Assessment of Ultra-Early-Stage Liver Fibrosis in Human Non-Alcoholic Fatty Liver Disease by Second-Harmonic Generation Microscopy
Abstract
:1. Introduction
2. Results
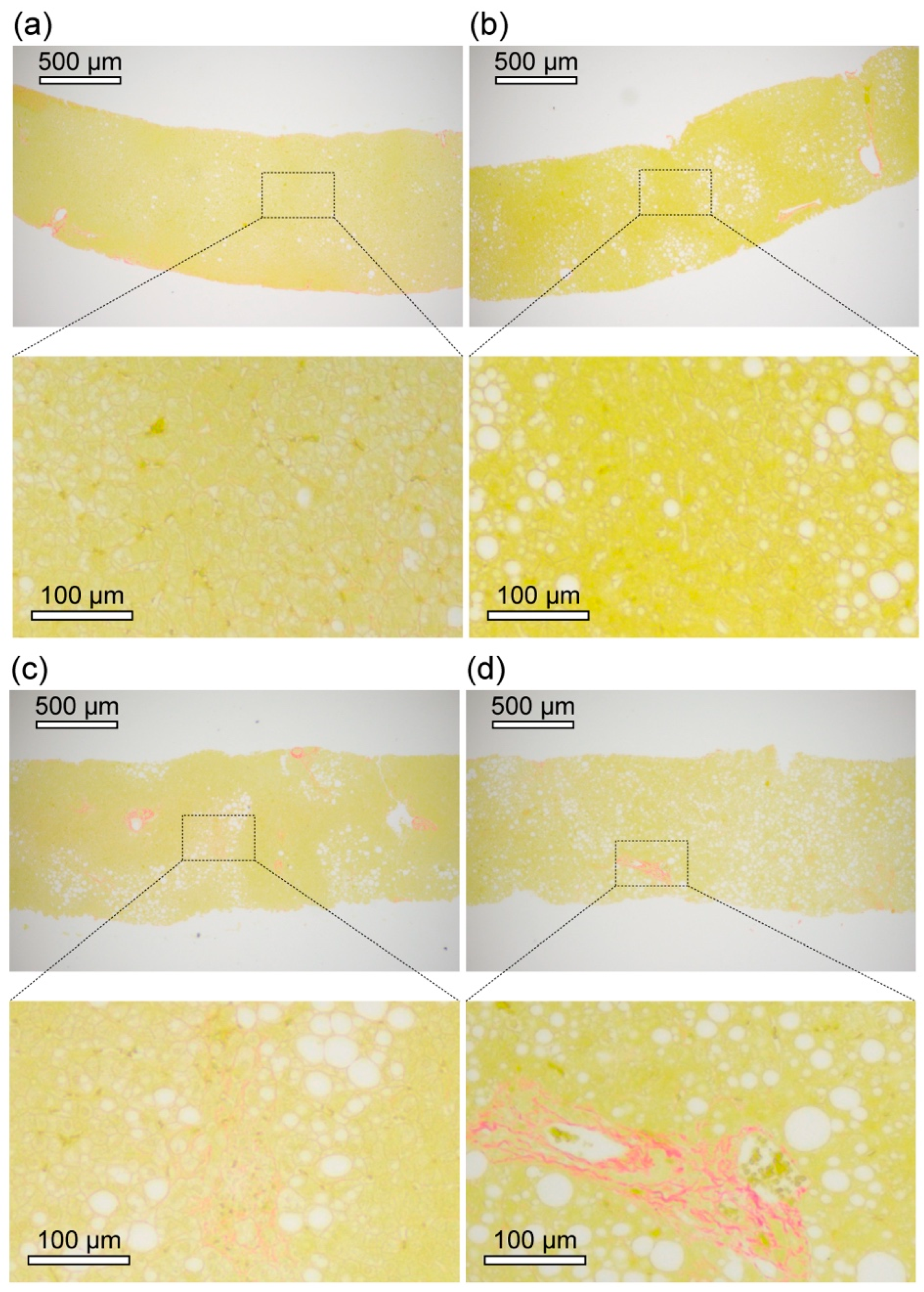
2.1. Clinical and Histopathological Examination of Livers of NAFLD Patients
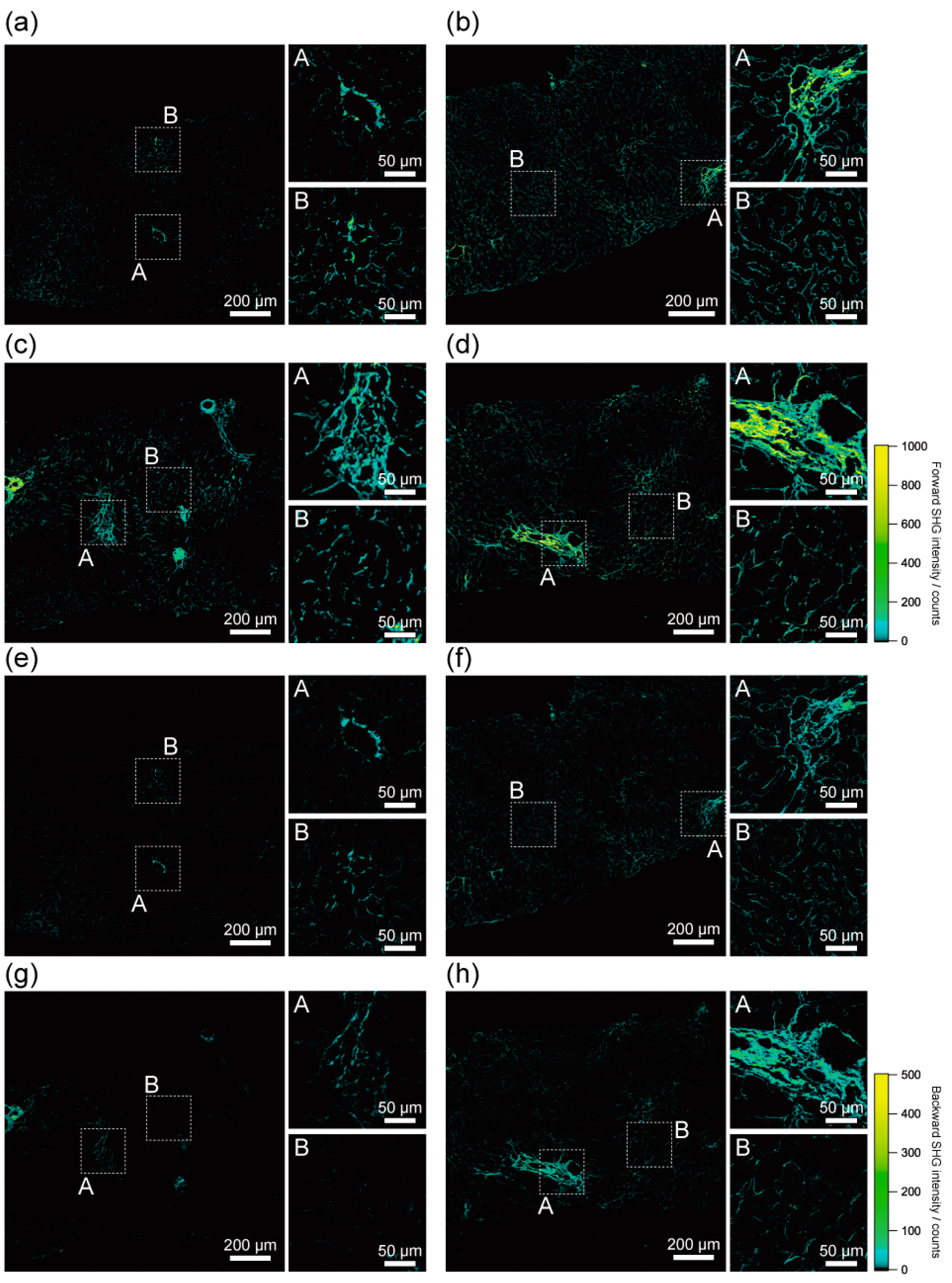
2.2. Histological Assessment of Liver Fibrosis by SHG Imaging
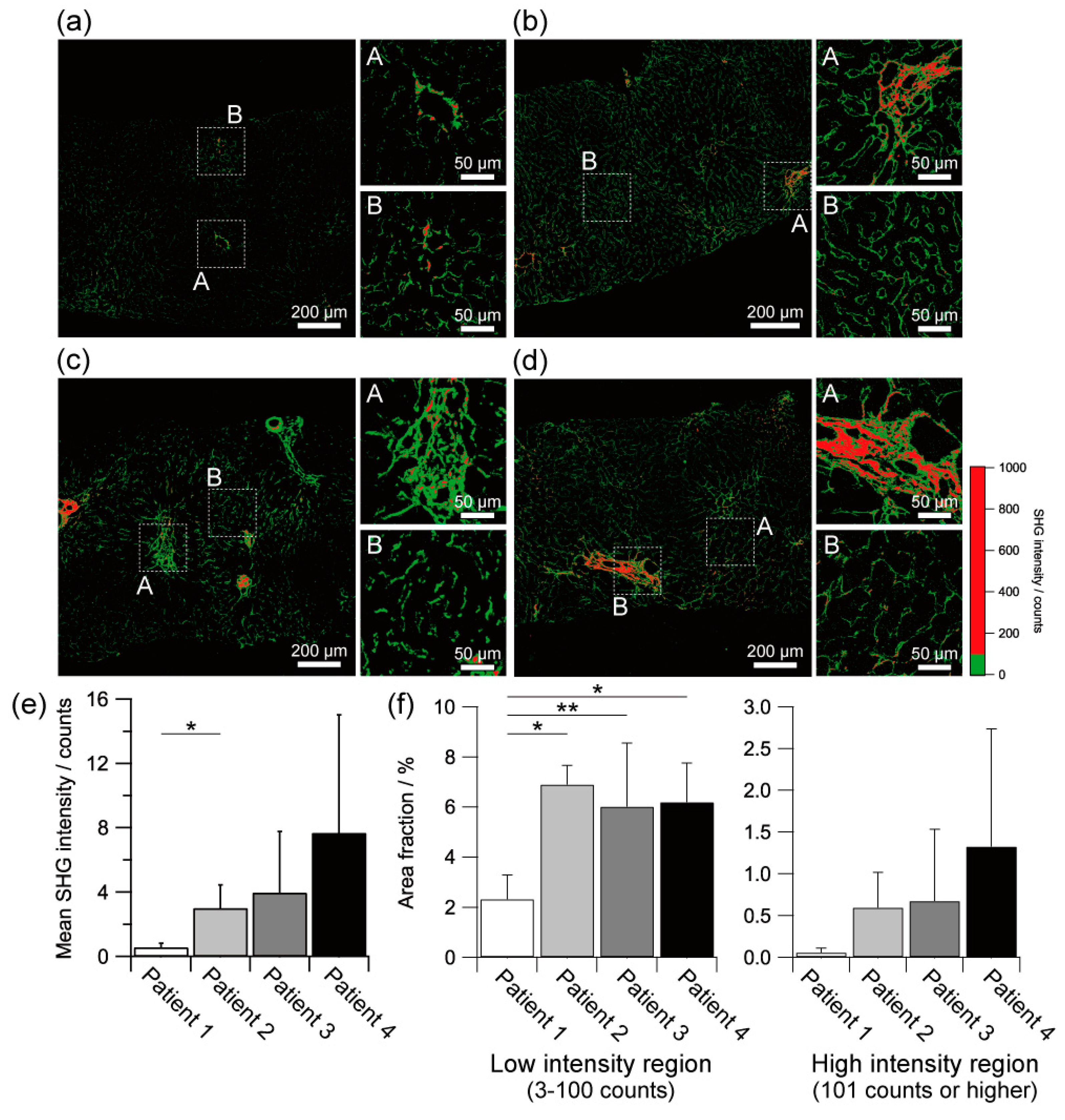
2.3. Quantitative Assessment of Liver Fibrosis by SHG Intensity
2.4. Molecular Maturation Assessment of Collagenous Fibre by SHG Emission Directionality Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Preparation
4.3. Clinical and Histopathological Examinations
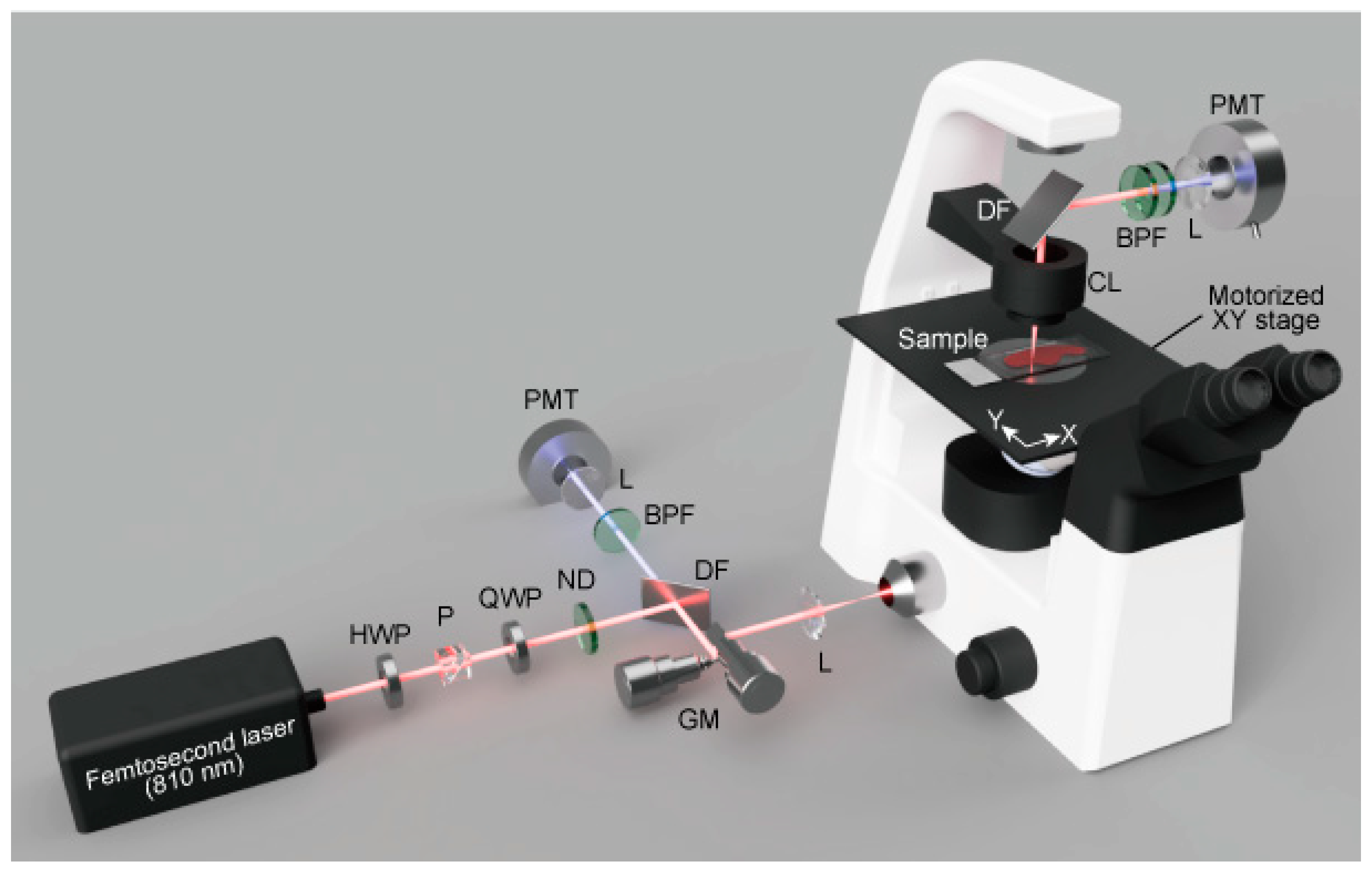
4.4. SHG Microscopy
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Byrne, C.D. Nonalcoholic fatty liver disease: A novel cardiometabolic risk factor for type 2 diabetes and its complications. J. Clin. Endocrinol. Metab. 2013, 98, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus, P. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R. Role of imaging-based biomarkers in NAFLD: Recent advances in clinical application and future research directions. J. Hepatol. 2018, 68, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V.; Group, L.S. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef]
- Wong, V.W.; Adams, L.A.; de Ledinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Ono, M.; Hyogo, H.; Fujii, H.; Sumida, Y.; Yamada, M.; Mori, K.; Tanaka, S.; Maekawa, T.; Ebisutani, Y.; et al. Use of Mac-2 binding protein as a biomarker for nonalcoholic fatty liver disease diagnosis. Hepatol. Commun. 2017, 1, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, M.C.; Michalak, S.; Dupre, F.; Croue, A.; Bedossa, P.; Saint-Andre, J.P.; Cales, P.; Hepatitis, N. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology 2005, 41, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nadiarynkh, O.; Plotnikov, S.; Campagnola, P.J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 2012, 7, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chang, S.; Tai, D.; Tan, N.; Xiao, G.; Tang, H.; Yu, H. Nonlinear optical microscopy: Use of second harmonic generation and two-photon microscopy for automated quantitative liver fibrosis studies. J. Biomed. Opt. 2008, 13, 064010. [Google Scholar] [CrossRef] [PubMed]
- James, D.S.; Jambor, A.N.; Chang, H.Y.; Alden, Z.; Tilbury, K.B.; Sandbo, N.K.; Campagnola, P.J. Probing ECM remodeling in idiopathic pulmonary fibrosis via second harmonic generation microscopy analysis of macro/supramolecular collagen structure. J. Biomed. Opt. 2019, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.P.; Norris, G.; McConnell, G.; Currie, S. A novel approach for assessing cardiac fibrosis using label-free second harmonic generation. Int. J. Card. Imaging 2013, 29, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Gailhouste, L.; Le Grand, Y.; Odin, C.; Guyader, D.; Turlin, B.; Ezan, F.; Desille, Y.; Guilbert, T.; Bessard, A.; Fremin, C.; et al. Fibrillar collagen scoring by second harmonic microscopy: A new tool in the assessment of liver fibrosis. J. Hepatol. 2010, 52, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Campagnola, P. Second harmonic generation imaging microscopy: Applications to diseases diagnostics. Anal. Chem. 2011, 83, 3224–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnola, P.J.; Dong, C.Y. Second harmonic generation microscopy: Principles and applications to disease diagnosis. Laser Photonics Rev. 2011, 5, 13–26. [Google Scholar] [CrossRef]
- Strupler, M.; Pena, A.M.; Hernest, M.; Tharaux, P.L.; Martin, J.L.; Beaurepaire, E.; Schanne-Klein, M.C. Second harmonic imaging and scoring of collagen in fibrotic tissues. Opt. Express 2007, 15, 4054–4065. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Langohr, I.M.; Locker, M.J.; Sturek, M.; Cheng, J.X. Label-free molecular imaging of atherosclerotic lesions using multimodal nonlinear optical microscopy. J. Biomed. Opt. 2007, 12, 054007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hase, E.; Sato, K.; Yonekura, D.; Minamikawa, T.; Takahashi, M.; Yasui, T. Evaluation of the histological and mechanical features of tendon healing in a rabbit model with the use of second-harmonic-generation imaging and tensile testing. Bone Joint Res. 2016, 5, 577–585. [Google Scholar] [CrossRef]
- Sato, K.; Matsubara, O.; Hase, E.; Minamikawa, T.; Yasui, T. Quantitative in situ time-series evaluation of osteoblastic collagen synthesis under cyclic strain using second-harmonic-generation microscopy. J. Biomed. Opt. 2019, 24, 031019. [Google Scholar] [CrossRef]
- Lin, S.-J.; Wu, R., Jr.; Tan, H.-Y.; Lo, W.; Lin, W.-C.; Young, T.-H.; Hsu, C.-J.; Chen, J.-S.; Jee, S.-H.; Dong, C.-Y. Evaluating cutaneous photoaging by use of multiphoton fluorescence and second-harmonic generation microscopy. Opt. Lett. 2005, 30, 2275–2277. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Tohno, Y.; Araki, T. Characterization of collagen orientation in human dermis by two-dimensional second-harmonic-generation polarimetry. J. Biomed. Opt. 2004, 9, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.Y.; Yu, H.C.; Wang, I.J.; Sun, C.K. Infrared-based third and second harmonic generation imaging of cornea. J. Biomed. Opt. 2009, 14, 044012. [Google Scholar] [CrossRef] [PubMed]
- Zoumi, A.; Yeh, A.; Tromberg, B.J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl. Acad. Sci. USA 2002, 99, 11014–11019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, C.-Y.; Teng, X.; Su, T.-H.; Lee, P.-H.; Kao, J.-H.; Huang, K.-W. Improved second harmonic generation and two-photon excitation fluorescence microscopy-based quantitative assessments of liver fibrosis through auto-correction and optimal sampling. Quant. Imaging Med. Surg. 2021, 11, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Tan, C.Z.; Cheng, C.A.; Hung, J.T.; Chen, S.Y. Improving resolution of second harmonic generation microscopy via scanning structured illumination. Biomed. Opt. Express 2018, 9, 6081–6090. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhuo, S.; So, P.T.C.; Yu, H. Improving liver fibrosis diagnosis based on forward and backward second harmonic generation signals. Appl. Phys. Lett. 2015, 106, 083701. [Google Scholar] [CrossRef] [Green Version]
- Lacomb, R.; Nadiarnykh, O.; Campagnola, P.J. Quantitative second harmonic generation imaging of the diseased state osteogenesis imperfecta: Experiment and simulation. Biophys. J. 2008, 94, 4504–4514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadiarnykh, O.; LaComb, R.B.; Brewer, M.A.; Campagnola, P.J. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajeti, V.; Nadiarnykh, O.; Ponik, S.M.; Keely, P.J.; Eliceiri, K.W.; Campagnola, P.J. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: Implications for probing stromal alterations in human breast cancer. Biomed. Opt. Express 2011, 2, 2307–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Oshima, Y.; Saitou, T.; Watanabe, T.; Miyake, T.; Yoshida, O.; Tokumoto, Y.; Abe, M.; Matsuura, B.; Hiasa, Y.; et al. Quantitative imaging of fibrotic and morphological changes in liver of non-alcoholic steatohepatitis (NASH) model mice by second harmonic generation (SHG) and auto-fluorescence (AF) imaging using two-photon excitation microscopy (TPEM). Biochem. Biophys. Rep. 2016, 8, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.E.; Goh, G.B.B.; Leow, W.Q.; Shen, L.; Lim, K.H.; Tan, C.K. Second harmonic generation microscopy provides accurate automated staging of liver fibrosis in patients with non-alcoholic fatty liver disease. PLoS ONE 2018, 13, e0199166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhao, J.M.; Rao, H.Y.; Yu, W.M.; Zhang, W.; Theise, N.D.; Wee, A.; Wei, L. Second harmonic generation reveals subtle fibrosis differences in adult and pediatric nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 2017, 148, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Qu, H.; Yang, W.; Liu, J.; Wang, F.; Liu, Y.; Ding, J.; Shi, J. Comparing hepatic steatosis distribution patterns between non-alcoholic fatty liver disease and fatty liver disease with chronic hepatitis B by second-harmonic generation/two-photon excited fluorescence method. Ann. Hepatol. 2020, 19, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Pirhonen, J.; Arola, J.; Sadevirta, S.; Luukkonen, P.; Karppinen, S.M.; Pihlajaniemi, T.; Isomaki, A.; Hukkanen, M.; Yki-Jarvinen, H.; Ikonen, E. Continuous grading of early fibrosis in NAFLD using label-free imaging: A proof-of-concept study. PLoS ONE 2016, 11, e0147804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, G.B.; Leow, W.Q.; Liang, S.; Wan, W.K.; Lim, T.K.H.; Tan, C.K.; Chang, P.E. Quantification of hepatic steatosis in chronic liver disease using novel automated method of second harmonic generation and two-photon excited fluorescence. Sci. Rep. 2019, 9, 2975. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Ledinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.D.; Hooker, J.; Tu, X.; Bettencourt, R.; et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: A systematic review and pooled analysis of individual participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.M.; Zipfel, W.R.; Webb, W.W. Interpreting second-harmonic generation images of collagen I fibrils. Biophys. J. 2005, 88, 1377–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.; Giese, G.; Bille, J. Second harmonic generation imaging of collagen fibrils in cornea and sclera. Opt. Express 2005, 13, 5791–5797. [Google Scholar] [CrossRef]
- LaComb, R.; Nadiarnykh, O.; Townsend, S.S.; Campagnola, P.J. Phase matching considerations in second harmonic generation from tissues: Effects on emission directionality, conversion efficiency and observed morphology. Opt. Commun. 2008, 281, 1823–1832. [Google Scholar] [CrossRef] [Green Version]
- Hristu, R.; Eftimie, L.G.; Stanciu, S.G.; Tranca, D.E.; Paun, B.; Sajin, M.; Stanciu, G.A. Quantitative second harmonic generation microscopy for the structural characterization of capsular collagen in thyroid neoplasms. Biomed. Opt. Express 2018, 9, 3923–3936. [Google Scholar] [CrossRef]
- Nadiarnykh, O.; Lacomb, R.B.; Campagnola, P.J.; Mohler, W.A. Coherent and incoherent SHG in fibrillar cellulose matrices. Opt. Express 2007, 15, 3348–3360. [Google Scholar] [CrossRef]
- Bateman, E.D.; Turner-Warwick, M.; Adelmann-Grill, B.C. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax 1981, 36, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gay, S.; Fietzek, P.P.; Remberger, K.; Eder, M.; Kuhn, K. Liver cirrhosis: Immunofluorescence and biochemical studies demonstrate two types of collagen. Klin. Wochenschr. 1975, 53, 205–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Akins, M.L.; Murari, K.; Xi, J.; Li, M.J.; Luby-Phelps, K.; Mahendroo, M.; Li, X. A compact fiber-optic SHG scanning endomicroscope and its application to visualize cervical remodeling during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 109, 12878–12883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Kang, C.H.; Gou, X.; Peng, Q.; Yan, J.; Zhuo, S.; Cheng, C.L.; He, Y.; Kang, Y.; Xia, W.; et al. Quantification of liver fibrosis via second harmonic imaging of the Glisson’s capsule from liver surface. J. Biophoton. 2016, 9, 351–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.; Rivera, D.; Ouzounov, D.; Webb, W.; Xu, C.; Pavlova, I.; Williams, W.; Mohanan, S. In vivo imaging of unstained tissues using a compact and flexible multiphoton microendoscope. J. Biomed. Opt. 2012, 17, 040505. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Wu, H.Y.; Sun, C.K. In vivo harmonic generation biopsy of human skin. J. Biomed. Opt. 2009, 14, 060505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llewellyn, M.E.; Barretto, R.P.; Delp, S.L.; Schnitzer, M.J. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 2008, 454, 784–788. [Google Scholar] [CrossRef]
- Avila, F.J.; Gambin, A.; Artal, P.; Bueno, J.M. In vivo two-photon microscopy of the human eye. Sci. Rep. 2019, 9, 10121. [Google Scholar] [CrossRef] [Green Version]
- Hristu, R.; Stanciu, S.G.; Dumitru, A.; Paun, B.; Floroiu, I.; Costache, M.; Stanciu, G.A. Influence of hematoxylin and eosin staining on the quantitative analysis of second harmonic generation imaging of fixed tissue sections. Biomed. Opt. Express 2021, 12, 5829–5843. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The controlled attenuation parameter (CAP): A novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, J.F.; Morin, S.; Taylor-Robinson, S.D. Transient elastography for the assessment of chronic liver disease: Ready for the clinic? World J. Gastroenterol. 2007, 13, 4791–4797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]

| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age | 41 | 42 | 70 | 59 |
| Sex | Male | Female | Female | Male |
| Blood test | ||||
| AST/IU L−1 | 43 | 20 | 29 | 63 |
| ALT/IU L−1 | 69 | 31 | 31 | 102 |
| PLT/×104 µL−1 | 21.7 | 23.7 | 20.0 | 22.6 |
| FIB-4 index | 1.00 (L) | 0.94 (L) | 1.88 (I) | 1.66 (I) |
| Ultrasound elastography | ||||
| CAP/dB m−1 | 201 (S0) | 242 (S0) | 253 (S1) | 322 (S3) |
| Liver stiffness/kPa | 3.8 (F0) | 4.2 (F0) | 5.7 (F0) | 13.1 (F4) |
| FAST score | 0.186 (L) | 0.052 (L) | 0.177(L) | 0.728 (H) |
| Histopathology | ||||
| Steatosis | + | + | + | ++ |
| Hepatitis | - | - | + | + |
| Fibrosis | F0 | F0 | F0 | F2 |
| NAS score | 1 | 2 | 2 | 5 |
| Total diagnosed stage | Ultra-early NAFLD | Ultra-early NAFLD | Early NASH | Advanced NASH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minamikawa, T.; Hase, E.; Ichimura-Shimizu, M.; Morimoto, Y.; Suzuki, A.; Yasui, T.; Nakamura, S.; Tsutsui, A.; Takaguchi, K.; Tsuneyama, K. Assessment of Ultra-Early-Stage Liver Fibrosis in Human Non-Alcoholic Fatty Liver Disease by Second-Harmonic Generation Microscopy. Int. J. Mol. Sci. 2022, 23, 3357. https://doi.org/10.3390/ijms23063357
Minamikawa T, Hase E, Ichimura-Shimizu M, Morimoto Y, Suzuki A, Yasui T, Nakamura S, Tsutsui A, Takaguchi K, Tsuneyama K. Assessment of Ultra-Early-Stage Liver Fibrosis in Human Non-Alcoholic Fatty Liver Disease by Second-Harmonic Generation Microscopy. International Journal of Molecular Sciences. 2022; 23(6):3357. https://doi.org/10.3390/ijms23063357
Chicago/Turabian StyleMinamikawa, Takeo, Eiji Hase, Mayuko Ichimura-Shimizu, Yuki Morimoto, Akihiro Suzuki, Takeshi Yasui, Satoko Nakamura, Akemi Tsutsui, Koichi Takaguchi, and Koichi Tsuneyama. 2022. "Assessment of Ultra-Early-Stage Liver Fibrosis in Human Non-Alcoholic Fatty Liver Disease by Second-Harmonic Generation Microscopy" International Journal of Molecular Sciences 23, no. 6: 3357. https://doi.org/10.3390/ijms23063357
APA StyleMinamikawa, T., Hase, E., Ichimura-Shimizu, M., Morimoto, Y., Suzuki, A., Yasui, T., Nakamura, S., Tsutsui, A., Takaguchi, K., & Tsuneyama, K. (2022). Assessment of Ultra-Early-Stage Liver Fibrosis in Human Non-Alcoholic Fatty Liver Disease by Second-Harmonic Generation Microscopy. International Journal of Molecular Sciences, 23(6), 3357. https://doi.org/10.3390/ijms23063357






