MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets
Abstract
1. miRNAs Effects on Aortic Stenosis and Mitral Prolapse Necessity of Novel Biomarkers
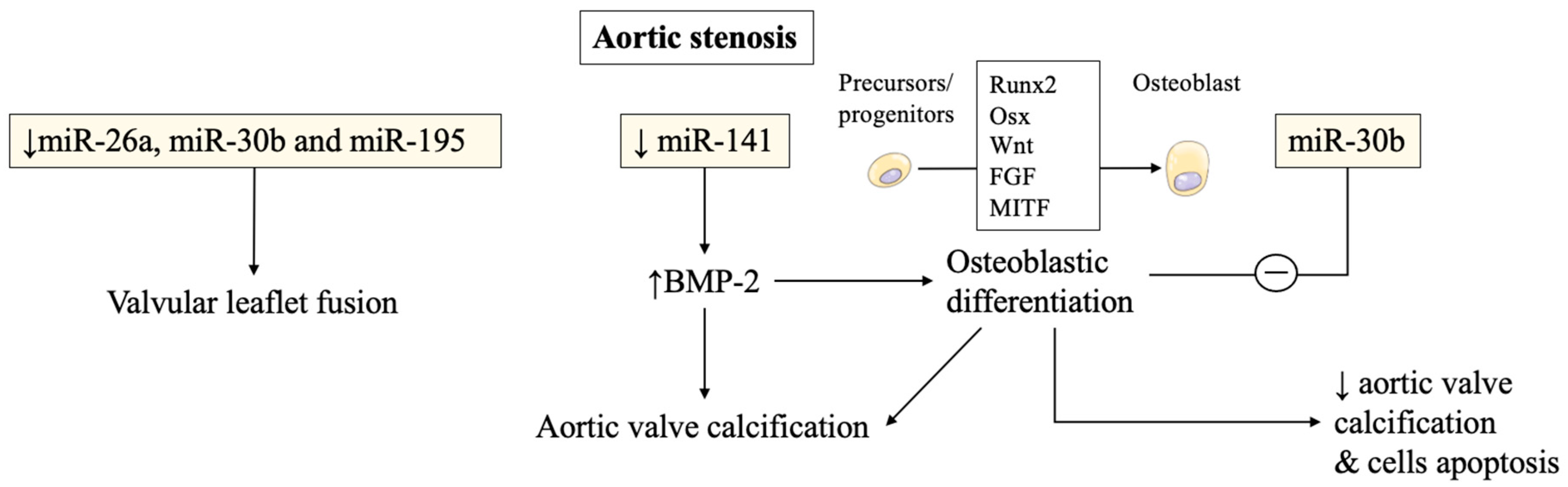
1.1. miRNAs Involvement in Aortic Stenosis Pathogenesis and Calcification Progression
1.2. The Importance of Discovering Diagnostic Biomarkers for Mitral Valve Prolapse
2. Pathophysiology of Valvular Calcification Pathways, from Preclinical Models to Clinical Perspectives
2.1. Complex Interplays between miRNAs and Intracellular Osteogenic Signals
2.2. Over and Underexpression of miRNAs from In Vivo Animal Experimentations
2.3. Diagnostic and Prognostic Relevance of miRNAs in Mitral Valve Diseases
3. Altered miRNAs Expression in Congenital Valve Disorders and Cardiogenetic Processes
4. Novel Therapeutical Strategies: miRNAs Targeting to Suppress or Activate Them
4.1. Results from In-Vivo and In-Vitro Testing for Aortic Valvular Stenosis
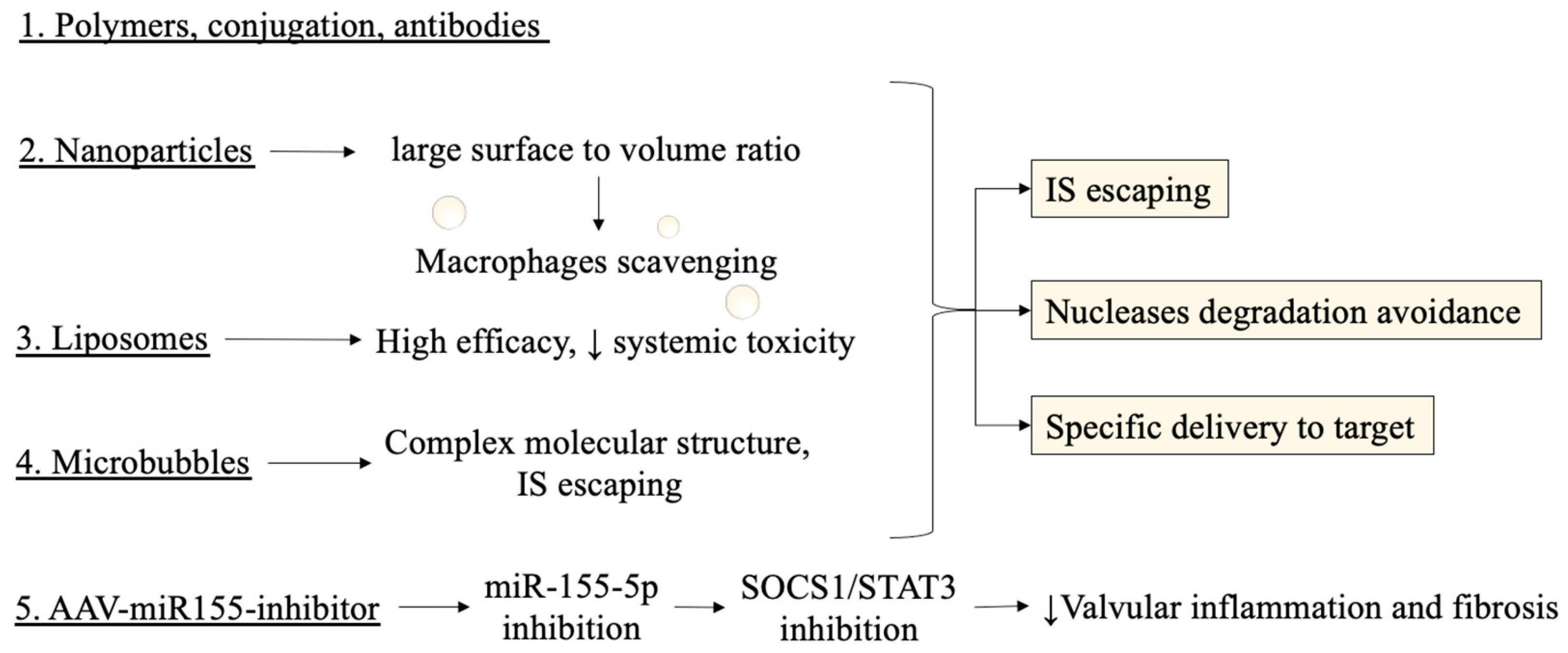
4.2. Technical Concerns on Stability and Efficacy of miRNAs as Therapeutical Targets
4.3. Disadvantages in Pharmacokinetics and Proposed Mechanisms for Delivery Vehicles
5. Conclusions: Future Directions and Clinical Relevance of Preclinical Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| ACP5 | acid phosphatase 5 |
| ACVIM | American College of Veterinary Internal Medicine |
| ADAMTS-7 | a disintegrin and metalloproteinase with thrombospondin motifs-7 |
| AGEs | advanced glycation end products |
| Ap | alkaline phosphatase |
| APF | autophagy promoting factor |
| APPAT | atherosclerotic plaque pathogenesis associated transcript |
| AR | aortic regurgitation |
| ASD | atrial septal defects |
| ASOs | antisense nucleotides |
| ATF4 | activator transcription factor 4 |
| AUROC | Area Under the Receiver Operating Characteristics |
| AVICS | aortic valve interstitial cells |
| BAV | bicuspid aortic valve |
| BMPs | bone morphogenetic proteins |
| BMP-2 | Bone morphogenetic protein 2 |
| CAIF | cardiac autophagy inhibitory factor |
| CARL | cardiac apoptosis-related lncRNA |
| CAVS | calcific aortic valve stenosis |
| Chast | cardiac hypertrophy-associated transcript |
| CHD | Congenital heart diseases |
| CHRF | cardiac hypertrophy-related factor; |
| CircRIC3 | circular RNA |
| CoA | coarctation of the aorta |
| CPLL | combinatorial peptide ligand library |
| DPP4 | dipeptidyl peptidase-4 |
| EGFR | Epidermal growth factor receptor |
| ESI-MS/MS | electrospray ionization mass spectrometry |
| ETA | endothelin type A |
| FGF | Fibroblast growth factor |
| GAS5 | growth block specificity 5 |
| GATA6 | GATA Binding Protein 6 |
| Giver | growth factor-and proinflammatory cytokine-induced vascular cell-expressed lncRNA |
| Gpx1 | Glutathione Peroxidase 1 |
| HCD | high cholesterol diet |
| HDAC4 | histone deacetylase 4 |
| HIF1A-AS1 | HIF1 alpha-antisense RNA1 |
| HOTAIR | HOX antisense intergenic RNA |
| HOXA2 | Homeobox A2 |
| hVICs | human valvular interstitial cells |
| IGF-1 | insulin-growth factor 1 |
| IS | immune system |
| iTRAQ | Isobaric Tags for Relative and Absolute Quantitation |
| JAK-STAT | Janus kinase-signal transducer and activator of transcription |
| lincRNA-p21 | long intergenic noncoding RNA-p21 |
| LNA | locked nucleic acid MEG3: maternally expressed gene 3 |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| Mhrt | myosin heavy chain associated RNA transcripts |
| MIAT | myocardial infarction–associated transcript |
| miRNA | microRNA |
| MITF | Microphthalmia-associated transcription factor |
| MMPs | Matrix metalloproteinase |
| MMVD | myxomatous mitral valve disease |
| MR | mitral regurgitation |
| MTCR | mitral chordae tendineae rupture |
| MV | mitral valve |
| MVP | mitral valve prolapse |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| Neat1 | nuclear-enriched abundant transcript 1 |
| NRAS | neuroblastoma RAS |
| OC | osteocalcin |
| OPG/RANK/RANKL | osteoprotegerin/receptor activator of nuclear factor-kB and its ligand |
| OPN | Osteopontin |
| OSX | osterix factor |
| Ph | phosphorus |
| PIK3R2 | phosphoinositide-3-kinase regulatory subunit 2 |
| Plekhm1 | pleckstrin homology domain-containing protein family M member 1 |
| PPP3CA | Protein Phosphatase 3 Catalytic Subunit Alpha |
| PPP3R1 | Protein Phosphatase 3 Regulatory Subunit B, Alpha |
| Prdx1 | Peroxiredoxin 1 |
| PTGS 2 | Prostaglandin-Endoperoxide Synthase 2 |
| PVA | pulmonary valve atresia |
| RHD | rheumatic heart disease |
| ROS | reactive oxygen species |
| RT-qPCR | Real-time quantitative PCR |
| Runx2 | RUNX Family Transcription Factor 2 |
| SATB2 | Special AT-rich sequence-binding protein 2 |
| siRNAs | short interfering RNAs |
| SIRT1 | Sirtuin 1 |
| SM | smooth muscle |
| SM22α | smooth muscle 22 alpha |
| SOCS1 | suppressor of cytokine signaling 1 |
| SOD1 | superoxide dismutase 1 |
| SOD2 | superoxide dismutase 2 |
| SRF | serum response factor |
| STAT3 | signal transducer and activator of transcription 3 |
| S1PR1 | Sphingosine-1-Phosphate Receptor 1 |
| TA | tricuspid atresia |
| TAV | tricuspid aortic valve |
| TGFB1 | tumor growth factor-beta 1 |
| TGFBR2 | transforming growth factor beta receptor 2 |
| TIMP-2 | Tissue inhibitor of metalloproteinases 2 |
| TNF-α | Tumor necrosis factor-alpha |
| TOF | tetralogy of Fallot |
| TRPV1 | transient receptor potential vanilloid type 1 |
| TUG1 | taurine upregulated 1 |
| UCA1 | urothelial carcinoma-associated |
| VC | valvular calcification |
| VEGFA | vascular endothelial growth factor alpha |
| VSD | ventricular septal defects |
| VSMCs | vascular smooth muscle cells |
| XIST | X-inactive specific transcript |
| α-SMA | alpha-smooth muscle actin |
| 2D | bi-dimensional |
| 2-OMe | 2-O-methyl |
| 3D | three-dimensional |
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Tüfekci, K.U.; Oner, M.G.; Meuwissen, R.L.; Genç, S. The role of microRNAs in human diseases. Methods Mol. Biol. 2014, 1107, 33–50. [Google Scholar]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRX 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Nigam, V.; Sievers, H.H.; Jensen, B.C.; Sier, H.A.; Simpson, P.C.; Srivastava, D.; Mohamed, S.A. Altered microRNAs in bicuspid aortic valve: A comparison between stenotic and insufficient valves. J. Heart Valve Dis. 2010, 19, 459–465. [Google Scholar]
- Yanagawa, B.; Lovren, F.; Pan, Y.; Garg, V.; Quan, A.; Tang, G.; Singh, K.K.; Shukla, P.C.; Kalra, N.P.; Peterson, M.D.; et al. miRNA-141 is a novel regulator of BMP-2-mediated calcification in aortic stenosis. J. Thorac. Cardiovasc. Surg. 2012, 144, 256–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Zhang, X.; Song, Z.; Han, L.; He, Y.; Xu, Z. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2014, 147, 1073–1080.e2. [Google Scholar] [CrossRef]
- Song, R.; Fullerton, D.A.; Ao, L.; Zhao, K.; Reece, T.B.; Cleveland, J.C.; Meng, X. Altered MicroRNA Expression Is Responsible for the Pro-Osteogenic Phenotype of Interstitial Cells in Calcified Human Aortic Valves. J. Am. Hear. Assoc. 2017, 6, e005364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Narang, R.; Sreenivas, V.; Rastogi, V.; Bhatia, J.; Saluja, D.; Srivastava, K. Circulatory miR-133b and miR-21 as Novel Biomarkers in Early Prediction and Diagnosis of Coronary Artery Disease. Genes 2020, 11, 164. [Google Scholar] [CrossRef]
- Ikeda, S.; Kong, S.W.; Lu, J.; Bisping, E.; Zhang, H.; Allen, P.D.; Golub, T.R.; Pieske, B.; Pu, W.T. Altered microRNA expression in human heart disease. Physiol. Genom. 2007, 31, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, Y.; Lu, Y.; Li, P.; Yu, H.; Diao, F.-R.; Tang, W.-D.; Hou, P.; Zhao, X.-X.; Shi, C.-Y. High level of circulating microRNA-142 is associated with acute myocardial infarction and reduced survival. Ir. J. Med. Sci. 2020, 189, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Wicik, Z.; De Rosa, S.; Eyileten, C.; Jakubik, D.; Spaccarotella, C.; Mongiardo, A.; Postula, M.; Indolfi, C. MicroRNAs fingerprint of bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 134, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Songia, P.; Branchetti, E.; Parolari, A.; Myasoedova, V.; Ferrari, G.; Alamanni, F.; Tremoli, E.; Poggio, P. Mitral valve endothelial cells secrete osteoprotegerin during endothelial mesenchymal transition. J. Mol. Cell. Cardiol. 2016, 98, 48–57, 386–387. [Google Scholar] [CrossRef]
- Songia, P.; Porro, B.; Chiesa, M.; Myasoedova, V.; Alamanni, F.; Tremoli, E.; Poggio, P. Identification of Patients Affected by Mitral Valve Prolapse with Severe Regurgitation: A Multivariable Regression Model. Oxidative Med. Cell. Longev. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Tan, H.T.; Ling, L.H.; Dolor-Torres, M.C.; Yip, J.W.-L.; Richards, A.M.; Chung, M.C. Proteomics discovery of biomarkers for mitral regurgitation caused by mitral valve prolapse. J. Proteom. 2013, 94, 337–345. [Google Scholar] [CrossRef]
- Hulanicka, M.; Garncarz, M.; Parzeniecka-Jaworska, M.; Jank, M. Plasma miRNAs as potential biomarkers of chronic degenerative valvular disease in Dachshunds. BMC Veter. Res. 2014, 10, 205. [Google Scholar] [CrossRef]
- Li, Q.; Freeman, L.M.; Rush, J.E.; Laflamme, D.P. Expression Profiling of Circulating MicroRNAs in Canine Myxomatous 410 Mitral Valve Disease. Int. J. Mol. Sci. 2015, 16, 14098–14108. [Google Scholar] [CrossRef]
- Bulent Vatan, M.; Kalayci Yigin, A.; Akdemir, R.; Tarik Agac, M.; Akif Cakar, M.; Aksoy, M.; Tatli, E.; Kilic, H.; Gunduz, H.; Guzel, D.; et al. Altered Plasma MicroRNA Expression in Patients with Mitral Chordae Tendineae Rupture. J. Heart Valve Dis. 2016, 25, 580–588. [Google Scholar]
- Hayek, E.; Gring, C.N.; Griffin, B.P. Mitral valve prolapse. Lancet 2005, 365, 507–518. [Google Scholar] [CrossRef]
- Delling, F.N.; Vasan, R.S. Epidemiology and Pathophysiology of Mitral Valve Prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef]
- Barlow, J.B.; Pocock, W.A. Billowing, floppy, prolapsed or flail mitral valves? Am. J. Cardiol. 1985, 55, 501–502. [Google Scholar] [CrossRef]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and Clinical Outcome of Mitral-Valve Prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. The changing spectrum of valvular heart disease pathology. In Harrison’s Advances in Cardiology; McGraw-Hill: New York, NY, USA, 2002; pp. 317–323. [Google Scholar]
- Avierinos, J.F.; Inamo, J.; Grigioni, F.; Gersh, B.; Shub, C.; Enriquez-Sarano, M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann. Intern. Med. 2008, 149, 787–795. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef]
- Lang, R.M.; Adams, D.H. 3D Echocardiographic Quantification in Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2012, 5, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Levine, R.A.; Hua, L.; Morris, E.L.; Kang, Y.; Flaherty, M.; Morgan, N.V.; Hung, J. Diagnostic Value of Vena Contracta Area in the Quantification of Mitral Regurgitation Severity by Color Doppler 3D Echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef]
- Carpentier, A.; Chauvaud, S.; Fabiani, J.N.; Deloche, A.; Relland, J.; Lessana, A.; D’Allaines, C.; Blondeau, P.; Piwnica, A.; Dubost, C. Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J. Thorac. Cardiovasc. Surg. 1980, 79, 338–358. [Google Scholar] [CrossRef]
- Yacoub, M.; Halim, M.; Radley-Smith, R.; McKay, R.; Nijveld, A.; Towers, M. Surgical treatment of mitral regurgitation caused by floppy valves: Repair versus replacement. Circulation 1981, 64, II-210–II-216. [Google Scholar]
- Verma, S.; Mesana, T.G. Mitral-Valve Repair for Mitral-Valve Prolapse. N. Engl. J. Med. 2009, 361, 2261–2269. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. EVEREST II Investigators Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Ryu, J.; Ahn, Y.; Kook, H.; Kim, Y.-K. The roles of non-coding RNAs in vascular calcification and opportunities as therapeutic targets. Pharmacol. Ther. 2021, 218, 107675. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Isaia, G.C. The osteoprotegerin/RANK/RANKL system: A bone key to vascular disease. J. Endocrinol. Investig. 2009, 32, 6–9. [Google Scholar]
- Min, H.; Morony, S.; Sarosi, I.; Dunstan, C.; Capparelli, C.; Scully, S.; Van, G.; Kaufman, S.; Kostenuik, P.J.; Lacey, D.L.; et al. Osteoprotegerin Reverses Osteoporosis by Inhibiting Endosteal Osteoclasts and Prevents Vascular Calcification by Blocking a Process Resembling Osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef]
- Agharazii, M.; St-Louis, R.; Gautier-Bastien, A.; Ung, R.-V.; Mokas, S.; Larivière, R.; Richard, D. Inflammatory Cytokines and Reactive Oxygen Species as Mediators of Chronic Kidney Disease-Related Vascular Calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, C.; Liu, Z.; Wang, L.; Liu, B.; He, F.; Zhang, T.; Wang, Y.; Wang, X.; Xu, M.; et al. Upregulation of a Disintegrin and Metalloproteinase With Thrombospondin Motifs-7 by miR-29 Repression Mediates Vascular Smooth Muscle Calcification. Arter. Thromb. Vasc. Biol. 2012, 32, 2580–2588. [Google Scholar] [CrossRef]
- Balderman, J.A.; Lee, H.Y.; Mahoney, C.E.; Handy, D.E.; White, K.; Annis, S.; Lebeche, D.; Hajjar, R.J.; Loscalzo, J.; Leopold, J.A. Bone Morphogenetic Protein-2 Decreases MicroRNA-30b and MicroRNA-30c to Promote Vascular Smooth Muscle Cell Calcification. J. Am. Hear. Assoc. 2012, 1, e003905. [Google Scholar] [CrossRef]
- Liao, X.-B.; Zhang, Z.-Y.; Yuan, K.; Liu, Y.; Feng, X.; Cui, R.-R.; Hu, Y.-R.; Yuan, Z.-S.; Gu, L.; Li, S.-J.; et al. MiR-133a Modulates Osteogenic Differentiation of Vascular Smooth Muscle Cells. Endocrinology 2013, 154, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.-R.; Li, S.-J.; Liu, L.-J.; Yi, L.; Liang, Q.-H.; Zhu, X.; Liu, G.-Y.; Liu, Y.; Wu, S.-S.; Liao, X.-B.; et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc. Res. 2012, 96, 320–329. [Google Scholar] [CrossRef]
- Qiao, W.; Chen, L.; Zhang, M. MicroRNA-205 Regulates the Calcification and Osteoblastic Differentiation of Vascular Smooth Muscle Cells. Cell. Physiol. Biochem. 2014, 33, 1945–1953. [Google Scholar] [CrossRef]
- Lin, X.; Zhan, J.K.; Zhong, J.Y.; Wang, Y.J.; Wang, Y.; Li, S.; Liu, Y.S. lncRNA-ES3/miR-34c-5p/BMF axis is involved in regulating high-glucose-induced calcification/senescence of VSMCs. Aging 2019, 11, 523–535. [Google Scholar] [CrossRef]
- Wei, J.; Shi, Y.; Zheng, L.; Zhou, B.; Inose, H.; Wang, J.; Guo, X.E.; Grosschedl, R.; Karsenty, G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J. Cell Biol. 2012, 197, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, L.; Cong, G.; Ren, L.; Hao, L. MicroRNA-34b/c inhibits aldosterone-induced vascular smooth muscle cell calcification via a SATB2/Runx2 pathway. Cell Tissue Res. 2016, 366, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Schinke, T.; Karsenty, G. The Osteoblast: A Sophisticated Fibroblast under Central Surveillance. Science 2000, 289, 1501–1504. [Google Scholar] [CrossRef]
- Sudo, R.; Sato, F.; Azechi, T.; Wachi, H. MiR-29-mediated elastin down-regulation contributes to inorganic phosphorus-induced osteoblastic differentiation in vascular smooth muscle cells. Genes Cells 2015, 20, 1077–1087. [Google Scholar] [CrossRef]
- Panizo, S.; Naves-Díaz, M.; Carrillo-López, N.; Martínez-Arias, L.; Fernández-Martín, J.L.; Ruiz-Torres, M.P.; Cannata-Andía, J.B.; Rodríguez, I. MicroRNAs 29b, 133b, and 211 Regulate Vascular Smooth Muscle Calcification Mediated by High Phosphorus. J. Am. Soc. Nephrol. 2016, 27, 824–834. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Tang, H.; Fang, X.; Wang, F.; Ding, Y.; Huang, H.; Zhang, H. Differentially expressed microRNA profiles in exosomes from vascular smooth muscle cells associated with coronary artery calcification. Int. J. Biochem. Cell Biol. 2019, 118, 105645. [Google Scholar] [CrossRef]
- Ryu, J.; Kwon, D.-H.; Choe, N.; Shin, S.; Jeong, G.; Lim, Y.-H.; Kim, J.; Park, W.J.; Kook, H.; Kim, Y.-K. Characterization of Circular RNAs in Vascular Smooth Muscle Cells with Vascular Calcification. Mol. Ther.-Nucleic Acids 2019, 19, 31–41. [Google Scholar] [CrossRef]
- Songia, P.; Chiesa, M.; Alfieri, V.; Massaiu, I.; Moschetta, D.; Myasoedova, V.; Valerio, V.; Fusini, L.; Gripari, P.; Zanobini, M.; et al. Putative Circulating MicroRNAs Are Able to Identify Patients with Mitral Valve Prolapse and Severe Regurgitation. Int. J. Mol. Sci. 2021, 22, 2102. [Google Scholar] [CrossRef]
- Deroyer, C.; Magne, J.; Moonen, M.; Le Goff, C.; Dupont, L.; Hulin, A.; Radermecker, M.; Colige, A.; Cavalier, E.; Kolh, P.; et al. New biomarkers for primary mitral regurgitation. Clin. Proteom. 2015, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saporiti, F.; Piacentini, L.; Alfieri, V.; Bono, E.; Ferrari, F.; Chiesa, M.; Colombo, G.I. Melanocortin-1 Receptor Positively Regulates Human Artery Endothelial Cell Migration. Cell. Physiol. Biochem. 2019, 52, 1339–1360. [Google Scholar] [CrossRef] [PubMed]
- Wylie-Sears, J.; Aikawa, E.; Levine, R.A.; Yang, J.-H.; Bischoff, J. Mitral Valve Endothelial Cells With Osteogenic Differentiation Potential. Arter. Thromb. Vasc. Biol. 2011, 31, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, E.; Aikawa, M.; Stone, J.R.; Fukumoto, Y.; Libby, P.; Schoen, F.J. Activated Interstitial Myofibroblasts Express Catabolic Enzymes and Mediate Matrix Remodeling in Myxomatous Heart Valves. Circulation 2001, 104, 2525–2532. [Google Scholar] [CrossRef]
- Roberts, W.C.; Vowels, T.J.; Ko, J.M.; Hebeler, R.F., Jr. Gross and Histological Features of Excised Portions of Posterior Mitral Leaflet in Patients Having Operative Repair of Mitral Valve Prolapse and Comments on the Concept of Missing (=Ruptured) Chordae Tendineae. J. Am. Coll. Cardiol. 2014, 63, 1667–1674. [Google Scholar] [CrossRef]
- Sainger, R.; Grau, J.B.; Branchetti, E.; Poggio, P.; Seefried, W.F.; Field, B.C.; Acker, M.A.; Gorman, R.C.; Gorman, J.H.; Hargrove, C.W., 3rd; et al. Human myxomatous mitral valve prolapse: Role of bone morphogenetic protein 4 in valvular interstitial cell activation. J. Cell. Physiol. 2012, 227, 2595–2604. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X.; Yang, A.; Xu, A.; Huang, T.; You, H. miRNA-150-5p promotes hepatic stellate cell proliferation and sensitizes hepatocyte apoptosis during liver fibrosis. Epigenomics 2020, 12, 53–67. [Google Scholar] [CrossRef]
- Liu, F.; Di Wang, X. miR-150-5p represses TP53 tumor suppressor gene to promote proliferation of colon adenocarcinoma. Sci. Rep. 2019, 9, 6740. [Google Scholar] [CrossRef]
- Zhu, J.; Han, S. miR-150-5p promotes the proliferation and epithelial-mesenchymal transition of cervical carcinoma cells via targeting SRCIN1. Pathol.-Res. Pr. 2019, 215, 738–747. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Lu, L.; Zheng, Y.; Zhang, P. Congenital Heart Disease: Causes, Diagnosis, Symptoms, and Treatments. Cell Biophys. 2015, 72, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Dolbec, K.; Mick, N.W. Congenital heart disease. Emerg. Med. Clin. N. Am. 2011, 29, 811–827. [Google Scholar] [CrossRef]
- Higgins, S.S.; Reid, A. Common congenital heart defects. Long-term follow-up. Nurs. Clin. N. Am. 1994, 29, 233–248. [Google Scholar]
- Chen, J.-F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.-Z. The Role of MicroRNA-1 and MicroRNA-133 in Skeletal Muscle Proliferation and Differentiation. Nat. Genet. 2005, 38, 228–233. [Google Scholar] [CrossRef]
- Yin, V.P.; Lepilina, A.; Smith, A.; Poss, K.D. Regulation of zebrafish heart regeneration by miR-133. Dev. Biol. 2012, 365, 319–327. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Ferro, M.D.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kamińska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, B.; Yang, Y.-X.; Jia, Q.-J.; Zhang, A.; Qi, Z.-W.; Zhang, J.-P. Long Noncoding RNAs in Pathological Cardiac Remodeling: A Review of the Update Literature. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.A.; Zilberberg, J.; Davies, P.F. A rapid, reliable method to isolate high quality endothelial RNA from small spatially-defined locations. Ann. Biomed. Eng. 2004, 32, 1453–1459. [Google Scholar] [CrossRef]
- Simmons, C.A.; Grant, G.; Manduchi, E.; Davies, P. Spatial Heterogeneity of Endothelial Phenotypes Correlates With Side-Specific Vulnerability to Calcification in Normal Porcine Aortic Valves. Circ. Res. 2005, 96, 792–799. [Google Scholar] [CrossRef]
- Scolari, F.L.; Faganello, L.S.; Garbin, H.I.; e Mattos, B.P.; Biolo, A. A systematic review of microRNAs in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2020, 327, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.V.; Merino, D.; Wenner, M.; Llano, M.; Cobo, M.; Montalvo, C.; García, R.; Martín-Durán, R.; Hurlé, J.M.; A Hurlé, M.; et al. Myocardial gene expression of microRNA-133a and myosin heavy and light chains, in conjunction with clinical parameters, predict regression of left ventricular hypertrophy after valve replacement in patients with aortic stenosis. Heart 2011, 97, 1132–1137. [Google Scholar] [CrossRef]
- García, R.; Villar, A.V.; Cobo, M.; Llano, M.; Martín-Durán, R.; Hurlé, M.A.; Nistal, J.F. Circulating Levels of miR-133a Predict the Regression Potential of Left Ventricular Hypertrophy After Valve Replacement Surgery in Patients With Aortic Stenosis. J. Am. Hear. Assoc. 2013, 2, e000211. [Google Scholar] [CrossRef]
- Dixit, G.; Schanz, W.; Pappas, B.; Maretzky, T. Members of the Fibroblast Growth Factor Receptor Superfamily Are Proteolytically Cleaved by Two Differently Activated Metalloproteases. Int. J. Mol. Sci. 2021, 22, 3165. [Google Scholar] [CrossRef]
- Rathan, S.; Ankeny, C.J.; Arjunon, S.; Ferdous, Z.; Kumar, S.; Esmerats, J.F.; Heath, J.M.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Identification of side- and shear-dependent microRNAs regulating porcine aortic valve pathogenesis. Sci. Rep. 2016, 6, 25397. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.M.; Esmerats, J.F.; Khambouneheuang, L.; Kumar, S.; Simmons, R.; Jo, H. Mechanosensitive microRNA-181b Regulates Aortic Valve Endothelial Matrix Degradation by Targeting TIMP3. Cardiovasc. Eng. Technol. 2017, 9, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Pinilla-Vera, M.; Blackwell, T.S.; Baron, R.M.; et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar] [CrossRef] [PubMed]
- Larivière, R.; Gauthier-Bastien, A.; Ung, R.-V.; St-Hilaire, J.; Mac-Way, F.; Richard, D.; Agharazii, M. Endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease. J. Hypertens. 2017, 35, 376–384. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zhang, B.H.; Pan, C.S.; Jiang, H.F.; Pang, Y.Z.; Tang, C.S.; Qi, Y.F. Endothelin-1 is a potent regulator in vivo in vascular calcification and in vitro in calcification of vascular smooth muscle cells. Peptides 2003, 24, 1149–1156. [Google Scholar] [CrossRef]
- Henein, M.; Granåsen, G.; Wiklund, U.; Schmermund, A.; Guerci, A.; Erbel, R.; Raggi, P. High dose and long-term statin therapy accelerate coronary artery calcification. Int. J. Cardiol. 2015, 184, 581–586. [Google Scholar] [CrossRef]
- Toshima, T.; Watanabe, T.; Narumi, T.; Otaki, Y.; Shishido, T.; Aono, T.; Goto, J.; Watanabe, K.; Sugai, T.; Takahashi, T.; et al. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signaling. Cardiovasc. Res. 2019, 116, 983–994. [Google Scholar] [CrossRef]
- Wang, Y.; Han, D.; Zhou, T.; Zhang, J.; Liu, C.; Cao, F.; Dong, N. Melatonin ameliorates aortic valve calcification via the regulation of circular RNA CircRIC3/miR-204-5p/DPP4 signaling in valvular interstitial cells. J. Pineal. Res. 2020, 69, e12666. [Google Scholar] [CrossRef] [PubMed]
- Van der Ven, C.F.; Wu, P.J.; Tibbitt, M.W.; van Mil, A.; Sluijter, J.P.; Langer, R.; Aikawa, E. In vitro 3D model and miRNA drug delivery to target calcific aortic valve disease. Clin. Sci. 2017, 131, 181–195. [Google Scholar] [CrossRef]
- Ebert, M.S.; Neilson, J.R.; A Sharp, P. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Ma, L.; Reinhardt, F.; Pan, E.; Soutschek, J.; Bhat, B.; Marcusson, E.G.; Teruya-Feldstein, J.; Bell, G.W.; A Weinberg, R. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol. 2010, 28, 341–347. [Google Scholar] [CrossRef]
- Davis, S.; Lollo, B.; Freier, S.; Esau, C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006, 34, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Kanasty, R.L.; A Whitehead, K.; Vegas, A.J.; Anderson, D.G. Action and Reaction: The Biological Response to siRNA and Its Delivery Vehicles. Mol. Ther. 2012, 20, 513–524. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R. Anderson DG Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Tafer, H.; Ameres, S.L.; Obernosterer, G.; A Gebeshuber, C.; Schroeder, R.; Martinez, J.; Hofacker, I. The impact of target site accessibility on the design of effective siRNAs. Nat. Biotechnol. 2008, 26, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Chen, M.Y.; Hoffer, A.; Morrison, P.F.; Hamilton, J.F.; Hughes, J.; Schlageter, K.S.; Lee, J.; Kelly, B.R.; Oldfield, E.H. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J. Neurosurg. 2005, 103, 311–319. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Davis, M.E. Physicochemical and Biological Characterization of Targeted, Nucleic Acid-Containing Nanoparticles. Bioconjugate Chem. 2007, 18, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Majoros, I.J.; Orr, B.G.; Baker, J.R.; Holl, M.M.B. The Binding Avidity of a Nanoparticle-Based Multivalent Targeted Drug Delivery Platform. Chem. Biol. 2007, 14, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, T.; Luan, Y.; Renaud, G.; van der Steen, A.F.; Versluis, M.; de Jong, N.; Kooiman, K. Non-linear response and viscoelastic properties of lipid-coated microbubbles: DSPC versus DPPC. Ultrasound Med. Biol. 2015, 41, 1432–1445. [Google Scholar] [CrossRef]
- Langeveld, S.; Beekers, I.; Collado-Lara, G.; van der Steen, A.; de Jong, N.; Kooiman, K. The Impact of Lipid Handling and Phase Distribution on the Acoustic Behavior of Microbubbles. Pharmaceutics 2021, 13, 119. [Google Scholar] [CrossRef]
- Daeichin, V.; van Rooij, T.; Skachkov, I.; Ergin, B.; Specht, P.A.; Lima, A.; Ince, C.; Bosch, J.G.; van der Steen, A.F.; de Jong, N.; et al. Microbubble Composition and Preparation for High-Frequency Contrast-Enhanced Ultrasound Imaging: In Vitro and In Vivo Evaluation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 555–567. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V.; Gupta, G.; Khanna, N. Effect of PEGylation on performance of protein microbubbles and its comparison with lipid microbubbles. Mater. Sci. Eng. C 2017, 71, 425–430. [Google Scholar] [CrossRef]
- Gilam, A.; Conde, J.; Weissglas-Volkov, D.; Oliva-Jorge, N.; Friedman, E.; Artzi, N.; Shomron, N. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat. Commun. 2016, 7, 12868. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva-Jorge, N.; Artzi, N. Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E1278–E1287. [Google Scholar] [CrossRef]
- Segovia, N.; Pont, M.; Oliva, N.; Ramos, V.; Borrós, S.; Artzi, N. Hydrogel Doped with Nanoparticles for Local Sustained Release of siRNA in Breast Cancer. Adv. Heal. Mater. 2014, 4, 271–280. [Google Scholar] [CrossRef]
- Conde, J.; Oliva-Jorge, N.; Atilano, M.; Song, H.S.; Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 2015, 15, 353–363. [Google Scholar] [CrossRef]
- Rita Balistreri, C.; Allegra, A.; Crapanzano, F.; Pisano, C.; Ruvolo, G. Matrix Metalloproteinases (MMPs), Their Genetic Variants and miRNA in Mitral Valve Diseases: Potential Biomarker Tools and Targets for Personalized Treatments. J. Heart Valve Dis. 2016, 25, 463–474. [Google Scholar] [PubMed]
- Spadaccio, C.; Rainer, A.; Mozetic, P.; Trombetta, M.; Dion, R.A.; Barbato, R.; Nappi, F.; Chello, M. The role of extracellular matrix in age-related conduction disorders: A forgotten player? J. Geriatr. Cardiol. 2015, 12, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Mozetic, P.; Nappi, F.; Nenna, A.; Sutherland, F.; Trombetta, M.; Chello, M.; Rainer, A. Cells and extracellular matrix interplay in cardiac valve disease: Because age matters. Basic Res. Cardiol. 2016, 111, 16. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Taffon, C.; Nenna, A.; Crescenzi, A.; Chello, M.; Trombetta, M.; Gambardella, I.; et al. Implantation of a Poly-l-Lactide GCSF-Functionalized Scaffold in a Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Chello, M.; Acar, C. The Ross procedure: Underuse or under-comprehension? J. Thorac. Cardiovasc. Surg. 2015, 149, 1463–1464. [Google Scholar] [CrossRef][Green Version]
- Nappi, F.; Fraldi, M.; Spadaccio, C.; Carotenuto, A.R.; Montagnani, S.; Castaldo, C.; Chachques, J.C.; Acar, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. Part A 2016, 104, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Nataf, P.; Guettier, C.; Bourbon, A.; Nappi, F.; Lima, L.; Dorent, R.; Pavie, A.; Gandjbakhch, I. Influence of arterial allograft preparation techniques on chronic vascular rejection: A histological study. Transplant. Proc. 1996, 28, 2890–2892. [Google Scholar]
- Chen, A.; Wen, J.; Lu, C.; Lin, B.; Xian, S.; Huang, F.; Wu, Y.; Zeng, Z. Inhibition of miR-155-5p attenuates the valvular damage induced by rheumatic heart disease. Int. J. Mol. Med. 2020, 45, 429–440. [Google Scholar] [CrossRef]

| Study Groups Hulanicka et al. [13], Li et al. [14] | miR-30b, miR-29b | miR-133b, miR-21, miR-126, miR-423, miR-125, miR-208a, miR-208b | cfa-miR-302d, cfa-miR-380, cfa-miR-874, cfa-miR-582, cfa-miR-490, cfa-miR-329b, and cfa-miR-487b | cfa-miR-103, cfa-miR-98, cfa-let-7b, and cfa-let-7c |
|---|---|---|---|---|
| ACVIM stage B HF [17] | downregulated | normal | not tested | not tested |
| ACVIM stage C HF [17] | downregulated | downregulated | not tested | not tested |
| Group B (stage B1, B2) [18] | not tested | not tested | downregulated | upregulated |
| Group C (stage C, D) [18] | not tested | not tested | downregulated | upregulated |
| Cardiovascular Diseases | 140-3p | 150-5p | 210-3p | 451a | 487a-3p | 223-3p | 323a-3p | 361-5p | 340-5p |
|---|---|---|---|---|---|---|---|---|---|
| MVP versus controls | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ |
| MCTR versus controls | / | ↓ | / | / | / | ↓ | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Iervolino, A.; Avtaar Singh, S.S.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132. https://doi.org/10.3390/ijms222212132
Nappi F, Iervolino A, Avtaar Singh SS, Chello M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. International Journal of Molecular Sciences. 2021; 22(22):12132. https://doi.org/10.3390/ijms222212132
Chicago/Turabian StyleNappi, Francesco, Adelaide Iervolino, Sanjeet Singh Avtaar Singh, and Massimo Chello. 2021. "MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets" International Journal of Molecular Sciences 22, no. 22: 12132. https://doi.org/10.3390/ijms222212132
APA StyleNappi, F., Iervolino, A., Avtaar Singh, S. S., & Chello, M. (2021). MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. International Journal of Molecular Sciences, 22(22), 12132. https://doi.org/10.3390/ijms222212132






