, M.M. Fecal Microbiota Transplantation, Commensal Escherichia coli and Lactobacillus johnsonii Strains Differentially Restore Intestinal and Systemic Adaptive Immune Cell Populations Following Broad-spectrum Antibiotic Treatment. Front. Microbiol. 2017, 8, 2430. [Google Scholar] [CrossRef] [PubMed]
- Hrdý, J.; Alard, J.; Couturier-Maillard, A.; Boulard, O.; Boutillier, D.; Delacre, M.; Lapadatescu, C.; Cesaro, A.; Blanc, P.; Pot, B.; et al. Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci. Rep. 2020, 10, 5345. [Google Scholar] [CrossRef]
- Lin, D.; Yang, L.; Wen, L.; Lu, H.; Chen, Q.; Wang, Z. Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal Immunol. 2021, 14, 1247–1258. [Google Scholar] [CrossRef]
- Jia, L.; Wu, R.; Han, N.; Fu, J.; Luo, Z.; Guo, L.; Su, Y.; Du, J.; Liu, Y. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin. Transl. Immunol. 2020, 9, e1213. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, X.; Guan, H.; Wu, L.; Yu, M.; Hou, D.; Yan, Y.; Fang, X. Lactobacillus acidipiscis Induced Regulatory Gamma Delta T Cells and Attenuated Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2021, 12, 623451. [Google Scholar] [CrossRef]
- Dolpady, J.; Sorini, C.; Di Pietro, C.; Cosorich, I.; Ferrarese, R.; Saita, D.; Clementi, M.; Canducci, F.; Falcone, M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J. Diabetes Res. 2016, 2016, 7569431. [Google Scholar] [CrossRef]
- Jia, H.; Ren, S.; Wang, X. Heat-killed probiotic regulates the body’s regulatory immunity to attenuate subsequent experimental autoimmune arthritis. Immunol. Lett. 2019, 216, 89–96. [Google Scholar] [CrossRef]
- Chae, C.-S.; Kwon, H.-K.; Hwang, J.-S.; Kim, J.-E.; Im, S.-H. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PLoS ONE 2012, 7, e52119. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetta, P.; Ormelli, M.; Amoruso, A.; Pane, M.; Azzimonti, B.; Squarzanti, D.F. Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New? Int. J. Mol. Sci. 2022, 23, 3489. https://doi.org/10.3390/ijms23073489
Zanetta P, Ormelli M, Amoruso A, Pane M, Azzimonti B, Squarzanti DF. Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New? International Journal of Molecular Sciences. 2022; 23(7):3489. https://doi.org/10.3390/ijms23073489
Chicago/Turabian StyleZanetta, Paola, Margherita Ormelli, Angela Amoruso, Marco Pane, Barbara Azzimonti, and Diletta Francesca Squarzanti. 2022. "Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New?" International Journal of Molecular Sciences 23, no. 7: 3489. https://doi.org/10.3390/ijms23073489
APA StyleZanetta, P., Ormelli, M., Amoruso, A., Pane, M., Azzimonti, B., & Squarzanti, D. F. (2022). Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New? International Journal of Molecular Sciences, 23(7), 3489. https://doi.org/10.3390/ijms23073489









