CCL2 Inhibition of Pro-Resolving Mediators Potentiates Neuroinflammation in Astrocytes
Abstract
:1. Introduction
2. Results
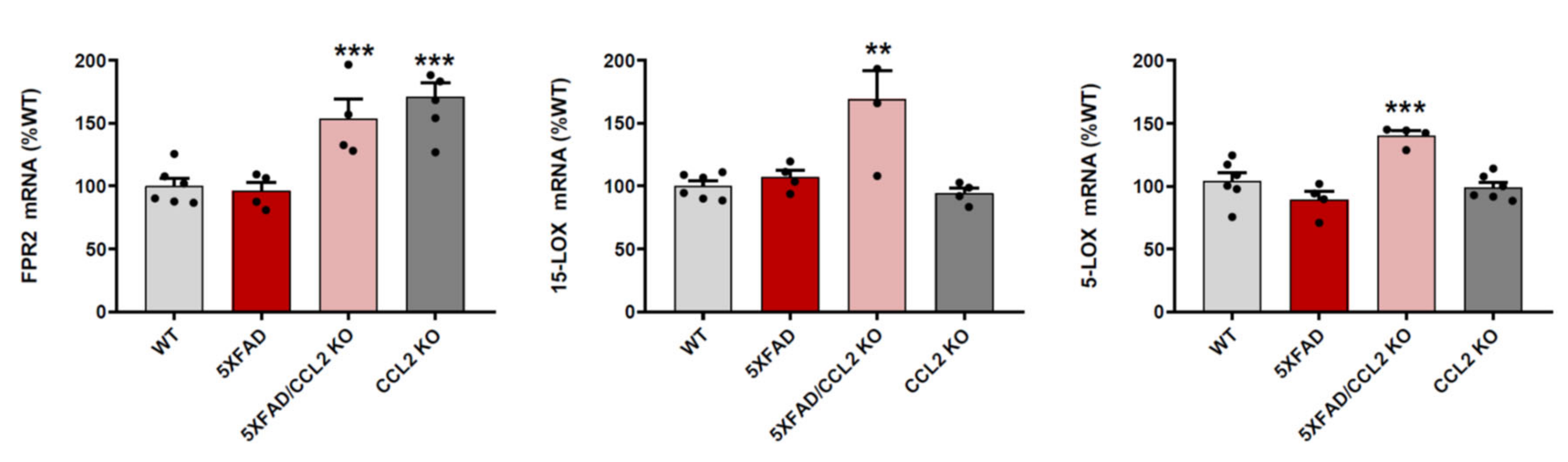
2.1. CCL2 Deletion Increases FPR2, 15-LOX and 5-LOX mRNA Expression in 5xFAD Mice
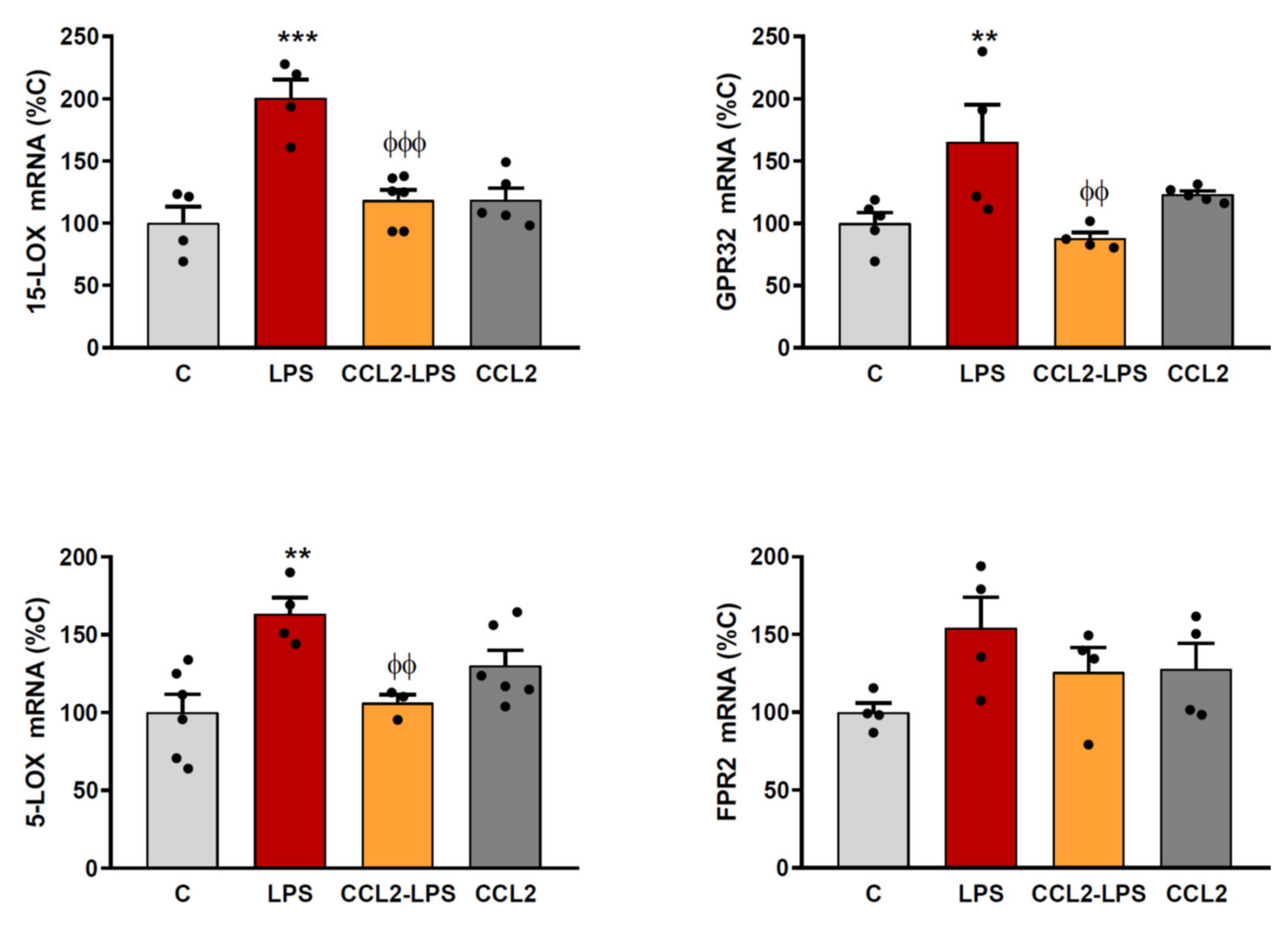
2.2. CCL2 Prevents the Expression of RvD1 Synthesizing Enzymes and Receptors
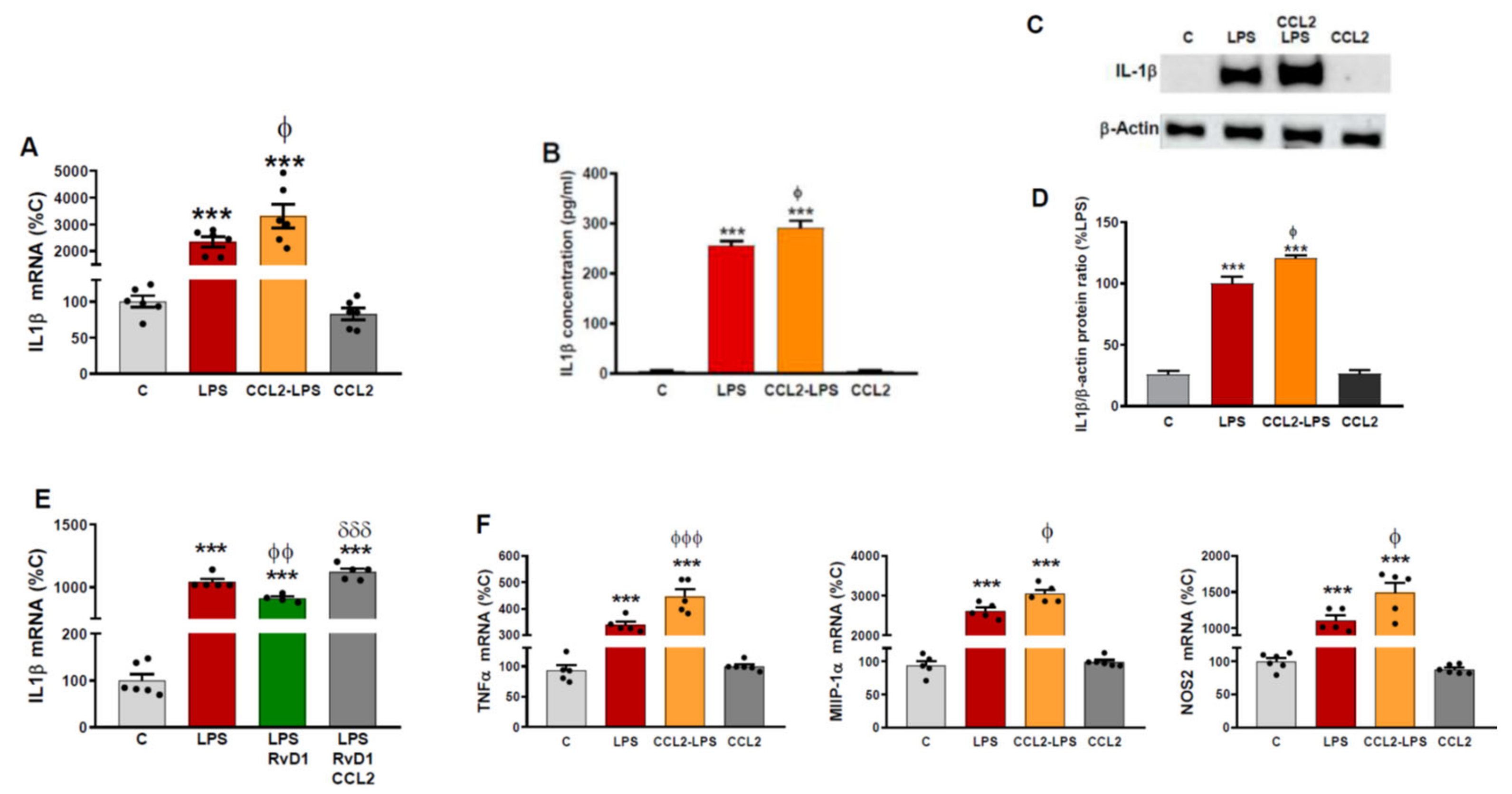
2.3. CCL2 Potentiates the Production of Inflammatory Markers Induced by LPS in Astrocytes
3. Discussion
4. Materials and Methods
4.1. Mouse Models and Brain Samples Collection
4.2. Cell Cultures
4.3. mRNA Analysis
| Mouse FPR2: | CTGGGCTCAAACTGATGAAGA/CGTAAAGGACGGCTGGAATTA |
| Mouse 15-LOX: | GGGACAATGGACACCGTTATTA/CCAGGTACTGCTGACTACAAAG |
| Mouse 5-LOX: | TCAAGCAGCACAGACGTAAA/GCCATCCAGTAGCTCGTAATC |
| Rat 15-LOX: | CTCCACTACAAGACCGACAAAG/GTGCATTAGGAACCCAGTAGAA |
| Rat 5-LOX: | CTGGTAGCCCATGTGAGGTT/GCACAGGGAGGAATAGGTCA |
| Rat GPR32: | CCTTTCTGGTTCTCACCTTCTT/GTGATGGCCTGTCTCTCTTTC |
| Rat FPR2: | CGCTGTCAAGATCCACAGAA/CTCCAAACTGGAAGGCAGAG |
| Rat IL-1β: | ACCTGCTAGTGTGTGATGTTCCCA/AGGTGGAGAGCTTTCAGCTCACAT |
| RatMIP-1α: | CAGAACATTCCTGCCACCTGCAAA/AGGAATGTGCCCTGAGGTCTT TCA |
| Rat TNFα: | CTGGCCAATGGCATGGATCTCAAA/AGCCTTGTCCCTTGAAGAGAA CCT |
| Rat NOS2: | AGCACATTTGGCAATGGAGACTGC/AGCAAAGGCACAGAACTGAGG GTA |
| Rat/mouse GAPDH: | TGCACCACCAACTGCTTAGA/GGCATGGACTGTGGTCATGAG |
4.4. Western Blot
4.5. IL-1β Measurement
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madrigal, J.L.; Caso, J.R. The chemokine (C-C motif) ligand 2 in neuroinflammation and neurodegeneration. Adv. Exp. Med. Biol. 2014, 824, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, I.L.; Gonzalez-Prieto, M.; Caso, J.R.; Garcia-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. Reboxetine Treatment Reduces Neuroinflammation and Neurodegeneration in the 5xFAD Mouse Model of Alzheimer’s Disease: Role of CCL2. Mol. Neurobiol. 2019, 56, 8628–8642. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.X.; Arumugam, T.V.; Gelderblom, M.; Magnus, T.; Drummond, G.R.; Sobey, C.G. Role of CCR2 in inflammatory conditions of the central nervous system. J. Cereb. Blood Flow Metab. 2014, 34, 1425–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, J.; Jiang, X.; Qian, W.; Yang, T.; Sun, X.; Chen, Z.; Zhu, Q. Neuron-derived CCL2 contributes to microglia activation and neurological decline in hepatic encephalopathy. Biol. Res. 2017, 50, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khoury, J.; Toft, M.; Hickman, S.E.; Means, T.K.; Terada, K.; Geula, C.; Luster, A.D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007, 13, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Naert, G.; Rivest, S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6208–6220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittington, R.A.; Planel, E.; Terrando, N. Impaired Resolution of Inflammation in Alzheimer’s Disease: A Review. Front. Immunol. 2017, 8, 1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, K.; Fukunaga, H.; Tatewaki, Y.; Takano, Y.; Yamamoto, S.; Mutoh, T.; Taki, Y. Alzheimer’s Disease and Specialized Pro-Resolving Lipid Mediators: Do MaR1, RvD1, and NPD1 Show Promise for Prevention and Treatment? Int. J. Mol. Sci. 2020, 21, 5783. [Google Scholar] [CrossRef] [PubMed]
- Schmitz Nunes, V.; Rogerio, A.P.; Abrahao, O., Jr. Insights into the Activation Mechanism of the ALX/FPR2 Receptor. J. Phys. Chem. Lett. 2020, 11, 8952–8957. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front. Pharm. 2020, 11, 612. [Google Scholar] [CrossRef]
- Glabinski, A.R.; Balasingam, V.; Tani, M.; Kunkel, S.L.; Strieter, R.M.; Yong, V.W.; Ransohoff, R.M. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J. Immunol. 1996, 156, 4363–4368. [Google Scholar] [PubMed]
- Schmid, M.; Gemperle, C.; Rimann, N.; Hersberger, M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J. Immunol. 2016, 196, 3429–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dartt, D.A.; Hodges, R.R.; Li, D.; Shatos, M.A.; Lashkari, K.; Serhan, C.N. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J. Immunol. 2011, 186, 4455–4466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.J.; Liao, Y.C.; Wang, Y.F.; Lin, I.F.; Wang, S.J.; Fuh, J.L. Plasma MCP-1 and Cognitive Decline in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: A Two-year Follow-up Study. Sci. Rep. 2018, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Fredman, G.; Serhan, C.N. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 2012, 180, 2018–2027. [Google Scholar] [CrossRef] [Green Version]
- Tiberi, M.; Chiurchiu, V. Specialized Pro-resolving Lipid Mediators and Glial Cells: Emerging Candidates for Brain Homeostasis and Repair. Front. Cell Neurosci. 2021, 15, 673549. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Stratoulias, V.; Venero, J.L.; Tremblay, M.E.; Joseph, B. Microglial subtypes: Diversity within the microglial community. EMBO J. 2019, 38, e101997. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Schultzberg, M.; Hjorth, E. Differential regulation of resolution in inflammation induced by amyloid-beta42 and lipopolysaccharides in human microglia. J. Alzheimers Dis. 2015, 43, 1237–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiffany, H.L.; Lavigne, M.C.; Cui, Y.H.; Wang, J.M.; Leto, T.L.; Gao, J.L.; Murphy, P.M. Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J. Biol. Chem. 2001, 276, 23645–23652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.V.; Dalli, J.; Flower, R.J.; Serhan, C.N.; Perretti, M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: Receptor-dependent actions. Arter. Thromb. Vasc. Biol. 2012, 32, 1970–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, W.Y.; Chua, J.J.E. Role of formyl peptide receptor 2 (FPR2) in the normal brain and in neurological conditions. Neural. Regen. Res. 2019, 14, 2071–2072. [Google Scholar] [CrossRef] [PubMed]
- Oldekamp, S.; Pscheidl, S.; Kress, E.; Soehnlein, O.; Jansen, S.; Pufe, T.; Wang, J.M.; Tauber, S.C.; Brandenburg, L.O. Lack of formyl peptide receptor 1 and 2 leads to more severe inflammation and higher mortality in mice with of pneumococcal meningitis. Immunology 2014, 143, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; Dello Russo, C.; Gavrilyuk, V.; Feinstein, D.L. Effects of noradrenaline on neuronal NOS2 expression and viability. Antioxid Redox Signal. 2006, 8, 885–892. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, I.L.; Novellino, F.; Caso, J.R.; García-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. CCL2 Inhibition of Pro-Resolving Mediators Potentiates Neuroinflammation in Astrocytes. Int. J. Mol. Sci. 2022, 23, 3307. https://doi.org/10.3390/ijms23063307
Gutiérrez IL, Novellino F, Caso JR, García-Bueno B, Leza JC, Madrigal JLM. CCL2 Inhibition of Pro-Resolving Mediators Potentiates Neuroinflammation in Astrocytes. International Journal of Molecular Sciences. 2022; 23(6):3307. https://doi.org/10.3390/ijms23063307
Chicago/Turabian StyleGutiérrez, Irene L., Fabiana Novellino, Javier R. Caso, Borja García-Bueno, Juan C. Leza, and José L. M. Madrigal. 2022. "CCL2 Inhibition of Pro-Resolving Mediators Potentiates Neuroinflammation in Astrocytes" International Journal of Molecular Sciences 23, no. 6: 3307. https://doi.org/10.3390/ijms23063307
APA StyleGutiérrez, I. L., Novellino, F., Caso, J. R., García-Bueno, B., Leza, J. C., & Madrigal, J. L. M. (2022). CCL2 Inhibition of Pro-Resolving Mediators Potentiates Neuroinflammation in Astrocytes. International Journal of Molecular Sciences, 23(6), 3307. https://doi.org/10.3390/ijms23063307






