miRNAs in Cardiac Myxoma: New Pathologic Findings for Potential Therapeutic Opportunities
Abstract
1. miRNAs: The Clinical Concept
2. miRNA and Cardiac Myxomas
3. Biological Implications
4. Clinical Implications
5. Future Therapeutic Implications
6. Gaps in Evidence
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nappi, F.; Iervolino, A.; Singh, S.S.A.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Condorelli, G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ. Res. 2015, 116, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.; Choong, A.M.; Woo, C.C.; Foo, R.; Sorokin, V. Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2020, 21, 6334. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Elucidating the contributory role of microRNA to cardiovascular diseases (a review). Vasc. Pharmacol. 2019, 114, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wu, Y.; Zhang, H.; Guo, Y.; Cui, H.; Wei, S.; Zhao, Y.; Wang, R. Identify the critical protein-coding genes and long noncoding RNAs in cardiac myxoma. J. Cell. Biochem. 2019, 120, 13441–13452. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Liu, D.; Cui, C.; Wang, X. Endothelial-to-Mesenchymal Transition: Role in Cardiac Fibrosis. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 3–11. [Google Scholar] [CrossRef]
- Kalayinia, S.; Arjmand, F.; Maleki, M.; Malakootian, M.; Singh, C.P. MicroRNAs: Roles in cardiovascular development and disease. Cardiovasc. Pathol. 2021, 50, 107296. [Google Scholar] [CrossRef]
- Gholaminejad, A.; Zare, N.; Dana, N.; Shafie, D.; Mani, A.; Javanmard, S.H. A meta-analysis of microRNA expression profiling studies in heart failure. Heart Fail. Rev. 2021, 26, 997–1021. [Google Scholar] [CrossRef]
- Parikh, M.; Pierce, G. A Brief Review on the Biology and Effects of Cellular and Circulating microRNAs on Cardiac Remodeling after Infarction. Int. J. Mol. Sci. 2021, 22, 4995. [Google Scholar] [CrossRef]
- Kura, B.; Bacova, B.S.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef] [PubMed]
- Izarra, A.; Moscoso, I.; Cañón, S.; Carreiro, C.; Fondevila, D.; Martín-Caballero, J.; Blanca, V.; Valiente, I.; Díez-Juan, A.; Bernad, A. miRNA-1 and miRNA-133a are involved in early commitment of pluripotent stem cells and demonstrate antagonistic roles in the regulation of cardiac differentiation. J. Tissue Eng. Regen. Med. 2017, 11, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Wei, K. miRNA in cardiac development and regeneration. Cell Regen. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, J.; Wu, Q.; Chen, L. Specific miRNA expression profile in the blood serum of cardiac myxoma patients. Oncol. Lett. 2018, 16, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Viggiani, G.; Chen, Y.-W.; Coulis, G.; Castaldi, A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21, 4370. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. A Review of the Molecular Mechanisms Underlying Cardiac Fibrosis and Atrial Fibrillation. J. Clin. Med. 2021, 10, 4430. [Google Scholar] [CrossRef]
- Scalise, M.; Torella, M.; Marino, F.; Ravo, M.; Giurato, G.; Vicinanza, C.; Cianflone, E.; Mancuso, T.; Aquila, I.; Salerno, L.; et al. Atrial myxomas arise from multipotent cardiac stem cells. Eur. Heart J. 2020, 41, 4332–4345. [Google Scholar] [CrossRef]
- Pucci, A.; Mattioli, C.; Matteucci, M.; Lorenzini, D.; Panvini, F.; Pacini, S.; Ippolito, C.; Celiento, M.; De Martino, A.; Dolfi, A.; et al. Cell differentiation in cardiac myxomas: Confocal microscopy and gene expression analysis after laser capture microdissection. Heart Vessel. 2018, 33, 1403–1410. [Google Scholar] [CrossRef]
- Colpaert, R.M.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef]
- Tome, M.E.; López-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2010, 18, 985–995. [Google Scholar] [CrossRef]
- Tomé, M.; Sepúlveda, J.C.; Delgado, M.; Andrades, J.A.; Campisi, J.; González, M.A.; Bernad, A. miR-335 Correlates with Senescence/Aging in Human Mesenchymal Stem Cells and Inhibits Their Therapeutic Actions Through Inhibition of AP-1 Activity. Stem Cells 2014, 32, 2229–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Xu, H. miR-217 suppresses proliferation and promotes apoptosis in cardiac myxoma by targeting Interleukin-6. Biochem. Biophys. Res. Commun. 2017, 490, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Dong, P.; Wang, Y.; Zhang, J.; Shi, X.; Wang, Y. miR-218 suppresses cardiac myxoma proliferation by targeting myocyte enhancer factor 2D. Oncol. Rep. 2015, 33, 2606–2612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, Y.; Yang, J.; Bian, S.; Chen, G.; Yu, J. PPARgamma suppresses the proliferation of cardiac myxoma cells through downregulation of MEF2D in a miR-122-dependent manner. Biochem. Biophys. Res. Commun. 2016, 474, 560–565. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Yuan, T.; Krishnan, J. Non-coding RNAs in Cardiac Regeneration. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Rai, A.K.; Lee, B.; Gomez, R.; Rajendran, D.; Khan, M.; Garikipati, V.N.S. Current Status and Potential Therapeutic Strategies for Using Non-coding RNA to Treat Diabetic Cardiomyopathy. Front. Physiol. 2021, 11. [Google Scholar] [CrossRef]
- Abkhooie, L.; Sarabi, M.M.; Kahroba, H.; Eyvazi, S.; Montazersaheb, S.; Tarhriz, V.; Hejazi, M.S. Potential Roles of MyomiRs in Cardiac Development and Related Diseases. Curr. Cardiol. Rev. 2021, 17, 66–79. [Google Scholar] [CrossRef]
- Tolouei, S.E.; Curi, T.Z.; Klider, L.M.; Junior, A.G. MicroRNA-30 and 145 as Targets for the Treatment of Cardiovascular Diseases: Therapeutic Feasibility and Challenges. Curr. Pharm. Des. 2021, 27, 3858–3870. [Google Scholar] [CrossRef]
- Momin, M.Y.; Gaddam, R.R.; Kravitz, M.; Gupta, A.; Vikram, A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells 2021, 10, 3097. [Google Scholar] [CrossRef]
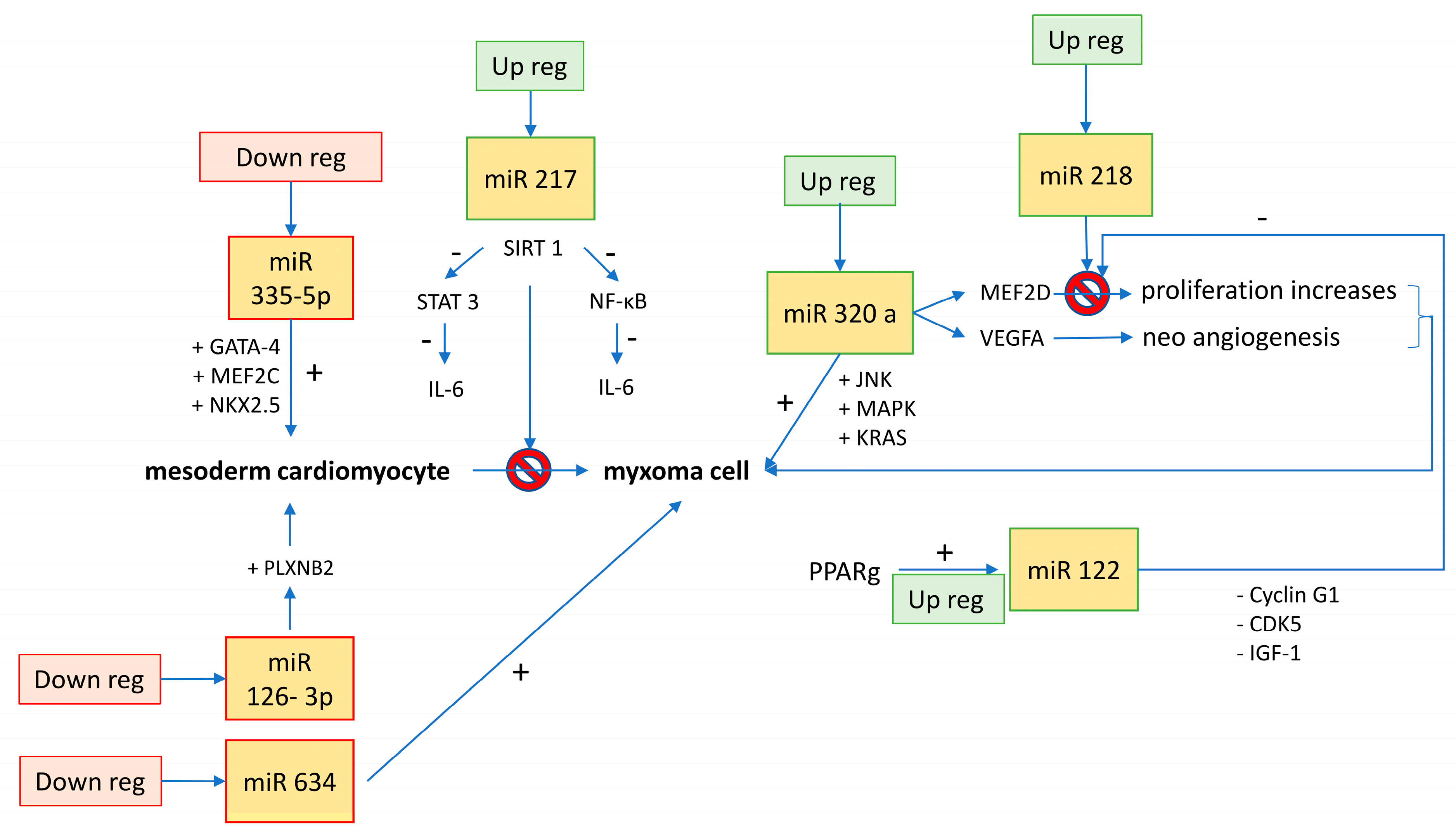
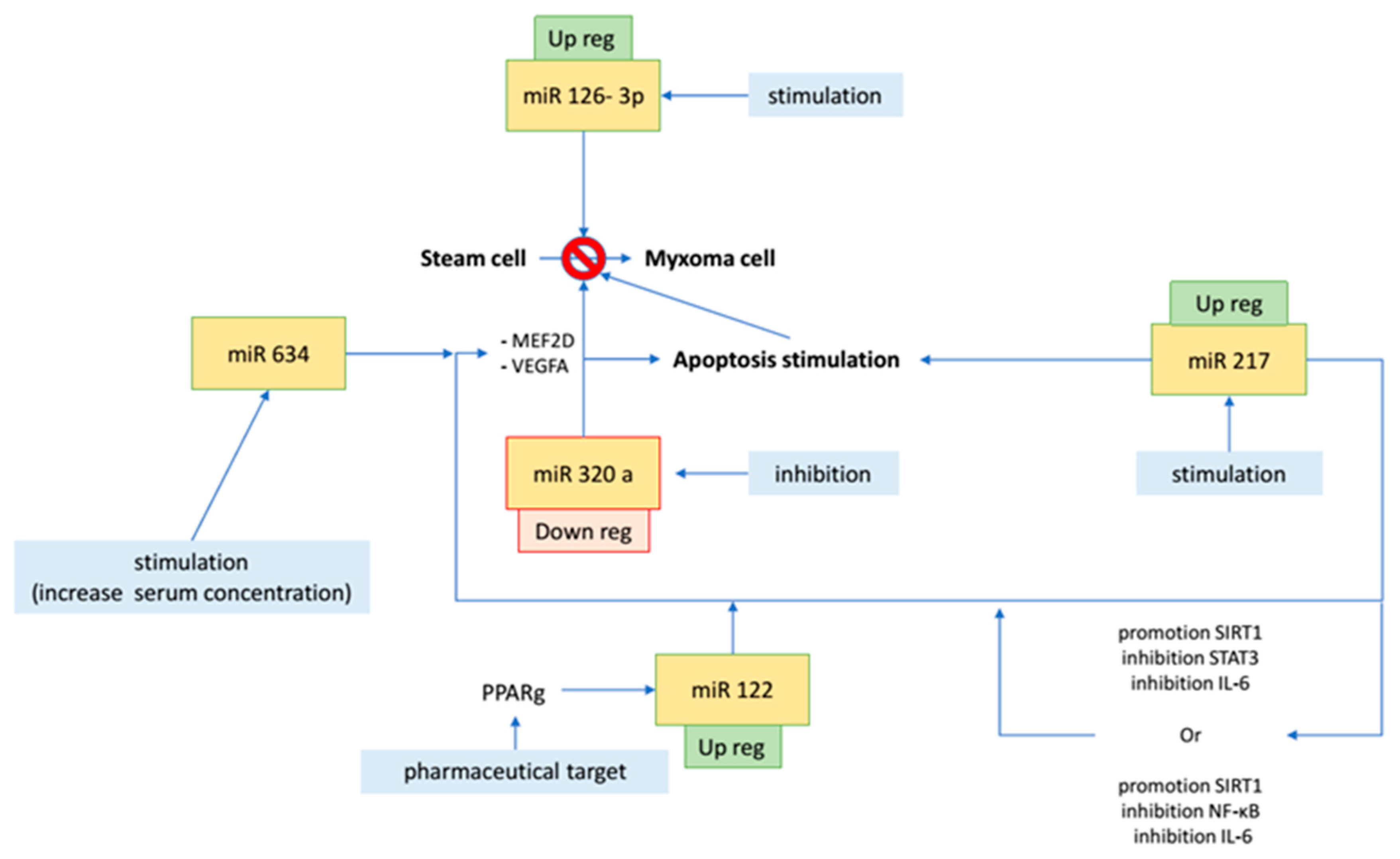
| MiRNAs | Biological Effect | Biological Control | Macroscopic Effect | Potential Therapeutic Implication | Reference |
|---|---|---|---|---|---|
| miR-335-5p | downregulation increases the expression of cardiac transcription factors (GATA-4, MEF2C, and NKX2.5) | ? | differentiation from embryonic stem cell to mesoderm cardiomyocyte | inhibition could enhance new myocyte formation from stem and progenitor cells | [17] |
| miR-126-3p | downregulation increases cell cycle trigger factors such as PLXNB2 | ? | cell growth | delay in cell overgrowth | [17] |
| miR-320a | overexpression induces MEF2D, VEGFA; interferes with the de novo biosynthesis of ceramide (apoptosis, cell differentiation, and proliferation) | ? | proliferation, cell invasion, apoptosis, and size increase (JNK, MAPK, KRAS); lipotoxicity in diabetic heart | ceramide-mediated apoptotic signaling pathways have been considered targets for anticancer therapies; inhibiting miR-320a unlocks the production of ceramides, promotes apoptosis, and inhibits MEF2D/VEGFA blocking cell proliferation and neoangiogenesis | [3,14] |
| miR-634 | ? | ? | oncosuppressor; inhibits cell proliferation and induces apoptosis | increases chemotherapy-induced cytotoxicity | [14] |
| miR-217 | suppresses production of IL-6 through different pathways: (1) promotion of SIRT-1 and inhibition of STAT-3 (cell cycle pathway); (2) promotion of SIRT-1 and inhibition of NF-kB (inflammatory pathway) | controlled by Ehmt1/Ehmt2 histone methylation (for cardiac hypertrophy) | inhibits proliferation and promotes apoptosis; implicated in cardiac hypertrophy | pharmacologically reduces the concentration of IL-6 through miR-217 stimulation | [19,22] |
| miR-122 | through PPARg pathway, promotion of miR-122 inhibits MEF2D | PPARg | blocks proliferation and induction of apoptosis or, in any case, blocks oncogenic factors (cyclin G1, CDK5, and IGF-1) | stimulation of PPARg to block MEF2D | [24] |
| miR-218 | MEF2D inhibitor | Dnmt3b DNA methylation | tumor suppressor when overexpressed (pro-apoptotic and reduces proliferation) | reduces cell proliferation through stimulation of miR-218/MEF2D pathway | [14,19,23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenna, A.; Loreni, F.; Giacinto, O.; Chello, C.; Nappi, P.; Chello, M.; Nappi, F. miRNAs in Cardiac Myxoma: New Pathologic Findings for Potential Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 3309. https://doi.org/10.3390/ijms23063309
Nenna A, Loreni F, Giacinto O, Chello C, Nappi P, Chello M, Nappi F. miRNAs in Cardiac Myxoma: New Pathologic Findings for Potential Therapeutic Opportunities. International Journal of Molecular Sciences. 2022; 23(6):3309. https://doi.org/10.3390/ijms23063309
Chicago/Turabian StyleNenna, Antonio, Francesco Loreni, Omar Giacinto, Camilla Chello, Pierluigi Nappi, Massimo Chello, and Francesco Nappi. 2022. "miRNAs in Cardiac Myxoma: New Pathologic Findings for Potential Therapeutic Opportunities" International Journal of Molecular Sciences 23, no. 6: 3309. https://doi.org/10.3390/ijms23063309
APA StyleNenna, A., Loreni, F., Giacinto, O., Chello, C., Nappi, P., Chello, M., & Nappi, F. (2022). miRNAs in Cardiac Myxoma: New Pathologic Findings for Potential Therapeutic Opportunities. International Journal of Molecular Sciences, 23(6), 3309. https://doi.org/10.3390/ijms23063309







