Agmatine Mitigates Inflammation-Related Oxidative Stress in BV-2 Cells by Inducing a Pre-Adaptive Response
Abstract
:1. Introduction
2. Results
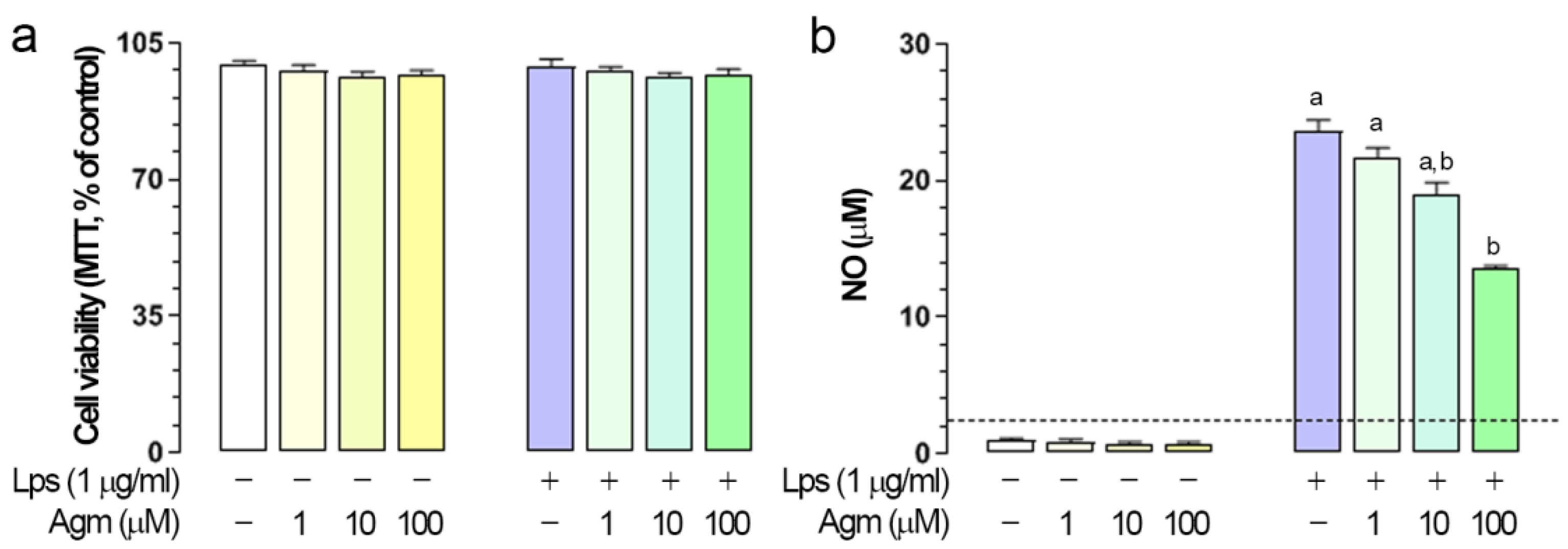
2.1. Effects of Agm on Viability and Lps-Induced NO Production in BV-2 Cells
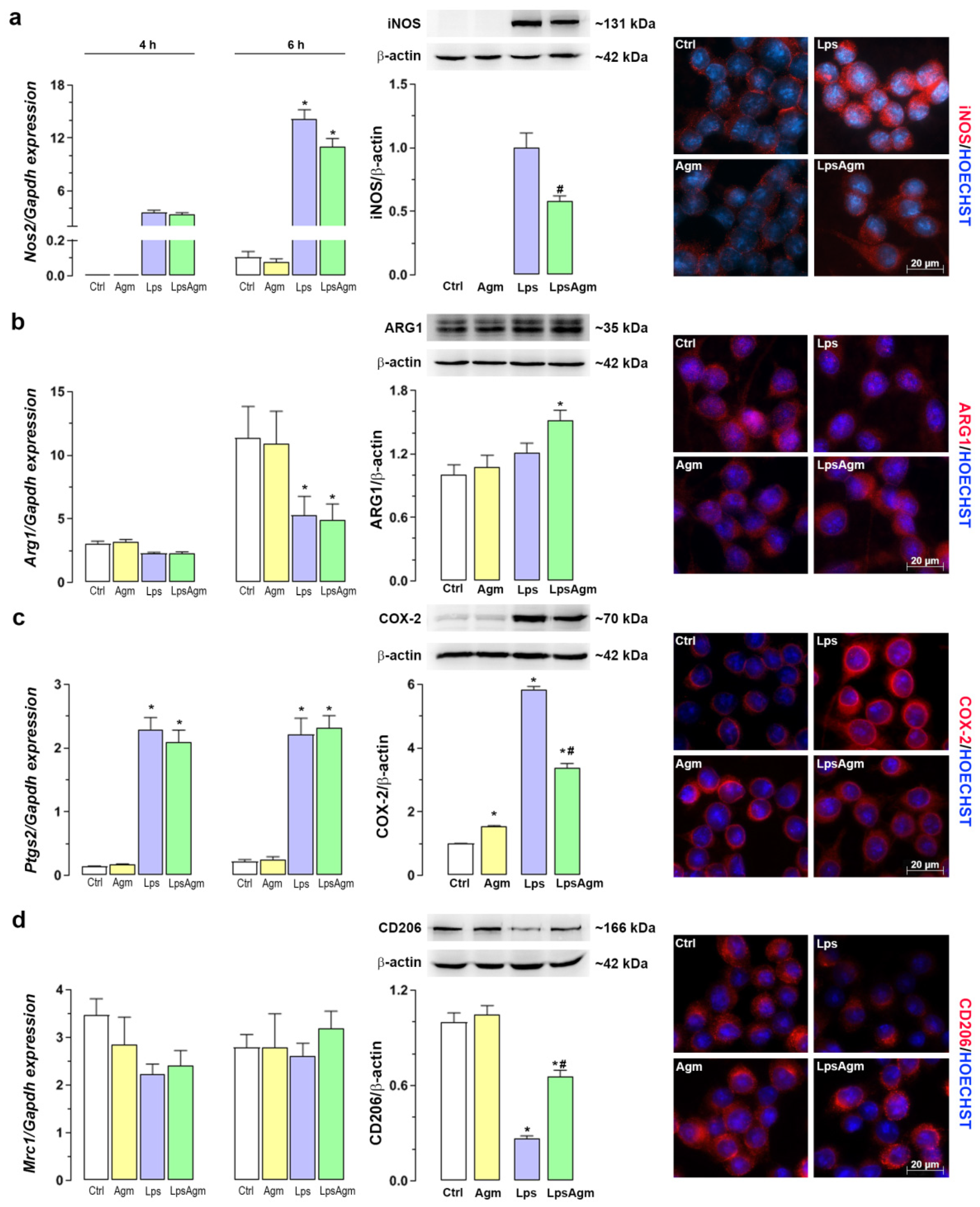
2.2. Effect of Agm on Arginine Catabolizing Enzymes: iNOS and ARG1 and Microglial Polarization
2.3. Agm Decreases Lps-Induced Production of O2− and Activity of XO
2.4. Agm Increases Biomarkers of Lipid Peroxidation
2.5. Agm Enhances the Antioxidant Capacity of Non-Stimulated and Lps-Stimulated BV-2 Cells
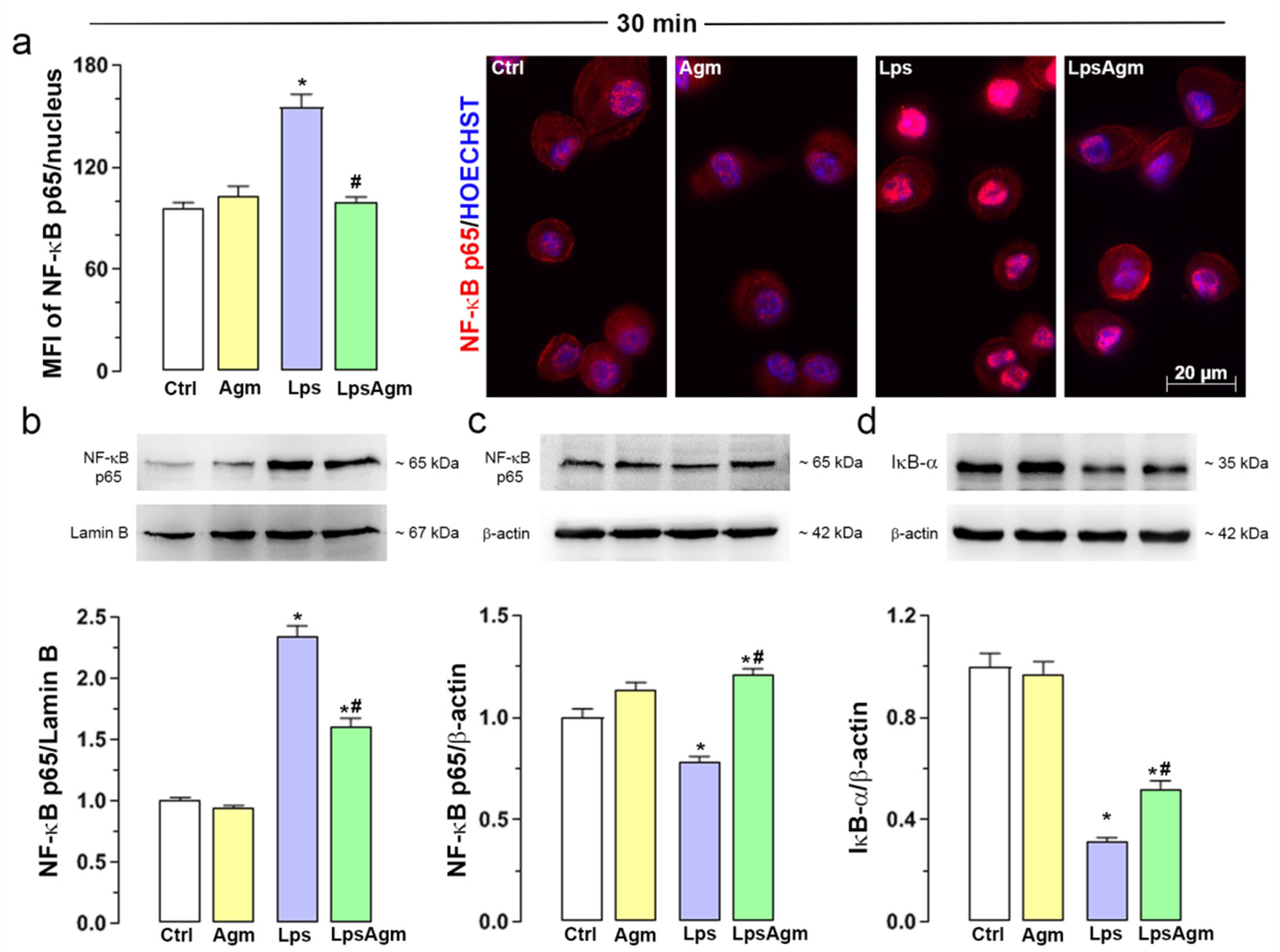
2.6. Agm Prevents Lps-Induced NF-κB Translocation to the Nucleus
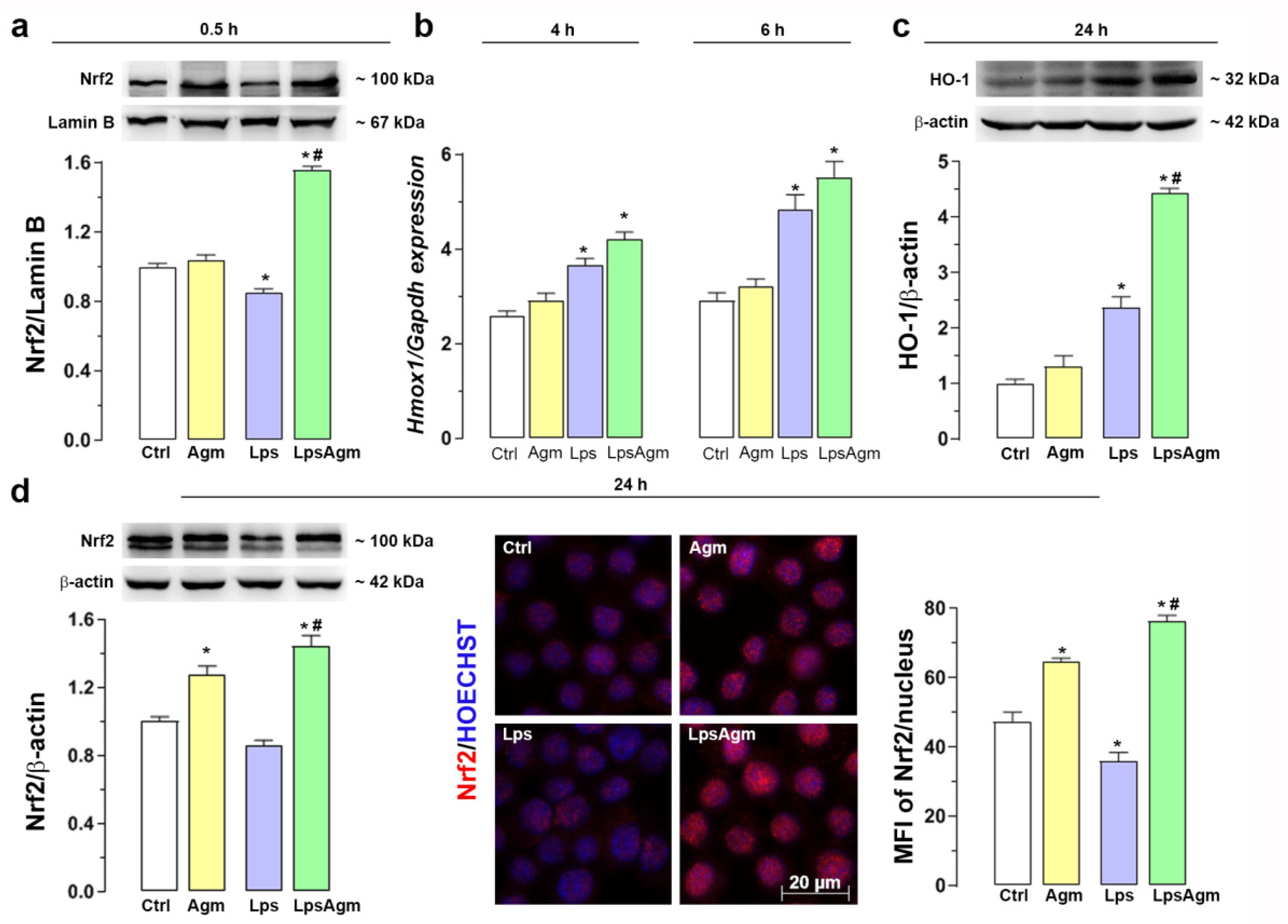
2.7. Agm Increases Nrf2 Nuclear Content and Enhances HO-1 Expression
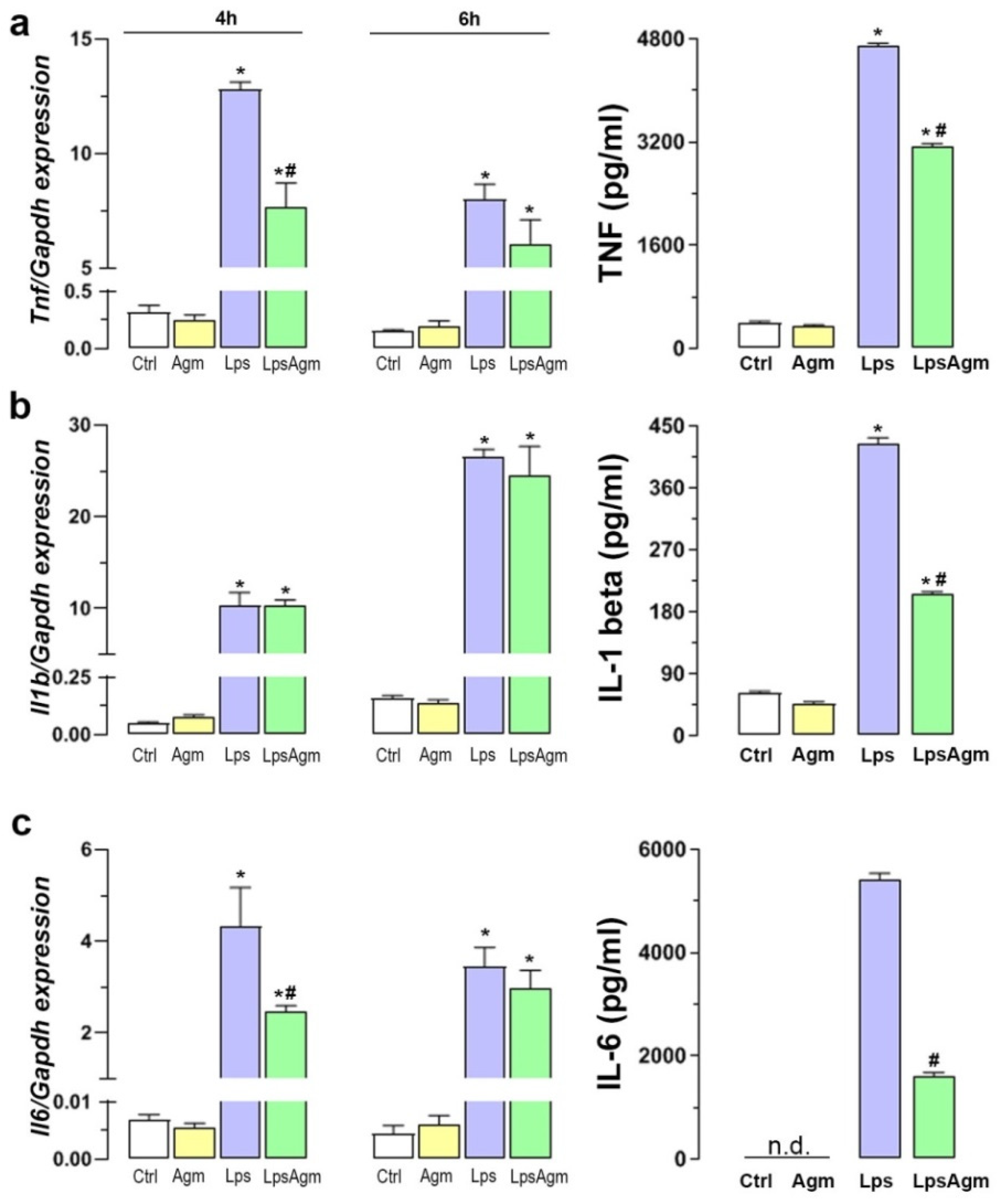
2.8. Agm Alleviates Proinflammatory Cytokines Release from Lps-Activated Microglia
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Viability Assay and Measurement of Nitric Oxide Release
4.3. RNA Extraction, Reverse Transcription, and Real-Time Polymerase Chain Reaction
4.4. Western Blot Analysis
4.5. Immunofluorescence Microscopy and Image Analysis
4.6. Spectrophotometric and Fluorometric Assays for Determination of Redox Status
4.7. Enzyme-Linked Immunosorbent Assay (ELISA) for Quantitative Detection of Proinflammatory Cytokines
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| Agm | Agmatine |
| ARG1 | Arginase 1 |
| CAT | Catalase |
| CD206 | Cluster of Differentiation 206 (mannose receptor) |
| COX-2 | Cyclooxygenase-2 |
| GCL | Glutamate-cysteine ligase |
| GPx | Gluthatione peroxidase |
| GR | Gluthatione reductase |
| GSH | Reduced glutathione |
| HO-1 | Heme oxygenase-1 |
| IκB | Nuclear factor kappa-B inhibitor |
| IL-1 beta | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| iNOS | Inducible nitric oxide synthase |
| Lps | Lipopolysaccharide |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NO | Nitric oxide |
| NOX | NADPH oxidase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| O2− | Superoxide radical anion |
| ONOO− | Peroxynitrite |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
| XO | Xanthine oxidase |
References
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [PubMed]
- Park, J.; Min, J.-S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH oxidases in oxidant production by microglia: Activating receptors, pharmacology and association with disease. Br. J. Pharmacol. 2017, 174, 1733–1749. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Tsunawaki, S.; Seguchi, H. Evaluation of the process for superoxide production by NADPH oxidase in human neutrophils: Evidence for cytoplasmic origin of superoxide. Redox Rep. Commun. Free Radic. Res. 2001, 6, 27–36. [Google Scholar] [CrossRef]
- Pei, Z.; Pang, H.; Qian, L.; Yang, S.; Wang, T.; Zhang, W.; Wu, X.; Dallas, S.; Wilson, B.; Reece, J.M.; et al. MAC1 mediates LPS-induced production of superoxide by microglia: The role of pattern recognition receptors in dopaminergic neurotoxicity. Glia 2007, 55, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.; Engels, M.; Voss, P.; Grune, T. Postanoxic damage of microglial cells is mediated by xanthine oxidase and cyclooxygenase. Free Radic. Res. 2007, 41, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N.; Simon, G.; Busso, N.; So, A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat. Commun. 2015, 6, 6555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Simonyi, A.; Kotzbauer, P.T.; Gu, Z.; Sun, G.Y. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J. Neuroinflamm. 2015, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Miljković, D.; Spasojević, I. Multiple sclerosis: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2013, 19, 2286–2334. [Google Scholar] [CrossRef] [PubMed]
- Reinert, A.; Morawski, M.; Seeger, J.; Arendt, T.; Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019, 20, 25. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [Green Version]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, Y.; Ren, X.; Liu, Q.; Wang, J.; Liu, X. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia. Mol. Cell. Biochem. 2014, 386, 153–165. [Google Scholar] [CrossRef]
- Salvemini, D.; Kim, S.F.; Mollace, V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: Relevance and clinical implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R473–R487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.J.; Yang, M.S.; Kim, S.U.; Jou, I.; Joe, E.H. Astrocytes induce hemeoxygenase-1 expression in microglia: A feasible mechanism for preventing excessive brain inflammation. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 1880–1887. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, Z.Y.; Wu, Y.; Schluesener, H.J. Lesional accumulation of CD163+ macrophages/microglia in rat traumatic brain injury. Brain Res. 2012, 1461, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [Green Version]
- Halaris, A.; Plietz, J. Agmatine: Metabolic pathway and spectrum of activity in brain. CNS Drugs 2007, 21, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Neis, V.B.; Rosa, P.B.; Olescowicz, G.; Rodrigues, A.L.S. Therapeutic potential of agmatine for CNS disorders. Neurochem. Int. 2017, 108, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Kotagale, N.R.; Taksande, B.G.; Inamdar, N.N. Neuroprotective offerings by agmatine. Neurotoxicology 2019, 73, 228–245. [Google Scholar] [CrossRef]
- Auguet, M.; Viossat, I.; Marin, J.G.; Chabrier, P.E. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn. J. Pharmacol. 1995, 69, 285–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galea, E.; Regunathan, S.; Eliopoulos, V.; Feinstein, D.L.; Reis, D.J. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem. J. 1996, 316 Pt 1, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satriano, J.; Schwartz, D.; Ishizuka, S.; Lortie, M.J.; Thomson, S.C.; Gabbai, F.; Kelly, C.J.; Blantz, R.C. Suppression of inducible nitric oxide generation by agmatine aldehyde: Beneficial effects in sepsis. J. Cell. Physiol. 2001, 188, 313–320. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, M.S.; Nader, M.A.; Sharawy, M.H. The inhibition of inducible nitric oxide synthase and oxidative stress by agmatine attenuates vascular dysfunction in rat acute endotoxemic model. Environ. Toxicol. Pharmacol. 2017, 55, 74–80. [Google Scholar] [CrossRef]
- Abe, K.; Abe, Y.; Saito, H. Agmatine suppresses nitric oxide production in microglia. Brain Res. 2000, 872, 141–148. [Google Scholar] [CrossRef]
- Ahn, S.K.; Hong, S.; Park, Y.M.; Choi, J.Y.; Lee, W.T.; Park, K.A.; Lee, J.E. Protective effects of agmatine on lipopolysaccharide-injured microglia and inducible nitric oxide synthase activity. Life Sci. 2012, 91, 1345–1350. [Google Scholar] [CrossRef]
- Ahn, S.K.; Hong, S.; Park, Y.M.; Lee, W.T.; Park, K.A.; Lee, J.E. Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res. 2011, 1373, 48–54. [Google Scholar] [CrossRef]
- Chai, J.; Luo, L.; Hou, F.; Fan, X.; Yu, J.; Ma, W.; Tang, W.; Yang, X.; Zhu, J.; Kang, W.; et al. Agmatine Reduces Lipopolysaccharide-Mediated Oxidant Response via Activating PI3K/Akt Pathway and Up-Regulating Nrf2 and HO-1 Expression in Macrophages. PLoS ONE 2016, 11, e0163634. [Google Scholar] [CrossRef]
- Sastre, M.; Galea, E.; Feinstein, D.; Reis, D.J.; Regunathan, S. Metabolism of agmatine in macrophages: Modulation by lipopolysaccharide and inhibitory cytokines. Biochem. J. 1998, 330 Pt 3, 1405–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, A.S.; Lee, J.K.; Nguyen, T.A.; Chang, J.; Ruhn, K.M.; Treviño, I.; Tansey, M.G. Regulation of microglia effector functions by tumor necrosis factor signaling. Glia 2012, 60, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, V.; Rossi, C.A.; Colombatto, S.; Grillo, M.A.; Toninello, A. Different behavior of agmatine in liver mitochondria: Inducer of oxidative stress or scavenger of reactive oxygen species? Biochim. Biophys. Acta 2007, 1768, 1147–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra de Souza, A.C.; Gonçalves, C.L.; de Souza, V.; Hartwig, J.M.; Farina, M.; Prediger, R.D. Agmatine attenuates depressive-like behavior and hippocampal oxidative stress following amyloid β (Aβ1-40) administration in mice. Behav. Brain Res. 2018, 353, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.E.; Bettio, L.E.B.; Neis, V.B.; Santos, D.B.; Ribeiro, C.M.; Rosa, P.B.; Farina, M.; Rodrigues, A.L.S. Agmatine abolishes restraint stress-induced depressive-like behavior and hippocampal antioxidant imbalance in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Bila, I.; Dzydzan, O.; Brodyak, I.; Sybirna, N. Agmatine Prevents Oxidative-nitrative Stress in Blood Leukocytes Under Streptozotocin-induced Diabetes Mellitus. Open Life Sci. 2019, 14, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: A toxic combination illuminated by redox proteomics studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H.; Niki, E. 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life 2006, 58, 372–373. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Ruiz, E.; Leake, D.S.; Unoki, H.; Yamamoto, M.; Mann, G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004, 94, 609–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikado, A.; Morino, K.; Nishio, Y.; Nakagawa, F.; Mukose, A.; Sono, Y.; Yoshioka, N.; Kondo, K.; Sekine, O.; Yoshizaki, T.; et al. 4-Hydroxy Hexenal Derived from Docosahexaenoic Acid Protects Endothelial Cells via Nrf2 Activation. PLoS ONE 2013, 8, e69415. [Google Scholar] [CrossRef]
- Lin, M.H.; Yen, J.H.; Weng, C.Y.; Wang, L.; Ha, C.L.; Wu, M.J. Lipid peroxidation end product 4-hydroxy-trans-2-nonenal triggers unfolded protein response and heme oxygenase-1 expression in PC12 cells: Roles of ROS and MAPK pathways. Toxicology 2014, 315, 24–37. [Google Scholar] [CrossRef] [PubMed]
- López-Bernardo, E.; Anedda, A.; Sánchez-Pérez, P.; Acosta-Iborra, B.; Cadenas, S. 4-Hydroxynonenal induces Nrf2-mediated UCP3 upregulation in mouse cardiomyocytes. Free Radic. Biol. Med. 2015, 88 Pt B, 427–438. [Google Scholar] [CrossRef]
- Yang, B.; Li, R.; Michael Greenlief, C.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J. Neuroinflamm. 2018, 15, 202. [Google Scholar] [CrossRef] [Green Version]
- Darley-Usmar, V.M.; Severn, A.; O’Leary, V.J.; Rogers, M. Treatment of macrophages with oxidized low-density lipoprotein increases their intracellular glutathione content. Biochem. J. 1991, 278 Pt 2, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Backos, D.S.; Fritz, K.S.; Roede, J.R.; Petersen, D.R.; Franklin, C.C. Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2011, 50, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, D.A.; Levonen, A.L.; Moellering, D.R.; Arnold, E.K.; Zhang, H.; Darley-Usmar, V.M.; Forman, H.J. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004, 37, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.E.; Egea, J.; Buendia, I.; Gómez-Rangel, V.; Parada, E.; Navarro, E.; Casas, A.I.; Wojnicz, A.; Ortiz, J.A.; Cuadrado, A.; et al. Agmatine, by Improving Neuroplasticity Markers and Inducing Nrf2, Prevents Corticosterone-Induced Depressive-Like Behavior in Mice. Mol. Neurobiol. 2016, 53, 3030–3045. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.E.; Egea, J.; Buendía, I.; Navarro, E.; Rada, P.; Cuadrado, A.; Rodrigues, A.L.; López, M.G. Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol. Neurobiol. 2015, 51, 1504–1519. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jobbagy, S.; Vitturi, D.A.; Salvatore, S.R.; Turell, L.; Pires, M.F.; Kansanen, E.; Batthyany, C.; Lancaster, J.R.; Freeman, B.A.; Schopfer, F.J. Electrophiles modulate glutathione reductase activity via alkylation and upregulation of glutathione biosynthesis. Redox Biol. 2019, 21, 101050. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. HNE as an inducer of COX-2. Free Radic. Biol. Med. 2017, 111, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Matsukawa, N.; Kaneko, Y.; Kusumi, Y.; Mitsumata, M.; Uchida, K. A lipid peroxidation-derived inflammatory mediator: Identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J. Biol. Chem. 2004, 279, 48389–48396. [Google Scholar] [CrossRef] [Green Version]
- Nomura, J.; Busso, N.; Ives, A.; Matsui, C.; Tsujimoto, S.; Shirakura, T.; Tamura, M.; Kobayashi, T.; So, A.; Yamanaka, Y. Xanthine Oxidase Inhibition by Febuxostat Attenuates Experimental Atherosclerosis in Mice. Sci. Rep. 2014, 4, 4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honorat, J.A.; Kinoshita, M.; Okuno, T.; Takata, K.; Koda, T.; Tada, S.; Shirakura, T.; Fujimura, H.; Mochizuki, H.; Sakoda, S.; et al. Xanthine oxidase mediates axonal and myelin loss in a murine model of multiple sclerosis. PLoS ONE 2013, 8, e71329. [Google Scholar] [CrossRef] [Green Version]
- Salvemini, D.; Misko, T.P.; Masferrer, J.L.; Seibert, K.; Currie, M.G.; Needleman, P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. USA 1993, 90, 7240–7244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minghetti, L.; Polazzi, E.; Nicolini, A.; Créminon, C.; Levi, G. Interferon-gamma and nitric oxide down-regulate lipopolysaccharide-induced prostanoid production in cultured rat microglial cells by inhibiting cyclooxygenase-2 expression. J. Neurochem. 1996, 66, 1963–1970. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Jin, H.; Fan, X.; Yang, X.; Tang, W.; Yan, J.; Liang, H. Agmatine protects against zymosan-induced acute lung injury in mice by inhibiting NF-κB-mediated inflammatory response. BioMed Res. Int. 2014, 2014, 583736. [Google Scholar] [CrossRef] [Green Version]
- Regunathan, S.; Piletz, J.E. Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann. N. Y. Acad. Sci. 2003, 1009, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Kim, D.; Paik, S.G.; Han, P.L. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic. Biol. Med. 2006, 41, 960–972. [Google Scholar] [CrossRef]
- Chan, P.H. Reactive Oxygen Radicals in Signaling and Damage in the Ischemic Brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.W.; Bates, T.E. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur. J. Neurosci. 2001, 13, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Noack, H.; Possel, H.; Wolf, G. Induction of nitric oxide synthesis lowers intracellular glutathione in microglia of primary glial cultures. Glia 2000, 29, 98–101. [Google Scholar] [PubMed]
- Bozic, I.; Savic, D.; Stevanovic, I.; Pekovic, S.; Nedeljkovic, N.; Lavrnja, I. Benfotiamine upregulates antioxidative system in activated BV-2 microglia cells. Front. Cell. Neurosci. 2015, 9, 351. [Google Scholar] [CrossRef] [Green Version]
- Lindenau, J.; Noack, H.; Asayama, K.; Wolf, G. Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia 1998, 24, 252–256. [Google Scholar] [PubMed]
- Brown, G.C. Reversible binding and inhibition of catalase by nitric oxide. Eur. J. Biochem. 1995, 232, 188–191. [Google Scholar] [CrossRef]
- Condello, S.; Currò, M.; Ferlazzo, N.; Caccamo, D.; Satriano, J.; Ientile, R. Agmatine effects on mitochondrial membrane potential andNF-κB activation protect against rotenone-induced cell damage in human neuronal-like SH-SY5Y cells. J. Neurochem. 2011, 116, 67–75. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Hua, Y.; Gong, J.; Ding, S.; Du, Y.; Wang, X.; Zheng, R.; Xu, H. Agmatine Alleviates Epileptic Seizures and Hippocampal Neuronal Damage by Inhibiting Gasdermin D-Mediated Pyroptosis. Front. Pharmacol. 2021, 12, 2034. [Google Scholar] [CrossRef]
- Ishihara, Y.; Takemoto, T.; Itoh, K.; Ishida, A.; Yamazaki, T. Dual role of superoxide dismutase 2 induced in activated microglia: Oxidative stress tolerance and convergence of inflammatory responses. J. Biol. Chem. 2015, 290, 22805–22817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.S.; Ota, A.; Nakashima, A.; Mori, K.; Nagatsu, I.; Nagatsu, T. Regulation of oxidative stress in long-lived lipopolysaccharide-activated microglia. Clin. Exp. Pharmacol. Physiol. 2012, 39, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, M.H.; Abdelrahman, R.S.; El-Kashef, D.H. Agmatine attenuates rhabdomyolysis-induced acute kidney injury in rats in a dose dependent manner. Life Sci. 2018, 208, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, I.; Ninkovic, M.; Stojanovic, I.; Ljubisavljevic, S.; Stojnev, S.; Bokonjic, D. Beneficial effect of agmatine in the acute phase of experimental autoimmune encephalomyelitis in iNOS-/- knockout mice. Chem. Biol. Interact. 2013, 206, 309–318. [Google Scholar] [CrossRef] [PubMed]
- McElroy, P.B.; Sri Hari, A.; Day, B.J.; Patel, M. Post-translational Activation of Glutamate Cysteine Ligase with Dimercaprol: A novel mechanism of inhibiting neuroinflammation in vitro. J. Biol. Chem. 2017, 292, 5532–5545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and Spiny Burs of Castanea Sativa from an Experimental Chestnut Grove: Metabolomic Analysis and Anti-Neuroinflammatory Activity. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]


| Primer | Sequence | Accession No. |
|---|---|---|
| Gapdh | f: GTTGTCTCCTGCGACTTCA r: TGGTCCAGGGTTTCTTACTC | NM_008084 |
| Nos2 | f: TTCACTCCACGGAGTAGCCT r: TGAGAACAGCACAAGGGGTT | NM_010927.4 |
| Arg1 | f: TAACCTTGGCTTGCTTCGG r: GTGGCGCATTCACAGTCAC | NM_007482.3 |
| Ptgs2 | f: TTCAACACACTCTATCACTGGC r: AGAAGCGTTTGCGGTACTCAT | NM_011198.4 |
| Mrc1 | f: GTTGTATTCTTTGCCTTTCCCAG r: CGTCTGAACTGAGATGGCACT | NM_008625.2 |
| Cybb | f: GGGAACTGGGCTGTGAATGA r:CAGTGCTGACCCAAGGAGTT | NM_000397.4 |
| Gsr | f: ACCGAGGAACTGGAGAATGC r: CACGGAAGTCACCACTTGGA | NM_010344.4 |
| Gpx1 | f: AGTCCACCGTGTATGCCTTCT r: GAGACGCGACATTCTCAATGA | NM_008160.6 |
| Cat | f: AGCGACCAGATGAAGCAGTG r: TCCGCTCTCTGTCAAAGTGTG | NM_012520.2 |
| Sod2 | f: CAGACCTGCCTTACGACTATGG r: CTCGGTGGCGTTGAGATTGTT | NM_013671.3 |
| Hmox1 | f: CCTCACTGGCAGGAAATCATC r: CTCGTGGAGACGCTTTACATA | NM_010442.2 |
| Tnf | f: GCCCACGTCGTAGCAAACCAC r: GGCTGGCACCACTAGTTGGTTGT | NM_013693.3 |
| Il1b | f: AAAAGCCTCGTGCTGTCGGACC r: TTGAGGCCCAAGGCCACAGGT | NM_008361.4 |
| Il6 | f: TAGTCCTTCCTACCCCAATTTCC r: TTGGTCCTTAGCCACTCCTTC | NM_012589.2 |
| Antibody | Source and Type | Dilution | Manufacturer |
|---|---|---|---|
| iNOS | Rabbit, polyclonal | 1:450 (IF) 1:500 (WB) | Abcam, ab15323 |
| ARG1 | Rabbit, polyclonal | 1:100 (IF) 1:1000 (WB) | Sigma Aldrich, AV45673 |
| COX-2 | Goat, polyclonal | 1:200 (IF) 1:1000 (WB) | Santa Cruz, sc-1745 |
| CD206 | Rabbit, polyclonal | 1:500 (IF) 1:1000 (WB) | Abcam, ab64693 |
| 4-HNE | Rabbit, polyclonal | 1:100 (IF) | Abcam, ab46545 |
| GR | Rabbit, polyclonal | 1:2500 (WB) | Abcam, ab16801 |
| GPx | Rabbit, polyclonal | 1:5000 (WB) | Abcam, ab22604 |
| CAT | Rabbit, polyclonal | 1:8000 (WB) | Sigma Aldrich, 219010 |
| SOD2 | Rabbit, polyclonal | 1:5000 (WB) | Abcam, ab13533 |
| NF-kB p65 | Rabbit, polyclonal | 1:200 (IF) 1:2000 (WB) | Santa Cruz, sc-372 |
| IkB-α | Rabbit, polyclonal | 1:1000 (WB) | Santa Cruz, sc-371 |
| Nrf2 | Rabbit, polyclonal | 1:200 (IF) 1:1000 (WB) | Santa Cruz, sc-722 |
| HO-1 | Goat, polyclonal | 1:1000 (WB) | Santa Cruz, sc-1796 |
| β-actin | Mouse, monoclonal | 1:5000 (WB) | Sigma Aldrich, A5316 |
| Lamin B | Goat, polyclonal | 1:1000 (WB) | Santa Cruz, sc-6217 |
| Anti-rabbit IgG AlexaFluor 568 | Donkey | 1:250 (IF) | Invitrogen, A10042 |
| Anti-rabbit IgG AlexaFluor 555 | Donkey | 1:250 (IF) | Invitrogen, A31572 |
| Anti-rabbit IgG-HRP | Goat | 1:5000 (WB) | Santa Cruz, sc-2004 |
| Anti-rabbit IgG-HRP | Donkey | 1:5000 (WB) | Santa Cruz, sc-2313 |
| Anti-mouse IgG-HRP | Donkey | 1:5000 (WB) | Santa Cruz, sc-2314 |
| Anti-goat IgG-HRP | Donkey | 1:5000 (WB) | Santa Cruz, sc-2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosevic, K.; Stevanovic, I.; Bozic, I.D.; Milosevic, A.; Janjic, M.M.; Laketa, D.; Bjelobaba, I.; Lavrnja, I.; Savic, D. Agmatine Mitigates Inflammation-Related Oxidative Stress in BV-2 Cells by Inducing a Pre-Adaptive Response. Int. J. Mol. Sci. 2022, 23, 3561. https://doi.org/10.3390/ijms23073561
Milosevic K, Stevanovic I, Bozic ID, Milosevic A, Janjic MM, Laketa D, Bjelobaba I, Lavrnja I, Savic D. Agmatine Mitigates Inflammation-Related Oxidative Stress in BV-2 Cells by Inducing a Pre-Adaptive Response. International Journal of Molecular Sciences. 2022; 23(7):3561. https://doi.org/10.3390/ijms23073561
Chicago/Turabian StyleMilosevic, Katarina, Ivana Stevanovic, Iva D. Bozic, Ana Milosevic, Marija M. Janjic, Danijela Laketa, Ivana Bjelobaba, Irena Lavrnja, and Danijela Savic. 2022. "Agmatine Mitigates Inflammation-Related Oxidative Stress in BV-2 Cells by Inducing a Pre-Adaptive Response" International Journal of Molecular Sciences 23, no. 7: 3561. https://doi.org/10.3390/ijms23073561
APA StyleMilosevic, K., Stevanovic, I., Bozic, I. D., Milosevic, A., Janjic, M. M., Laketa, D., Bjelobaba, I., Lavrnja, I., & Savic, D. (2022). Agmatine Mitigates Inflammation-Related Oxidative Stress in BV-2 Cells by Inducing a Pre-Adaptive Response. International Journal of Molecular Sciences, 23(7), 3561. https://doi.org/10.3390/ijms23073561






